Abstract
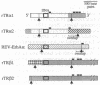
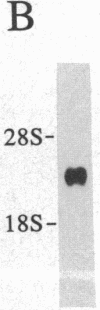
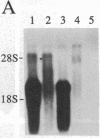
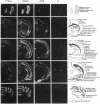
Multiple thyroid hormone receptor cDNAs have previously been identified in rat and are classified into alpha and beta subtypes. Alternative splicing of the alpha gene gives rise to the functional receptor, rTR alpha 1, and the non-thyroid hormone-binding isotype, rTR alpha 2. Recent evidence suggests the beta gene encodes two functional receptors, rTR beta 1, and the pituitary-specific receptor, rTR beta 2. By using synthetic DNA probes common to rTR beta transcripts and specific for rTR alpha 1 and rTR alpha 2 mRNAs, we mapped the expression of these transcripts in adult rat brain and pituitary by hybridization histochemistry. We also localized mRNAs encoding the putative nuclear receptor REV-ErbA alpha, a portion of which is derived from the opposite strand of the rTR alpha gene. rTR alpha 1 and rTR alpha 2 transcripts were widely distributed in a similar, if not identical, pattern. Highest levels of rTR alpha 1 and rTR alpha 2 transcripts were found in the olfactory bulb, hippocampus, and granular layer of the cerebellar cortex. REV-ErbA alpha and rTR beta mRNAs were found in more restricted patterns of expression distinct from those of rTR alpha 1 and rTR alpha 2. REV-ErbA alpha mRNA was highest in the neocortex. High levels of rTR beta transcripts in the anterior pituitary and the parvocellular part of the paraventricular hypothalamic nucleus suggest rTR beta gene products may mediate thyroid hormone feedback regulation of thyroid-stimulating hormone and thyrotropin-releasing hormone. Our results identify nuclei and structures in the mammalian central nervous system in which regulation of gene expression by specific thyroid hormone receptor subtypes may occur.
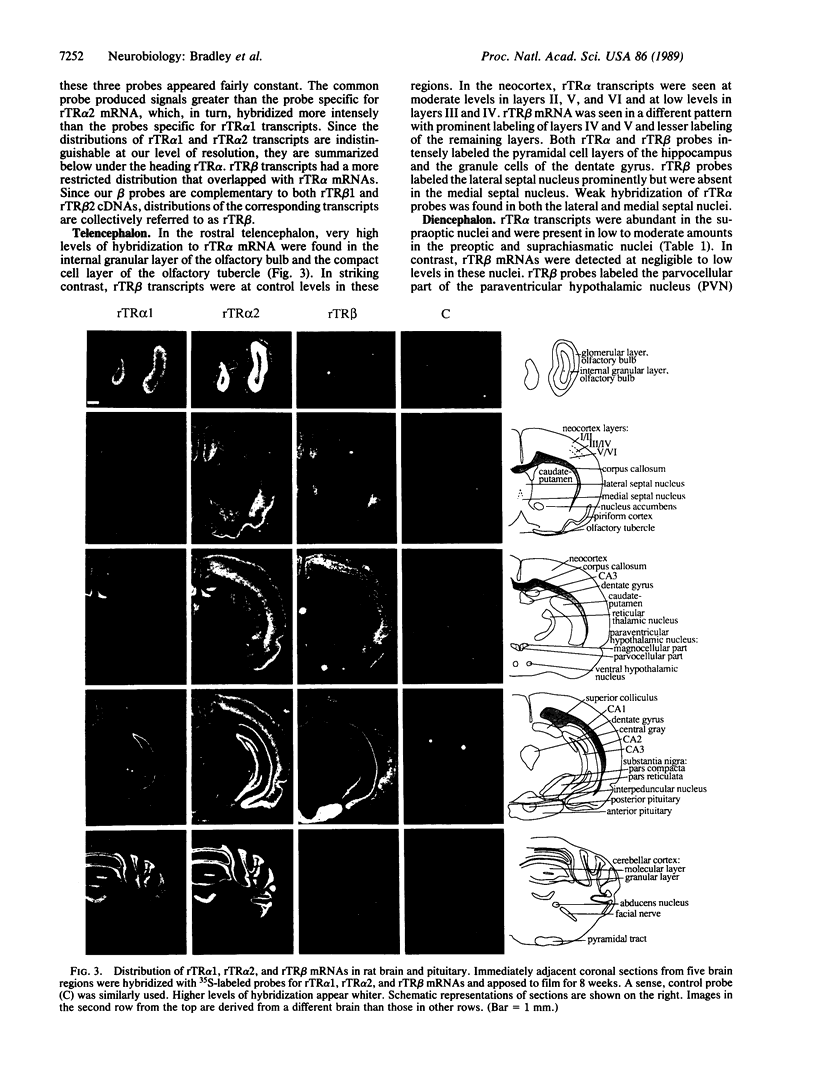
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D., Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987 Nov 6;238(4828):788–791. doi: 10.1126/science.3672126. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Need L. R., Chin W. W. Isolation and characterization of the rat thyrotropin beta-subunit gene. Differential regulation of two transcriptional start sites by thyroid hormone. J Biol Chem. 1987 Jan 25;262(3):981–987. [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman M. B., Crutchfield F. L., Futaesaku Y., Goldberger M. E., Murray M. [125I] triiodothyronine in the rat brain: evidence for neural localization and axonal transport derived from thaw-mount film autoradiography. J Comp Neurol. 1987 Jun 15;260(3):392–408. doi: 10.1002/cne.902600306. [DOI] [PubMed] [Google Scholar]
