Abstract
Glycemic disorders resolve following Roux-en-Y gastric bypass (RYGB) surgery, but early and longer-term mechanisms regarding effects on β-cell dysfunction as well as relationships with decreasing adiposity are not well understood. We evaluated longitudinal changes in peripheral insulin sensitivity (Si), the acute insulin response to glucose (AIRg), and the composite estimate of β-cell function, the disposition index (DI), over 24 mo via frequently sampled intravenous glucose tolerance testing in severely obese women who had fasting normoglycemia (n = 16) and hyperglycemia (n = 11) before RYGB surgery; homeostatic model assessment (HOMA-IR) estimated insulin resistance; air displacement plethysmography determined adipose tissue mass. At baseline, subjects with normoglycemia had adequate DI associated with elevated AIRg, but DI was markedly reduced in subjects with hyperglycemia. Within 1–6 mo post-RYGB, glycemic control was normalized in subjects with hyperglycemia related to reduced HOMA-IR (−54% at 1 mo, P < 0.005) and increased DI (23-fold at 6 mo vs. baseline, P < 0.05). Over 24 mo, DI improved in subjects with hyperglycemia (15-fold vs. baseline, P < 0.005) and also modestly in subjects with normoglycemia (58%, P < 0.05), due largely to increased Si. Decreasing adiposity correlated with longer-term HOMA-IR and Si values at 6 and 24 mo, respectively. In patients exhibiting fasting hyperglycemia before surgery, β-cell function improved early following RYGB, due largely to increases in insulin secretion. For both normoglycemic and hyperglycemic subjects, further improvement or stabilization of β-cell function over the 2 yr is due largely to improved Si associated with reduced adiposity.
Keywords: disposition index, weight loss, adipose tissue mass
severe obesity, defined as body mass index greater than 40 kg/m2, is associated with an increased prevalence of type 2 diabetes (T2DM) (5). Bariatric surgery is the most efficient method of producing substantial weight loss in severely obese patients (42). Surgical procedures induce weight loss by reducing the volume of the stomach, thereby restricting food intake, or by diverting the flow of ingested food to the ileum and bypassing the duodenum and the proximal jejunum, which promotes malabsoption (39). Roux-en-Y gastric bypass (RYGB) is a common and restrictive plus malabsorptive procedure; other procedures include adjustable gastric banding, a restrictive-only surgery, and biliopancreatic diversion, a primarily malabsorptive procedure that is less common in the US. (39).
As has been found in overweight and obese individuals (47), severely obese persons with prediabetes and diabetes have a decline in β-cell function such that insulin secretion is inadequate to compensate for reduced insulin sensitivity (Si) (19, 37). A special feature of bariatric surgery is its ability to induce remission of T2DM (9). Following adjustable gastric banding, remission of diabetes is strongly related to weight loss (15, 38). However, a greater effect of diabetes resolution is seen with malabsorptive surgeries RYGB and biliopancreatic diversion (9), which can induce normalization of glucose within weeks, before appreciable weight loss occurs. This suggests that rapid remission of diabetes is related to mechanisms that are independent of weight loss.
Diabetes remission early following surgery, observed exclusively following RYGB and biliopancreatic diversion, has been shown to be related to dramatic improvements in insulin secretion (25, 34, 41) and decreases in the HOMA-IR index (4, 34, 36), a surrogate of Si that primarily reflects hepatic insulin resistance. Improvement in β-cell function is also thought to be the basis for improvement in glucose levels following bariatric surgery, but little information currently exists on effects of bariatric surgery on β-cell function. β-Cell function can be assessed in vivo using the disposition index (DI) constant, which describes an individual's insulin secretion response for a prevailing level of Si. However, rarely have both insulin secretion and Si been measured longitudinally following bariatric surgery using detailed methods (19, 22, 41). In patients with prediabetes and diabetes undergoing RYGB and biliopancreatic diversion, improvement in the DI was found in subjects early (1–7 mo) following surgery (19, 41). The longer-term effects of bariatric surgery on patients with euglycemia and hyperglycemia remain unanswered.
Given the popularity of RYGB, the mechanisms responsible for its antidiabetic effects are needed. The purpose of this study was to determine the early (1 and 6 mo) and longer-term (24 mo) impact of RYGB on the DI, as a primary end point, in severely obese women who were normoglycemic or exhibited hyperglycemia (prediabetes and T2DM) at baseline. The contribution of decreasing adiposity to Si and secretion outcomes following surgery was also examined.
MATERIALS AND METHODS
Patients.
Subjects in the study were 27 consecutively enrolled severely obese female patients who had weight loss treatment at the Emory Bariatrics Center via laparoscopic RYGB surgery by a single surgeon (E. Lin), as has been described (28). Each patient served as her own control, and subjects were evaluated at baseline (before surgery) and at 1 mo (38 ± 2 days, n = 27), 6 mo (177 ± 12 days, n = 27), and 24 mo (823 ± 36 days, n = 15) following surgery. Based on baseline fasting blood glucose concentrations, subjects were categorized as having normoglycemia (<5.56 mmol/l, n = 16) or hyperglycemia [≥5.56 mmol/l, n = 11; 4 patients were categorized as having prediabetes, and 11 had T2DM (3)]. For the study's primary end point, the DI, a prior study of subjects with T2DM showed an improvement in DI of 83-fold during 7 mo following RYGB and BPD, suggesting a sample size of n = 6, β = 0.89, α = 0.05 (19). The Emory University Institutional Review Board approved the study, and all patients gave informed consent (IRB no. 333-2002). Subjects were excluded if they 1) were male, 2) were less than 18 or greater than 65 yr of age, 3) had a body mass index (BMI) < 40 kg/m2, or 4) smoked. Subjects were weight stable, defined as not undergoing a change in weight of more than 1 kg during the week of testing, at baseline (before surgery), and at 24 mo postsurgery. All medication use was monitored throughout the study.
