Abstract
N-methyl-d-aspartate (NMDA) receptors (NMDAR) are tetrameric amino acid receptors that act as membrane calcium channels. The presence of the receptor has been detected in the principal organs responsible for calcium homeostasis (kidney, bone, and parathyroid gland), pointing to a possible role in mineral metabolism. The aim of this study was to test the effect of NMDAR activation in the kidney and on 1,25(OH)2D3 synthesis. We determined the presence of NMDAR subunits in HK-2 (human kidney cells) cells and proved its functionality. NMDA treatment for 4 days induced a decrease in 1α-hydroxylase levels and 1,25(OH)2D3 synthesis through the activation of the MAPK/ERK pathway in HK-2 cells. In vivo administration of NMDA for 4 days also caused a decrease in blood 1,25(OH)2D3 levels in healthy animals and an increase in blood PTH levels. This increase in PTH induced a decrease in the urinary excretion of calcium and an increase in urinary excretion of phosphorous and sodium as well as in diuresis. Bone turnover markers also increased. Animals with 5/6 nephrectomy showed low levels of renal 1α-hydroxylase as well as high levels of renal glutamate compared with healthy animals. In conclusion, NMDAR activation in the kidney causes a decrease in 1,25(OH)2D3 synthesis, which induces an increase on PTH synthesis and release. In animals with chronic kidney disease, high renal levels of glutamate could be involved in the downregulation of 1α-hydroxylase expression.
Keywords: parathyroid hormone, mitogen-activated protein kinase, hyperparathyroidism
calcium homeostasis is a highly controlled process that is regulated mainly by two hormones, parathyroid hormone (PTH) and vitamin D. When levels of calcium are low, calcium-sensing receptor (CaSR) in the parathyroid gland (PTG) activates PTH secretion, which in turn increases vitamin D synthesis. PTH and vitamin D act in a coordinated way to normalize calcium (Ca) levels; when PTH is released, 1,25(OH)2D3 synthesis increases in the kidney, and intestinal absorption of calcium rises (14, 31). PTH also regulates renal transport of Ca and phosphorus (P) and bone turnover. At the end of this process, high levels of 1,25(OH)2D3 and Ca normalize PTH levels acting through the vitamin D receptor (VDR) and the CaSR, respectively.
PTH synthesis and release is a complex mechanism regulated by different factors (27, 32), such as Ca, which regulates PTH through the CaSR (44); P, which activates its secretion by a yet-unknown mechanism (1, 19, 46); fibroblast growth factor 23 (FGF23), which together with its cofactor klotho inhibits the synthesis and secretion of PTH (4, 21); and vitamin D itself through its receptor, the VDR (45). In addition, a recent paper from our laboratory has shown that activation of N-methyl-d-aspartate (NMDA) receptors (NMDAR) on the PTG can also decrease PTH release (34).
The active form of vitamin D, 1,25(OH)2D3, is synthesized mainly in the kidney by the action of the enzyme 1α-hydroxylase (14). On the one hand, this enzyme is regulated by the levels of 1,25(OH)2D3 itself, which like Ca, P, and FGF23 decrease its activity. On the other hand, PTH, interferon-γ, insulin-like growth factor I, and calcitonin exert the opposite effect (33).
In chronic kidney disease (CKD), there is a reduction in the functional renal parenchyma that causes a drop on glomerular filtration rate and in 1,25(OH)2D3 synthesis together with low serum calcium levels. All these changes stimulate the PTG, which, if this stimulus is persistent, can undergo hyperplasia and hypertrophy, developing the so-called secondary hyperparathyroidism (2HPT). Moreover, FGF23, hyperphosphatemia, metabolic acidosis, and uremic toxins also affect 1α-hydroxylase activity and 1,25(OH)2D3 synthesis (2).
NMDAR are tetrameric amino acid receptors that act as membrane calcium channels. The receptor has two subunits, R1 with the catalytic function and R2 with regulatory properties. There are four types of the subunit R2 (A, B, C, and D), and their presence is different depending on the distribution, properties, and regulation of the receptor. The receptor is gated by the binding of l-glutamate and its cofactor l-glycine, allowing calcium to enter the cell (28).
