Abstract
Low-carbohydrate, high-fat ketogenic diets (KD) have been suggested to be more effective in promoting weight loss than conventional caloric restriction, whereas their effect on hepatic glucose and lipid metabolism and the mechanisms by which they may promote weight loss remain controversial. The aim of this study was to explore the role of KD on liver and muscle insulin sensitivity, hepatic lipid metabolism, energy expenditure, and food intake. Using hyperinsulinemic-euglycemic clamps, we studied insulin action in mice fed a KD or regular chow (RC). Body composition was assessed by 1H magnetic resonance spectroscopy. Despite being 15% lighter (P < 0.001) than RC-fed mice because of a 17% increase in energy expenditure (P < 0.001), KD-fed mice manifested severe hepatic insulin resistance, as reflected by decreased suppression (0% vs. 100% in RC-fed mice, P < 0.01) of endogenous glucose production during the clamp. Hepatic insulin resistance could be attributed to a 350% increase in hepatic diacylglycerol content (P < 0.001), resulting in increased activation of PKCε (P < 0.05) and decreased insulin receptor substrate-2 tyrosine phosphorylation (P < 0.01). Food intake was 56% (P < 0.001) lower in KD-fed mice, despite similar caloric intake, and could partly be attributed to a more than threefold increase (P < 0.05) in plasma N-acylphosphatidylethanolamine concentrations. In conclusion, despite preventing weight gain in mice, KD induces hepatic insulin resistance secondary to increased hepatic diacylglycerol content. Given the key role of nonalcoholic fatty liver disease in the development of type 2 diabetes and the widespread use of KD for the treatment of obesity, these results may have potentially important clinical implications.
Keywords: nonalcoholic fatty liver disease, weight loss, diacylglycerol, ceramide, protein kinase Cε, FGF21
obesity is rising worldwide at an alarming rate (27). The clinical consequences of obesity are numerous and include nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes. Although caloric restriction promotes weight loss, long-term compliance with most diets is poor (7). In this regard, ketogenic diets (KD) have been suggested to be more efficacious than conventional caloric restriction by promoting a reduction in hunger (1).
Although some human studies have found KD to be effective in short-term weight loss (16, 43, 45), the effect of KD on glucose metabolism remains controversial (5, 8, 23, 30, 37). In most of these studies, insulin sensitivity was assessed by nonspecific indexes, such as the homeostasis model assessment of insulin resistance (HOMA-IR) (40) and the quantitative insulin sensitivity check index (QUICKI) (24), both of which rely on fasting plasma glucose and insulin concentrations. Recent studies in mice have found that KD improve whole body insulin sensitivity, as reflected by reductions in fasting plasma insulin concentrations and improved insulin tolerance tests, despite marked increases in hepatic steatosis (2, 25).
Despite the potential benefit of KD on weight loss, the reported development of NAFLD in mice and the controversies regarding its effect on insulin sensitivity led us to the hypothesis that KD could actually induce hepatic insulin resistance mediated by diacylglycerol (DAG) activation of PKCε (13, 28, 33, 35). The aim of this study was therefore to use the hyperinsulinemic-euglycemic clamp technique in combination with radiolabeled glucose to determine, in mice, the metabolic effects of a previously used KD (2–4, 25) on liver and muscle insulin responsiveness, as well as potential cellular mechanisms by which hepatic steatosis may cause hepatic insulin resistance. Finally, given recent studies that have identified N-acylphosphatidylethanolamine (NAPE), a gut-derived lipid metabolite, in mediating reduction in food intake (18), we examined whether a KD might cause an increase in plasma NAPE concentrations.
MATERIALS AND METHODS
Animals and diets.
Male C57BL/6J mice were purchased at 6 wk of age (Jackson Lab, Boston, MA) and individually housed under controlled temperature (23°C) and lighting (12:12-h light-dark cycle, lights on at 7 AM), with free access to water and food. After 1 wk of acclimatization, regular chow (RC; 2018S, Harlan Teklad, Madison, WI) was continued or the diet was changed to the KD (F3666, Bio-Serv, Frenchtown, NJ) for 5 wk. The proportion of calories derived from nutrients was as follows: 17% fat, 60% carbohydrate, 23% protein, and 3.3 kcal/g energy density for RC and 95.1% fat, 0.4% carbohydrate, 4.5% protein, and 7.456 kcal/g energy density for the KD. Body composition was assessed by 1H magnetic resonance spectroscopy using a Minispec analyzer (Bruker, The Woodlands, TX). Metabolic parameters and physical activity were measured using the Oxymax system (Columbus Instruments, Columbus, OH). All experiments were done in 6-h-fasted (6 AM to noon) animals. All procedures were approved by the Yale University Animal Care and Use Committee.
