The surveillance hypothesis of the immunologic control of malignancy has far-reaching clinical implications, of which two major and essentially opposite ones will be considered in this communication. The first concerns the growth of tumors in patients with surveillance failure. From the premise of Burnet (1957, 1963, 1967, 1970) and Thomas (1959), it could have been predicted and was (Thomas 1959, Starzl 1964b, Schwartz et al. 1966) that an increased incidence of de novo tumors would develop in people with naturally occurring immunologic deficiency diseases or in patients whose immune reactivity was deliberately depressed in order to permit their acceptance of organ homografts. The hazard of malignancy consequent to spontaneous deficiency is so well known (Page et al. 1963, Green et al. 1966, Dent et al. 1968, Huber 1968) that it will not be reviewed here. However, the analogous data in iatrogenically immunosuppressed transplant recipients that have accumulated since 1968 will be brought up to date. In addition, some comments will be made about the accidental transplantation of tumors to immunologically depressed humans and about the growth of metastases or residual tumor under these same conditions.
The second general topic of this report, and a far more interesting one from a therapeutic point of view, is the expectation that the artificial endowment of immunologic surveillance in a patient whose natural defense system had failed to prevent neoplasia might then have an inhibitory or even destructive effect upon the established growth. Of course, a necessary condition to evaluate this latter possibility is the successful transplantation of immunologically competent tissue such as spleen or bone marrow while at the same time avoiding the kind of fatal graft-versus-host reaction that has defeated essentially all efforts at bone marrow grafting for whatever purpose except between a few HL-A identical siblings. As a first step toward this previously unattainable goal, suggestions will be made for the application to the bone marrow problem of immunosuppressive regimens that have been evolved in the successful transplantation of whole organs such as the kidney, liver, and heart.
IMMUNOSUPPRESSION AND DE NOVO MALIGNANCIES
Although it was hardly surprising when it occurred, a convincing association between immunosuppression and new malignant neoplasms was not claimed until European and American meetings held respectively in March and April 1968 (Starzl et al. 1969, Starzl 1968). Prior to the first of these conferences, two reticulum cell sarcomas had been observed in our own patients and an anaplastic neoplasm (later judged to be a carcinoma) had been contributed to us by Dr. Claude Hitchcock of Minneapolis; in discussion of the paper from the floor, Woodruff added another reticulum cell sarcoma from his Edinburgh experience. By the time of the second meeting in Boston less than a month later, a third lymphoma had been encountered in our series. The five cases were discussed again at a Dundee, Scotland, cancer symposium on 31 May, 1968, and as a consequence were reported in an Annotation in The Lancet (1968).
When the subject was covered again the following September at the International Transplantation (Penn et al. 1969) and Sixth National Cancer (Starzl et al. 1970c) meetings, additional examples of both mesenchymal and epithelial malignancy were added from our own institution or were mentioned by interested audience members making the case total up to 13 (McKhann 1969). Since then, an unofficial registry of de novo malignancies has been kept in Denver to which observations have been fed in from all over the world and summarized on several occasions (Penn et al. 1969, 1971, Penn 1970, Penn & Starzl 1970, Starzl et al. 1970b). The following remarks will be based partly upon this donated material, partly upon the published reports of some of these cases from other institutions (Doak et al. 1968, Zukoski et al. 1968, Deodhar et al. 1969, Siegel et al. 1969, Kay et al. 1970, Pritzker et al. 1970, Simmons et al. 1970, Tallent et al. 1971, Walker et al. 1971) but principally upon the study of patients in our own transplantation program.
University of Colorado Series
It will be useful to record our own experience with post-transplantation neoplasia separately from that collected on a cooperative basis from elsewhere. For one thing, an accurate idea of the true incidence and severity of the problem cannot be obtained from the world experience, since neither the exact number of malignancies nor the numbers of patients at risk at various postoperative times are known. Moreover, there has been an obvious great variability in therapy from center to center, and it has not been easy to assess accurately or even to know about all the details of treatment. These limitations do not apply to our own series of patients (Table I).
TABLE I.
De novo malignancies in kidney transplant recipients at the University of Colorado
| No. | Age at time of transplantation | Immunosuppression |
Type of tumor | Time after transplantation (months) | Organs involved | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Donor | Splenectomy | Thymectomy | Azathioprine | Prednisone | ALG | ||||||
| 1 | 37 | F | Unrelated living donor | Yes | Yes | Yes | Yes | No | Squamous cell carcinoma in situ | 50 | Cervix of uterus | Alive, no recurrence after hysterectomy |
| 2 | 40 | M | Unrelated living donor | Yes | No | Yes | Yes | No | Superficial squamous cell carcinoma | 66 | Lower lip | Alive, no recurrence following excision |
| 3 | 39 | M | Brother | Yes | Yes | Yes | Yes | No | Superficial squamous cell carcinoma | 36 | Lower lip | Alive, no recurrence following excision |
| 4 | 40 | M | Unrelated living donor | Yes | Yes | Yes | Yes | No | Squamous cell carcinoma | 32 | Skin of ear | No recurrence after excision, died of other causes |
| 5 | 43 | M | Uncle | Yes | No | Yes | Yes | Yes | Basal cell carcinoma | 33 | Nasolabial fold | Alive, no recurrence following excision |
| 6 | 30 | M | Brother | Yes | Yes | Yes | Yes | No | Basal cell carcinoma | 75 | Nasolabial fold | Alive, no recurrence following excision |
| 7 | 34 | M | Sister | Yes | No | Yes | Yes | No | Squamous cell carcinoma | 74 | Both forearms, right arm, scalp | No recurrence after excision, died of other causes |
| 8 | 44 | F | Sister | Yes | No | Yes | Yes | Yes | Moderately differentiated adenocarcinoma | 31 | Lung | Cancer was incidental finding at autopsy |
| 9 | 22 | M | Cousin | Yes | No | Yes | Yes | No | Squamous cell carcinoma | 78 | Left hand | Alive, no recurrence following excision |
| 10 | 26 | F | Father | Yes | No | Yes | Yes | No | Squamous cell carcinoma in situ | 65 | Cervix of uterus | Alive, no recurrence following excision |
| 11 | 17 | F | Father | Yes | No | Yes | Yes | Yes | Squamous cell carcinoma in situ | 51 | Cervix of uterus | Alive, no recurrence following excision |
| 12 | 30 | M | Brother | Yes | No | Yes | Yes | Yes | Superficial squamous cell carcinoma | 36 | Face (3 areas) | Alive, no recurrence following excision |
| 13 | 14 | M | Mother | Yes | No | Yes | Yes | Yes | Reticulum cell sarcoma | 5½ | Brain | Died of tumor |
| 14 | 23 | M | Father | Yes | Yes | Yes | Yes | No | Reticulum cell sarcoma | 30 | Thyroid, liver, lung, stomach, prostate, pituitary, skin, psoas muscle | Died of tumor |
| 15 | 20 | F | Father | Yes | No | Yes | Yes | Yes | Unclassified lymphoma | 7 | Brain | Alive, no recurrence following radiotherapy |
Case Material and Immunosuppression
Our experience has been subdivided into two fractions according to whether the potential follow-up was greater or less than 3 years. Group I consisted of 195 consecutive recipients who were given renal homografts between November 1962 and 1 July 1968 (3 to 8 ⅔ years ago). Group II comprised 111 patients treated between 1 July 1968 and 1 January 1971 (6 months to 3 years ago).
The patients in Group I treated 5 years or longer ago had immunosuppression with a double drug program of azathioprine and prednisone (Starzl 1964b). All patients treated since June 1966, including the last members of Group I as well as all the recipients in Group II had horse ALG added as a third agent in what has been termed the triple drug regimen (Starzl 1969). Splenectomy was carried out in more than 90 per cent of cases of both groups, being omitted only in some of the cadaveric transplantations. Thymectomy was carried out in 36 patients of Group I prior to transplantation and at some time during the first year after transplantation in an additional 9 (Starzl et al. 1970b).
Incidence of Malignancy
Fifteen of the 195 consecutive recipients in Group I developed malignant tumors for an uncorrected incidence of 7.7 per cent (Table II). The incidence of the complication was approximately the same after unrelated (3/54) as opposed to related transplantation (12/141).
TABLE II.
University of Colorado, de novo malignancies
| Group |
||
|---|---|---|
| I | II | |
| Total number of patients | 195 | 111 |
| Related donor | 141 | 90 |
| Non-related donor, living | 35 | 0 |
| Non-related donor, cadaver | 19 | 21 |
| Patients with malignancies | 15 | 0 |
| Related donor | 12 | 0 |
| Non-related donor | 3 | 0 |
| Number of patients surviving more than 4 months | 154 | 92 |
| Incidence of malignancy | ||
| In all patients | 7.7 % | 0% |
| In patients surviving more than 4 months | 9.7 % | 0% |
Since 41 of the 195 patients of Group I died in less than 4 months, before they were exposed to a significant hazard of neoplasia, the actual number at risk was only 154. Within this culled series, the corrected incidence (15/154) was an extraordinary 9.7 per cent (Table II). Taken uncritically, this figure could be interpreted as being so high as to virtually cancel the usefulness of organ transplantation. However, as will be mentioned later, the majority of the tumors were easily treated. Only 2 of the patients died from the malignancy, both with reticulum cell sarcoma (Table I, Cases 13 and 14).
Moreover, not a single additional example of de novo malignancy has been encountered in the more recently treated 111 patients of Group II (Table II). If all the cases of Groups I and II are combined, the uncorrected incidence of malignant neoplasia is 4.9 per cent and excluding patients who died in the first 4 months, it corrects to 6.1 per cent.
The rate of tumor development in our combined series of generally young transplantation patients (3½ to 50 years, average approximately 28 years) compares with a yearly incidence of 58 per 100,000 (0.058 per cent) in a general East Coast population* of a comparable age range (Doll et al. 1966). In the foregoing state-wide statistics, carcinomas in situ were excluded. In our transplant series 3 of the 15 malignancies were of this kind. Omitting these, the incidence of malignant disease in the 306 patients of the combined Groups I and II is 3.9 per cent.