Glucose tolerance testing.
The insulin-modified frequently sampled intravenous glucose tolerance test (FSIGTT) (29) was chosen to assess insulin action in vivo, as it provides information about peripheral Si and first-phase insulin secretion (AIRg) for the calculation of the DI in a single test. Patients were admitted to the Emory General Clinical Research Center on the night before FSIGTT testing and fasted overnight (12 h). Before testing, diabetes medication use was adjusted so that baseline glucose levels were close to normal, but medications were withheld from patients on the morning of testing. Serum glucose was quantified at the Emory University Hospital Laboratory using the Beckman Coulter Alex 20 automated system; assay limit 0.17 mM (Beckman Coulter, Brea, CA). Insulin was measured by regular and ultrasensitive immunoassay; assay limits 1 μU/ml and 0.07 μU/ml, respectively with less than 1% cross-reactivity to proinsulin and C-peptide (Mercordia, Winston Salem, NC). Minimal-modeling analysis (6) was used to quantify Si, AIRg, and DI (MinMod Millennium, Los Angeles, CA; http://research.vet.upenn.edu/biomath/CurrentProjects/DiabetesGlucoseMetabolism/tabid/1622/Default.aspx). HOMA-IR was calculated using fasting insulin (mU/l) × fasting glucose (mM)/22.5 (32). To distinguish insulin secretion and hepatic insulin clearance, C-peptide concentrations were measured for a limited number of subjects during each of the time points for a 24-mo period (ARUP Laboratories, Salt Lake City, UT). The assay limit is 0.1 ng/ml, and the coefficient of variation is <10%. Insulin and C-peptide concentrations during first-phase insulin response to the IVGTT are plotted for a representative subject in Supplemental Fig. S3 (Supplemetal materials are found in the online version of this paper at the journal website). Responses of insulin and C-peptide in the first 10 min of the FSIGTT occurred in parallel, suggesting a close approximation between determined AIRg and insulin secretion. Comparisons with published values of reference controls (23, 44) were possible, as similar populations and FSIGTT methodology were used, and glucose/insulin assays had comparable specificity and sensitivity.
Anthropometry, body composition and fat distribution.
Body fat composition was measured by air plethysmography (BOD-POD; Life Measurement Instruments, Concord, CA). We found that the coefficient of variation and the measurement error were similar to published values (33). Waist circumference was obtained by tape measure at the smallest point of the torso below the most inferior rib. Body height was measured without shoes. Body weight was measured with subjects in light clothing, in the fasting state, and immediately after voiding in the morning.
Statistical analysis.
The statistical software packages STATISTICA (StatSoft, Tulsa, OK) was used for analysis. Analysis of variance with repeated measures was used to determine group differences and changes over time; post hoc comparisons used Tukey's test. Only when indicated in the text, a paired t-test was used to test for differences between time points within the same group, but this analysis is less conservative. Relationships among measurements were examined as standard multiple regression analysis, covariates tested were age, glycemic status, and race/ethnicity group at baseline. Data are presented separately for 0–6 mo for the entire group of 27 subjects and from 0–24 mo for the subgroup of 15 subjects, although similar trends were observed over the 0–6 mo period for all subjects; data were compared using unpaired t-tests. χ2 Analysis was used to compare baseline proportions race and glycemic status of 24-mo completers vs. noncompleters. Results are expressed as means ± SE.
RESULTS
Baseline patient characteristics.
Patient characteristics are described in Table 1. Twenty-seven subjects completed longitudinal analysis up to 6 mo postsurgery, and fifteen of these were evaluated again at 24 mo. The average age, BMI, postmenopausal status, and race/ethnicity were comparable between normoglycemic and hyperglycemic groups. Of the 12 patients not measured at 24 mo, two were ineligible due to pregnancy, two were undergoing serious illnesses unrelated to surgery, five were lost to follow-up due to moving from the area or inability to contact, and three had not reached the 24-mo time point. However, we found no differences in baseline or in 6-mo characteristics and measurements in subjects who completed vs. those who did not complete the 24-mo assessment. For example, there was no difference in weight loss at 6 mo for 24-mo completers vs. noncompleters (P = 0.51). Also, the glycemic status of completers vs. non completers was comparable (P = 0.48).
Table 1.
Baseline characteristics
| Normal Glycemia | Hyperglycemia | |
|---|---|---|
| n | 16 | 11 |
| Age, yr | 33.8 ± 2.1 | 38.3 ± 2.5 |
| Body mass index, kg/m2 | 47.4 ± 0.9 | 48.5 ± 1.3 |
| Ethnicity, %total population: black; white; Hispanic | 31; 63; 6 | 9; 72; 18 |
| Postmenopausal status, %total population | 13 | 18 |
| Antihyperglycemic medication use, %population | 0 | 64 |
Values are means ± SE. Baseline characteristics of 27 subjects completed longitudinal analysis ≤6 mo postsurgery, of these subjects, 15 were evaluated again at 24 mo. Patients exhibiting normal glycemia had fasting plasma glucose measures <5.6 mmol/l; patients exhibiting hyperglycemia had measures >5.6 mmol/l.
Changes in adiposity following weight loss surgery.