Plasma assays.
Blood samples were collected by cardiac puncture in heparinized syringes and centrifuged at 12,000 rpm for 2 min. Plasma was directly used or frozen at −20°C for further analysis. Plasma glucose was measured by a glucose oxidase method on a Glucose Analyzer II (Beckman Coulter, Brea, CA). Plasma fatty acids were determined with the NEFA C kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin and glucagon were measured by RIA kits (Millipore, Billerica, MA). Cholesterol panel, lactate, blood urea nitrogen, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and β-hydroxybutyrate were analyzed using COBAS Mira Plus (Roche, Indianapolis, IN). Plasma fibroblast growth factor-21 (FGF21) was measured using an ELISA kit (BioVendor, Candler, NC). NAPE was measured as previously described (18).
Liver lipids measurement.
Tissue triglycerides were extracted using the method of Bligh and Dyer (6) and measured using a DCL Triglyceride Reagent (Diagnostic Chemicals, Oxford, CT). DAG and ceramide were extracted as previously described (46). Total DAG content is expressed as the sum of individual species. All lipid measurements were done in animals under basal conditions.
Liver insulin signaling.
PKCε membrane translocation and insulin receptor substrate 2 (IRS-2) tyrosine phosphorylation were assessed in liver protein extracts as previously described (10).
Hyperinsulinemic-euglycemic clamp studies.
Jugular venous catheters were implanted 5–7 days prior to the hyperinsulinemic-euglycemic clamp experiments. To assess basal whole body glucose turnover, [3-3H]glucose (HPLC purified; Perkin Elmer Life Sciences, Boston, MA) was infused at a rate of 0.05 μCi/min for 2 h. After the basal period, the hyperinsulinemic-euglycemic clamp was conducted in awake mice for 140 min with a 4-min primed infusion (29 mU/kg) followed by a continuous infusion (3 mU·kg−1·min−1) of human insulin (Novolin, Novo Nordisk, Princeton, NJ), a continuous infusion of [3-3H]glucose (0.1 μCi/min), and a variable infusion of 20% dextrose to maintain euglycemia (100–120 mg/dl). Plasma samples were obtained from the tail at 0, 30, 50, 65, 80, 90, 100, 110, 120, 130, and 140 min. For measurement of tissue-specific glucose uptake, 2-deoxy-d-[1-14C]glucose (10 μCi; Perkin Elmer Life Sciences, Boston, MA) was injected as a bolus at 85 min. At the end of the clamp, mice were anesthetized with pentobarbital sodium injection (150 mg/kg), and all tissues were taken within 4 min, snap-frozen in liquid nitrogen using aluminum tongs, and stored at −80°C for subsequent analysis. Biochemical analysis and calculations were performed as previously described (32).
Total RNA preparation, real-time quantitative PCR analysis, and immunoblotting analysis.
Total RNA was extracted from frozen livers using the RNeasy 96 kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA was reverse-transcribed into cDNA with the use of Maloney's murine leukemia virus reverse transcriptase (New England Biolabs, Ipswich, MA). The abundance of transcripts was assessed by real-time PCR on a real-time PCR system (model 7500, Applied Biosystems, Foster City, CA) with a SYBR Green detection system. Samples were run in duplicate for the gene of interest and cyclophilin, and data were normalized for the efficiency of amplification, as determined by a standard curve included on each run. (Primers are shown in Supplemental Table S1 in Supplemental Material.) Liver proteins were extracted in RIA buffer (150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Na deoxycholate, and 50 mM Tris·HCl) containing a protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL), and protein concentration was determined by the bicinchoninic acid method (Pierce). Equal amounts of protein (30 μg) were separated on a 4–12% gradient polyacrylamide gel (Invitrogen, Carlsbad, CA) and subsequently transferred to polyvinylidene fluoride membranes (Millipore) using a semidry transfer cell (Bio-Rad, Hercules, CA). After they were blocked in Tris-buffered saline-Tween 20 containing 5% nonfat dry milk, the membranes were incubated overnight with primary antibodies for phosphoenolpyruvate carboxykinase [PEPCK (cytosolic isoform); a gift from Daryl Granner, Vanderbilt University Medical Center, Nashville, TN] or pyruvate carboxylase (PC; Abcam, Cambridge, MA). After further washes, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Bio-Rad) and visualized by enhanced chemiluminescent substrate (Pierce). Membranes were stripped and reblotted with anti-GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis.