The Timing of the Malignancies
As already mentioned, all 15 cases of de novo malignancy occurred in the patients of Group I. The diagnoses were made (Tables I and III) from 5½ to 78 months after transplantation (average 44.6 months) when the patients were 15 to 47 years old (average 34.4). Only 2 malignancies were identified within the first year (Cases 13 and 15), 6 more occurred in the second and third years (Cases 3–5, 8, 12 and 14), and the other 7 developed after from 50 to 78 months. The 3 most serious neoplasms, 2 reticulum cell sarcomas (Cases 13 and 14) and one lymphoma of unclassified type (Case 15), were the earliest to appear, after 5½, 30 and 7 months, respectively.
TABLE III.
De novo malignancies at University of Colorado and in collected series
| University of Colorado | All other | Total | |
|---|---|---|---|
| Average age of patient (years) | 30.6 | 33.0 | 32.3 |
| Average time of appearance of tumor (months after transplantation) | 44.6 | 25.0 | 30.1 |
| Splenectomy | 15/15 | 8/42 | 23/57 |
| Thymectomy | 5/15 | 0/42 | 5/51 |
| ALG treatment | 6/15 | 10/42 | 16/57 |
There has been no obvious explanation for the curious absence of additional de novo tumors in the Group II patients treated by transplantation under triple drug immunosuppression since July 1968.
Tumor Types and Organs Affected
In our original reports, the main attention was directed to the lymphorelicular neoplasms of which there were three. Three years later, there have been no new examples of such mesenchymal tumors which now constitute only 20 per cent of the case total. The other 12 patients (80 per cent) have had carcinomas (Table I) affecting in 11 instances the squamous epithelium of the lip, uterine cervix, and skin (especially that of the face and hand). Multiple primary skin cancers were found in two of these cases. The twelfth patient with an epithelial malignancy (Case 8) died of other causes and was found at autopsy to have a small adenocarcinoma of the lung.
Disseminated tumor (Figure 1) was found only in an organ recipient who had reticulum cell sarcoma in the brain, liver, lung, and five other locations (Table I, Case 14). The other reticulum cell sarcoma (Case 13) and an unclassified lymphoma (Case 15) also involved the brain (Figure 2) but were confined to this organ.
Figure 1.
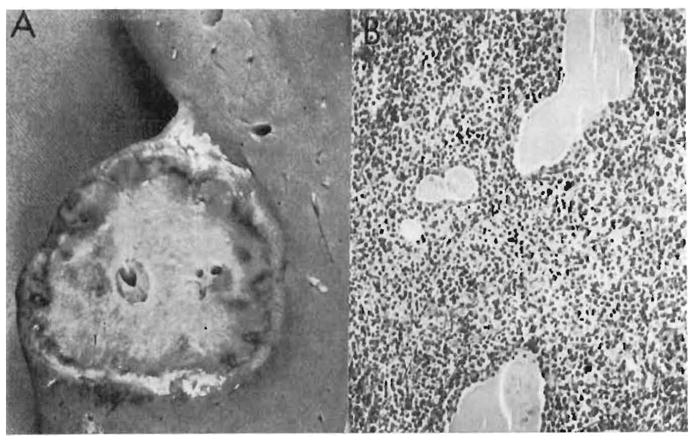
Disseminated reticulum cell sarcoma in a renal homograft recipient in our series (Table I, Case 14). A: This 5 cm nodule was one of several found in the liver. B: Malignant reticulum cells have massively infiltrated the thyroid, separating the follicles widely (× 80). (By permission of Transplant. Proc. 1, 106, 1969.)
Figure 2.
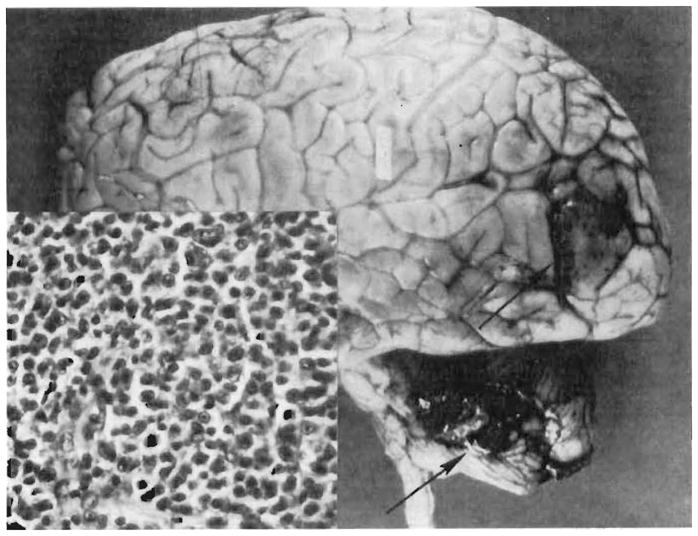
Case 15 in Colorado series (see Table I). Tumor nodules (arrows) in the left occipital lobe and cerebellum. The flattened gyri reflect increased intracranial pressure caused by the tumor. Insert – the large uniform cells with indistinct cytoplasm and round to oval nuclei are characteristic of reticulum cell sarcoma (× 350). (By permission of Transplant. Proc. 1, 106, 1969.)
Malignancies versus Immunosuppression
In experimental animals each of the main immunosuppressive agents, azathioprine (Casey 1968), prednisone (Foley & Silverstein 1951, Agosin et al. 1952, Toolan 1953, Baserga & Shubik 1954, Albert & Ziedman 1962), and ALG (Anigsten et al. 1966, Allison et al. 1967, Bremberg et al. 1967, Davis & Lewis 1967, Deodhar et al. 1968, Hellman et al. 1968, Hirsch & Murphy 1968, Fisher et al. 1969, Mandel & DeCosse 1969, Vredevoe & Hays 1969, Rabbat & Jeejeebhoy 1970) has been shown in animals either to (1) increase the incidence of spontaneous, virus-induced, or chemically initiated tumors; (2) facilitate the ease with which malignant cells can be transplanted; or (3) accelerate metastatic growth. All of our patients received two of the aforementioned three agents, namely azathioprine and prednisone. On the other hand, the first 110 recipients were not given horse ALG, whereas the subsequent 196 were also administered the equine globulin. Although almost two-thirds of the patients thus were treated with the triple drug schedule, only 6 of the 15 de novo malignancies developed under these conditions, including one fatal reticulum cell sarcoma. The other 9 tumors, including the other lethal reticulum cell sarcoma were distributed throughout the minority of recipients who were given only azathioprine and prednisone.
On the basis of these data, it is clear that ALG has not made a unique contribution to the complication of post-transplantation neoplasia. If anything, the problem has actually seemed less since the introduction of ALG, something which might have been expected if, as has been described (Starzl et al. 1967, Starzl 1969), globulin therapy permitted better control of rejection along with reduction in the quantities of the other two agents required for chronic maintenance. In view of these findings, it is difficult to understand the origin of the widespread misconception that the incidence of tumors in the posttransplantation period has increased since the use of ALG.
Thymectomy (Martinez et al. 1962, Vandeputte et al. 1963, Miller et al. 1964, Grant & Miller 1965, Allison et al. 1967, Davis & Lewis 1967, Law 1969) has also been shown in animals to promote tumor growth. Amongst the first 110 consecutive transplant patients treated at the University of Colorado, there were 45 whose thymus gland was excised. Five cases of de novo malignancies came from this special subpopulation, including a fatal reticulum cell sarcoma. However, there were four malignancies including the other lethal reticulum cell sarcoma in the 65 non-thymectomized recipients who were treated with transplantation during the same era of our experience. An authoritative opinion about the role of thymectomy in causing this complication in humans cannot be given, since the differences in incidence are not statistically significant.
Splenectomy as a factor in oncogenesis could not be studied from our experience since there were too small a number of non-splenectomy controls.
Treatment and Prognosis
Both of the patients with reticulum cell sarcoma died. In Case 14, the diagnosis was not made until autopsy. The other patient (Case 13) died a few days after craniotomy and biopsy.
The diagnosis in the recipient with an unclassified lymphoma was made by biopsy of the diencephalic lesion (Figure 3) with a stereotaxic instrument. The tumor was then irradiated with 5,650 rads. In addition, immunosuppressive therapy was lightened, despite which renal graft rejection did not follow (Figure 4). Except for some residual neurological impairment, she is well three years later, still with function of her transplanted kidney.
Figure 3.

Pneumoencephalogram in a 20-year-old woman who had been treated with renal homotransplantation a few months previously. Progressive hemiparesis had developed postoperatively. A mass was found, protruding into the right lateral ventricle. This was biopsied with stereotaxic apparatus and found to be a plasmacytoma. (By permission of Radiology 95, 1, 1970.)
Figure 4.
The clinical course of the patient whose pneumoencephalogram is shown in Figure 3. When the diencephalic tumor was diagnosed, immunosuppression was drastically reduced, and the intracranial neoplasm was irradiated with a total of 5,650 R. Kidney function was well maintained, and tumor growth is apparently arrested since the patient has been well for more than three subsequent years. (By permission of Transplant. Proc. 1, 106, 1969.)
Three patients with epithelial tumors also died, but from complications of uremia or vascular disease rather than from cancer. Two of these three recipients had squamous cell carcinomas of the skin (Cases 4 and 7) that were completely excised. The third patient (Case 8) was the one with an incidental carcinoma of the lung found at autopsy.
The other nine recipients with de novo epithelial malignancies were all successfully treated by standard and relatively conservative excisions. With follow-ups of 1 month to 2 years there have been no recurrences.
Cases from Other Transplantation Centers
Data concerning additional de novo malignancies in one cardiac and 41 renal homograft recipients have been collected from transplantation centers throughout the world. These are summarized in Table IV. As in the Colorado series, the patients tended to be young (age range 8 to 58 years, average 33 years) and all had received immunosuppression with azathioprine and prednisone.
TABLE IV.