Anthropometric values at baseline and changes following RYGB for normoglycemic and hyperglycemic subjects are shown in Table 2 for 27 subjects followed out to 6 mo and in Supplemental Table S1 for the 15 subjects followed out to 24 mo. At baseline, there were no differences in adiposity between the normoglycemic and hyperglycemic groups. For all subjects, body weight, body mass index, and body fat mass decreased as early as 1 mo and continued to decrease at 6 and 24 mo postsurgery (P < 0.05 for 1 mo, P < 0.005 for 6 and 24 mo). For both groups, decreases in waist circumference were evident at 6 and 24 mo following surgery (both P < 0.005). Body fat mass decreased similarly among normoglycemic and hyperglycemic subjects (average −12.3% and −14.2%, respectively, at 1 mo, and −43.1% and −43.2% at 6 mo, vs. baseline). Also, over the 24-mo period, the amount of fat lost by the normoglycemic (−59.0 ± 5.4%) and hyperglycemic groups (−46.0 ± 7.2%) groups were comparable (P < 0.17).
Table 2.
Anthropometric and glycemic responses during 6 mo following RYGB
| Baseline | 1 mo | Δ 1 mo | 6 mo | Δ 6 mo | ||
|---|---|---|---|---|---|---|
| BMI, kg/m2 | Normal glycemia | 47.4 ± 0.9 | 42.8 ± 0.9‡ | −4.5 ± 0.4 | 34.1 ± 1.0‡ | −13.3 ± 0.6 |
| Hyperglycemia | 48.5 ± 1.3 | 43.6 ± 1.4‡ | −4.8 ± 0.4 | 35.0 ± 1.8‡ | −13.5 ± 0.9 | |
| Body weight, kg | Normal glycemia | 126.6 ± 2.7 | 114.4 ± 2.6‡ | −12.2 ± 1.0 | 91.1 ± 2.8‡ | −35.5 ± 1.5 |
| Hyperglycemia | 125.3 ± 3.7 | 112.7 ± 3.5‡ | −12.6 ± 1.3 | 90.6 ± 5.0‡ | −34.7 ± 2.3 | |
| Body fat mass, kg | Normal glycemia | 71.2 ± 2.4 | 63.1 ± 2.3‡ | −12.0 ± 3.3 | 40.7 ± 2.2‡ | −30.4 ± 1.3 |
| Hyperglycemia | 71.8 ± 3.0 | 61.5 ± 2.7‡ | −10.3 ± 1.2 | 41.5 ± 4.0‡ | −30.3 ± 2.1 | |
| Waist circumference, cm | Normal glycemia | 132.0 ± 4.1 | 126.6 ± 4.3 | −4.9 ± 1.3 | 110.4 ± 4.2‡ | 21.5 ± 2.3 |
| Hyperglycemia | 132.4 ± 4.9 | 126.7 ± 3.7 | −5.7 ± 2.3 | 110.0 ± 5.3‡ | −22.4 ± 2.1 | |
| Glucose, mmol/l | Normal glycemia | 4.63 ± 0.11a | 3.94 ± 0.11a* | −0.68 ± 0.11 | 4.03 ± 0.09 | −0.60 ± 0.11 |
| Hyperglycemia | 6.94 ± 0.33b | 5.15 ± 0.38b‡ | −1.79 ± 0.44 | 4.51 ± 0.25‡ | −2.43 ± 0.42 | |
| Hemoglobin A1c, % | Normal glycemia | 5.2 ± 0.2a | 5.1 ± 0.0 | −0.1 ± 0.2 | 5.1 ± 0.1 | −0.1 ± 0.1 |
| Hyperglycemia | 7.6 ± 0.7b | 6.8 ± 0.4 | −0.9 ± 0.5 | 5.8 ± 0.2* | −1.8 ± 0.8 | |
| Insulin, mU/l | Normal glycemia | 13.49 ± 1.44 | 6.00 ± 0.77‡ | −7.50 ± 1.23 | 3.47 ± 0.43‡ | −10.03 ± 1.53 |
| Hyperglycemia | 13.63 ± 1.29 | 7.99 ± 0.94‡ | −5.63 ± 1.52 | 5.74 ± 1.37‡ | −7.89 ± 1.52 | |
| HOMA-IR, mU/l·mM | Normal glycemia | 2.79 ± 0.32a | 1.06 ± 0.14‡ | −1.74 ± 0.28 | 0.62 ± 0.08‡ | −2.17 ± 0.35 |
| Hyperglycemia | 4.27 ± 0.50b | 1.95 ± 0.38‡ | −2.32 ± 0.57 | 1.28 ± 0.43‡ | −2.99 ± 0.58 |
Values are means ± SE. Parameters were determined in 27 subjects with normal glycemia (n = 16) and hyperglycemia (n = 11) who were measured during 6 mo, following Roux-en-Y gastric bypass (RYGB) surgery.
P < 0.05, †P < 0.01,
P < 0.005 vs. baseline.
Changes in glycemic control following surgery.
At baseline,7 of 11 subjects in the hyperglycemic group were using various antihyperglycemic medications, including oral biguanides, sulfonylureas, thiazolidinediones, glucagon-like peptide-1 agonists, and insulin, although medications were withheld on the morning of intravenous glucose tolerance testing. At 1 and 6 mo following surgery, 2 of 11 subjects continued medication therapy, and at 24 mo following surgery, one subject continued medication therapy. Patients with normoglycemia had fasting plasma glucose and hemoglobin A1c concentrations in the normal range at baseline, and these did not change following surgery (Table 2 and Supplemental Table S1 for subjects followed to 24 mo). Despite medication use at baseline, subjects with hyperglycemia exhibited poor glucose control at baseline, having fasting blood glucose concentrations of 6.94 ± 0.33 mmol/l and hemoglobin A1c concentrations of 7.6 ± 0.7%. Except for one hyperglycemic subject, blood glucose concentrations were normalized at 1 mo (mean, 5.2 ± 0.4 mmol/l, P < 0.005 vs. baseline), and values continued to decrease at 6 and 24 mo following surgery; hemoglobin A1c concentrations were normalized at 6 mo following surgery (mean, 5.8 ± 0.2%, P < 0.05 vs. baseline). At baseline, subjects with hyperglycemia had higher HOMA-IR values than normoglycemic subjects (P < 0.05), and both groups had higher than reference values (40). Normalization of HOMA-IR occurred at 1 mo, associated with dramatic decreases in values with further decreases occurring at 6 and 24 mo following surgery (all time points, P < 0.005 vs. baseline).