Values are means ± SE. Results were assessed using two-tailed unpaired Student's t-test or one-way ANOVA (Prism 5, GraphPad, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
KD causes hepatic insulin resistance.
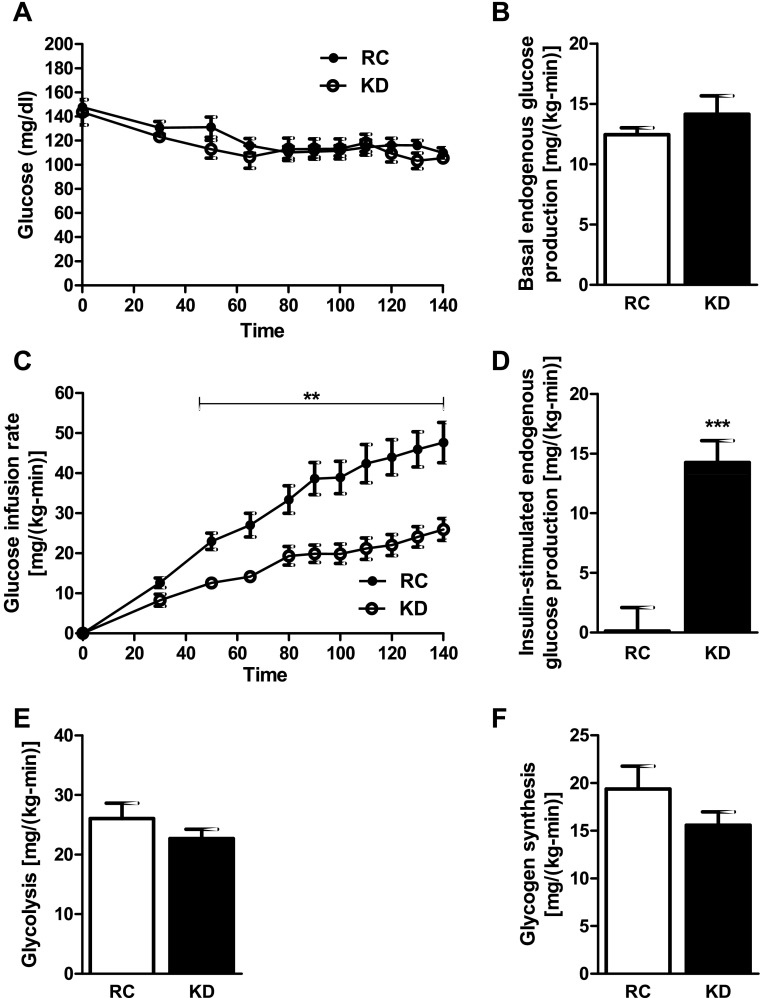
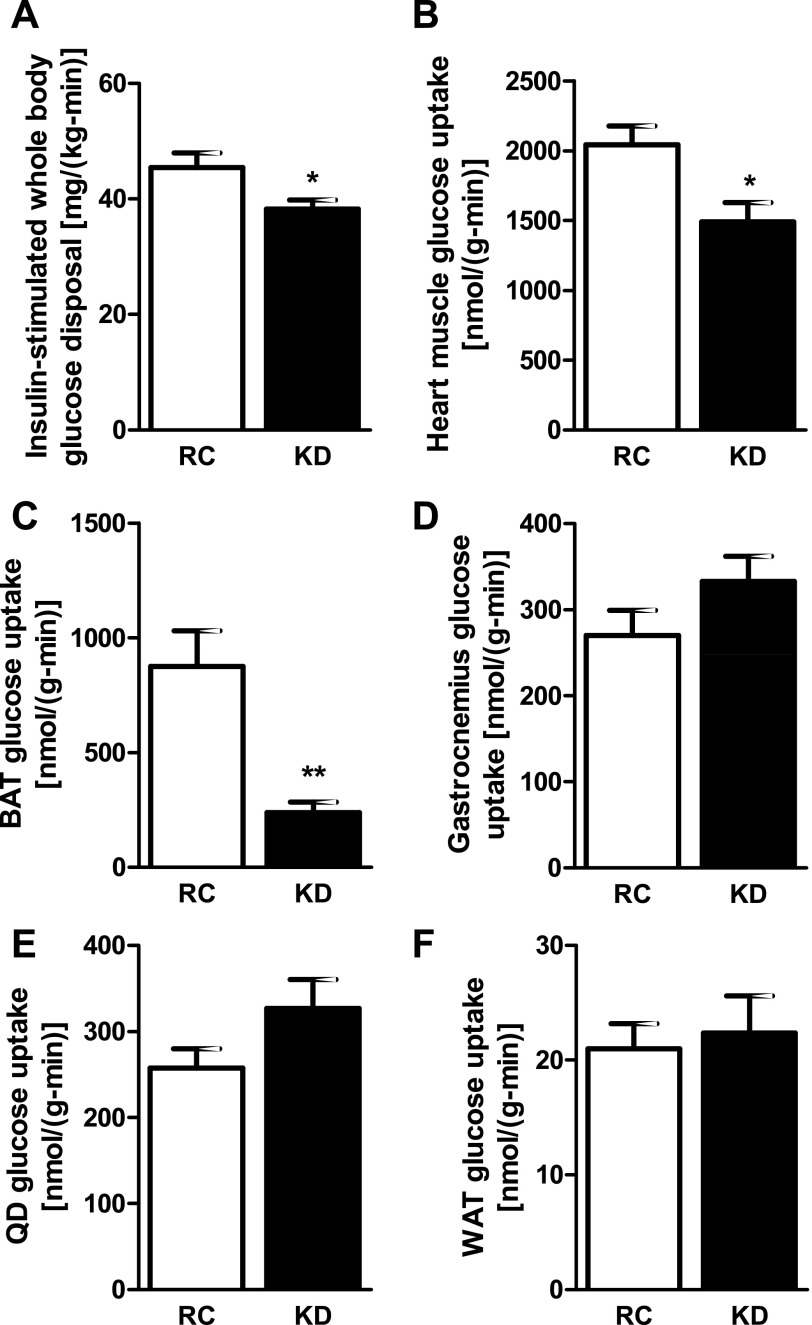
Basal plasma glucose and insulin concentrations were significantly lower in KD- than RC-fed mice, leading to HOMA-IR and QUICKI scores that suggest greater insulin sensitivity (Table 1). Interestingly, plasma glucagon concentrations were also significantly lower in KD-fed mice (Table 1). To assess hepatic and peripheral insulin sensitivity, we performed hyperinsulinemic-euglycemic clamps. Glucose infusion rates required to maintain euglycemia during the clamp (Fig. 1A) were 47% lower in the KD- than RC-fed mice, demonstrating whole body insulin resistance (Fig. 1C). Although basal endogenous glucose production was not different between groups (Fig. 1B), the ability of insulin to suppress endogenous glucose production was significantly impaired in the KD-fed mice (Fig. 1D). Although plasma insulin levels were slightly lower in the KD-fed mice at the end of the clamp, this difference was not significant and was probably due to the fact that plasma insulin levels were significantly lower in the KD-fed mice before the clamp (Table 1). KD changed neither insulin-stimulated