Abstract
Background
Hemodynamic and neurohumoral function can affect the efficacy of diuretic therapy in congestive heart failure (CHF). Arginine vasopressin increases water reabsorption via the V2 receptor in the collecting duct, whereas B-type natriuretic peptide (BNP) decreases sodium reabsorption in the collecting duct. We hypothesized that combining BNP to the V2-receptor antagonist tolvaptan (TLV) would enhance renal excretory function by augmenting sodium excretion together with aquaresis without adversely affecting renal hemodynamics in experimental CHF.
Methods and Results
CHF was induced in three groups (all n=6) of dogs by tachypacing. An acute experiment was done after 10 days. After baseline measurements, study groups received a 0.1 mg/kg IV bolus of TLV alone (TLV), in combination with BNP (50 ng/kg/min; TLV+BNP), or BNP alone (BNP). *p<0.05 among groups. Mean arterial pressure increased with TLV, was unchanged with TLV+BNP, but decreased with BNP (+5±1 vs. −1±1 mmHg vs. −15±1 mmHg*). Renal blood flow and glomerular filtration rate were preserved with all regimens. Urine flow increased in all three groups, but significantly more so with TLV+BNP (TLV: +0.4±0.1 vs. TLV+BNP: +2.4±0.5 vs. BNP: +0.8±0.3 mL/min*). Only TLV+BNP and BNP were natriuretic* while only TLV and TLV+BNP increased electrolyte-free water excretion*. Compared to TLV alone, TLV+BNP prevented an increase in aldosterone*.
Conclusions
Coadministration of TLV and BNP in experimental HF resulted in a beneficial profile of renal, neurohumoral, and hemodynamic actions, specifically potent diuresis with natriuresis, neutral effect on mean arterial pressure, and lack of aldosterone activation.
Keywords: diuretics, heart failure, kidney, natriuretic peptides, pharmacology, V2 receptor antagonist, experimental model
INTRODUCTION
Arginine vasopressin (AVP, also called antidiuretic hormone, ADH) is a 9-amino acid peptide secreted from the posterior pituitary in response to high plasma osmolality and hypotension. Its major actions are to reduce free water excretion and maintain blood pressure. The latter is mediated via V1A receptors in the vasculature, while the former is mediated by V2 receptors in the renal inner medullary collecting duct. Binding to the V2 receptor via adenylate cyclase generates the second messenger cyclic adenosine monophosphate (cAMP), which promotes translocation of the water channel aquaporin 2 into the luminal membrane and thus increases water permeability and water reabsorption. In congestive heart failure (CHF) AVP secretion is increased, which can lead to hyponatremia and congestion.1 Both combined V1A/V2 as well as selective V2 receptor antagonists have been developed.
Tolvaptan (TLV) is a V2 receptor antagonist and has recently been approved by the FDA for the treatment of euvolemic and hypervolemic hyponatremia. Indeed, the large multicenter Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) reported that chronic TLV was safe and induced an aquaresis as demonstrated by a reduction in body weight during the initial hospitalization for acute decompensated HF. Associated with this was an improvement in dyspnea although TLV neither improved nor worsened the primary outcome variable, which was mortality.2,3 While V2 receptor antagonism can aid in the management of congestion through aquaresis, it lacks natriuretic actions and its cardiovascular actions remain incompletely defined.
The 32-amino acid cardiac hormone B-type natriuretic peptide (BNP), like AVP, targets the renal inner medullary collecting duct but rather than increasing water reabsorption it reduces sodium reabsorption contributing to natriuresis especially if administered at non-hypotensive doses. Studies have established that mature BNP is a 32- amino acid peptide derived primarily from the heart and that its circulating levels increase in the setting of cardiac overload and ventricular stretch. BNP activates the guanylate cyclase A receptor (GC-A; also called natriuretic peptide A receptor (NPR-A)) and the second messenger cGMP is produced; ultimately, resulting in vasodilatation, renin and aldosterone suppression, and natriuresis. It was approved for the treatment of acute decompensated heart failure (ADHF) in 2001 but concerns regarding excessive hypotension have been advanced as a limitation to its use in CHF.4-6 Its use in CHF however has been advocated especially at non-hypotensive doses as increased immunoreactive BNP in plasma in patients with CHF may represent altered molecular forms of BNP with reduced biological actions.7-9
Recognizing the pure aquaretic and nonvasodilating properties of TLV and the natriuretic and hypotensive actions of BNP, one could hypothesize an important synergistic action between the two when infused together in CHF. Here we tested the hypothesis that the addition of BNP to TLV would evoke a natriuretic action together with TLV's diuretic action in experimental CHF. We also sought to define in experimental CHF the acute hemodynamic actions of TLV specifically on arterial pressure, systemic vascular resistance and cardiac filling pressures and the modulating actions of BNP. Here we hypothesized that TLV, which does not block the V1A-mediated vascular actions of AVP, would have no vasodilating actions and thus not reduce cardiac filling pressures while producing an aquaresis. We further hypothesized that the addition of BNP would attenuate any vasoconstriction by V1A activation by AVP that was displaced during TLV administration, but without the hypotension frequently seen with BNP alone. Therefore, the goal of the current study in experimental CHF was to define for the first time the interactions of these two sodium and water regulating therapeutic agents when acutely infused simultaneously in experimental CHF.