De novo malignancies in organ transplant recipients at other centers
| No. | Transplant Center | Age at time of transplantation | Sex | Donor | Immunosuppression |
Type of tumor | Time after transplantation (months) | Organs involved | Outcome | Contributing physician | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Splenectomy | Thymectomy | Azathioprine | Prednisone | ALG | ||||||||||
| 1 | Edinburgh, Scotland | 26 | F | Mother | No | No | Yes | Yes | Yes | Reticulum cell sarcoma | 25 | Lymph nodes, pleura, spleen, liver, ovary, adrenal, bone marrow, and transplanted kidney | Dead | Prof. M. Woodruff |
| 2 | Cleveland | 32 | M | Cadaver | No | No | Yes | Yes | Yes | Reticulum cell sarcoma | 22 | Buttock, lungs, aortic lymph nodes | Dead | Dr. S. D. Deodhar |
| 3 | Richmond | 38 | M | Cadaver | No | No | Yes | Yes | No | Reticulum cell sarcoma | 31 | Lung, aortic lymph nodes | Dead | Dr. J. Pierce |
| 4 | Auckland, New Zealand | 34 | M | a) Cadaver b) Cadaver c) Cadaver |
No | No | Yes | Yes | No | Reticulum cell sarcoma | 7 | Tongue, esophagus, liver | Dead | Dr. P. Doak |
| 5 | Auckland, New Zealand | 46 | F | Cadaver | No | No | Yes | Yes | No | Reticulum cell sarcoma | 9 | Brain | Dead | Dr. P. Doak |
| 6 | New York | 18 | M | Uncle | No | No | Yes | Yes | No | Reticulum cell sarcoma | 9 | Brain | Dead | Dr. R. Porro |
| 7 | New York | 36 | M | Cadaver | No | No | Yes | Yes | No | Reticulum cell sarcoma | 10 | Brain | Dead | Dr. F. Veith |
| 8 | Richmond | 29 | M | Brother | Yes | No | Yes | Yes | No | Reticulum cell sarcoma | 67 | Widespread lymph nodes, liver, vertebrae | Dead | Dr. H. Lee |
| 9 | Little Rock, Arkansas | 21 | M | Father | Yes | No | Yes | Yes | No | Reticulum cell sarcoma | 24 | Brain | Dead | Dr. C. Araoz |
| 10 | San Francisco | 39 | F | Sister | Yes | No | Yes | Yes | No | Reticulum cell sarcoma | 14 | Brain, lungs | Dead | Dr. F. O. Belzer |
| 11 | Richmond | 18 | M | Father | Yes | No | Yes | Yes | No | Reticulum cell sarcoma | 73 | Widespread lymph nodes, liver, pancreas, bone marrow, meninges, bladder, testes, transplanted kidney, accessory spleen, sciatic nerve, lung | Dead | Dr. H. M. Lee |
| 12 | New York | 35 | F | Cadaver | No | No | Yes | Yes | No | Visceral Kaposi’s sarcoma | 10 | Lungs, esophagus, stomach, urinary bladder, mediastinal and abdominal lymph nodes | Dead | Dr. J. H. Siegel |
| 13 | Boston | 34 | M | a) Cadaver b) Half-sister |
No | No | Yes | Yes | No | Leiomyosarcoma | 47 | Stomach, perigastric lymph node, peritoneum, bowel, liver, lungs, vertebrae and ribs | Dead | Dr. R. E. Wilson |
| 14 | Montreal | 36 | M | Cadaver | No | No | Yes | Yes | No | Leiomyosarcoma | 51 | Small bowel, liver, pancreas | Dead | Dr. L. D. MacLean |
| 15 | Israel | 28 | M | Cadaver | No | No | Yes | Yes | No | Kaposi’s sarcoma | 9 | Skin, mucosa of mouth | Alive | Dr. B. Myers |
| 16 | Israel | 38 | F | Cadaver | No | No | Yes | Yes | No | a) Reticulum cell sarcoma a) Kaposi’s sarcoma |
9 | a) Brain b) Skin |
Alive | Dr. B. Myers |
| 17 | Oklahoma City | 19 | F | Cadaver | No | No | Yes | Yes | No | Reticulum cell sarcoma | 13 | Lungs, liver, spleen, rectum, transplanted kidney, mesentery, bone marrow | Dead | Dr. Y. Muto |
| 18 | Chapel Hill* | 39 | M | Cadaver | No | No | Yes | Yes | Yes | Synovial sarcoma | 12 | Right popliteal fossa, lungs, liver, small bowel | Dead | Dr. W. B. Blythe |
| 19 | Los Angeles | 23 | M | Cadaver | No | No | Yes | Yes | No | Unclassified lymphoma | 46 | Brain | Dead | Dr. R. Goldman |
| 20 | Chicago | 52 | M | Cadaver | No | No | Yes | Yes | No | Reticulum cell sarcoma | 7½ | Brain | Dead | Dr. N. Levine |
| 21 | San Francisco | 24 | M | 1) Brother 2) Cadaver 3) Cadaver 4) Cadaver |
Yes | No | Yes | Yes | No | Reticulum cell sarcoma | 41 | Brain, cervical lymph nodes | Dead | Dr. F. O. Belzer |
| 22 | Minneapolis | 28 | F | Brother | No | No | Yes | Yes | No | Squamous cell carcinoma in situ | 30 | Cervix of uterus | Alive, no recurrence after hysterectomy | Dr. R. L. Simmons |
| 23 | Montreal | 38 | F | Cadaver | No | No | Yes | Yes | Yes | Squamous cell carcinoma in situ | 6 | Cervix of uterus | Alive, no recurrence after cryosurgery | Dr. K. Pritzker |
| 24 | Los Angeles | 38 | F | Mother | No | No | Yes | Yes | No | Squamous cell carcinoma in situ | 35 | Cervix of uterus, anterior wall of vagina | Alive, no recurrence after excision | Dr. A. Gordon |
| 25 | Richmond | 33 | F | Sister | No | No | Yes | Yes | No | Squamous cell carcinoma in situ | 36 | Cervix of uterus | Cone biopsy, patient being observed at regular intervals | Dr. H. Lee |
| 26 | Louisville | 35 | M | Brother | No | No | Yes | Yes | No | Squamous cell carcinoma | 8 | Lower lip | Alive, no recurrence following excision | Dr. D. Leb |
| 27 | Los Angeles | 27 | M | Mother | No | No | Yes | Yes | No | Squamous cell carcinoma | 25 | Lower lip | Alive, no recurrence following excision | Dr. R. Goldman |
| 28 | Los Angeles | 25 | F | Brother | No | No | Yes | Yes | No | Squamous cell carcinoma | 35 | Lower lip | Alive, no recurrence following excision | Dr. R. Goldman |
| 29 | Minneapolis | 27 | M | Brother | Yes | No | Yes | Yes | No | Undifferentiated carcinoma | 10 | Liver, brain, bone marrow | Dead | Dr. C. Hitchcock |
| 30 | Nashville | 47 | M | Cadaver | No | No | Yes | Yes | No | Undifferentiated carcinoma | 19 | Lung, mediastinal lymph nodes, brain, liver | Dead | Dr. C. Zukoski |
| 31 | San Francisco | 46 | M | a) Son b) Cadaver |
Yes | No | Yes | Yes | No | Alveolar cell carcinoma of lung | 9 | Lungs | Dead | Dr. S. Kountz |
| 32 | Minneapolis | 16 | F | Cadaver | No | No | Yes | Yes | No | Dysgerminoma | 32 | Ovary, peritoneum, mediastinal and axillary lymph nodes | Dead | Dr. W. Kelly |
| 33 | Ghent, Belgium | 53 | F | Cadaver | No | No | Yes | Yes | No | Adenocarcinoma | 35 | Sigmoid colon, liver | Dead | Dr. F. Derom |
| 34 | Nashville | 34 | M | Cadaver | No | No | Yes | Yes | No | Squamous cell carcinoma | 62 | Metastases in lymph nodes of neck, later widespread metastases. Primary site of tumor unknown | Dead | Dr. C. Zukoski |
| 35 | Louisville | 32 | M | Brother | Yes | No | Yes | Yes | No | Embryonal cell carcinoma | 2 | Testis, abdominal organs, ureter of transplanted kidney, lung | Dead | Dr. D. Leb |
| 36 | Cape Town** | 50 | M | Cadaver | No | No | Yes | Yes | Yes | Anaplastic small cell adenocarcinoma | 17 | Stomach, liver, mesentery, peritoneum | Dead | Dr. S. Bosman |
| 37 | Stockholm, Sweden | 58 | M | Cadaver | No | No | Yes | Yes | No | Multiple squamous cell carcinomas | 38 | Scalp | Alive | C. Franksson |
| 38 | Salt Lake City | 42 | M | Cadaver | No | No | Yes | Yes | Yes | Squamous cell carcinoma | 32 | Lip | Alive | Dr. L. Stevens |
| 39 | Sydney, Australia | 48 | F | Cadaver | No | No | Yes | Yes | No | Squamous cell carcinoma | 29 | Right forehead, right nasolabial fold | Alive | Dr. J. H. Stuart |
| 40 | Toronto*** | 21 | M | Cadaver | No | No | Yes | Yes | Yes | Highly anaplastic transitional cell tumor | 6 | Kidney, liver, brain, heart, lung | Dead | Dr. G. DeVeber |
| 41 | Montreal | 13 | M | Brother | No | No | Yes | Yes | No | Hepatocellular carcinoma | 32 | Liver | Dead | Dr. K. Pritzker |
| 42 | Toronto | 8 | F | Cadaver | No | No | Yes | Yes | Yes | Well differentiated hepatoma | 13 | Liver | Dead | Dr. A. Millner |
Tumor appeared after immunosuppression was discontinued.
Heart transplant recipient.
Tumor may have been present at the time of transplantation.
However, there were some quantitative differences between the local and collected series. For example, 21 of the contributed 42 cases (50 per cent) had some type of mesenchymal tumor, of which the most common by far was reticulum cell sarcoma, whereas mesenchymal malignancies accounted for only 3 of 15 of the Colorado cases (20 per cent). The disparity probably represents a reporting artifact in that there would be a tendency to report only the more florid and lethal malignancies to the registry with the dismissal of many skin tumors or in situ carcinomas of the cervix as relatively unimportant.