The receptor has been well described in neural tissue, where it has a fundamental role in the excitatory signal transmission of the central nervous system. Calcium entry through NMDAR has also been related with synaptic plasticity (7, 26) and long-term learning processes (18, 49). However, little is known about NMDAR in other tissues. Recently, the presence of this receptor has been shown in kidney (13), where it plays a role in the maintenance of basal arterial tone and stimulates proximal reabsorption and glomerular filtration (12), and in bone, where the antagonists of the receptor inhibit bone resorption (9, 35). Moreover, previous results of our group have shown the presence of the receptor in the PTG, which acutely decreases the secretion of PTH (34). Thus, the presence of NMDAR in the renal tubular cells, which are responsible mainly for 1,25(OH)2D3 production, suggests that NMDAR activation can play a role in renal control of Ca homeostasis.
Thus, the aim of the present work was to determine the effect of the stimulation of renal NMDAR in Ca regulatory pathways in the kidney and its effects on vitamin D and PTH levels.
MATERIALS AND METHODS
Animals and samples.
Experimental methods used on laboratory animals comply with Law 5 of June 21, 1995, by “Generalitat de Catalunya” of protection of animals used for experimentation and other scientific finalities and the Royal Decree 1201 of October 10, 2005, on the protection of animals used for experimentation and other scientific finalities. Moreover, the present work was approved by the Ethic Animal Experimentation Committee of the University of Lleida.
The study was performed in Sprague-Dawley rats (200–250 g, 50% male and 50% female) housed in groups and maintained under standard conditions. Food and water were available ad libitum. Room temperature was at 21°C with a 12:12-h dark-light cycle.
To assess the NMDAR function, we used NMDA, which activates specifically NMDAR and not the other glutamate receptors. For the in vivo experiments, blood and 24-h urine samples were obtained before and 4 days after daily NMDA intraperitoneal treatment (10 mg·kg−1·day−1). Some of the animals were also treated with calcitriol (10 ng/kg every other day) to determine the effect of replenishing active vitamin D levels on NMDA-treated animals.
At the end of the experiment, animals were euthanized with pentobarbital sodium (50 mg/kg), and kidney tissue was obtained after perfusion with PBS through abdominal aorta. Furthermore, tissue from untreated animals was also obtained to use as normal controls for 1α-hydroxylase expression levels and renal glutamate content.
Animals with reduced renal mass were obtained by subtotal nephrectomy, following the method described by Perez-Ruiz et al. (36). Briefly, animals were anesthetized with isoflurane and underwent 2/3 nephrectomy in the left kidney by ligation of both poles. One week later, animals underwent contralateral uninephrectomy. After 8 wk, animals were euthanized and renal tissue was collected.
Cell cultures.
For the in vitro experiments, we used the human kidney 2 (HK-2) cells (ATTC, no. CRL-2190), which are a cell line from normal human kidney. Cells were maintained in DMEM-Ham's F-12 medium (1:1) containing 5% FBS, 100 U/ml penicillin-streptomycin, 20 mM HEPES, 1.12 g of d-glucose anhydride, 5 μg/ml transferrine, 5 μg/ml insulin, 5.10−8 M dexametasone, 10−9 M triiodothyronine, 10 ng/ml EGF, and 60 nM selenium (Sigma). Cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Cells were treated with NMDA (500 μM) and glycine (500 μM) for 4 days, with the medium replaced every day. In some of the wells, U-O126 was also added (10 μM).
Fluo-4 method.
Cells were cultivated in coverslips and after 2 days were washed with Krebs solution (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 11 mM glucose at pH =7.5) and incubated with 5 μM of fluo 4-AM (Molecular Probes, PoortGebouw, The Netherlands) during 30 min at 37°C. The slides were assembled in a adaptor specific for the confocal microscope Nikon TE-200, and treatment with NMDA (500 μM) was added. Analysis was performed at 485 nm of stimulation and 528 nm of emission in the Nikon TE-200 (Nikon, Tokyo, Japan) microscope. We took pictures every minute with a ×100 objective lens. Controls were 10 μM ionomicine for the maximum level of fluorescence and 20 mM EGTA for the minimum.
PCR and RT-PCR.
Total RNA was extracted from tissue using the Trizol method. Reverse transcription was performed with First Strand cDNA Synthesis Kit for RT-PCR (AMV; Roche) followed by a Taqman real-time PCR amplification also with gene-specific primers (Applied Biosystems, Branchburg, NJ), using human glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) as a reference. Forty cycles at 95°C for 15 s and 60°C for 1 min were performed with an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Triplicate readings were taken, and the average was calculated. The relative RNA amount was calculated by standard formulae. The results referred to a randomly selected basal sample that we consider as value = 1. In the case of NMDAR subunits in HK-2 cells, PCR amplification with gene-specific primers (Table 1) and human GAPDH as a reference was performed. Forty cycles at 95°C for 30 s, 60°C for 1 min, and 72°C for 30 s were performed with an GeneAmp PCR System 2700 (Applied Biosystems).