METHODS
The current study was performed in male mongrel dogs (weight 20-28 kg) in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Animal Care and Use Committee.
Severe CHF was induced in three groups of dogs (n=6 per group) by rapid right ventricular pacing at 240 beats per minute as previously described and characterized in detail.10-13 On day 11 of pacing, cardiorenal parameters were assessed in an acute study under anesthesia with pentobarbital and fentanyl. Pacing was suspended for the induction of anesthesia and surgical preparation. Animals were intubated and mechanically ventilated with room air and supplemental oxygen (5 L/min). A flow-directed balloon-tipped thermodilution catheter was inserted via the right external jugular vein for hemodynamic measurements, and aortic pressure was assessed via a line inserted via the femoral artery. Cardiac output was assessed by the thermodilution method in triplicate and averaged (Cardiac output model 9510-A computer, American Edwards Laboratories, Irvine, CA). Via a left flank incision the left ureter was cannulated for urine collection. An electromagnetic flow probe was placed on the renal artery (Carolina Medical Electronics) to measure renal blood flow. At the end of the surgical preparation, pacing was restarted and inulin (1 mL/min; preceded by a weight adjusted bolus) and saline (1 mL/min) were continuously administered via lines in the femoral vein. After 60 minutes of equilibration, a 30-minute baseline clearance was done that included urine collection, blood sampling, and hemodynamic measurements. Pressure tracings and renal blood flow were recorded and analyzed digitally (Sonometrics Corporation, London, Ontario, Canada). After the baseline clearance one group received TLV alone (TLV), the second group received TLV and BNP (TLV+BNP), and the third group received BNP alone (BNP). Tolvaptan was administered as an intravenous bolus (0.1 mg/kg) and was a kind gift from Otsuka America Pharmaceutical, Rockville, MD, USA. Canine BNP was administered as an intravenous infusion (50 ng/kg/min; Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). Fifteen minutes after tolvaptan administration or start of BNP infusion, a second 30-minute clearance was started.
Assays
Electrolytes were measured by flame photometry (IL943, Instrumentation Laboratory, Lexington, MA). Plasma renin activity, aldosterone, atrial natriuretic peptide (ANP), and BNP were measured by radioimmunoassay as described previously.10 Glomerular filtration rate (GFR) was calculated by inulin clearance. Plasma and urine inulin were measured by the anthrone method.14 Osmolality was measured using a vapor pressure osmometer (VAPRO 5520, Wescor, Inc., Logan, UT, USA). Electrolyte-free water clearance (E-C-H2O) was calculated as:
with [Na]U, sodium concentration in urine, [K]U, potassium concentration in urine, [Na]P, sodium concentration in plasma.
Statistical Analysis
Data are provided as mean (standard deviation) if normally distributed or median (25th/75th percentile in tables, interquartile range in graphs) if not normally distributed. Parameters at baseline and with drug administration were compared within groups with paired t-test or, for not normally distributed data, with Wilcoxon signed-rank test. Groups were compared by analyzing changes from baseline to drug administration clearance with one-way analysis of variance and post-hoc Bonferroni test or, for not normally distributed data, with Kruskal-Wallis test and post-hoc Dunn's test. Statistical significance was accepted at p<0.05.
RESULTS
Cardiorenal parameters at baseline (Table 1) were consistent with a CHF phenotype with increased systemic and renal vascular resistances, decreased cardiac output, elevated cardiac filling pressures, decreased UNaV, and neurohumoral activation.11-13 There were some differences in baseline parameters among groups, mostly between the TLV and BNP groups. Changes from baseline within groups are shown in Table 2 and Figures 1-3.
Table 1.