As in our own cases, highly malignant tumors have tended to occur much earlier after transplantation than the relatively low grade lesions of skin, lip, and uterine cervix. Consequently, the average time of appearance of the tumor after transplantation in the 42 cases from other institutions was only 25 months as compared with 44.6 months in the Colorado series. The higher proportion of exceptionally malignant tumors also accounted for the relatively poor prognosis in the collected cases, only 12 of the 42 patients surviving at the time of reporting as compared with a 10 of 15 current survival in our own patients.
Other differences between the Colorado and the world series reflect primarily variations in management from institution to institution (Table III). Splenectomy was performed in all of our malignancy cases but in only 8 of 42 from elsewhere. The thymus gland had been removed from 5 of the 15 Colorado patients but not from any in the world series. ALG was given in 6 of 15 (40 per cent) of our cases and in only 10 of 42 (24 per cent) from other centers.
An unusual feature of the lymphomas both in the Colorado and the world series was the frequency with which the brain was involved. Of a total of 22 lymphomas (reticulum cell, Kaposi’s, and unclassified) in the combined series, the brain was involved in 11, and was the only organ affected in 8 of these patients (Schneck & Penn 1971).
IMMUNOSUPPRESSION AND THE ACCIDENTAL TRANSPLANTATION OF TUMORS
It was originally proposed by Burnet (1957) that the individuality of tumor cells, which allows their recognition as foreign, might be due to tumor-specific (T) antigens (Foley 1933, Gorer 1937, Prehn & Main 1957, Old & Boyse 1964, Southam 1964, Klein 1966, Isojima et al. 1969, Law 1969) not found in the normal host tissues. Such extra antigens are probably present on most if not all malignant cells but they are thought to be so weak that a surveillance mechanism that could detect them as ‘non-self’ must have exquisite discrimination. The presumed disruption of such a refined biologic apparatus by immunosuppression as an explanation for the de novo malignancies observed after transplantation is not difficult to envision.
With the transplantation of tumor homografts, the task of surveillance in a normal person should by no means be as subtle. Under this circumstance, T antigen(s) in the neoplastic tissue may help to elicit an immune reaction from a recipient but probably only to a very minor degree. The principal response as with any homograft is determined by histocompatibility differences between the donor and recipient. Since the vigor of homologous tumor rejection is thus governed in a general way by the same rules of histocompatibility to which normal tissues are subject (Jensen 1922, Gorer 1937, Snell 1952, Old & Boyse 1964, Sjögren 1965, Burnet 1967, Mogensen & Kissmeyer-Nielsen 1968, Law 1969), the repudiation of a transplanted neo plasm should be preventable by any treatment which is protective for other kinds of homografts.
This major point has been confirmed clinically by a small but revealing series of observations. A few years ago, several different transplantation teams employed renal homografts that had been obtained from cadaveric donors whose deaths were caused by carcinomas of the lung (Martin et al. 1965, Wilson et al. 1968), pyriform sinus (McIntosh et al. 1965), thyroid (Muizniks et al. 1968), breast (MacLean et al. 1965), or liver (Zukoski et al. 1970). In each instance the kidney was not thought to have been involved by tumor at the time of its excision. However, in two instances the homografts were removed within a few days after their transplantation and were found to contain microscopic metastases (MacLean et al. 1965, Muizniks et al. 1968). These observations were of interest since they showed how easily cancer cells could inadvertently be transferred under these dangerous conditions. The patients did not subsequently develop metastases.
However, the potentially tragic consequences of the practice of accepting cancer victims as donors soon became apparent in the four other patients who had the misfortune to achieve good initial renal function from their grafts and who, therefore, were placed on chronic immunosuppression. Four to 36 months later, malignancies of the same histologic type as that causing the death of the donor were found in the transplanted organs. In two of the four recipients, the tumor had already become autonomous in that it continued to flourish and caused fatal metastases even though immunosuppression was stopped and the renal portion of the composite graft was rejected (Martin et al. 1965, McIntosh et al. 1965). The local or distant metastases in the other two patients underwent involution and final complete disappearance after discontinuance of immunosuppression and loss of the renal homografts (Wilson et al. 1968, Zukoski et al. 1970). The spread in Zukoski’s remarkable case was very extensive, involving multiple areas in both lungs. Yet at autopsy, several months after stopping azathioprine and prednisone, there was no trace of tumor.
The relative ability of neoplastic tissue to selectively thrive as part of a composite homograft once it has achieved a foothold was also dramatically illustrated by a case of Tunner et al. (1971). A kidney containing a hypernephroma was rejected to the point of no function in 12 weeks in spite of immunosuppression. Three weeks later, after an unsuccessful second transplantation, the recipient died. At autopsy, the neoplastic tissue of the transplant was found to be free of histopathologic signs of rejection and to be vigorously invading adjacent structures. In contrast, the non-cancerous part of the kidney had numerous and severe findings of rejection. Conceivably, the ‘escape’ from control of the neoplastic cells at the same time as rejection of normal tissue from the same donor could be contributed to by the loss of histocompatibility antigens in malignant cells (Weiler 1959, Tyler 1960).
IMMUNOSUPPRESSION AND METASTASES
Some General Principles
Thus far, we have commented upon two kinds of clinical observations in immunosuppressed patients, first concerning de novo malignancies and then transplanted ones, that are unequivocal support of the hypothesis of immunologic surveillance. A third situation, and one about which the existing evidence is not so firm, will now be considered as it has also been studied in the organ transplantation environment.
As annotated earlier, acceleration of metastases has been reported in animals treated with prednisone, ALG, and azathioprine. The same thing has been described after the performance of splenectomy (Alford et al. 1966). Consequently, there is ample reason to suspect that effective immunosuppression in man might, by the further erosion of an already crumbling surveillance, facilitate the growth or spread of an established neoplasm or of residual tumor after incomplete destruction or extirpation. The thought is worth contemplating even by those in ordinary clinical practice since some of the most widely used cancer chemotherapeutic agents have indisputable immunosuppressive qualities in addition to their tumorocidal ones and might, therefore, theoretically be capable under some circumstances of an effect opposite to that which is desired.
To demonstrate the combination of tumorocidal plus immunosuppressive effects, the four examples of azathioprine, nitrogen mustard, methotrexate, and cyclophosphamide will suffice although numerous others could be cited. Cyclophosphamide is a particularly interesting drug since it has been one of the most extensively evaluated and highly thought of anticancer agents ever released for clinical trial; that it can inhibit the growth of some kinds of tumors is beyond reasonable doubt. However, it is also an immunosuppressant of such potency that it threatens to replace azathioprine as the cornerstone of therapy in our organ transplantation program (Starzl et al. 1971). Because of the latter fact, more attention will have to be paid in the future to the possible adverse effects just alluded to of which drugs like cyclophosphamide are not yet suspect, including the promotion of de novo or transplanted tumors as already described or even the fostering of metastases from certain types of primaries as will be discussed below.
Liver Transplantation For Hepatoma
In view of the potentially self-defeating aspects just described of systematic immunosuppression in patients with established cancer, it will come as no surprise that our efforts to treat extensive hepatomas by total hepatectomy and liver replacement have frequently failed due to recurrence of neoplasm in spite of the fact that no tumor was thought to have been left (Starzl 1969, Starzl et al. 1970). It might be persuasively argued that the frequency and seriousness of the recurrences were approximately predictable from what is already known (Gustafson 1937, Spatt & Grayzel 1948, Berman 1959) about the highly unfavorable natural history of primary hepatic malignancies. The alternative opinion that growth of residual microscopic deposits was actually enhanced by immunosuppression is equally unprovable. Both views could be consistent with the actual observations, which were as follows.
The preoperative diagnosis of hepatoma was the reason for proceeding with liver transplantation in 12 patients at our institution. Six of these 12 recipients had underlying cirrhosis of the Laennec, postnecrotic, and biliary varieties in 3, 2, and 1 instances, respectively. The biliary cirrhosis was in a 10-year-old child with biliary atresia. An additional unsuspected small hepatoma (Figure 5) was found in the liver of another child treated for intra-hepatic biliary atresia, making a total of 13 cases available for observation.
Figure 5.

A small hepatoma (surrounded by arrows) in a liver that was replaced at the age of 3 years, 10 months, because of biliary atresia.
Seven of the 12 patients with prospectively diagnosed primary cancer of the liver died early, from 6½ to 39 days after orthotopic hepatic homo-transplantation. Five of these cases were amongst the first 7 in our experience; an important contributory cause of failure was the use of livers that had been badly damaged by ischemia. The other 2 patients who were treated at a later time died of technical accidents with subphrenic abscesses and bile peritonitis, respectively. At autopsy, an exhaustive search was made to determine whether any metastases had been missed with the preoperative survey and the operative examination. No residual tumor was found in 6 of the 7 recipients. In the other patient, who died 7½ days after transplantation, there had been previously unsuspected spread to a lumbar vertebra.
The other 6 recipients including the one with an incidental hepatoma lived through the immediate effects of the operation and became available for longer-term studies. In all 6, hepatic cell carcinoma (hepatoma) was the histologic diagnosis. Five of the patients died 76, 143, 339, 400, and 432 days after operation (Table V). Recurrent neoplasm was responsible for the death of 4 of the recipients. The fifth (OT 26) died of other causes but had developed a metastasis to the left lung. The most common sites of spread (Table V) were the lungs and the liver homografts (4 examples each). In some cases, the rate of secondary growth was amazingly rapid (Figure 6).
TABLE V.