- Dussault J. H., Ruel J. Thyroid hormones and brain development. Annu Rev Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Gullo D., Sinha A. K., Woods R., Pervin K., Ekins R. P. Triiodothyronine binding in adult rat brain: compartmentation of receptor populations in purified neuronal and glial nuclei. Endocrinology. 1987 Jan;120(1):325–331. doi: 10.1210/endo-120-1-325. [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Jansson M., Philipson L., Vennström B. Isolation and characterization of multiple human genes homologous to the oncogenes of avian erythroblastosis virus. EMBO J. 1983;2(4):561–565. doi: 10.1002/j.1460-2075.1983.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. J., Wolff R. S., Warden M. K., Zoeller R. T. Thyroid hormones regulate levels of thyrotropin-releasing-hormone mRNA in the paraventricular nucleus. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7329–7333. doi: 10.1073/pnas.84.20.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. B., Zilz N. D., McCreary N. L., MacDonald M. J., Towle H. C. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988 Sep 5;263(25):12770–12777. [PubMed] [Google Scholar]
- Nakai A., Sakurai A., Bell G. I., DeGroot L. J. Characterization of a third human thyroid hormone receptor coexpressed with other thyroid hormone receptors in several tissues. Mol Endocrinol. 1988 Nov;2(11):1087–1092. doi: 10.1210/mend-2-11-1087. [DOI] [PubMed] [Google Scholar]
- Nakai A., Seino S., Sakurai A., Szilak I., Bell G. I., DeGroot L. J. Characterization of a thyroid hormone receptor expressed in human kidney and other tissues. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2781–2785. doi: 10.1073/pnas.85.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Ruel J., Faure R., Dussault J. H. Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons. J Endocrinol Invest. 1985 Aug;8(4):343–348. doi: 10.1007/BF03348511. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988 Apr;81(4):957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Nuclear triiodothyronine receptor sites in brain: probable identity with hepatic receptors and regional distribution. Endocrinology. 1978 Jul;103(1):267–273. doi: 10.1210/endo-103-1-267. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Ontogenesis of 3,5,3'-triiodothyronine receptors in neonatal rat brain: dissociation between receptor concentration and stimulation of oxygen consumption by 3,5,3'-triiodothyronine. Endocrinology. 1978 Sep;103(3):943–948. doi: 10.1210/endo-103-3-943. [DOI] [PubMed] [Google Scholar]
- Segerson T. P., Kauer J., Wolfe H. C., Mobtaker H., Wu P., Jackson I. M., Lechan R. M. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science. 1987 Oct 2;238(4823):78–80. doi: 10.1126/science.3116669. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986 Dec;387(3):231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]