Cardiorenal and neurohumoral function in the three experimental groups at baseline.
| TLV | TLV+BNP | BNP | p-value* | |
|---|---|---|---|---|
| Hemodynamic function | ||||
| Mean arterial pressure (mmHg) | 110 (10) | 110 (13) | 108 (10) | 0.90 |
| Systemic vascular resistance (mmHg·L−1·min) |
68 (6) | 58 (17) | 43 (14) | 0.02b |
| Cardiac output (L/min) | 1.49 (0.13) | 1.85 (0.51) | 2.52 (0.79) | 0.02b |
| Right atrial pressure (mmHg) | 9.8 (2.6) | 8.3 (2.2) | 8.4 (3.2) | 0.58 |
| Pulmonary capillary wedge pressure (mmHg) |
24 (2) | 24 (4) | 22 (4) | 0.41 |
| Renal perfusion pressure (mmHg) | 101 (11) | 101 (12) | 101 (6) | 0.99 |
| Renal blood flow (mL/min) | 94 (32) | 102 (34) | 236 (136) | 0.02b |
| Renal vascular resistance (mmHg·L−1·min) |
1170 (367) | 924 (428) | 520 (220) | 0.02b |
| Renal function | ||||
| Glomerular filtration rate (mL/min) | 25.2 (12.5) | 31.5 (6.2) | 37.0 (7.3) | 0.12 |
| Urine flow (mL/min) | 0.07 (0.02) | 0.17 (0.16) | 0.33 (0.33) | 0.13 |
| Urinary Na+ excretion (μEq/min) | 1.4 (0.4/3.0) | 0.6 (0.5/1.9) | 4.3 (2.9/7.7) | 0.02 |
| Urinary K+ excretion (μEq/min) | 7 (5/19) | 9 (4/19) | 22 (19/25) | 0.02c |
| Electrolyte-free water excretion (mL/min) |
−1.03 (0.73) | −0.37 (0.45) | 0.15 (0.30) | 0.005b |
| Filtration fraction | 0.50 (0.34) | 0.47 (0.23) | 0.30 (0.15) | 0.34 |
| Urinary cGMP excretion (pmol/min) | 1487 (1110/2301) |
1446 (1152/1945) |
2187 (1640/2934) |
0.18 |
| Urine osmolality (mosm/L) | 1137 (621) | 1118 (468) | 694 (174) | 0.15 |
| Humoral function | ||||
| Hematocrit (%) | 39 (4) | 38 (6) | 41 (4) | 0.50 |
| Plasma Na+ (mmol/L) | 145 (6) | 159 (9) | 149 (7) | 0.006a |
| Plasma K+ (mmol/L) | 4.7 (0.4) | 4.9 (0.5) | 4.4 (0.3) | 0.18 |
| Plasma Osm (mOsm/L) | 305 (14) | 301 (6) | 320 (15) | 0.055 |
| BNP (pg/mL) | 69 (59/75) | 82 (59/137) | 32 (27/40) | 0.01b |
| cGMP (pmol/mL) | 26 (5) | 21 (3) | 22 (4) | 0.07 |
| Plasma renin activity (ng·mL−1·h) | 19 (4) | 15 (8) | 18 (7) | 0.32 |
| Angiotensin II (pg/mL) | 104 (49/136) | 44 (24/163) | 75 (34/84) | 0.21 |
| Aldosterone (ng/dL) | 63 (28) | 61 (48) | 44 (27) | 0.67 |
Values are mean (SD) for normally distributed data or median (25th/75th percentile) for not normally distributed data.
p-value for comparison of baseline values between groups.
P<0.05 in post-hoc tests is indicated as follows:
TLV vs. TLV+BNP
TLV vs. BNP
TLV+BNP vs. BNP.
BNP, B-type natriuretic peptide, TLV, tolvaptan.
Table 2.
Changes in cardiorenal and neurohumoral function from baseline in the three experimental groups with tolvaptan alone, tolvaptan plus BNP, or BNP alone, respectively.