Changes in insulin action following surgery.
Peripheral Si was below normal values (23) in all subjects, indicating severe insulin resistance, and there were no differences between normoglycemic and hyperglycemic groups (Table 3 for subjects followed for 6 mo following surgery; Supplemental Table S2 for subjects followed for 24 mo). However, consistent with poorer glucose control, subjects with hyperglycemia showed dramatically reduced AIRg values compared with normoglycemic subjects (P < 0.005), as well as to reference values from healthy controls (44). Associated with inadequate AIRg, DI was reduced in the hyperglycemic group (P < 0.005), showing that β-cell function was inadequate for the severity of insulin resistance. In contrast, DI was normal in the normoglycemic group, primarily related to the increased AIRg response (44).
Table 3.
Indexes of insulin secretion and Si at baseline and during 6-mo weight loss following RYGB
| Baseline |
1 mo |
6 mo |
||||
|---|---|---|---|---|---|---|
| Normal Glycemia | Hyperglycemia | Normal Glycemia | Hyperglycemia | Normal Glycemia | Hyperglycemia | |
| AIRg | 750 ± 100a | 28 ± 15b | 780 ± 180a | 154 ± 50b | 430 ± 40* | 203 ± 48 |
| Si | 1.58 ± 0.14 | 1.87 ± 0.24 | 1.95 ± 0.30 | 2.56 ± 0.40 | 2.92 ± 0.25‡ | 3.00 ± 0.46* |
| DI | 1110 ± 130a | 23 ± 28b | 1070 ± 130a | 403 ± 170b | 1190 ± 100a | 522 ± 89*b |
Values are means ± SE. Acute insulin response to glucose (AIRg, μU·ml−1·min−1), β-cell function, disposition index (DI), and peripheral insulin sensitivity (Si, min−1·μU−1·ml−1) were measured as described in materials and methods. Changes over 6 mo (n = 27) following surgical intervention were measured in patients who exhibited normal glycemia (n = 16) and those with glycemic disorders (n = 11);
P < 0.05,
P < 0.01 vs. baseline; values with different letters (a, b) are significantly different vs. normal glycemia group, P < 0.05.
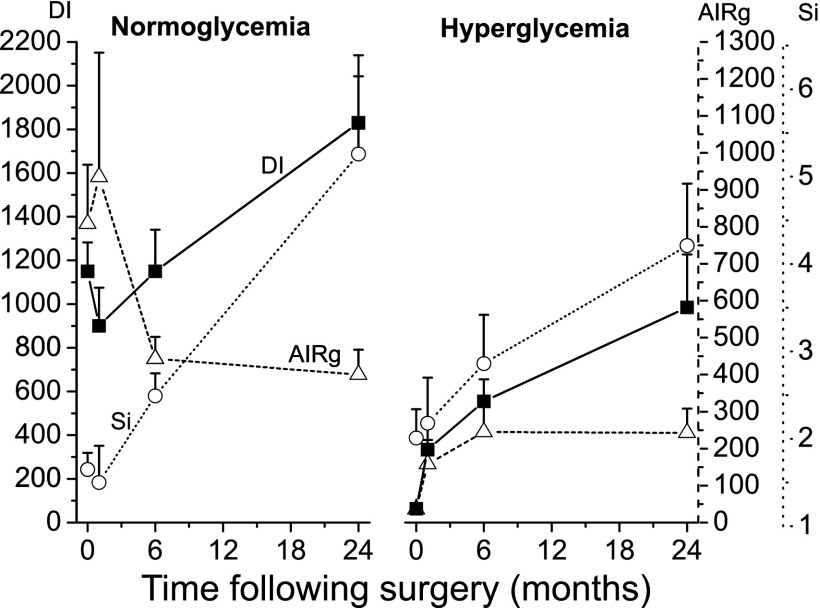
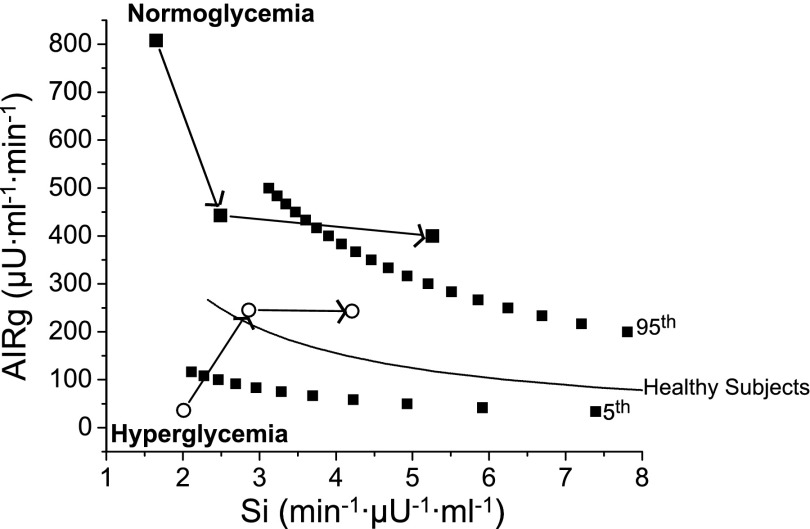
Longitudinal changes in AIRg, peripheral Si, as well as the composite measure DI are shown in Table 3 (for subjects followed for 6 mo) and in Fig. 1 and Supplemental Table S2 (for subjects followed for 24 mo). In each group, there were generally progressive improvements in Si at 6 mo (by 85% for the normoglycemic group, P < 0.005; by 60% for the hyperglycemic group, P < 0.05) and at 24 mo (3.2-fold in the normoglycemic group, P < 0.005; 2.0-fold in the hyperglycemic group, P < 0.05). AIRg fell in the normoglycemic group over 6 mo (by −43%, P < 0.05) but tended to improve in the hyperglycemic group at 1 and at 6 mo (5.5- and 7.3-fold, P = 0.02 and P = 0.002, respectively, using paired t-test). The reciprocal relationship between Si and insulin secretion represented by the DI is well described (7, 24); changes to the DI result from increases in AIRg or Si or both. Graphic representation of this relationship based on longitudinal measurements of both parameters is presented in Fig. 2 (for subjects followed out to 24 mo). For subjects with normoglycemia, DI changed little over 6 mo, reflecting reciprocal changes in Si and AIRg. In contrast, DI improved over 6 mo in subjects with hyperglycemia (23-fold vs. baseline, P < 0.05), largely associated with improved AIRg and Si. Over 24 mo, DI improved further, as seen by shifts to the right in the curve in both groups (by 58% vs. baseline, P < 0.05 in the normoglycemic group; 15-fold in the hyperglycemic group, P < 0.005), reflecting consistent improvement in Si without further change in AIRg.