Fate of six patients who received liver replacement for indication of hepatoma and lived long enough after operation to permit observations about course of malignancy
| Patient number | Metastases first detected (days postop.) | Location first metastases | Treatment of metastases | Metastases to homograft | Organs ultimately involved | Cause of death and time |
|---|---|---|---|---|---|---|
| OT 8 | 90 | Lungs | Vincristine sulfate; 5 FU; surgical excision of intra-abdominal masses; local X-ray therapy to pelvis | Yes | Brain, lungs, liver other abdominal organs | Carcinomatosis, 400 days |
| OT 14 | 380 | Diaphragm, liver, retroperitoneal space | — | Yes | Mediastinum, pleural cavity, diaphragm, retroperitoneal space, liver, pancreas, other abdominal organs | Carcinomatosis; disrupted cholecystoduodenostomy, 432 days* |
| OT 15 | 60 | Lungs | — | Yes | Lungs, liver, diaphragm | Carcinomatosis, 339 days |
| OT 23 | 29 | Lungs | — | Yes | Brain, lungs, liver, retroperitoneal space | Carcinomatosis, 143 days |
| OT 26 | 59 | Lungs | — | No | Lungs | Gastrointestinal hemorrhage, 76 days |
| OT 33** | — | None found | — | No | None | Alive, 18 months |
Retransplantation had been carried out at 380 days, at which time the first metastases were discovered. By the time of death 52 days later, the extension of the metastases was pronounced.
The diagnosis was an incidental finding in the liver specimen which had been removed because of biliary atresia.
Figure 6.
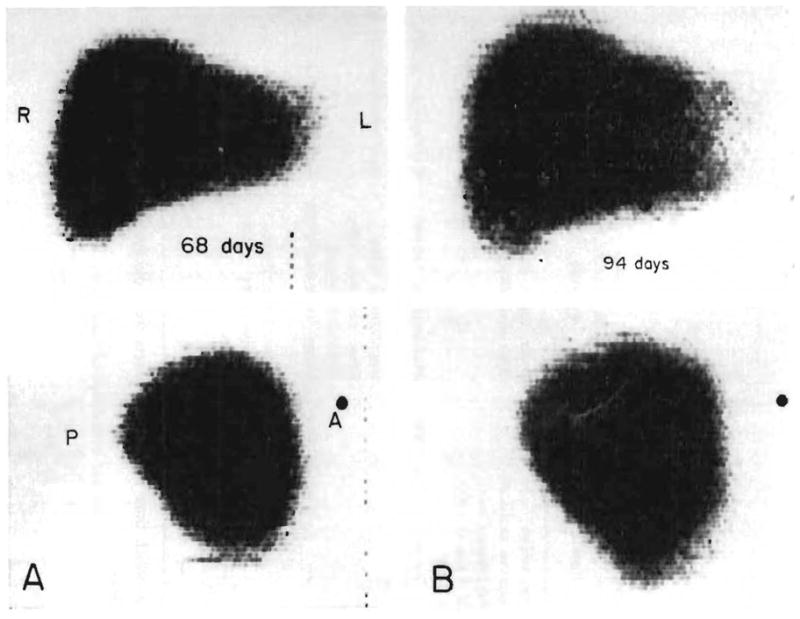

Destruction of an orthotopic liver homograft by tumor recurrence. The posteroanterior and lateral liver scans were obtained with technetium. A: 68 days. The scan is normal. B: 94 days. The patient had become jaundiced. Hepatomegaly is evident. C: 101 days. Multiple areas of poor isotope concentration are now visible. D: 111 days. The process has continued its rapid progression. By the time of death one month later, the homograft was almost completely replaced with carcinoma. (By permission of W. B. Saunders Co., 1969.)
The only patient in our series with hepatoma still surviving is the 4-year-old girl with biliary atresia whose tumor was not known in advance to be present (Figure 5). In this case sera were examined for the presence of the fetoprotein (Alpert et al. 1971) which when found essentially always signifies the diagnosis of hepatoma (Alpert et al. 1968). Fetoglobulin was found in the preoperative sample. After transplantation, the abnormal protein persisted for more than 5 months, but in diminishing quantities until it finally disappeared. The child is in excellent condition 18 months postoperative and apparently has been cured of the hepatoma. A probable cure of hepatoma in one of Calne’s patients has also been reported (Evans & Lehmann 1971). However, both Calne and Fortner et al. (1970) have also encountered metastases in patients with an otherwise successful liver transplantation.
Because of the high rate of recurrent carcinoma in our own experience and in that of others, it has become our policy in cases of hepatoma to consider liver replacement only under the most exceptional circumstances.
Renal Transplantation For Cancer
As already emphasized, the role, if any, of immunosuppression in promoting the metastases described in the preceding section after liver transplantation could not be proved.
The results of renal transplantation at the same time or at varying times after nephrectomy for Wilm’s tumor or renal cell carcinoma have been equally inconclusive. In 9 bona fide cases collected from other centers (Penn 1970), mostly from personal communication, there were 5 patients who developed metastases. Two more recipients died too soon after transplantation to provide meaningful follow-up, but the final two lived long enough to be considered cured of their original cancers.
THE ENDOWMENT OF IMMUNOLOGIC SURVEILLANCE
It was not long after the notion of immunologic surveillance became widely known that efforts were made to exploit the concept for the treatment of cancer. The following remarks will be concerned with our own reflections about the matter (Marchioro et al. 1964, Starzl 1964a, Starzl et al. 1965) and not with an attempt to review even a fraction of the enormous literature bearing on this possibility. Nevertheless, it is worth mentioning that the idea of endowing a new surveillance apparatus has been a major theme in the clinical work of Dr. George Mathé (1971) and Dr. George Moore (1970) during much of the past decade.
The rationale of all such proposals can best be appreciated by directly quoting Thomas’ (1959) view of surveillance as he expressed it in his discussion of a paper by Sir Peter Medawar given at a symposium on hypersensitive states in New York City in 1957. In commenting on the possible biologic significance of homograft rejection, he speculated:
… (Among) functions that leap to the mind … One has to do with the universal requirement of multicell ular organisms to preserve uniformity of cell type and to prevent mutant cells from colonizing and flourishing. This may be a real hazard, if we can judge from the readiness of bacterial cells to undergo mutation, or the weird cell forms of malignant appearance that develop and multiply, and outgrow and replace, normal epithelial cells in tissue cultures. Perhaps, in short, the phenomenon of homograft rejection will turn out to represent a primary mechanism for natural defense against neoplasia. The numerous analogies between this reaction and the immunologically induced destruction of experimental tumors (or their prolonged preservation after flooding an animal with tumor antigen) are consistent with this line of speculation.
If this were indeed the case, one might expect that a defect in the mechanism of delayed-type hypersensitivity would result in special susceptibility to neoplasia …
It is not difficult to recognize the following thought, written in 1963 (Starzl 1964a), to be a corollary of Thomas’ statement:
Immunology may play a significant role in future cancer therapy … One unexplored approach may be to change the cancer victim’s immunologic pattern by replacing it with the reticuloendothelial (lymphoreticular) system of a donor. Since cancer cannot ordinarily be transplanted from one patient to another, there is reason to believe that complete alteration of the immune potential of the patient might cause the tumor to be rejected. Such an approach awaits perfection of techniques of homologous tissue transplantation. In simplified terms, it would be necessary to destroy the patients reticuloendothelial system, and replace it with donated bone marrow or spleen, and perhaps other reticuloendothelial components.
Early Efforts at Promoting a Graft-Versus-Tumor Reaction With Spleen Grafts
In February, April, and May 1963, 4 patients were given whole organ spleen homografts which had been sensitized against the tumor tissue of the eventual recipient by the scheme shown in Figure 7 (Marchioro et al. 1964, Starzl et al. 1965). In essence, fragments of the carcinoma were removed, killed by emulsification, mixed with Freund’s adjuvant, and injected subcutaneously into the next cancer victim, who 2 to 8 weeks later became the donor of a ‘preinstructed’ spleen. The splenic transplantations were to the extra peritoneal space of the recipients’ iliac fossa, anastomosing the splenic vessels to the hypogastric artery and the external iliac vein.
Figure 7.
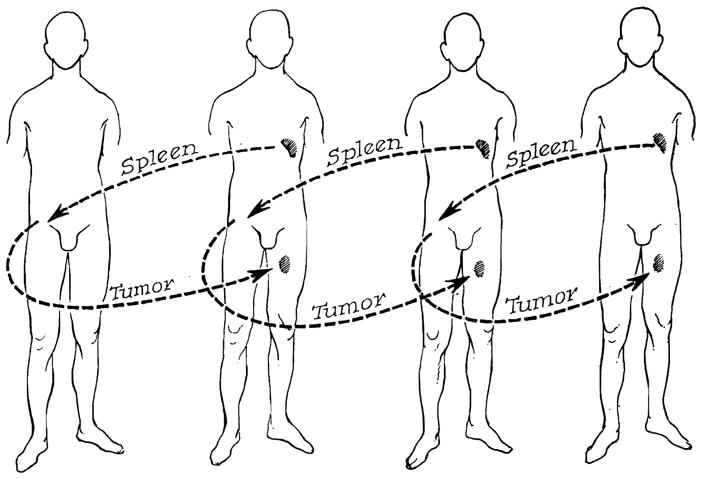
Experimental procedure in patients with disseminated malignancies who were treated with splenic homotransplantation. Note that the donor is sensitized with the recipient’s tumor in each case. (By permission of Sth. med. J. 58, 131, 1965.)
The types of malignancies were alveolar cell carcinoma of the lung and carcinomas of the stomach (2 cases), and kidney. Postoperative immunosuppression was given with azathioprine and prednisone. One of the recipients developed an acute hemolytic anemia, probably because of a breach of red cell group compatibility (tissue transfer from Group 0 to A). This patient ruptured his new spleen and died 10 days postoperatively of aspiration while on the way to the operating room. The other 3 recipients died of metastases after 2, 3, and 21 months. By the time of autopsy, the splenic homografts had involuted.
The two organs that had been in residence for 2 and 3 months weighed 400 and 240 grams, respectively. They were very firm. Microscopically, broad bands of fibrous connective tissue and/or necrotic areas replaced large amounts of parenchyma (Figure 8). Scattered throughout were small patches of hemorrhage and some aggregates of lymphocytes. White pulp was not identified. The patient who lived for 21 months had immunosuppression stopped 9 months post-transplantation. By the time of death from carcinomatosis, one year later, the residual spleen graft had become a first-sized mass of connective tissue; the splenic artery and vein were thrombosed.