| Δ with TLV |
Δ with TLV+BNP |
Δ with BNP |
p-value† | |
|---|---|---|---|---|
| Hemodynamic function | ||||
| Cardiac output (L/min) | −0.05 (0.07) | −0.08 (0.23) | +0.51 (1.13) | 0.26 |
| Right atrial pressure (mmHg) | −0.3 (0.8) | −0.7 (0.9) | −1.3 (0.6)* | 0.15 |
| Pulmonary capillary wedge pressure (mmHg) | −0.9 (2.0) | −1.2 (0.8)* | −4.0 (1.0)* | 0.0029b,c |
| Renal perfusion pressure (mmHg) | +6 (3) | −1 (2) | −14 (3) | <0.0001a,b,c |
| Renal blood flow (mL/min) | +3 (9) | +14 (30) | +24 (34) | 0.40 |
| Renal vascular resistance (mmHg·L−1·min) |
+69 (210) | +43 (583) | −124 (85)* | 0.56 |
| Renal function | ||||
| Urinary potassium excretion (μEq/min) | +5 (1/7) | +15 (8/21)* | +24 (15/39)* | 0.0027b |
| Filtration fraction | −0.04 (0.29) | +0.08 (0.32) | −0.03 (0.14) | 0.67 |
| Urinary cGMP excretion (pmol/min) | +348 (−507/1120) | +5356 (2320/8142)* | +6369 (4196/7682)* | 0.0022a,b |
| Urine osmolality (mosm/L) | −708 (657)* | −1013 (468)* | −310 (92)* | 0.13 |
| Humoral function | ||||
| Hematocrit (%) | +1 (3) | 0 (2) | 0 (1) | 0.83 |
| Plasma Na+ (mmol/L) | +5 (8) | −1 (7) | −3 (6) | 0.12 |
| Plasma K+ (mmol/L) | −0.1 (0.2) | −0.1 (0.3) | −0.3 (0.2)* | 0.26 |
| Plasma Osm (mOsm/L) | +3 (10) | +2 (8) | −19 (17) | 0.015b,c |
| Angiotensin II (pg/mL) | +5 (−20/18) | −15 (−79/−5) | −29 (−39/−21)* | 0.39 |
Values are mean (SD) for normally distributed data or median (25th/75th percentile) for not normally distributed data.
indicates p<0.05 vs. respective baseline level.
p-value for comparison of changes from baseline induced by TLV vs. TLV+BNP vs. BNP.
P<0.05 in post-hoc tests is indicated as follows:
TLV vs. TLV+BNP
TLV vs. BNP
TLV+BNP vs. BNP.
BNP, B-type natriuretic peptide, TLV, tolvaptan; Δ, change from baseline level.
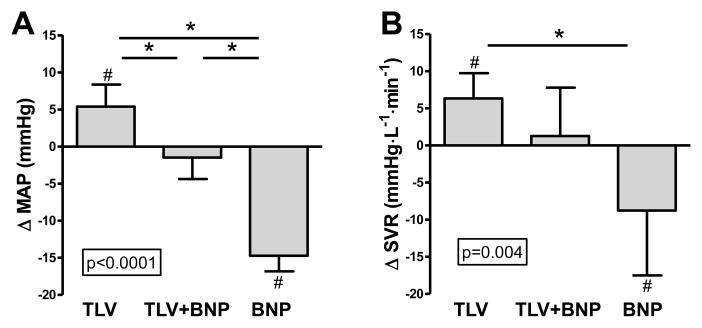
Figure 1.
Change in mean arterial pressure (A) and systemic vascular resistance (B) with TLV, TLV+BNP, and BNP (A). P-value in box is from ANOVA for comparison of groups. *p<0.05 between groups. #p<0.05 vs. respective baseline. B-type natriuretic peptide, MAP, mean arterial pressure, SVR, systemic vascular resistance, TLV, tolvaptan. Data is mean (standard deviation).
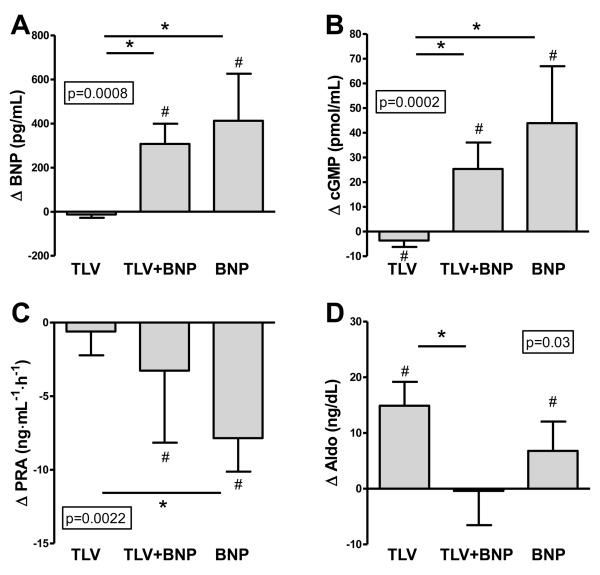
Figure 3.
Changes in plasma BNP (A), plasma cGMP (B), plasma renin activity (C), and plasma aldosterone (D) with TLV, TLV+BNP, and BNP (A). P-value in box is from ANOVA for comparison of groups. *p<0.05 between groups. #p<0.05 vs. respective baseline. BNP, B-type natriuretic peptide, cGMP, cyclic guanosine monophosphate, PRA, plasma renin activity, TLV, tolvaptan. Data is median (interquartile range) except for (B), which is mean (standard deviation).