whole body glycolysis (Fig. 1E) nor glycogen synthesis (Fig. 1F). Insulin-stimulated whole body glucose disposal (Fig. 2A) was decreased by ∼16% in the KD-fed mice: 45.4 ± 2.5 and 38.3 ± 1.5 mg·kg−1·min−1 for RC- and KD-fed mice, respectively (P < 0.05). This decrease in insulin-stimulated whole body glucose disposal could mostly be attributed to a 16% decrease in lean body mass (Table 1). Indeed, when normalized for lean body mass, the difference in insulin-stimulated whole body glucose disposal was no longer significantly different between the groups: 48.8 ± 2.6 and 43.5 ± 1.7 mg·kg lean body mass−1·min−1 for RC- and KD-fed mice, respectively (P = 0.11). The reduction in insulin-stimulated whole body glucose disposal in the KD-fed mice could also be attributed to a 27% decrease and a 70% decrease in insulin-stimulated 2-deoxy-d-[1-14C]glucose uptake in heart (Fig. 2B) and brown adipose tissue (BAT; Fig. 2C), respectively. There were no differences in insulin-stimulated skeletal muscle [gastrocnemius (Fig. 2D) and quadriceps (Fig. 2E)] or white adipose tissue (WAT; Fig. 2F) glucose uptake. The ability of insulin to decrease plasma fatty acid concentrations during the hyperinsulinemic-euglycemic clamp was also significantly lower in the KD-fed mice (Table 1), demonstrating that insulin suppression of WAT lipolysis was also decreased in these mice.