Figure 8.

Photomicrograph of splenic homograft obtained at autopsy 2 operatively. Note the widespread necrosis with complete loss of architecture (hematoxylin and eosin, × 80). (By permission of Ann. N.Y. Acad. Sci. 120, 626, 1964.)
The natural history of the malignancies was not altered in any obvious way. In retrospect, it must be conceded that the trials could not be properly evaluated since rejection of the homografts was not prevented in any of the three longest surviving cases. The two homografts retrieved at autopsy after 2 and 3 months retained gross characteristics of spleen, but microscopic study showed architectural disruptions so extensive that it is difficult to conceive of functional integrity of the organs in terms of a chronic graft-versus-tumor reaction.
The use of whole spleen homotransplantation for the treatment of malignant neoplasm has not been described by any other group. However, Woodruff attempted the intravenous infusion of spleen cell suspensions (Woodruff & Nolan 1963). Apparently the course of the widespread tumors in his patients was not substantially affected, inasmuch as there have been no follow-ups to the mildly optimistic preliminary communication.
Bone Marrow and Graft-Versus-Tumor Reactions
The Barrier of the Graft-Versus-Host Reaction
There are two sequential problems that must be solved before the transplantation of immunocompetent tissue can be evaluated for the treatment of human malignancy. The first, and one toward which major progress has been made, is to prevent rejection of the graft by the host. The second is to prevent fatal rejection of the host by the graft while at the same time retaining enough graft-versus-host activity to permit killing of the neoplasm.
Speculation about a differential lethal effect upon a tumor in combination with a sparing of normal recipient tissues and organs may require the special kind of optimism that strays beyond logic. However, the potential role of T-antigens in promoting such a specific vulnerability of neoplastic cells was discussed earlier. In addition, the possibility cannot be excluded that neoplastic tissue might present a spectacularly susceptible target to a graft-versus-host reaction for strictly non-immunologic reasons. The relative ease with which tumor tissue can be damaged is the essential reason for the success of many other kinds of anticancer treatment including irradiation.
For the moment, the question of a differential graft-versus-host reaction must remain moot since the simple fact is that the satisfactory test conditions to provide an answer have not yet been fulfilled. A few successful bone marrow transplantations have been performed, but essentially always between siblings with identity of the major histocompatibility (HL-A) system (Bach et al. 1968, Gatti et al. 1968, Mathé et al. 1970, Santos et al. 1970, Speck et al. 1970), and even then usually for the indication of one of the immune deficiency diseases. In the event of a compatibility so perfect that no adverse consequences are seen, much of the therapeutic potential offered by a vigorous graft-versus-host reaction would be predicted to be lost. Yet, with all other kinds of donors, including those from within the family, the outcome has been a fatal graft-versus-host reaction.
It is probable that the exceedingly limited reference in which bone marrow transplantation has had any chance at all of success is partly due to the kind of immunosuppression that has been employed. In the first trials for patients not suffering from spontaneous immunologic deficiency, total body irradiation was used (Van Bekkum & DeVries 1967, Mathé et al. 1971). More recently, cyclophosphamide, other drugs, or antilymphocyte globulin (ALG) have been employed. With all these agents, the principle has been to cripple the recipient immunologically in advance of grafting. For example, cyclophosphamide which is presently enjoying a wave of popularity has been given for a few days before the marrow infusion in the prodigious daily quantities of 45–200 mg/kg (Bortin et al. 1971, Santos 1971). After transplantation, little or no therapy has been provided. What has been asked in such cases is the almost instant induction of tolerance in the recipient in order to make possible graft acceptance but with the added proviso that there be good enough histocompatibility to avoid the fatal graft-versus-host reaction that would otherwise ensue without follow-up treatment.
To achieve more than isolated successes after bone marrow transplantation even with the highly favorable double haplotype identical sibling combinations, it would seem reasonable to give less extreme but more protracted treatment such as that used after whole organ transplantation. When this is done after transplantation of kidneys, livers, and hearts, a characteristic cycle transpires during which the host-graft relationship is altered and after which the degree of immunosuppression required to retain the graft is reduced in successful cases (Starzl 1964b, 1969). This kind of immunosuppressive regimen, which would simultaneously treat the immunocompetent cells of the host as well as those of the graft with combinations of agents, has not been evaluated for bone marrow transplantation.
A Justification for Hope
Earlier, a possible element of circular reasoning was mentioned in any scheme to exploit controlled graft-versus-host reactions to treat human tumors. The circle begins with the firm evidence that immunosuppression emasculates surveillance and, therefore, is a factor in oncogenesis. It could close with the acknowledgment that effective and probably chronic immunosuppression must be used to prevent and/or combat complicating and lethal graft-versus-host reactions that must be dealt with if bone marrow is to be given to patients in the hope of suppressing neoplasia. It is possible that the appropriate juggling of all the biological and therapeutic factors to achieve the desired effect upon neoplastic growth will prove to be an impracticality.
If so, an important prospect for therapy will be lost since there is considerable evidence, recently summarized by Santos (1971), that bone marrow grafts can have a profoundly inhibitory effect upon malignancies. In more than a dozen cases, patients have had remissions of leukemia lasting from several weeks to as long as 8 months after bone marrow transplantation. Moreover, other kinds of malignancy have been noted to regress or disappear for shorter intervals in animals which died from graft-versus-host reaction, infection, or other causes. As an example in dogs, Epstein et al. (1971) of Seattle recently reported tumor involution in a series of sarcomas and one anaplastic carcinoma. Eight of their animals lived for more than a week after succesful bone marrow transplantation with maximum survival of 60 days. None of these dogs had any residual tumor at the time of its death.
SUMMARY
The hypothesis that immunologic surveillance is nature’s way of preventing and controlling malignant neoplasia has received three kinds of support from clinical observations after organ transplantation under continuous immunosuppression. First, de novo epithelial or mesenchymal tumors have been observed throughout the world in 57 chronic survivors of whole organ transplantation, including 15 of our own patients, for an incidence far exceeding that in a normal population. Second, malignant tumors have been accidently transplanted along with renal homografts, have become autonomous and invasive once well established, but have been rejected in two instances after stopping immunosuppression. Finally and least conclusively, the environment for metastatic growth of residual tumor may have been made more permissive after total hepatectomy or nephrectomy followed by liver or kidney transplantation.
If the surveillance hypothesis is accepted and if it is conceded that clinically evident cancer represents a failure of a fundamental biologic control system, it becomes natural to consider means of conferring new immunologic defenses upon patients with uncontrolled malignancy. This has been attempted by us with whole organ spleen transplantation and by others with transplantation of bone marrow or other immunocompetent tissue. Long-term cures have not been obtained, but there have been some indications of an antitumor effect of such grafts. Further testing of this approach will require better regulation of the consequences of graft-versus-host reactions, possibly by applying some principles of immunosuppression that have been well standardized with the transplantation of whole organs.
Acknowledgments
This work was supported by research grants from the Veterans Administration, by grants RR-00051, and RR-00069 from the general clinical research centers program of the Division of Research Resources, National Institutes of Health and by grants AI-10176-01, AI-AM-08898, AM-07772, GM-01686, HE-09110 of the United States Public Health Service.
Footnotes
Cancer Registry, State of Connecticut, 1960 to 1962. The statistics from New York are essentially the same.