Table 1.
Primer sequences (5′-3′) for PCR for the different NMDAR subunits and GAPDH
| Gene (Human) | Sequence (5′-3′) |
|---|---|
| NMDA R1A | |
| Forward | AGACGTGGGTTCGGTATCAG |
| Reverse | AGGACCCATCAGTGTCCTTG |
| NMDA R2A | |
| Forward | GTCCTTCTCCGACTGTGAGC |
| Reverse | ACTGCCCGTTGATAGACCAC |
| NMDA R2B | |
| Forward | GCCTGAGCGACAAAAAGTTC |
| Reverse | CATCTCCCCATCTCCAAAGA |
| NMDA R2C | |
| Forward | CGCTGGTCTTCAACAACTCA |
| Reverse | GTCCTTGCCTGCCATGTAGT |
| NMDA R2D | |
| Forward | TTCACCATTGGGAAATCCAT |
| Reverse | GGATAGTTGCTGCGGATGTT |
| GADPH | |
| Forward | GAAGGTGAAGGTCGGAGT |
| Reverse | GAAGATGGTGATGGGATTTC |
NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor.
Western blot.
Protein extracts from renal and cell tissue were obtained as described previously (11). Briefly, 20 μg of protein was separated in an 8% polyacrylamide-SDS gel. After running and transfer to PDVF membranes (Immobilon-P; Millipore, Bedford, MA), blots were incubated in 3% nonfat milk in Tris-buffered saline-Tween 20 (TBST) for 1 h. Then, primary antibody for NMDAR1 (1:1,000; Affinity Bioreagents, Golden, CO), 1α-hydroxilase (1:1,000; The Binding Site, Birmingham, UK), pERK (1:1,000; Cell Signaling Technology, Boston, MA), total ERK (1:1,000; Cell Signaling Technology), or tubuline (1:30,000, Sigma) was added, and incubation was performed overnight at 4°C. After washing with TBST, HRP-conjugated secondary antibody (1:12,500; Amersham Biosciences) was added for an extra hour. Binding was detected with the ECL Advance Western Blotting Detection Kit (Amersham Biosciences) and the VersaDoc Imaging system Model 4000 (Bio-Rad Laboratories, Munich, Germany).
Analytical determinations.
Serum and urine biochemistries were analyzed using a multichannel autoanalyzer (Roche/Hitachi Modular Analytics). The method used for calcium analysis was the ocresolphthalein complexone method, for phosphate the ammonium molybdate method, and for creatinine the Jaffe reaction. For sodium ions we used a selective electrode; the determination of the osmotic concentrations in urine was obtained by the automatic measurement of the freezing point with the Fiske One-Ten Osmometer.
PTH concentration was measured using a Rat Intact PTH Elisa Kit (Immutopics, San Clemente, CA). For 1,25(OH)2D3 level analysis, we used the IDS 1,25-dihydroxy vitamin D EIA (Immunodiagnostics Systems) commercial kit. Collagen type I fragments in urine were from RatLaps Elisa of Nordic Bioscience Diagnostics (Herlev, Denmark). To measure bone formation, we analyzed the presence of osteocalcin in serum through the Rat-Mid Osteocalcin ELISA kit of Nordic Bioscience Diagnostics.
1,25(OH)2D3 production analysis.
Cells were cultured and treated with NMDA (500 μM) for 4 days. On the last day, we added to the medium 200 ng/ml of 25-hydroxyvitamin D3 (Sigma) for 1 h. 25-hydroxyvitamin D3 was converted to 1,25(OH)2D3, which was measured in the medium by ELISA.
Statistical analysis.
Differences between single groups were assessed by paired Student's t-test. Multiple groups were analyzed by ANOVA followed by Dunnett's post hoc test. P < 0.05 was considered statistically significant.