Cardiovascular function
Mean arterial pressure increased with TLV, remained unchanged with TLV+BNP, and decreased with BNP, and this was significant between groups (Fig. 1A). The same was true for renal perfusion pressure, which was calculated as mean arterial pressure minus right atrial pressure. Systemic vascular resistance, likewise, increased with TLV, was unchanged with TLV+BNP, and decreased with BNP, but this was significant between TLV and BNP only (Fig. 1B). Cardiac output (CO) and renal blood flow were unchanged in all groups. Right atrial pressure and renal vascular resistance decreased only with BNP, but this was not significant compared to the other groups. In contrast, pulmonary artery pressure and pulmonary capillary wedge pressure decreased with BNP and this was significant compared to TLV or TLV+BNP.
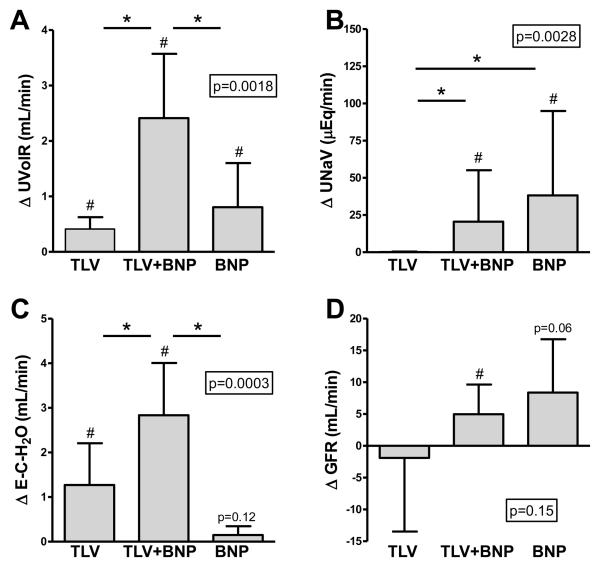
Renal function
Urine flow (UVolR) increased significantly with TLV alone, with TLV+BNP, and with BNP alone, but significantly more so with TLV+BNP (Fig. 2A). In contrast, urinary sodium excretion (UNaV; Fig. 2B) and potassium excretion were increased with BNP alone and TLV+BNP, but not with TLV alone. Urine osmolality decreased with all three regimens. Electrolyte-free water clearance increased in both TLV and TLV+BNP, but significantly more so with TLV+BNP (Fig. 3C), and this was highly significant compared to TLV and BNP alone. Glomerular filtration rate (GFR) was unchanged with TLV, tended to increase with BNP (p=0.06), and significantly increased with TLV+BNP with no difference between groups (Fig. 2D). Consistent with the administration of the GC-A agonist BNP, urinary excretion of the second messenger cGMP (UcGMPV) increased with BNP alone and with TLV+BNP, and this was significant compared to TLV alone.
Figure 2.
Change in urine flow (A), urinary sodium excretion (B), electrolyte-free water clearance (C), and glomerular filtration rate (D) with TLV, TLV+BNP, and BNP (A). P-value in box is from ANOVA for comparison of groups. *p<0.05 between groups. #p<0.05 vs. respective baseline. BNP, B-type natriuretic peptide, TLV, tolvaptan. Data is mean (standard deviation) except for (B), which is median (interquartile range).
Humoral function
There were no significant differences among groups with regard to changes in plasma sodium, potassium, and hematocrit. Plasma osmolality decreased with BNP compared to TLV and TLV+BNP. As expected with the infusion of exogenous BNP, BNP levels increased in the BNP alone and TLV+BNP groups but were unchanged in the TLV group (Fig. 3A). Plasma cGMP decreased with TLV and increased with BNP alone and with TLV+BNP (Fig. 3B). Plasma renin activity was unchanged with TLV but decreased with TLV+BNP and with BNP, and this was significant between TLV and BNP (Fig. 3C). Angiotensin II levels followed a similar pattern (levels tended to decrease with TLV+BNP, p=0.06), but this was not significant among groups. Aldosterone increased significantly from baseline in both the TLV alone and BNP alone groups, but was unchanged in TLV+BNP, even though this group had the largest diuresis (Fig. 3D).
A qualitative summary of the major changes in the three experimental groups is shown in Table 3.
Table 3.
Qualitative summary of major changes in the three experimental groups.
| Parameter | TLV | TLV+BNP | BNP |
|---|---|---|---|
| Hemodynamic Function | |||
| Mean arterial pressure | ↑ | ↔ | ↓ |
| Systemic vascular resistance | ↑ | ↔ | ↓ |
| Renal perfusion pressure | ↑ | ↔ | ↓ |
| Renal function | |||
| Urine flow | ↑ | ↑ ↑ | ↑ |
| Urinary sodium excretion | ↔ | ↑ | ↑ |
| Electrolyte-free water excretion | ↑ | ↑ ↑ | ↔ |
| Humoral function | |||
| Plasma renin activity | ↔ | ↓ | ↓ |
| Aldosterone | ↑ | ↔ | ↑ |
Changes are within-group changes. Differences between groups were mostly but not always significant (see Table 1 and 2 and Figures for details). ↑, increase, ↓, decrease, ↔, no change, ↑↑, increase greater than ↑.
