Abstract
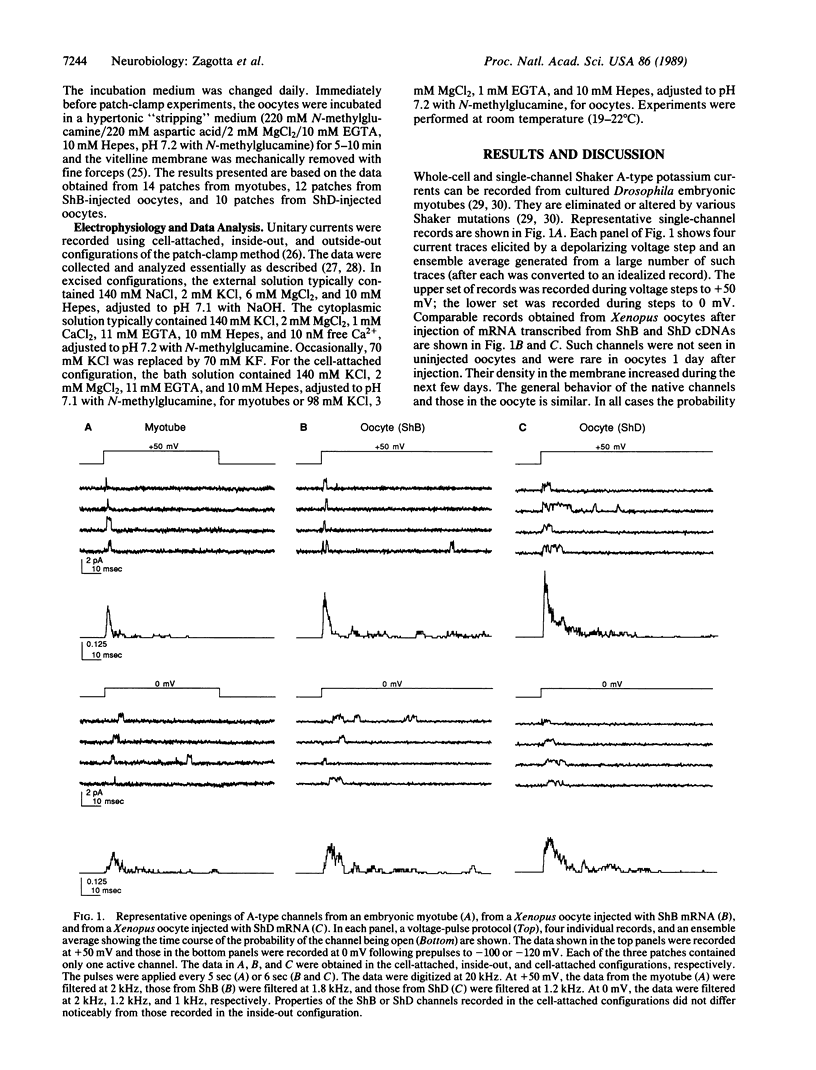
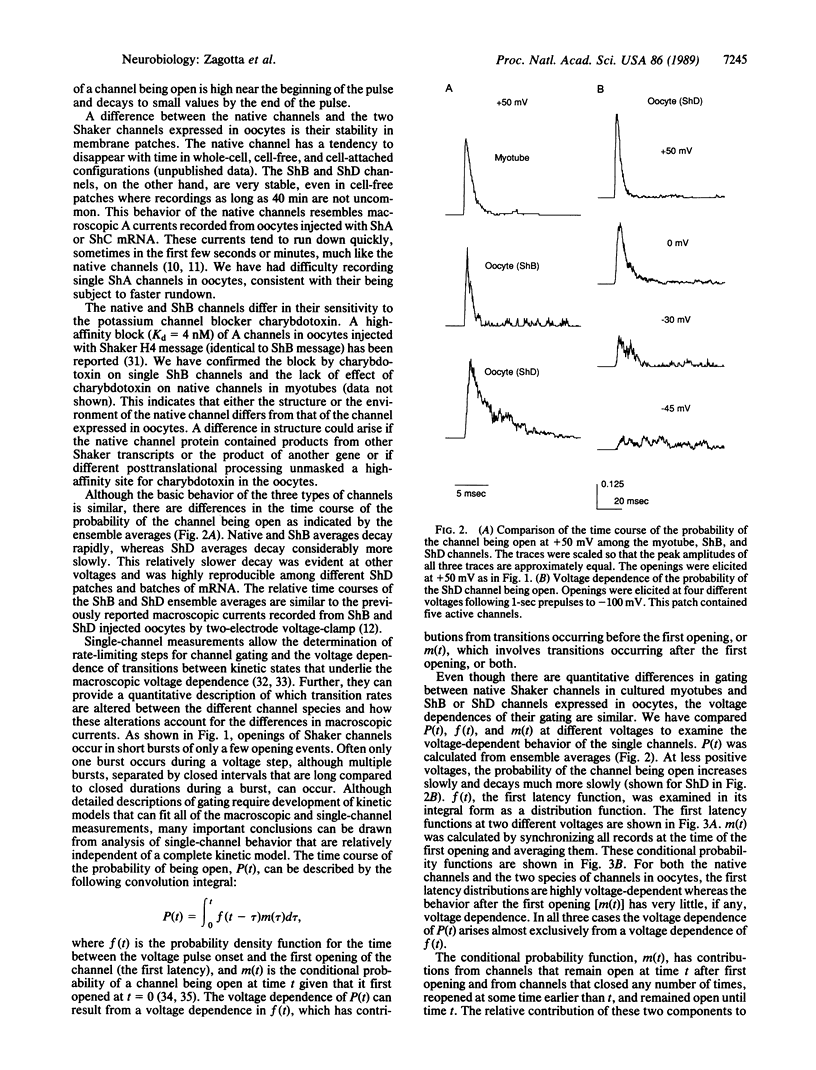
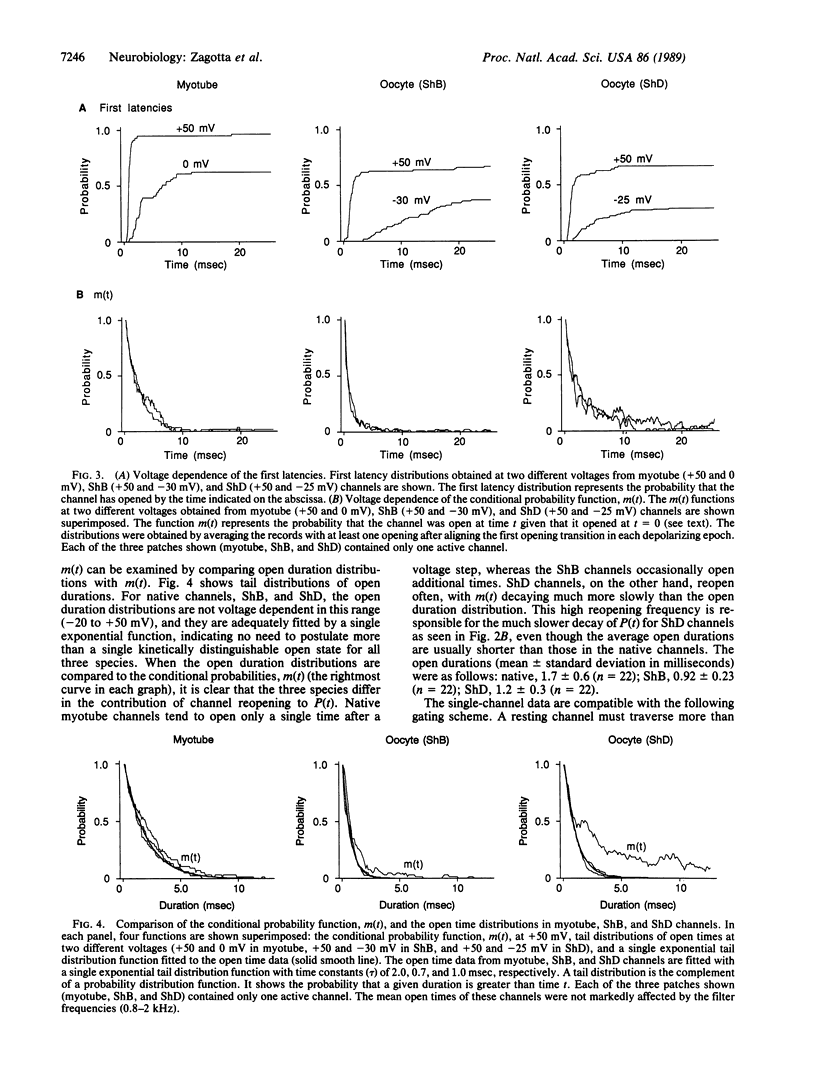
The voltage-dependent gating mechanism of single A-type potassium channels coded for by the Shaker locus of Drosophila was studied by single-channel recording. A-type channels expressed in Xenopus oocytes injected with Shaker B and Shaker D mRNA exhibited gating and voltage dependence that were qualitatively similar to those of the native Shaker A-types channels from embryonic myotubes. In all three channel types the molecular transition rates leading to the first opening were voltage-dependent, whereas all transitions after the first opening, including inactivation, were independent of voltage. While these channels exhibit some quantitative differences in their transition rates that account for the observed differences in macroscopic currents, in all three cases the voltage dependence of the macroscopic currents is determined by a voltage dependence in the time to first opening. This gating mechanism is similar to that of the vertebrate voltage-gated sodium channel and, together with the sequence similarities in the S4 region of the proteins, suggests a conserved mechanism for activation and inactivation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Baumann A., Krah-Jentgens I., Müller R., Müller-Holtkamp F., Seidel R., Kecskemethy N., Casal J., Ferrus A., Pongs O. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an I(A) channel transcript with homology to vertebrate Na channel. EMBO J. 1987 Nov;6(11):3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt R. E., Blatt Y., Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricus channel primary structure. FEBS Lett. 1985 Dec 2;193(2):125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci U S A. 1986 Jan;83(2):508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Gating kinetics of four classes of voltage-dependent K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):107–131. doi: 10.1085/jgp.91.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Voltage-dependent K+ currents and underlying single K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):73–106. doi: 10.1085/jgp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. E., Tanouye M. A., Lester H. A., Davidson N., Rudy B. A-type potassium channels expressed from Shaker locus cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5723–5727. doi: 10.1073/pnas.85.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Iverson L. E., Tanouye M. A. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987 Jul 31;50(3):405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Kamb A., Tseng-Crank J., Tanouye M. A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Kinetic properties of single sodium channels in rat heart and rat brain. J Gen Physiol. 1989 Jan;93(1):85–99. doi: 10.1085/jgp.93.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Reinhart P. H., White M. M. Charybdotoxin block of Shaker K+ channels suggests that different types of K+ channels share common structural features. Neuron. 1988 Dec;1(10):997–1001. doi: 10.1016/0896-6273(88)90156-0. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Jan Y. N., Jan L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987 Aug 14;237(4816):749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Butler A., Wei A., Scavarda N., Giffen K., Ifune C., Goodman R., Mandel G. Genomic organization and deduced amino acid sequence of a putative sodium channel gene in Drosophila. Science. 1987 Aug 14;237(4816):744–749. doi: 10.1126/science.2441469. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Zagotta W. N., Aldrich R. W. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987 May 29;236(4805):1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Jan Y. N., Jan L. Y. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988 Oct;1(8):659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988 Jan 14;331(6152):143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Brainard M. S., Aldrich R. W. Single-channel analysis of four distinct classes of potassium channels in Drosophila muscle. J Neurosci. 1988 Dec;8(12):4765–4779. doi: 10.1523/JNEUROSCI.08-12-04765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