References
- Agosin M, Christen R, Badinez O, Gasic G, Neghme A, Pizarro O, Jarpa A. Cortisone induced metastases of adenocarcinoma in mice. Proc Soc Exp Bioi Med. 1952;80:128. doi: 10.3181/00379727-80-19544. [DOI] [PubMed] [Google Scholar]
- Albert D, Ziedman I. Relation of glucocorticoid activity of steroids to number of metastases. Cancer Res. 1962;22:1297. [PubMed] [Google Scholar]
- Alford TC, Stoneburner LL, Holinshead AC. Effect of spleen and lymph node removal on adenovirus tumor growth. Arch Surg. 1966;93:971. doi: 10.1001/archsurg.1966.01330060115013. [DOI] [PubMed] [Google Scholar]
- Allison AC, Berman LD, Levey RH. Increased tumor induction by adenovirus type 12 in thymectomized mice and mice treated with antilymphocyte serum. Nature (Land) 1967;215:185. doi: 10.1038/215185a0. [DOI] [PubMed] [Google Scholar]
- Alpert E, Starzl TE, Schur PH, Isselbacher KJ. Serum alpha-fetoprotein in hepatoma patients after liver transplantation. Gastroenterology. 1971 In press. [PubMed] [Google Scholar]
- Alpert ME, Uriel J, de Nechaud B. Alpha-fetoglobulin in the diagnosis of human hepatoma. New Engl J Med. 1968;278:984. doi: 10.1056/NEJM196805022781804. [DOI] [PubMed] [Google Scholar]
- Anigsten L, Anigsten DM, Rennels EG, O’Steen WK. Induced alteration of resistance to transplantable mammary adenocarcinoma in mice neonatally innoculated with rat thymus antiserum. Cancer Res. 1966;26:1867. [PubMed] [Google Scholar]
- Bach FH, Albertini RJ, Anderson JL, Joo P, Bortin M. Bone-marrow transplantation in a patient with Wiskott-Aldrich syndrome. Lancet. 1968;2:136. doi: 10.1016/s0140-6736(68)92672-x. [DOI] [PubMed] [Google Scholar]
- Baserga R, Shubik P. Action of cortisone on disseminated tumor cells after removal of the primary growth. Science. 1954;121:100. doi: 10.1126/science.121.3134.100. [DOI] [PubMed] [Google Scholar]
- Berman C. Primary carcinoma of the liver. Bull NY Acad Sci. 1959;35:275. [PMC free article] [PubMed] [Google Scholar]
- Bortin MM, Saltzstein EC, Waisbren BA, Kay SA, Hong R, Bach F. Bone-marrow transplantation for aplastic anemia, Establishment of chimerism using multiple HL-A identical donors following pretreatment with cyclophosphamide. Transplantation. 1971;11:573. [PubMed] [Google Scholar]
- Bremberg S, Klein E, Stjernsward J. Effects of heterologous antilymphoid-cell serum on tumor isografts and viral leukemogenesis. Cancer Res. 1967;27:2113. [PubMed] [Google Scholar]
- Burnet FM. Cancer: A biological approach. Brit med J. 1957;1:779–841. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. The evolution of bodily defence. Med J Austr. 1963;2:817. doi: 10.5694/j.1326-5377.1963.tb18118.x. [DOI] [PubMed] [Google Scholar]
- Burnet FM. Immunologic aspects of malignant disease. Lancet. 1967;1:1171. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- Burnet FM. The evolution of adaptive immunity in vertebrates. Acta path microbiol scand. 1969;76:1. doi: 10.1111/j.1699-0463.1969.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Burnet FM. The concept of immunological surveillance. Progr exp Tumor Res. 1970;13:1. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Casey TP. Azathioprine (Imuran) administration and the development of lymphomas in N.Z.B. mice. Clin exp Immunol. 1968;3:305. [PMC free article] [PubMed] [Google Scholar]
- Davis RC, Lewis J., Jr Effect of thymectomy on an antilymphocyte serum treated human tumor xenograft. Surg Forum. 1967;18:229. [Google Scholar]
- Dent PB, Peterson RDA, Good RA. The relationship between immunologic function and oncogenesis. In: Good RA, Bergsma D, editors. Immunologic Deficiency Diseases in Man. National Foundation Press; 1968. p. 443. (Birth Defects Original Article Series, Vol. 4) [Google Scholar]
- Deodhar SD, Crik G, Jr, Schofield PF. Immunosuppression in allogeneic murine tumor system: A model for the study of antilymphocyte serum. Lancet. 1968;1:168. doi: 10.1016/s0140-6736(68)92562-2. [DOI] [PubMed] [Google Scholar]
- Deodhar SD, Kuklinea AG, Vidt DG, Robertson AL, Hazard JB. Development of reticulum cell sarcoma at the site of antilymphocyte globulin injection in a patient with renal transplant. New Engl J Med. 1969;280:1104. doi: 10.1056/NEJM196905152802007. [DOI] [PubMed] [Google Scholar]
- Doak PB, Montgomerie JZ, North JDK, Smith F. Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Brit med J. 1968;2:746. doi: 10.1136/bmj.4.5633.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Payne P, Waterhouse J, editors. Cancer Incidence in Five Continents. A Technical Report. (International Union against Cancer) Springer; New York: 1966. [Google Scholar]
- Epstein RB, Graham TC, Storb R, Thomas ED. Studies of marrow transplantation, chemotherapy and cross circulation in canine lymphosarcoma. Blood. 1971;37:349. [PubMed] [Google Scholar]
- Evans DB, Lehmann H. Pseudocholinesterase activity in liver transplantation. Lancet. 1971;1:1040. doi: 10.1016/s0140-6736(71)91604-7. [DOI] [PubMed] [Google Scholar]
- Fisher ER, Soliman O, Fisher B. Effect of antilymphocyte serum on parameters of growth of MCA-induced tumours. Nature (Lond) 1969;221:287. doi: 10.1038/221287a0. [DOI] [PubMed] [Google Scholar]
- Foley EJ. Antigenic properties of methyl cholanthrene induced tumors in mice of the strain of origin. Cancer Res. 1933;13:835. [PubMed] [Google Scholar]
- Foley EJ, Silverstein R. Progressive growth of C3H mouse lymphosarcoma in CF1 mice treated with cortisone acetate. Proc Soc Exp Biol (NY) 1951;77:713. doi: 10.3181/00379727-77-18902. [DOI] [PubMed] [Google Scholar]
- Fortner JG, Beattie EJ, Jr, Shiu MH, Kowano N, Howland WS. Orthotopic and heterotopic liver homografts in man. Ann Surg. 1970;172:23. [PMC free article] [PubMed] [Google Scholar]
- Gatti RA, Menwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiencies. Lancet. 1968;2:1366. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- Gorer PA. The genetic and antigenic basis of tumor transplantation. J Pathol Bact. 1937;44:691. [Google Scholar]
- Grant GA, Miller JFAP. Effect of neonatal thymectomy in the induction of sarcomata in C57BL mice. Nature (Lond) 1965;205:1124. doi: 10.1038/2051124a0. [DOI] [PubMed] [Google Scholar]
- Green I, Litwin S, Adlersberg R, Rubin I. Hypogammaglobulinemia with late development of lymphosarcoma. Arch intern Med. 1966;118:592. [PubMed] [Google Scholar]
- Gustafson EG. An analysis. of 62 cases of primary carcinoma of the liver based on 24,400 necropsies at Bellevue Hospital. Ann intern Med. 1937;11:889. [Google Scholar]
- Hellman K, Hawkins RI, Whitecross S. Antilymphocytic serum and tumour dissemination. Brit med J. 1968;2:533. doi: 10.1136/bmj.2.5604.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch MS, Murphy FA. Effects of antithymocyte serum on Rauscher virus infection of mice. Nature (Lond) 1968;218:478. doi: 10.1038/218478a0. [DOI] [PubMed] [Google Scholar]
- Huber J. Experience with various immunologic deficiencies in Holland. In: Bergsma D, Good RA, editors. Immunologic Deficiency Diseases in Man. National Foundation Press; 1968. p. 53. (Birth Defects Original Article Series, Vol. 4) [Google Scholar]
- Immunology and Cancer. Lancet. 1968;1:1298. [PubMed] [Google Scholar]
- Isojima S, Yagi Y, Pressman D. Antigens common to rat hepatomas induced with 2-acetylamino fluorene. Cancer Res. 1969;29:140. [PubMed] [Google Scholar]
- Jensen CO. Experimentelle Untersuchungen uber Krebs bei Mäusen. Zbl Bakt. 1922;34:28. [Google Scholar]
- Kay S, Frable WJ, Hume DM. Cervical dysplasia and cancer developing in woman on immunosuppression therapy for renal homotransplantation. Cancer. 1970;26:1048. doi: 10.1002/1097-0142(197011)26:5<1048::aid-cncr2820260512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Klein G. Tumor antigens. Ann Rev Microbiol. 1966;20:223. doi: 10.1146/annurev.mi.20.100166.001255. [DOI] [PubMed] [Google Scholar]
- Law LW. Studies of the significance of tumor antigens in induction and repression of neoplastic disease. Presidential address. Cancer Res. 1969;29:1. [PubMed] [Google Scholar]
- MacLean LD, Dossetor JB, Gault MH, Oliver JA, Inglis FG, MacKinnon KJ. Renal transplantation using cadaver donors. Arch Surg. 1965;91:288. [Google Scholar]
- McIntosh DA, McPhaul JJ, Jr, Peterson EW, Harvin JS, Smith JR, Cook FE, Jr, Humphreys JW., Jr Homotransplantation of a cadaver neoplasm and a renal homograft. Letter to the editor. J Amer med Ass. 1965;192:1171. doi: 10.1001/jama.1965.03080260059024. [DOI] [PubMed] [Google Scholar]
- McKhann CF. Primary malignancy in patients undergoing immunosuppression for renal transplantation. A request for information. Transplantation. 1969;8:209. doi: 10.1097/00007890-196908000-00033. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Decosse JJ. Effect of antithymocyte sera on the growth of plasma cell tumors in mice. Surg Forum. 1969;20:109. [PubMed] [Google Scholar]
- Marchioro TL, Fudenberg H, Rowlands D, Rifkind D, Waddell WR, Starzl TE. Splenic homotransplantation. Ann NY Acad Sci. 1964;120:626. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DC, Rubini M, Rosen VJ. Cadaveric renal homotransplantation with inadvertent transplantation of carcinoma. J Amer med Ass. 1965;192:82. doi: 10.1001/jama.1965.03080220016003. [DOI] [PubMed] [Google Scholar]
- Martinez C, Dalmasso AP, Good RA. Acceptance of tumor homografts by thymectomized mice. Nature (Lond) 1962;194:1289. [Google Scholar]
- Mathé G, Amiel JL, Schwarzenberg L. Bone Marrow Transplantation and Leukocyte Transfusion. Charles C. Thomas; Springfield, Ill: 1971. [Google Scholar]
- Mathé G, Amiel JL, Schwarzenberg L, Choay J, Trolard P, Schneider M, Hayat M, Schlumberger JR, Jasmin C. Bone marrow graft in man after conditioning by antilymphocytic serum. Transplant Proc. 1970;3:325. [PubMed] [Google Scholar]