DISCUSSION
We report for the first time the cardiorenal actions of co-administration of the V2-antagonist TLV and the GC-A agonist BNP in experimental HF. TLV alone as expected was aquaretic, increased blood pressure and SVR and, in this model of experimental HF, activated aldosterone. BNP alone acted as a natriuretic, decreased blood pressure and SVR, but increased aldosterone, perhaps secondary to hypotension and diuresis. Addition of BNP to TLV augmented free water excretion and produced a natriuresis. Importantly, BNP+TLV had a neutral effect on blood pressure and SVR, consistent with the changes in opposing directions produced by TLV alone or BNP alone, respectively, and similar results were seen for renal perfusion pressure. There was no activation of the renin-angiotensin-aldosterone system (RAAS) with TLV+BNP. Thus, co-administration of TLV together with BNP may be a compelling strategy to optimize water and sodium excretion in CHF without RAAS activation and without reducing renal perfusion pressure.
With regard to renal function, both TLV alone and BNP alone significantly increased urine flow, but TLV+BNP increased urine flow substantially more so. In contrast, TLV did not increase urinary sodium excretion, while BNP alone and TLV+BNP did. These findings are in keeping with TLV acting as a pure aquaretic via V2-receptor antagonism, while the natriuresis with TLV+BNP can be explained by the known natriuretic actions of BNP.15-17 Interestingly, electrolyte-free water clearance was significantly higher with TLV+BNP as compared to TLV alone or BNP alone, which can in part be due to increased delivery of sodium and water to the collecting duct through reductions in proximal sodium and water reabsorption previously reported with both ANP and BNP. Also, while GFR remained unchanged with TLV alone, it increased with TLV+BNP and tended to increase with BNP; however, this was not significant among groups. The GFR-enhancing action of BNP could be due to an increase in the coefficient of ultrafiltration or a differential effect on the afferent and efferent glomerular arteriole so as to increase glomerular hydrostatic pressure.18 Also, in cultured inner medullary collecting duct cells ANP, which like BNP activates GC-A, has been reported to promote retrieval of AQP2 from the cell membrane to the cytosol, which may be able to enhance the aquaretic actions of TLV.19
Diuretic therapy is frequently associated with the activation of sodium retaining neurohumoral systems, which can counteract the diuretic and natriuretic actions and reduce GFR.20-23 Particularly, diuretics may potently activate the RAAS with deleterious actions. In this study, TLV alone did not change plasma renin activity or angiotensin II but significantly increased aldosterone. The mechanism of the increase may be further stimulation of the unblocked V1A receptor in the adrenals. Indeed, it has been reported that in mice AVP induced aldosterone release from adrenal gland cells via the V1A receptor and that AVP activated the RAAS via the V1A receptor in macula densa cells.24,25 In contrast, addition of BNP to TLV significantly reduced plasma renin activity and prevented the increase in aldosterone. Again, these differential changes occurred despite the fact that TLV+BNP induced a greater diuresis and natriuresis. These findings are in keeping with the direct suppressing actions of GC-A activation on renin and aldosterone secretion.15-17 However, BNP alone in this study also caused an increase in plasma aldosterone, presumably secondary to hypotension because when the hypotension was negated by combining TLV with BNP, this increase in aldosterone was not seen even though the diuretic response was greater. These results emphasize that aldosterone secretion is determined by a complex interplay of factors that include V1A receptor activation, GC-A activation, and blood pressure.