RESULTS
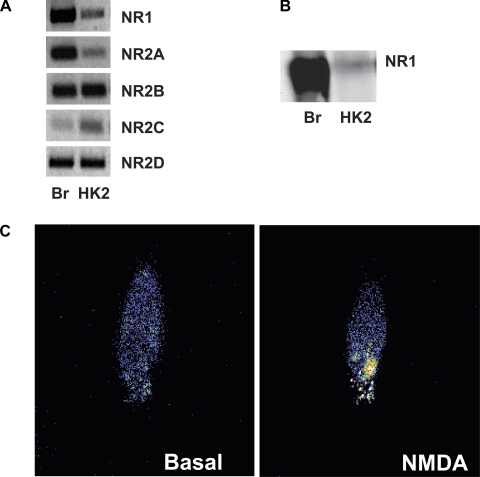
To use HK-2 cells for the study of NMDAR activity in the kidney, first we wanted to assess whether the receptor is expressed in this cell line and its functionality. In Fig. 1A, we show PCR results with specific primers for all NMDAR subunits, R1, R2A, R2B, R2C, and R2D, in the HK-2 cells compared with levels present in brain (taken as reference). Moreover, we detected the presence of the NMDAR1 protein by Western blot techniques (fig. 1B). To determine whether the receptor present in the HK-2 cells is functional, we performed fluo-4 analysis (Fig. 1C). When kidney cells were treated with NMDA (500 μM), intracellular calcium levels increased. This increment measured as fluorescence intensity indicates that the NMDAR channels present in the HK-2 cells are fully functional.
Fig. 1.
A: PCR for N-methyl-d-aspartate (NMDA) receptor (NMDAR) subunits in human kidney 2 (HK-2) cells. Results show the presence of R1 and all R2 subunits. We used brain (Br) as a positive control. B: representative Western blot analysis showing NMDA R1 protein in HK-2 cells. C: fluo-4 determination of NMDAR activation. The entry of calcium caused by the activation of NMDAR can be visualized by the fluorochrome activation. Intracellular calcium levels are higher (measured as color intensity) after 1 min of NMDA treatment (500 μM).
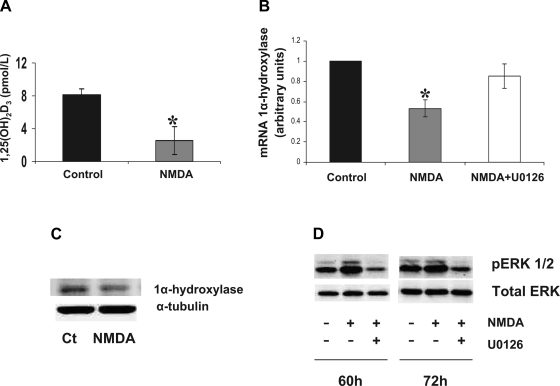
The activation of the NMDAR in this model induced modifications on the vitamin D synthesis pathway. Analyzing the synthesis of 1,25(OH)2D3 in the in vitro model, we observed that NMDA treatment decreased 1,25(OH)2D3 production (Fig. 2A). We analyzed 1α-hydroxylase levels on HK-2 (Fig. 2, B and C), and we detected that 1α-hydroxylase expression was decreased after 4 days of daily NMDA treatment (500 μM/day), in parallel to an increase in ERK 1/2 phosphorilation (Fig. 2D). The decrease on 1α-hydroxylase was abolished when we added the ERK1/2 inhibitor U0126 (10 μM) to the medium (Fig. 2B), indicating a role of the MAPK/ERK pathway mediating the decrease in 1α-hydroxylase induced by NMDA.
Fig. 2.
A: 1,25(OH)2D3 production by HK-2 cells after 4 days of NMDA treatment (500 μM/day). B: 1α-hydroxylase mRNA levels in HK-2 cells after 4 days of treatment with NMDA (500 μM/day; gray bars) with respect to the control ones (black bars). The inhibitory effect of NMDA treatment was abolished with the coincubation with the MAPK/ERK inhibitor U-0126 (white bar). Values are means ± SE. *P < 0.05 vs. control. C: representative Western blot for 1α-hydroxylase in HK-2 cells, control (Ct) or incubated with NMDA (500 μM). We used α-tubuline as load control. D: representative Western blot showing the activation of ERK1/2 on HK-2 cells after 60 and 72 h of NMDA (500 μM) incubation compared with the untreated cells or cells coincubated with U-0126 (10 μM). We used total ERK antibody as load control.
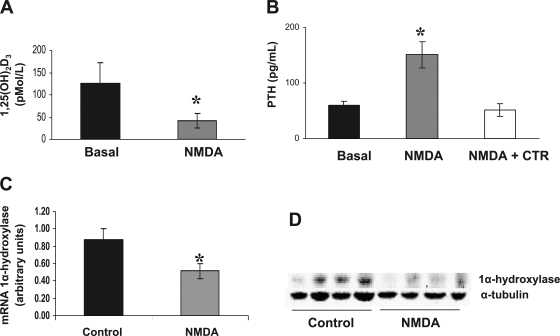
We further investigated the effect of NMDAR activation on renal function, using an in vivo model where normal rats were treated during 4 days with NMDA (10 mg·kg−1·day−1). In these animals, treatment with NMDA induced a decrease of 1,25(OH)2D3 levels compared with the same animals before the treatment (Fig. 3A). In those rats, levels of renal 1α-hydroxylase were lower than in control animals both at mRNA and at protein level (Fig. 3, C and D, respectively). NMDA-treated animals also showed higher levels of PTH with respect to control (Fig. 3B). When we replenished 1,25(OH)2D3 levels in those animals by administration of calcitriol (10 ng/kg every other day), PTH levels did not increase (Fig. 3B).