- Miller JFAP, Ting RC, Law LW. Influence of thymectomy on tumor induction by polyoma virus in C57BL mice. Proc Soc Exp Biol Med. 1964;116:323. doi: 10.3181/00379727-116-29237. [DOI] [PubMed] [Google Scholar]
- Mogensen B, Kissmeyer-Nielsen F. Histocompatibility antigens on the HL-A locus in generalized gestational choriocarcinoma. Lancet. 1968;1:721. doi: 10.1016/s0140-6736(68)92166-1. [DOI] [PubMed] [Google Scholar]
- Moore GE. Tumors. Surg Gynec Obstet. 1970;130:322. [PubMed] [Google Scholar]
- Muizniks HW, Berg JW, Lawrence W, Jr, Randall HT. Suitability of donor kidneys from patients with cancer. Surgery. 1968;64:871. [PubMed] [Google Scholar]
- Old LJ, Boyse EA. Immunology of experimental tumors. Ann Rev Med. 1964;15:167. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- Page AR, Hansen AE, Good RA. Occurrences of leukemia and lymphoma in patients with agammaglobulinemia. Blood. 1963;21:197. [PubMed] [Google Scholar]
- Penn I. Malignant Tumors in Organ Transplant Recipients. Springer-Verlag; New York: 1970. [Google Scholar]
- Penn I, Halgrimson CG, Starzl TE. De novo malignant tumors in organ transplant recipients. Transplant Proc. 1971;3:773. [PMC free article] [PubMed] [Google Scholar]
- Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106. [PMC free article] [PubMed] [Google Scholar]
- Penn I, Starzl TE. Malignant lymphomas in transplantation patients. A review of the world experience. Int J clin Pharmacol Ther Toxicol. 1970;3:49. [PMC free article] [PubMed] [Google Scholar]
- Prehn RT, Main JM. Immunity to methylcholanthrene induced sarcomas. J nat Cancer Inst. 1957;18:769. [PubMed] [Google Scholar]
- Pritzker KPH, Huang SN, Marshall KG. Malignant tumors following immunosuppressive therapy. Canad med Ass J. 1970;103:1362. [PMC free article] [PubMed] [Google Scholar]
- Rabbat AG, Jeejeebhoy HF. Heterologous antilymphocyte serum (ALS) hastens the appearance of methylcholanthrene induced tumours in mice. Transplantation. 1970;9:164. [PubMed] [Google Scholar]
- Santos GW. Application of marrow grafts in human disease: Its problems and potential. In: Hanna MG Jr, editor. Current Problems in Immunobiology. Plenum Publ. Co; 1971. In press. [Google Scholar]
- Santos GW, Sensenbrenner LL, Burke PJ, Colvin OM, Owens AH, Jr, Bins WB, Slavin RE. Marrow transplantation in man following cyclophosphamide. Transplant Proc. 1970;3:400. [PubMed] [Google Scholar]
- Schneck SA, Penn I. De novo cerebral neoplasms in renal transplant recipients. Lancet. 1971;1:983. doi: 10.1016/s0140-6736(71)91384-5. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Schwartz JA, Armstrong MYK, Beldotti L. Neoplastic sequelae of allogenic disease. I. Theoretical considerations and experimental design. Ann NY Acad Sci. 1966;129:804. [Google Scholar]
- Siegel JH, Janis R, Alper JC, Schutte H, Robbins L, Blaufox MD. Disseminated visceral Kaposi’s sarcoma appearing after human renal allografting. J Amer med Ass. 1969;207:1493. [PubMed] [Google Scholar]
- Simmons RL, Kelly WD, Tallent MB, Najarian JS. Cure of dysgerminoma with widespread metastases appearing after renal transplantation. New Engl J Med. 1970;283:190. doi: 10.1056/NEJM197007232830409. [DOI] [PubMed] [Google Scholar]
- Sjögren HO. Transplantation methods as a tool for detection of tumor specific antigens. Progr exp Tumor Res. 1965;6:289. [PubMed] [Google Scholar]
- Snell GD. The immunogenetics of tumor transplantation. Cancer Res. 1952;12:543. [PubMed] [Google Scholar]
- Southam CM. Host defense mechanisms and human cancer. Ann Inst Pasteur (Paris) 1964;107:585. [PubMed] [Google Scholar]
- Spatt SD, Grayzel DM. Primary carcinoma of the liver. Amer J Med. 1948;5:570. doi: 10.1016/0002-9343(48)90107-7. [DOI] [PubMed] [Google Scholar]
- Speck B, Dooren LJ, DeKoning J, van Bekkum DW, Eernisse JG, Elkerbout F, Vossen JM, van Rood JJ. Clinical experience with bone marrow transplantation: Failure and success. Transplant Proc. 1970;3:409. [PubMed] [Google Scholar]
- Starzl TE. Frontiers of surgery. In: Davis L, editor. Christopher’s Textbook of Surgery. W. B. Saunders Co; Philadelphia: 1964a. p. 1429. [Google Scholar]
- Starzl TE. Experience in Renal Transplantation. W. B. Saunders Co; Philadelphia: 1964b. [Google Scholar]
- Starzl TE. Discussion of Murray, J. E., Wilson, R. E., Tilney, N. L., Merrill, J. P., Cooper, W. C., Birtch, A G., Carpenter, C. B., Hager, E. B., Dammin, G. J. & Harrison, J. H.: Five years experience in renal transplantation with immunosuppressive drugs: Survival, function, complications, and the role of lymphocyte depletion by thoracic duct fistula. (Presented at the American Surgical Assoc., Boston, Mass., April 17, 1968) Ann Surg. 1968;168:416. doi: 10.1097/00000658-196809000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE. Experience in Hepatic Transplantation. W. B. Saunders Co; Philadelphia: 1969. with the assistance of C. W. Putnam. [Google Scholar]
- Starzl TE, Giles G, Lilly J, Takagi H, Martineau G, Schroter G, Halgrimson CG, Penn I, Putnam CW. Indications for orthotopic liver transplantation; with particular reference to hepatoma, biliary atresia, cirrhosis, Wilson’s disease and serum hepatitis. Transplant Proc. 1970;3:308. [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Groth CG, Brettschneider L, Smith GV, Penn I, Kashiwagi N. Perspectives in organ transplantation. (Presented at the Swiss Society of Immunology, Davos, Switzerland, March 27, 1968) Antibiot Chemother (Basel) 1969;15:349. doi: 10.1159/000386790. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Halgrimson CG, Penn I, Martineau G, Schroter G, Amemiya H, Putnam CW, Groth CG. Cyclophosphamide and human organ transplantation. Lancet. 1971;2:70. doi: 10.1016/s0140-6736(71)92046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Marchioro TL, Rifkind D, Rowlands DT, Jr, Waddell WR. Clinical experience with organ transplantation. Sth med J (Bgham, Ala) 1965;58:131. doi: 10.1097/00007611-196502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Porter KA, Halgrimson CG, Andres G, Hurwitz R, Giles G, Terasaki PI, Penn I, Lilly J, Starkie SJ, Schroter GPJ, Putnam CW. Long term survival after renal transplantation in humans. With special reference to histocompatibility matching, thymectomy, homograft glomerulone-nephritis, heterologous ALG, and recipient malignancy. Ann Surg. 1970b;172:437. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Porter KA, Iwasaki Y, Marchioro TL, Kashiwagi N. The use of antilymphocyte globulin in human renal homotransplantation. In: Wolstenholme GEW, O’Connor M, editors. Antilymphocytic Serum. J. and A Churchill Ltd; London: 1967. pp. 4–34. [Google Scholar]
- Starzl TE, Putnam CW, Brettschneider L, Penn I. The prospect of organ transplantation in cancer surgery. Proceedings of the Sixth National Cancer Conference; Presented at the Sixth National Cancer Conference; Denver, Colorado. September 18, 1968; New York: J. P. Lippincott Co; 1970c. pp. 45–55. [PubMed] [Google Scholar]
- Tallent MB, Simmons RL, Najarian JS. Primary carcinoma of the cervix appearing in immunosuppressed renal transplant recipient. Amer J Obstet Gynec. 1971;109:663. doi: 10.1016/0002-9378(71)90644-2. [DOI] [PubMed] [Google Scholar]
- Thomas L. Discussion of paper by P. B. Medawar (1957) Reactions to homologous tissue antigens in relation to hypersensitivity, March 12 and 13, 1957, New York City. (Symposium No. 9, New York Academy of Science) In: Lawrence HS, editor. Cellular and Humoral Aspects of the Hypersensitive States. Paul Hoeber, Inc; New York: 1959. p. 530. [Google Scholar]
- Toolan HW. Growth of human tumors in cortisone-treated laboratory animals, the possibility of obtaining permanently transplantable human tumors. Cancer Res. 1953;13:389. [PubMed] [Google Scholar]
- Tunner WS, Goldsmith EI, Whitsell JC., II Human homotransplantation of normal and neoplastic tissue from the same organ. J Urol. 1971;105:18. doi: 10.1016/s0022-5347(17)61451-4. [DOI] [PubMed] [Google Scholar]
- Tyler A. An immunological analysis of cancer. J nat Cancer Inst. 1960;25:1197. [Google Scholar]
- Van Bekkum DW, DeVries MJ. Radiation Chimeras. Academic Press; New York: 1967. [Google Scholar]
- Vandeputte M, Denys P, Leyten R, Desomer P. The oncogenic activity of polyoma virus in thymectomized rats. Life Sci. 1963;2:475. doi: 10.1016/0024-3205(63)90135-8. [DOI] [PubMed] [Google Scholar]
- Vredevoe DL, Hays EF. Effect of antilymphocytic and antithymocytic sera on the development of mouse lymphoma. Cancer Res. 1969;29:1685. [PubMed] [Google Scholar]
- Walker D, Gill TJ, III, Corson JM. Leiomyosarcoma in a renal allograft recipient treated with immunosuppressive drugs. J A mer med Ass. 1971;215:2084. [PubMed] [Google Scholar]
- Weiler E. Ciba Foundation Symposium on Carcinogenesis. Churchill; London: 1959. Loss of specific cell antigen in relation to carcinogenesis; p. 165. [Google Scholar]
- Wilson RE, Hager EB, Hampers CL, Carson JM, Merrill JP, Murray JE. Immunologic rejection of human cancer transplanted with a renal allograft. New Engl J Med. 1968;278:479. doi: 10.1056/NEJM196802292780904. [DOI] [PubMed] [Google Scholar]
- Woodruff MFA, Nolan B. Preliminary observations on treatment of advanced cancer by injection of allogeneic spleen cells. Lancet. 1963;2:426. doi: 10.1016/s0140-6736(63)92171-8. [DOI] [PubMed] [Google Scholar]
- Zukoski CF, Killen DA, Ginn E, Matter B, Lucas DO, Seigler HF. Transplanted carcinoma in an immunosuppressed patient. Transplantation. 1970;9:71. doi: 10.1097/00007890-197001000-00021. [DOI] [PubMed] [Google Scholar]
- Zukoski CF, Simmons JL, Killen DA, Ginn E, Matter B, Lucas D, Seigler H, Crews D. Cancer in patients on immunosuppressive therapy. Transplanted and spontaneous. J Amer med Ass. 1968;204:537. [Google Scholar]