With respect to hemodynamic actions, TLV increased mean arterial pressure and systemic vascular resistance, while cardiac output remained unchanged. As previously noted, TLV acts by blocking V2 receptors in the collecting duct cells but it leaves V1A receptors in the vasculature readily accessible to endogenous AVP. Therefore, the increase in MAP and SVR may be explained by increased binding to the V1A receptor by AVP blocked from the V2 receptor. To our knowledge, this is the first invasive hemodynamic study of acute V2 receptor antagonism with TLV in a model of CHF which then documents an acute vasoconstriction and blood pressure elevating action. Addition of BNP with its vasodilating actions prevented this increase in MAP and SVR, thus resulting in a neutral effect. We also observed that cardiac filling pressures did not acutely decrease with TLV despite the aquaresis. It is possible that the increase in SVR and afterload offset an unloading action of TLV. Also, a more prolonged period of observation and diuresis may have demonstrated a decrease in pulmonary capillary wedge pressure. Indeed, Udelson et al reported that in the ECLIPSE trial TLV dose-dependently increased urine output and also decreased PCWP compared to placebo.26 No significant differences were reported for the secondary endpoints of systolic blood pressure, systemic or pulmonary vascular resistance, cardiac index, or heart rate. Of note, most of these patients were on concomitant medication and it is unclear to what extent the volume loss could have offset a V1A-mediated increase in blood pressure.26 Nonetheless, in our study renal hemodynamics were preserved despite the increase in systemic vascular resistance as GFR, renal blood flow and renal vascular resistance remained unchanged. Addition of BNP to TLV also did not decrease right atrial pressure, whereas pulmonary capillary wedge pressure decreased with TLV+BNP but this was not significant as compared to TLV. Similar to TLV alone, TLV+BNP did not change renal blood flow and renal vascular resistance. BNP alone reduced mean arterial pressure compared to TLV and TLV+BNP. The same was true for pulmonary capillary wedge pressure, which was probably due to the larger decrease in afterload rather than a diuretic effect, which was higher in TLV+BNP. Importantly, corresponding to the reduction in mean arterial pressure, BNP alone decreased renal perfusion pressure, which may offset some of the direct renal enhancing actions of BNP.
A major therapeutic aim in patients with CHF is to induce renal excretion of water and sodium to reduce congestion without impairing renal function. Conventional diuretics such as loop diuretics and thiazides act primarily as saluretics by blocking sodium channels in the luminal membrane of tubular cells, thus increasing intraluminal electrolyte concentration and ultimately, for osmotic reasons, water excretion is then increased. Disadvantages are that increased sodium in the tubule can reduce GFR via tubuloglomerular feedback, promote hypertrophy of tubular cells in more distant nephron segments, and the potential for electrolyte imbalance such as hyponatremia if the electrolyte loss is high relative to the water excretion. Indeed, renal dysfunction in terms of decreased GFR as well as hyponatremia are important complications in the treatment of CHF and are associated with increased morbidity and mortality.27-31 Therefore, the development of strategies to enhance water and sodium excretion without inducing renal dysfunction and electrolyte imbalance is a high priority.
Tolvaptan represents the first selective and orally available V2 receptor antagonist and as mentioned above has recently been evaluated in patients hospitalized with acute decompensated CHF in the EVEREST trial.2,3 A recombinant form of human BNP, nesiritide, was approved for the treatment of acute decompensated HF in the US in 2001 and is also being evaluated for other indications. There is still controversy regarding the value of BNP, particularly its renal actions, which may in part be secondary to a dosing issue as some studies used regimens with doses high enough to induce significant hypotension.4-7,32,33 In contrast, BNP in lower doses was associated with improved renal function.7,32 While a small number of patients in the EVEREST trial received both TLV and BNP, to the best of our knowledge our study is the first to assess formally co-administration of TLV and BNP which again co-target the inner medullary collecting duct cells in the control of sodium and water homeostasis. Co-administration of TLV and BNP may be a beneficial strategy to mobilize congestion in patients with CHF. The tendency of TLV to increase MAP and SVR may prevent some of the hypotension reported with BNP.
There are several limitations to this study. The neurohumoral and renal alterations observed in this pacing model may not perfectly correspond to the alterations in CHF with e.g. longer duration, atherosclerotic disease, and preexisting renal disease. The baseline differences, primarily between the TLV alone and BNP alone groups, could have affected the response to the drugs. Also, we only investigated the short-term effects of the drugs.
In summary, addition of BNP augmented the diuretic actions of TLV and attenuated some of the adverse hemodynamic and neurohumoral effects seen with either TLV or BNP alone. Co-administration of TLV and BNP may represent a beneficial strategy to induce enhanced water and sodium excretion while maintaining renal perfusion pressure in CHF and suppressing the RAAS thus representing a more physiologic therapy for sodium and water retention in CHF. Further studies are required to assess whether these acute findings in experimental CHF translate into improved outcomes in human CHF patients.
Acknowledgements
We are very grateful to Denise M. Heublein and Sharon M. Sandberg for their technical assistance.
Funding sources
This research was supported by grants HL-36634 (JCB) and HL07111 (GB) from the National Institutes of Health, the Mayo Foundation, and the Marriott Foundation.