Fig. 3.
A: 1,25(OH)2D3 levels in normal animals before (black bars) and after NMDA treatment (10 mg·kg−1·day−1 for 4 days; gray bars). Values are means ± SE. *P < 0.05 vs. basal. B: PTH levels in normal rats before (black bars) and after NMDA treatment (10 mg·kg−1·day−1 for 4 days; gray bars) or NMDA plus calcitriol (NMDA + CTR; 10 ng/kg every other day). Values are means ± SE. *P < 0.05 vs basal. C: 1α-hydroxylase mRNA levels in kidney tissue from normal animals (control) and from animals treated for 4 days with NMDA (10 mg·kg−1·day−1). Values are means ± SE. *P < 0.05 vs. control. D: representative Western blot of kidney tissue showing a decrease in 1α-hydroxylase protein levels in 3 different animals treated with NMDA (10 mg·kg−1·day−1 for 4 days). We used α-tubulin as load control.
NMDA treatment induced several changes on renal function. Table 2 shows that NMDA treatment decreased the urinary excretion of calcium, whereas urinary excretion of phosphorous and sodium increased. Urine volume also was higher on NMDA-treated animals, and creatinine clearance decreased. Bone resorption (measured as amount of collagen type I fragments) and bone formation (estimated by osteocalcin levels) both increased. Furthermore, blood Ca levels were lower in NMDA-treated animals.
Table 2.
Effects of NMDA treatment (10 mg·kg−1·day−1) for 4 days in rats
| Basal | NMDA (4 days) | P Value | |
|---|---|---|---|
| Urine volume, ml/day | 8 ± 1.5 | 30 ± 3.7 | P < 0.05 |
| Serum calcium, mg/dl | 11.42 ± 0.07 | 9.87 ± 0.13 | P < 0.05 |
| Serum P, mg/dl | 9.48 ± 0.39 | 9.41 ± 0.35 | NS |
| Serum Na, mEq/l | 138 ± 0.5 | 140 ± 0.2 | NS |
| Creatinine clearance, ml/min | 1.48 ± 0.12 | 1.11 ± 0.08 | P < 0.05 |
| Urinary excretion of Ca, mg/day | 4.8 ± 1.57 | 1.2 ± 0.17 | P < 0.05 |
| Urinary excretion of P, mg/day | 2.04 ± 0.91 | 19.6 ± 1.43 | P < 0.05 |
| Urinary excretion of Na, mEq/day | 0.45 ± 0.11 | 1.2 ± 0.14 | P < 0.05 |
| Osmolarity, mOsm/kg | 1,440 ± 192 | 396 ± 40 | P < 0.05 |
| Collagen type I fragments, ng/day | 3,127 ± 786 | 8,022 ± 156 | P < 0.05 |
| Osteocalcin, ng/ml | 98.4 ± 106 | 178.2 ± 135 | P < 0.05 |
Values are means ± SE; n = 10. P, phosphorous; Na, sodium; NS, not significant.
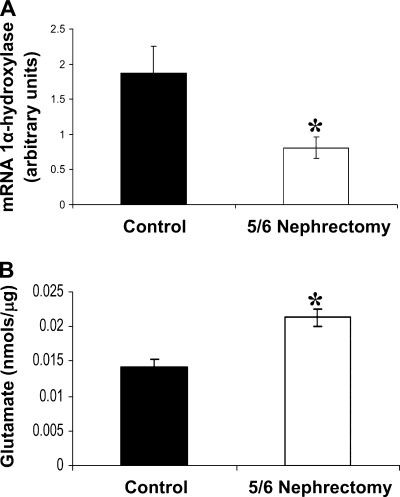
We wanted to analyze whether glutamate could have a role in the decrease in 1α-hydroxylase levels in animals with reduced renal mass. The levels of 1α-hydroxylase in kidney tissue of animals with CKD are lower than in the control ones (Fig. 4A). Moreover, levels of glutamate in the kidneys of these animals were higher than in control kidneys (Fig. 4B).