Fig. 1.
Baseline insulin action and responses following Roux-en-Y gastric bypass (RYGB) surgery in patients with normoglycemia and hyperglycemia. The impact of RYGB on peripheral insulin sensitivity (Si, μU·min−1·ml−1, ○ and dotted line), the acute insulin response to glucose (AIRg, μU·ml−1·min−1, ▵ and dashed line) and the disposition index (DI, min−1, ■ and solid line) were measured via intravenous glucose tolerance test from baseline to 24 mo in severely obese women (n = 15) with normoglycemia and hyperglycemia at baseline, as described in materials and methods.
Fig. 2.
Graphic representation of the relationship between Si and insulin secretion during the 24-mo intervention period. Relationships are depicted between Si and AIRg in severely obese women with normoglycemia (■) and hyperglycemia at baseline (○) from baseline to 6 and to 24 mo (depicted by arrows) following RYGB (n = 15). Data of the same relationship in healthy controls was provided by Kahn et al (24); the mean relationship as well as the 5th and 95th percentiles are presented. Hyperglycemic subjects experienced normalization of the relationship, as shown by upward and rightward shifts of their dots at 6 mo following surgery. Normoglycemic subjects were in the normal range of response of the DI at baseline and at 6 mo but experienced improvement, signified by a rightward shift of their dot, at 24 mo following surgery.
Associations among adiposity and measures of insulin action.
We tested for cross-sectional and longitudinal associations between adiposity and in vivo measurements of insulin action using multiple regression analysis. At baseline, no cross-sectional relationships were found between fat mass and AIRg, Si, or DI. Associations between fat mass and Si grew stronger as time progressed following surgery, so that at 24 mo a significant negative association was observed (β = −0.56, P = 0.04; Table 4). Also, a greater adipose tissue decrease over 24 mo negatively predicted Si at that time point (β = −0.58, P = 0.038). These relationships remained significant after adjustment for race and baseline glycemic status, but not age (data not shown). As found for Si, associations between HOMA-IR and fat mass grew progressively stronger with weight loss (Table 5), with significant cross-sectional associations independent of baseline age, race, and glycemic status found at 6 mo (β = 0.60, P = 0.001). At 24 mo, the cross-sectional association between adipose tissue mass and HOMA-IR was also significant (β = 0.71, P = 0.004), but this was not independent of age, race, and glycemic status. Greater decrease in adipose tissue mass at 6 and 24 mo was a determinant of HOMA-IR values at those respective time points (β = 0.53, P < 0.004, independent of age, race, and glycemic status; β = 0.61, P = 0.02, respectively). No cross-sectional or longitudinal relationships were observed between fat mass and indexes of insulin secretion (data not shown).
Table 4.
Relationships between fat mass and peripheral Si
| Fat Baseline | Fat 1 mo | Fat 6 mo | Fat 24 mo | Δ Fat 24 mo | |
|---|---|---|---|---|---|
| Si baseline | −0.34 | ||||
| 0.87 | |||||
| Si 1 mo | −0.06 | ||||
| 0.79 | |||||
| Si 6 mo | −0.18 | ||||
| 0.37 | |||||
| Si 24 mo | −0.56 | −0.58 | |||
| 0.04 | 0.04 |
Cross-sectional associations between fat mass and peripheral Si at baseline and 1, 6, and 24 mo following surgery were determined using multiple regression analysis; β-statistic, and associated P values are presented (n = 27). Longitudinal association between change (Δ) in fat mass and the final value of Si at 24 mo following surgery. Significant associations are highlighted in boldface (n = 15). Associations remained significant after adjusting for race and glycemic status at baseline, but not age.
Table 5.
Relationships between fat mass and HOMA-IR
| Fat Baseline | Fat 1 mo | Fat 6 mo | Fat 24 mo | Δ Fat 6 mo | Δ Fat 24 mo | |
|---|---|---|---|---|---|---|
| HOMA-IR baseline | −0.13 | |||||
| 0.52 | ||||||
| HOMA-IR 1 mo | 0.37 | |||||
| 0.064 | ||||||
| HOMA-IR 6 mo | 0.60 | 0.53 | ||||
| 0.001 | 0.004 | |||||
| HOMA-IR 24 mo | 0.71 | 0.61 | ||||
| 0.004 | 0.02 |
Cross-sectional associations between fat mass and HOMA-IR at baseline and 1, 6, (n = 27) and 24 mo (n = 15) following surgery were determined using multiple regression analysis; β-statistic and associated P values are presented. Significant associations are highlighted in boldface. Also shown are significant longitudinal associations between change in fat mass and the final value of Si at 24 mo following surgery. Significant associations between fat mass and HOMA-IR at 6 mo, but not 24 mo, remained after adjusting for age, race, and glycemic status at baseline.
