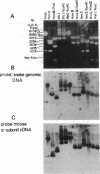
Abstract
The acetylcholine receptor (AcChoR) at the neuromuscular junction of elapid snakes binds cholinergic ligands but unlike other muscle AcChoRs does not bind alpha-bungarotoxin. Numerous studies indicate that the ligand-binding site of the AcChoR includes cysteine residues at positions 192 and 193 of the alpha subunit. We have previously shown that a synthetic dodecapeptide corresponding to residues 185-196 of the Torpedo AcChoR alpha subunit contains the essential elements of the ligand-binding site. In an attempt to elucidate the structural basis for the precise binding properties of snake AcChoR, we sequenced a portion of the snake AcChoR alpha subunit. First, a mouse AcChoR alpha-subunit cDNA probe was used to screen a size-selected snake (Natrix tessellata) genomic library. A genomic clone was isolated and was found to contain sequences homologous to the exon including the first two cysteines (Cys-128 and -142) of AcChoR alpha subunit. The domain of the alpha subunit from Natrix and cobra AcChoR (amino acid residues 119-222), which contains the four extracellular cysteines (128, 142, 192, and 193), was amplified by reverse transcription of mRNA and the polymerase chain reaction and then sequenced. The deduced amino acid sequence showed that the snake alpha subunit contains the two tandem cysteines at positions 192 and 193, resembling all other AcChoR alpha subunits. Sequence comparison revealed that the cloned region of the snake alpha subunit is highly homologous (75-80%) to other muscle AcChoRs and not to neuronal AcChoR, which also does not bind alpha-bungarotoxin. In the presumed ligand-binding site, in the vicinity of Cys-192 and Cys-193, four major substitutions occur in the snake sequence--at positions 184 (Trp----Phe), 185 (Lys----Trp), 187 (Trp----Ser), and 194 (Pro----Leu). In addition, Asn-189 is a putative N-glycosylation site, present only in the snake. These changes, or part of them, may explain the lack of alpha-bungarotoxin-binding to snake AcChoR.
Full text
PDF
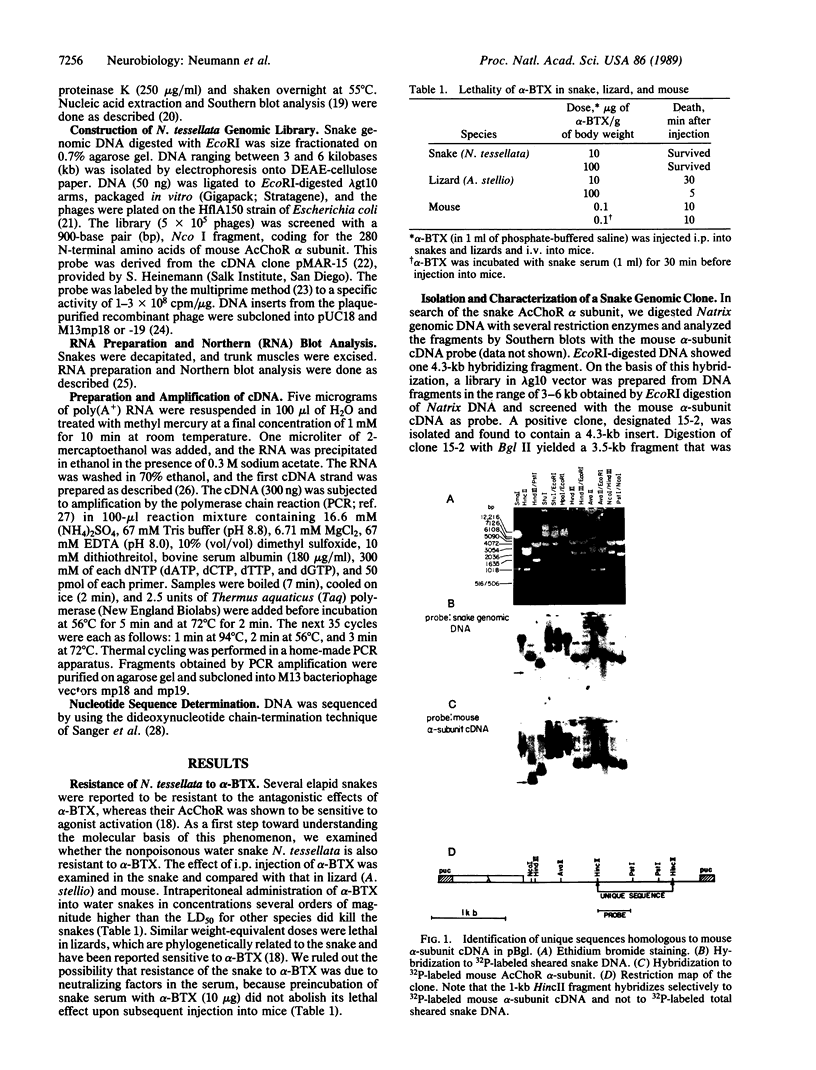


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronheim A., Eshel Y., Mosckovitz R., Gershoni J. M. Characterization of the binding of alpha-bungarotoxin to bacterially expressed cholinergic binding sites. J Biol Chem. 1988 Jul 15;263(20):9933–9937. [PubMed] [Google Scholar]
- Asher O., Neumann D., Fuchs S. Increased levels of acetylcholine receptor alpha-subunit mRNA in experimental autoimmune myasthenia gravis. FEBS Lett. 1988 Jun 20;233(2):277–281. doi: 10.1016/0014-5793(88)80442-3. [DOI] [PubMed] [Google Scholar]
- Ballivet M., Nef P., Stalder R., Fulpius B. Genomic sequences encoding the alpha-subunit of acetylcholine receptor are conserved in evolution. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):83–87. doi: 10.1101/sqb.1983.048.01.011. [DOI] [PubMed] [Google Scholar]
- Barkas T., Mauron A., Roth B., Alliod C., Tzartos S. J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987 Jan 2;235(4784):77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. J., Hartzell H. C., Yoshikami D. Acetylcholine receptors at neuromuscular synapses: phylogenetic differences detected by snake alpha-neurotoxins. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3245–3249. doi: 10.1073/pnas.72.8.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M. Expression of the alpha-bungarotoxin binding site of the nicotinic acetylcholine receptor by Escherichia coli transformants. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4318–4321. doi: 10.1073/pnas.84.12.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Hawrot E., Lentz T. L. Binding of alpha-bungarotoxin to isolated alpha subunit of the acetylcholine receptor of Torpedo californica: quantitative analysis with protein blots. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4973–4977. doi: 10.1073/pnas.80.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C., Mazzola G., Longhi R., Fornasari D., Clementi F. The binding site for alpha-bungarotoxin resides in the sequence 188-201 of the alpha-subunit of acetylcholine receptor: structure, conformation and binding characteristics of peptide [Lys] 188-201. Neurosci Lett. 1987 Nov 10;82(1):113–119. doi: 10.1016/0304-3940(87)90180-7. [DOI] [PubMed] [Google Scholar]
- Haggerty J. G., Froehner S. C. Restoration of 125I-alpha-bungarotoxin binding activity to the alpha subunit of Torpedo acetylcholine receptor isolated by gel electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1981 Aug 25;256(16):8294–8297. [PubMed] [Google Scholar]
- Kao P. N., Karlin A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J Biol Chem. 1986 Jun 25;261(18):8085–8088. [PubMed] [Google Scholar]
- Kochva E. The origin of snakes and evolution of the venom apparatus. Toxicon. 1987;25(1):65–106. doi: 10.1016/0041-0101(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Lecky B. R., Morgan-Hughes J. A. Kinetic analysis of acetylcholine receptors (AChR) in normal and myasthenic human muscle. Ann N Y Acad Sci. 1981;377:61–76. doi: 10.1111/j.1749-6632.1981.tb33724.x. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Atassi M. Z. Segment alpha 182-198 of Torpedo californica acetylcholine receptor contains second toxin-binding region and binds anti-receptor antibodies. FEBS Lett. 1986 Apr 7;199(1):68–74. doi: 10.1016/0014-5793(86)81225-x. [DOI] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Fridkin M., Fuchs S. Analysis of ligand binding to the synthetic dodecapeptide 185-196 of the acetylcholine receptor alpha subunit. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9250–9253. doi: 10.1073/pnas.83.23.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Safran A., Gershoni J. M., Fuchs S. Mapping of the alpha-bungarotoxin binding site within the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):3008–3011. doi: 10.1073/pnas.83.9.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Pedersen S. E., Dreyer E. B., Cohen J. B. Location of ligand-binding sites on the nicotinic acetylcholine receptor alpha-subunit. J Biol Chem. 1986 Oct 15;261(29):13735–13743. [PubMed] [Google Scholar]
- Reiner O., Wigderson M., Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988 Mar;7(2):107–116. doi: 10.1089/dna.1988.7.107. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Luther M., Lindstrom J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett. 1988 Jan 4;226(2):235–240. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilson P. T., Lentz T. L., Hawrot E. Determination of the primary amino acid sequence specifying the alpha-bungarotoxin binding site on the alpha subunit of the acetylcholine receptor from Torpedo californica. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8790–8794. doi: 10.1073/pnas.82.24.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]