Fig. 4.
A: 1α-Hydroxylase protein levels in kidney tissue from healthy animals (black bars; control, n = 10) and from animals with 5/6 nephrectomy (white bars; n = 10). Values are means ± SE. *P < 0.05 vs. control. B: glutamate levels in kidney tissue from healthy rats (black bars) and from animals with 5/6 nephrectomy (white bars). Values are expressed as nmol of glutamate for μg of tissue protein. Values are means ± SE. *P < 0.05 vs. basal.
DISCUSSION
NMDAR is a potent calcium channel gated by the binding of glutamate and glycine (28). The role of NMDAR has been widely investigated in the central nervous system, but recent studies have shown the presence of NMDAR in kidney (13), where it plays a role in the maintenance of basal arterial tone; in bone, where it stimulates bone resorption (9, 35); and in the parathyroid gland (34), where it takes part in the regulation of PTH release. Thus, the presence and role of this receptor outside the nervous system is a new field of study.
We have determined the presence of different subunits of the receptor (NR1, NR2A, NR2B, NR2C, and NR2D) needed for its functionality in a cell line of human kidney cells. Furthermore, we have shown that the receptor is functional because its activation in vitro provokes an increase in free intracellular Ca. Intracellular calcium is an important secondary messenger and is essential for the normal physiological activity of all living cells (10).
In a previous study (34), we determined that the acute activation of the NMDAR in the PTG inhibits PTH release. In this study, we wanted to analyze the influence of the sustained activation of the NMDAR on 1,25(OH)2D3 synthesis and in the regulation of PTH secretion. Thus, NMDA treatment for 4 days causes downregulation of 1α-hydroxylase expression both in vivo and in vitro. This downregulation results in a drop in 1,25(OH)2D3 synthesis and in the blood levels of 1,25(OH)2D3. It is well known that low levels of 1,25(OH)2D3 strongly affect the PTG, stimulating the synthesis and release of PTH. Thus, the lack of inhibitory effect of vitamin D on the PTG in sustained treatments, together with the hypocalcemia induced by the same treatment, prevails over the direct effect of NMDAR activation on the parathyroid gland, provoking a net increase of PTH levels. Indeed, when we treated animals with both NMDA and 1,25(OH)2D3, PTH levels did not rise, confirming that a decrease in circulating 1,25(OH)2D3 levels is, at least in part, responsible for the increase in PTH induced by NMDA treatment.
1,25(OH)2D3 is synthesized through 25(OH)D3 by the action of renal 1α-hydroxylase (14), the levels of which are tightly regulated. 1α-Hydroxylase promoter has response elements for different transcription factors, such as AP-1 and AP-2, Sp1, and NF-κB (20, 39), and for the MAPK activation pathway (37, 43). Perwad et al. (37) showed that ERK1/2 pathway activation caused a drop in the renal expression of 1α-hydroxylase in renal cells. The activation of the MAPK pathway by the NMDAR is a well-described process in the nervous system (16, 17, 30). Indeed, our results showed that NMDA treatment causes the activation of the MAPK/ERK pathway together with a decrease in 1α-hydroxylase levels. The inhibition of the pathway with U-O126 blunted both the increase in the activation of the MAPK/ERK pathway and the decrease in 1α-hydroxylase levels. This suggests that NMDAR activation decreases 1α-hydroxylase levels by activating the MAPK/ERK pathway.
One of the most important actions of vitamin D is the regulation of bone turnover (14); thus, when 1,25(OH)2D3 levels are low, the expression of genes related to calcium absorption in the gut (like TRPV6 and calbindin-D9K) is downregulated, and calcium absorption decreases (39). This effect would explain the hypocalcemia in animals treated with NMDA. Vitamin D deficit (signaling through the VDR) together with low calcium, which activates the CaSR (27, 32) in the PTG, would increase the synthesis and secretion of PTH in those animals. Moreover, vitamin D deficit causes hyperplasia of the PTG and an increase of PTH synthesis and release (14). In the kidney, PTH increases calcium and magnesium reabsorption and decreases tubular reabsorption of phosphate (5, 29, 38). Results obtained in animals treated 4 days with NMDA agree with previous studies, because the increase on PTH produced by NMDA treatment caused a rise in renal absorption of calcium and a decrease in the absorption of phosphorous. Furthermore, PTH also activates bone turnover (3, 6, 8), as shown by the increase in fragments of collagen type I. However, NMDAR is also present in bone cells, where it is necessary for the its correct function (9, 35). In bone, NMDAR activation is involved in the control of osteoblast maturation, causes an increase in calcium deposition, and upregulates osteocalcin expression (25). Thus, after NMDA administration, we observed a high bone turnover state by the increase in bone formation (measured as osteocalcin levels) and the increase in bone resorption (revealed by the increase in collagen type I fragments in urine).