BIBLIOGRAPHY
- 1.Goldsmith SR, Francis GS, Cowley AW, Jr., Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 2.Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Konstam MA, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 4.VMAC-Investigators Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 5.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 6.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:I134–138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 8.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr., Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederkofler E, Kiernan U, O'Rear J, Menon S, Saghir S, Protter A, Nelson R, Schellenberger U. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1:258–264. doi: 10.1161/CIRCHEARTFAILURE.108.790774. [DOI] [PubMed] [Google Scholar]
- 10.Lisy O, Lainchbury JG, Leskinen H, Burnett JC., Jr. Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension. 2001;37:1089–1094. doi: 10.1161/01.hyp.37.4.1089. [DOI] [PubMed] [Google Scholar]
- 11.Chen HH, Schirger JA, Chau WL, Jougasaki M, Lisy O, Redfield MM, Barclay PT, Burnett JC., Jr. Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation. 1999;100:2443–2448. doi: 10.1161/01.cir.100.24.2443. [DOI] [PubMed] [Google Scholar]
- 12.Cataliotti A, Boerrigter G, Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM, Chen HH, Malatino LS, Burnett JC., Jr. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]
- 13.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 14.Fuhr J, Kaczmarczyk K, Kruttgen C. Eine einfache colorimetrische Methode zur Inulinbestimmung fuer Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955;33:729–733. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986;83:4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasue H, Yoshimura M. Natriuretic peptides in the treatment of heart failure. J Card Fail. 1996;2:S277–285. doi: 10.1016/s1071-9164(96)80088-1. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi K, Nawata H, Kato K, Ibayashi H, Matsuo H. Alpha-human atrial natriuretic polypeptide inhibits steroidogenesis in cultured human adrenal cells. J Clin Endocrinol Metab. 1986;62:941–944. doi: 10.1210/jcem-62-5-941. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Hirata Y, Nanba S, Tojo A, Matsuoka H, Sugimoto T. Effects of atrial natriuretic peptide on renal arterioles: morphometric analysis using microvascular casts. Am J Physiol. 1990;259:F936–944. doi: 10.1152/ajprenal.1990.259.6.F936. [DOI] [PubMed] [Google Scholar]
- 19.Klokkers J, Langehanenberg P, Kemper B, Kosmeier S, von Bally G, Riethmuller C, Wunder F, Sindic A, Pavenstadt H, Schlatter E, Edemir B. Atrial natriuretic peptide and nitric oxide signaling antagonizes vasopressin-mediated water permeability in inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2009;297:F693–703. doi: 10.1152/ajprenal.00136.2009. [DOI] [PubMed] [Google Scholar]
- 20.Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- 23.Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, Burnett JC., Jr. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–278. doi: 10.1152/ajprenal.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birumachi J, Hiroyama M, Fujiwara Y, Aoyagi T, Sanbe A, Tanoue A. Impaired arginine-vasopressin-induced aldosterone release from adrenal gland cells in mice lacking the vasopressin V1A receptor. Eur J Pharmacol. 2007;566:226–230. doi: 10.1016/j.ejphar.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Aoyagi T, Izumi Y, Hiroyama M, Matsuzaki T, Yasuoka Y, Sanbe A, Miyazaki H, Fujiwara Y, Nakayama Y, Kohda Y, Yamauchi J, Inoue T, Kawahara K, Saito H, Tomita K, Nonoguchi H, Tanoue A. Vasopressin regulates the renin-angiotensin-aldosterone system via V1a receptors in macula densa cells. Am J Physiol Renal Physiol. 2008;295:F100–107. doi: 10.1152/ajprenal.00088.2008. [DOI] [PubMed] [Google Scholar]
- 26.Udelson JE, Orlandi C, Ouyang J, Krasa H, Zimmer CA, Frivold G, Haught WH, Meymandi S, Macarie C, Raef D, Wedge P, Konstam MA, Gheorghiade M. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:1540–1545. doi: 10.1016/j.jacc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 28.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 29.Kearney MT, Fox KA, Lee AJ, Prescott RJ, Shah AM, Batin PD, Baig W, Lindsay S, Callahan TS, Shell WE, Eckberg DL, Zaman AG, Williams S, Neilson JM, Nolan J. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. J Am Coll Cardiol. 2002;40:1801–1808. doi: 10.1016/s0735-1097(02)02490-7. [DOI] [PubMed] [Google Scholar]
- 30.Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr., Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111:2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 32.Mentzer RM, Jr., Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr., Luber JM, Jr., Smedira NG. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Wang DJ, Dowling TC, Meadows D, Ayala T, Marshall J, Minshall S, Greenberg N, Thattassery E, Fisher ML, Rao K, Gottlieb SS. Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine. Circulation. 2004;110:1620–1625. doi: 10.1161/01.CIR.0000141829.04031.25. [DOI] [PubMed] [Google Scholar]