DISCUSSION
The major new findings in this study are that the DI, a measure of β-cell function, improved when measured longitudinally over 24 mo following RYGB in subjects with both normal and glycemic disorders at baseline, subsequent to early increases insulin secretion and later improvement in Si. Decreasing adiposity was associated with improvements in Si but not changes in insulin secretion. These findings extend previous observations of early improvement in β-cell function in subjects undergoing RYGB to those followed over the longer term.
Using the FSIVGTT, we simultaneously estimated in vivo peripheral Si and insulin secretion in severely obese patients before and during 24 mo following RYGB. The findings highlight the role of β-cell function, whereby pancreatic β-cells adjust insulin secretion relative to changes in peripheral insulin resistance for the maintenance of euglycemia. Before undergoing surgery, all severely obese individuals in the present study exhibited impaired peripheral Si, which is in line with previous evidence suggesting that insulin resistance is a primary consequence of severe obesity (11, 19), but subjects varied in capacity for β-cell function. Despite having peripheral insulin resistance, glucose tolerance was maintained in subjects having normal glycemia, associated with insulin hypersecretion compared with a published reference group (44). This observation is consistent with the hyperbolic relationship between Si and insulin secretion described by the DI; having intact β-cell function largely explains the avoidance of dysglycemic disorders by the normoglycemic group (7, 24). In contrast, subjects who had fasting hyperglycemia exhibited profound β-cell dysfunction. Similar diminished insulin secretion in severely obese patients with T2DM has been demonstrated (19, 37). Moreover, data in overweight and mildly obese individuals have shown a major role for decreased β-cell function in the development of glucose intolerance and diabetes (14, 18, 47, 48). As has been shown for individuals with lesser obesity (2, 16), hepatic insulin resistance as suggested by elevated HOMA-IR also contributed to hyperglycemia. Taken all together, the preoperative findings highlight the roles of β-cell dysfunction, as well as insulin resistance, in the pathophysiology of T2DM in severely obese individuals and are consistent with findings from individuals with lesser obesity.
In light of the importance of β-cell function as a determinant of glucose tolerance, various studies have estimated changes in this parameter following biliopancreatic diversion (8, 19, 21, 37, 41) and RYGB surgery (19, 25, 27, 46) as well as lifestyle intervention (13, 43) and pharmacotherapy (10, 45). However, only a few longitudinal studies of bariatric surgery (19, 21, 41) have simultaneously measured both insulin secretion and peripheral Si and thus derived the DI, an estimate that adjusts insulin secretory response to the prevailing level of insulin resistance. Consistent with these studies (19, 21, 41) the present data show, within 6 mo post-RYGB surgery, an acute improvement in β-cell function in subjects with hyperglycemia, primarily related to an increase in insulin secretion. Improvements in Si occurred as subjects continued to lose weight, which in subjects with normoglycemia led to a compensatory decrease in insulin secretion, necessary to prevent hypoglycemia, consistent with previous studies of normoglycemic patients undergoing bariatric surgery (11, 26, 37).
The present study extends the period of observation following surgery to 24 mo, which enabled longer-term trends in insulin secretion and insulin action over the entire period of weight loss to become evident. Our findings show that further decreases in adiposity induced an improvement in Si without a further decrease in insulin secretion, thus resulting in enhanced β-cell function, which might also be considered as an increase in “β-cell reserve”. Compared with other studies investigating the response of β-cell function following therapy, our finding is novel in that it shows that improvement in β-cell function is sustained in patients with varying presurgery glycemic status. Previous findings in normoglycemic subjects at 24 mo following biliopancreatic diversion did not show changes in β-cell glucose sensitivity measured by OGTT (12, 30). The reasons for this discrepancy are unclear, since the increase in peripheral Si in the present study was similar in magnitude to that observed in patients undergoing biliopancreatic diversion (11). It is likely that variables measured by OGTT may provide different characteristics of β-cell function than those estimated by IVGTT (31). Improvement in DI was demonstrated in subjects treated with the dipeptidyl peptidase-4 inhibitor vildagliptin for 6 wk (45) and also with exenatide for 1 yr (10), but these effects were not sustained after washout of the drugs. No change in β-cell function was observed in subjects with impaired glucose tolerance following lifestyle intervention for 24 mo (13). A recent study demonstrated improved DI in normoglycemic subjects who underwent exercise training for 8 mo (43); since very little weight loss was reported in this study, a longer follow-up of these individuals would be important to determine whether these improvements were maintained. In the present study, improvements in β-cell function and expansion of β-cell reserve appear to be sustained over the long term, associated with dramatic weight loss and improvements in peripheral insulin action. This finding therefore highlights the unique ability for bariatric surgery to protect against future risk of diabetes.
The early improvement in glycemic status observed following RYGB surgery was also related to decreases in HOMA-IR in all groups. Parallel decreases were observed over the first 6 mo in normo- and hyperglycemic subjects, and steady states were achieved by 24 mo. Early and long-term decreases in HOMA-IR following various bariatric surgeries have been frequently reported (4, 8, 34, 36). Although fat mass decreased by −13% from baseline to 1 mo following surgery, cross-sectional and longitudinal associations between fat mass and HOMA-IR over that time period were not observed, suggesting that mechanisms responsible for improvement in hepatic Si may be related to caloric restriction rather than decreasing adiposity (17). Laferrere et al. (25) demonstrated equivalent decreases in HOMA-IR in severely obese individuals following weight loss of 9% (initial body weight) via RYGB or dietary restriction; thus, mechanisms responsible for improvement in HOMA-IR may not be limited to gastric bypass surgery.