PTH also modifies renal sodium reabsorption. Previous studies had shown that PTH treatment is associated with increases in diuresis and natriuresis (40, 42) by causing modifications on activity, traffic, and expression of renal sodium transporters (47). Water reabsorption is bound to sodium reabsorption. Thus in our experimental model, high PTH levels could be the responsible for the increase in dieresis and natriuresis. Moreover, increased sodium delivery to the macula densa causes the activation of the tubuloglomerular feedback and the subsequent decrease in glomerular filtration rate to avoid sodium losses.
The activation of the NMDAR on kidney cells could also have importance on CKD and the consequent 2HPT. A common consequence of CKD is metabolic acidosis. To eliminate the great amount of H+, there is an increase of the production of NH4+ (41). It is well known that NH4+ is produced through the metabolism of amino acids, mainly glutamine, with the production of NH4+ and glutamate (48). This excess of glutamate could activate the renal NMDAR and contributes to the suppression of the renal 1α-hydroxylase activity. In animals, acute metabolic acidosis was found to decrease 1,25(OH)2D3 synthesis through a decrease in 1α-hydroxylase activity (23, 24). However, chronic metabolic acidosis was repeatedly shown to increase 1,25(OH)2D3 and to concomitantly decrease PTH concentrations in humans (22). Indeed, chronic metabolic acidosis leads to a decrease in renal glutamate levels (15), which agrees again with a role of glutamate regulating 1α-hydroxylase levels.
In our study, we measured renal glutamate levels in kidneys from animals with CKD. We found that those levels were high, together with a decreased expression of 1α-hydroxylase levels. Although this fact does not imply causality, and both findings could be parallel and unrelated, our in vitro studies suggest that a link could be present. Further and more extensive studies, beyond the scope of the present work, are needed to prove an association between the increase in glutamate levels in kidneys of animals with CKD and the decrease in 1α-hydroxylase expression levels.
In conclusion, sustained NMDAR activation in the kidney causes an increase in PTH synthesis and release due to a decrease in 1α-hydroxylase synthesis and activity through the MAPK activation and the following decrease on 1,25(OH)2D3 synthesis. In CKD, an increase in renal glutamate levels could be involved in the decrease in renal 1α-hydroxylase activity and the related decrease in 1,25(OH)2D3 levels.
GRANTS
This work was supported by FIS PI09/0299, FIS PI07/0427, and REDINREN (16/06).
DISCLOSURES
B. Coll holds a contract from the Miguel Servet program.
ACKNOWLEDGMENTS
We thank Ana Martinez and Montse Freixenet (Institut de Recerca Biomèdica de Lleida) for their help and cooperation in the laboratory.
REFERENCES
- 1.Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11: 970–976, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int 69: 33–43, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Barnicot NA. The local action of the parathyroid and other tissues on bone in intracerebral grafts. J Anat 82: 233–248, 1948 [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutner EH, Munson PL. Time course of urinary excretion of inorganic phosphate by rats after parathyroidectomy and after injection of parathyroid extract. Endocrinology 66: 610–616, 1960 [DOI] [PubMed] [Google Scholar]
- 6.Bingham PJ, Brazell IA, Owen M. The effect of parathyroid extract on cellular activity and plasma calcium levels in vivo. J Endocrinol 45: 387–400, 1969 [DOI] [PubMed] [Google Scholar]
- 7.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Chang HY. Grafts of parathyroid and other tissues to bone. Anat Rec 111: 23–47, 1951 [DOI] [PubMed] [Google Scholar]
- 9.Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE. Calcium signaling. Cell 80: 259–268, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Deng A, Munger KA, Valdivielso JM, Satriano J, Lortie M, Blantz RC, Thomson SC. Increased expression of ornithine decarboxylase in distal tubules of early diabetic rat kidneys: are polyamines paracrine hypertrophic factors? Diabetes 52: 1235–1239, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Deng A, Thomson SC. Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am J Physiol Renal Physiol 296: F976–F982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng A, Valdivielso JM, Munger KA, Blantz RC, Thomson SC. Vasodilatory N-methyl-d-aspartate receptors are constitutively expressed in rat kidney. J Am Soc Nephrol 13: 1381–1384, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 289: F8–F28, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol 210: 661–666, 1966 [DOI] [PubMed] [Google Scholar]
- 16.Haddad JJ. N-methyl-d-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: a revolving neurochemical axis for therapeutic intervention? Prog Neurobiol 77: 252–282, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Gu Z, Zhang G, Jing G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res 857: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Li ST, Zorumski CF. Modulation of long-term potentiation induction in the hippocampus by N-methyl-d-aspartate-mediated presynaptic inhibition. Neuroscience 92: 1261–1272, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kilav R, Silver J, Naveh-Many T. Parathyroid hormone gene expression in hypophosphatemic rats. J Clin Invest 96: 327–333, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong XF, Zhu XH, Pei YL, Jackson DM, Holick MF. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3–1alpha-hydroxylase gene. Proc Natl Acad Sci USA 96: 6988–6993, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Krapf R, Vetsch R, Vetsch W, Hulter HN. Chronic metabolic acidosis increases the serum concentration of 1,25-dihydroxyvitamin D in humans by stimulating its production rate. Critical role of acidosis-induced renal hypophosphatemia. J Clin Invest 90: 2456–2463, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langman CB. Calcitriol metabolism during chronic metabolic acidosis. Semin Nephrol 9: 65–71, 1989 [PubMed] [Google Scholar]
- 24.Lee SW, Russell J, Avioli LV. 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis. Science 195: 994–996, 1977 [DOI] [PubMed] [Google Scholar]
- 25.Lin TH, Yang RS, Tang CH, Wu MY, Fu WM. Regulation of the maturation of osteoblasts and osteoclastogenesis by glutamate. Eur J Pharmacol 589: 37–44, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci 16: 521–527, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem 273: 5253–5259, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology 34: 1219–1237, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Munson PL. Studies on the role of the parathyroids in calcium and phosphorus metabolism. Ann NY Acad Sci 60: 776–796, 1955 [DOI] [PubMed] [Google Scholar]
- 30.Murphy TH, Blatter LA, Bhat RV, Fiore RS, Wier WG, Baraban JM. Differential regulation of calcium/calmodulin-dependent protein kinase II and p42 MAP kinase activity by synaptic transmission. J Neurosci 14: 1320–1331, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev 26: 78–113, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Naveh-Many T, Silver J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J Clin Invest 86: 1313–1319, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr 22: 139–166, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Parisi E, Almadén Y, Ibarz M, Panizo S, Cardús A, Rodriguez M, Fernandez E, Valdivielso JM. N-methyl-d-aspartate receptors are expressed in rat parathyroid gland and regulate PTH secretion. Am J Physiol Renal Physiol 296: F1291–F1296, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM. Expression of an N-methyl-d-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 22: 645–649, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Perez-Ruiz L, Ros-Lopez S, Cardús A, Fernandez E, Valdivielso JM. A forgotten method to induce experimental chronic renal failure in the rat by ligation of the renal parenchyma. Nephron Exp Nephrol 103: e126–e130, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin d-1α-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293: F1577–F1583, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Pullman TN, Lavender AR, Aho I, Rasmussen H. Direct renal action of a purified parathyroid extract. Endocrinology 67: 570–582, 1960 [DOI] [PubMed] [Google Scholar]
- 39.Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med 44: 237–273, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Schneider EG. Effect of parathyroid hormone secretion on sodium reabsorption by the proximal tubule. Am J Physiol 229: 1170–1173, 1975 [DOI] [PubMed] [Google Scholar]
- 41.Schoolwerth AC. Regulation of renal ammoniagenesis in metabolic acidosis. Kidney Int 40: 961–973, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Schor N, Ichikawa I, Brenner BM. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int 20: 442–451, 1981 [DOI] [PubMed] [Google Scholar]
- 43.Shankar K, Liu X, Singhal R, Chen JR, Nagarajan S, Badger TM, Ronis MJ. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3–24-hydroxylase (CYP24A1). Endocrinology 149: 1748–1756, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver J, Kilav R, Naveh-Many T. Mechanisms of secondary hyperparathyroidism. Am J Physiol Renal Physiol 283: F367–F376, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci USA 82: 4270–4273, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ. Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, Sanderson JE. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 15: 2186–2194, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Watford M. Hepatic glutaminase expression: relationship to kidney-type glutaminase and to the urea cycle. FASEB J 7: 1468–1474, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Youssef F, Stone TW, Addae JI. Interactions of glutamate receptor agonists with long-term potentiation in the rat hippocampal slice. Eur J Pharmacol 398: 349–359, 2000 [DOI] [PubMed] [Google Scholar]