Although the negative relationship between adiposity and Si is well described (1), we did not find that body fat mass was correlated with HOMA-IR or Si values at baseline. An explanation may be that effects of adiposity on Si may be nonlinear in the severely obese state (35). We found that correlations among body fat and both peripheral and hepatic Si grew stronger as subjects approached normal weight, suggesting that other mediators confound the relationship when fat mass is excessive. Consistent with other studies (25, 35, 41), our findings demonstrate that during weight loss early improvements in Si and insulin secretion do not appear to be related to loss of adipose tissue. Subjects who experienced greater loss of adipose tissue at 6 and 24 mo had a lower HOMA-IR and higher peripheral Si at those respective time points, which suggests that decreasing adipose tissue had longer-term effects on both hepatic and peripheral Si.
Our study has limitations. Only 56% of initial subjects were followed to 24 mo, and this is a substantial limitation of the study, as it may have led to the error of selection bias. However, at baseline and 1- and 6-mo time points, there were no differences in study end points between patients studied at 6 mo and those followed up to 24 mo. For example, subjects who were followed up at 24 mo did not experience greater weight loss at 6 mo than those who were not followed. Also, similar proportions of normoglycemic and hyperglycemic subjects were followed for the entire study. Finally, given the individual reasons presented for lack of follow-up, it does not appear that some members were more likely to be measured than others. We did not determine the contribution of an incretin effect over the short term following RYGB, although an increase compared with baseline measurements was observed in glucagon-like peptide-1 at 24 mo following surgery, and this was not correlated to changes in AIRg, Si, or DI (data not shown). Glycemic status at baseline was assessed using fasting glucose and not by OGTT, which could distinguish patients who were truly glucose tolerant from those who had impaired glucose tolerance but normal fasting glucose (2). Although our study was adequately powered to measure changes in the DI (19), apparent increases in AIRg early following surgery might not have reached significance due to small sample size. We did not subject patients to a substantial washout of diabetes medications; thus, improvements in insulin action may be underestimated. Changes in diet may contribute to improvements in insulin action following RYGB surgery; although we have reported decreases in energy intake following RYGB (20), we did not collect dietary information on subjects in the current study. Our findings may also be limited to subjects who are severely obese and perhaps only to females within this population. However, the study population is fairly representative of those undergoing bariatric surgery in the United States in terms of sex, ethnicity, and ranges in glucose tolerance. Other study strengths are the use of detailed measurements of insulin action and body fat mass at early and long-term periods following RYGB.
In summary, in this study we demonstrate an improvement in β-cell function in subjects who had euglycemia or hyperglycemia over a period of 24 mo following Roux-en-Y gastric bypass surgery, resulting primarily from early increases in insulin secretion (in subjects with glycemic disorders) and later increases in peripheral insulin sensitivity (in all subjects). Improvement in disposition index, along with reduced HOMA-IR, led to improvement of glucose control by 6 mo postsurgery. Increases in insulin sensitivity over the long term were related to changes in adiposity; however, the early antidiabetic mechanisms of Roux-en-Y gastric bypass surgery appear to be independent of decreasing adiposity and remain to be determined.
GRANTS
This work was supported by National Institutes of Health Grants R03 DK-067167 and R21 DK-075745 (to N. Gletsu Miller), K24 RR-023356 (to T. R. Ziegler), DK-066204 (to L. S. Phillips), General Clinical Research Center Grant M01 RR-00039, the Atlanta Clinical and Translational Science Institute Grant UL1 RR-025008, and the Veterans' Association HSR&D Awards SHP 08-144 and IIR 07-138 (to L. S. Phillips).
DISCLOSURES
No conflicts of interest are reported by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the study participants. Adeola T. Ayeni MD assisted with clinical research coordination of the study participants.
REFERENCES
- 1. Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes 45: 1684–1693, 1996. [DOI] [PubMed] [Google Scholar]
- 2. Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance. Results from the Veteran's Administration Genetic Epidemiology Study. Diabetes 55: 1430–1435, 2006. [DOI] [PubMed] [Google Scholar]
- 3. American-Diabetes-Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 30: S42–S47, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Ballantyne GH, Farkas D, Laker S, Wasilewski A. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg 16: 1189–1197, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bays HE, Chapman RH, Grandy S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract 61: 737–747, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergman RN. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38: 1512–1527, 1989. [DOI] [PubMed] [Google Scholar]
- 7. Bergman RN, Ader M, Huecking K, Van Critters G. Accurate assessment of beta-cell function. The hyperbolic correction. Diabetes 51: S212–S220, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Briatore L, Salani B, Andraghetti G, Danovaro C, Sferrazzo E, Scopinaro N, Adami GF, Maggi D, Cordera R. Restoration of acute insulin response in T2DM subjects 1 month after biliopancreatic diversion. Obesity 16: 77–81, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories WJ, Fahrbach K, Schoelles K. Bariatric surgery. A systemic reveiw and meta-analysis. JAMA 292: 1724–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Bunck MC, Diamant M, Corner A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Jarvinen H, Heine RJ. One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients. Diabetes Care 32: 762–768, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camastra S, Manco M, Mari A, Baldi S, Gastaldelli A, Greco AV, Mingrone G, Ferrannini E. B-cell function in morbidly obese subjects during free living. Long-term effects of weight loss. Diabetes 54: 2382–2389, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Camastra S, Manco M, Mari A, Greco AV, Frascerra S, Mingrone G, Ferrannini E. B-cell function in severely obese type 2 diabetic patients. Diabetes Care 30: 1002–1004, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Carr DB, Utzschneider KM, Boyko EJ, Asberry PJ, Hull RL, Kodama K, Callahan HS, Matthys CC, Leonetti DL, Schwartz RS, Kahn SE, Fujimoto WY. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not b-cell function. Diabetes 54: 340–347, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE. Progressive loss of B-cell function leads to worsening glucose tolerance in first-degree relative of subjects with type 2 diabetes. Diabetes Care 30: 677–682, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299: 316–323, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Faerch K, Vaag A, Holst JJ, Hansen T, Jorgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance—the Inter99 study. Diabetes Care 32: 439–444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care 32: 514–520, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Festa A, Williams K, D'Agostino R, Wagenknecht LE, Haffner SM. The natural course of b-cell function in nondiabetic and diabetes individuals. The Insulin Resistance Atherosclerosis Study. Diabetes 55: 1114–1120, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Fuentes E, Garcia-Almeida JM, Garcia-Arnes J, Rivas-Marin R, Gallego-Perales JL, Gonzalez-Jimenez B, Cardona I, Garcia-Serrano S, Garriga MJ, Gonzalo M, Ruiz-de-Adana MS, Soriguer F. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg 16: 1179–1188, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Gletsu-Miller N, Hansen JM, Jones DP, Go YM, Torres WE, Ziegler TR, Lin E. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight loss surgery. Obesity 17: 439–446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 55: 2025–2031, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Guldstrand M, Ahren B, Adamson U. Improved β-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 284: E557–E565, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Haffner SM, Howard G, Mayer E, Bergman RN, Savage PJ, Rewers M, Mykkanen L, Karter AJ, Hamman R, Saad MF. Insulin sensitivity and acute insulin response in African Americans, Non-Hispanic Whites, and Hispanics with NIDDM: The Insulin Resistance Atherosclerosis Study. Diabetes 46: 63–69, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D., Jr Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993. [DOI] [PubMed] [Google Scholar]
- 25. Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Letiexhe MR, Desaive C, Lefebvre PJ, Scheen AJ. Intact cross-talk between insulin secretion and insulin action after postgastroplasty recovery of ideal body weight in severely obese patients. Int J Obes 28: 821–823, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Lin E, Davis SS, Srinivasan J, Sweeney JF, Ziegler TR, Phillips LS, Gletsu-Miller N. Dual mechanism for type-2 diabetes resolution after Roux-en-Y gastric bypass. Am Surgeon 6: 498–503, 2009. [PMC free article] [PubMed] [Google Scholar]
- 28. Lin E, Gletsu N, Fugate K, McClusky D, Ziegler TR, Gu LH, Papanicolaou DA, Ramshaw BJ, Smith CD. The effects of gastric division on systemic ghrelin concentrations in the morbidly obese. Arch Surg 139: 780–784, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Lin E, Phillips LS, Ziegler TR, Schmolzer B, Kongjun W, Gu LH, Khaitan L, Lynch SA, Torres WE, Smith CD, Gletsu-Miller N. Increases in adiponectin predict improved liver, but not peripheral, insulin sensitivity in severely obese women during weight loss. Diabetes 56: 735–742, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, Ferrannini E. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta-cell function. Diabetologia 49: 2136–2143, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Mari A, Tura A, Pacini G, Kautzky-Willer A, Ferrannini E. Relationships between insulin secretion after intravenous and oral glucose administration in subjects with glucose tolerance ranging from normal to overt diabetes. Diabet Med 25: 671–677, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 33. Minderico CS, Silva AM, Teixeira PJ, Sardinha LB, Hull HR, Fields DA. Validity of air-displacement plethysmography in the assessment of body composition changes in a 16-month weight loss program. Nutr Metab (Lond) 3: 32, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morinigo R, Lacy A, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 16: 1594–1601, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Muscelli E, Mingrove G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrrannini E. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med 118: 51–57, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Perugini RA, Quarfordt SH, Baker S, Czerniach DR, Litwin DEM, Kelly JJ. Metabolic characterization of nondiabetic severely obese patients undergoing roux-en-y gastric bypass on insulin-glucose homeostasis. J Gastrointest Surg 11: 1083–1090, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52: 1098–1103, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Pontiroli AE, Pizzocri P, Librenti MC, Vedani P, Marchi M, Cucchi E, Orena C, Paganelli M, Giacomelli M, Ferla G, Folli F. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab 87: 3555–3561, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab 93: S89–S96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radikova Z, Koska J, Huckova M, Ksinantova L, Imrich R, Vigas M, Trnovec T, Langer P, Sebokova E, Klimes I. Insulin sensitivity indices: a proposal of cut-off points for simple indentification of insulin-resistant subjects. Exp Clin Endocrinol Diabetes 115: 249–256, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 32: 375–380, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Engl J Med 351: 2683–2693, 2004. [DOI] [PubMed] [Google Scholar]
- 43. Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM, Houmard JA, Kraus WE. Effects of exercise training intensity on pancreatic β-cell function. Diabetes Care 32: 1807–1811, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Utzschneider KM, Carr DB, Hull RL, Kodama K, Shofer JB, Retzlaff BM, Knopp RH, Khan SE. Impact of intraabdominal fat and age on insulin sensitivity and β-cell function. Diabetes 53: 2867–2872, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM, Watson AD, Ligueros-Saylan MA, Foley JE, Holst JJ, Deacon CF, Kahn SE. The dipeptidyl peptidase-4 inhibitor vildagliptin improves β-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care 31: 108–113, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Vidal J, Nicolau J, Romero F, Casamitjana R, Mombian D, Conget I, Morinigo R, Lacy AM. Long-term effects of roux-en-y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 94: 884–891, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104: 787–794, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic b-cell function in Latino women at high risk for type 2 diabetes. Diabetes 55: 1074–1079, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.