Table 1.
Physiological parameters and plasma analyses
| Regular Chow | Ketogenic Diet | |
|---|---|---|
| Physiological parameters | ||
| Body wt after 5 wk, g | 28.4 ± 0.6 | 24.1 ± 0.5‡ |
| Body wt gain after 5 wk, g | 2.3 ± 0.4 | −0.7 ± 0.9* |
| Body fat | ||
| %Body wt | 9.3 ± 1.2 | 13.0 ± 1.0* |
| g | 2.6 ± 0.4 | 3.1 ± 0.2 |
| Lean body mass | ||
| %Body wt | 74.9 ± 0.8 | 73.9 ± 1.0 |
| g | 21.3 ± 0.5 | 17.9 ± 0.5‡ |
| Energy expenditure, kcal·h−1·mouse−1 | 0.47 ± 0.01 | 0.55 ± 0.01‡ |
| Caloric intake, kcal·h−1·mouse−1 | 0.58 ± 0.02 | 0.60 ± 0.02 |
| Food intake, g·h−1·mouse−1 | 0.18 ± 0.01 | 0.08 ± 0.00‡ |
| Water intake, ml·h−1·mouse−1 | 0.017 ± 0.001 | 0.016 ± 0.001 |
| Activity, counts | 74.8 ± 8.9 | 68.2 ± 9.0 |
| Plasma analyses | ||
| Glucose, mg/dl | 194.2 ± 21.1 | 121.5 ± 13.7* |
| Insulin, μU/ml | 16.5 ± 2.0 | 8.2 ± 1.9* |
| HOMA-IR | 8.1 ± 1.6 | 2.6 ± 0.7* |
| QUICKI | 0.29 ± 0.01 | 0.33 ± 0.01* |
| Glucagon, pg/ml | 47.9 ± 6.6 | 37.1 ± 6.9* |
| ALT, U/l | 10.4 ± 2.0 | 41.1 ± 9.1† |
| AST, U/l | 102.1 ± 32.5 | 160.4 ± 34.9 |
| Triglyceride, mg/dl | 84.0 ± 10.5 | 98.7 ± 11.6 |
| Cholesterol, mg/dl | ||
| Total | 107.8 ± 5.9 | 100.8 ± 4.8 |
| HDL | 54.3 ± 3.0 | 53.7 ± 6.1 |
| LDL (calculated) | 46.5 ± 5.1 | 46.8 ± 4.8 |
| FA, meq/l | ||
| Fasting | 0.8 ± 0.1 | 0.5 ± 0.0† |
| Insulin-stimulated (clamp) | 0.4 ± 0.1 | 0.3 ± 0.0 |
| Insulin suppression of FA (from fasting), % | 50.2 ± 6.0 | 23.5 ± 7.3* |
| Clamp insulin, μU/ml | 40.6 ± 5.0 | 33.6 ± 4.7 |
| NAPE, μM | 12.6 ± 5.6 | 39.7 ± 8.6* |
| β-Hydroxybutyrate, mmol/l | 0.29 ± 0.02 | 1.53 ± 0.2‡ |
| FGF21, ng/ml | 0.67 ± 0.14 | 6.15 ± 1.94* |
Values are means ± SE (n = 5–14 mice per group). HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FA, fatty acids; NAPE, N-acylphosphatidylethanolamine; FGF21, fibroblast growth factor-21.
P < 0.05;
P < 0.01;
P < 0.001 vs. regular chow.
Fig. 1.
Ketogenic diet (KD) causes hepatic insulin resistance in mice. A: euglycemia (100–120 mg/dl) was maintained throughout the clamp in mice fed KD and those fed regular chow (RC). B: basal endogenous glucose production was similar between groups. C: glucose infusion rates were significantly lower in KD-fed mice. D: ability of insulin to suppress clamp endogenous glucose production was significantly impaired in KD-fed mice. E and F: glycolysis and glycogen synthesis were similar between groups. Values are means ± SE (n = 9 per group). **P < 0.01; ***P < 0.001 vs. RC.
Fig. 2.
KD causes a decrease in insulin-stimulated whole body glucose disposal. A: insulin-stimulated whole body glucose disposal was significantly lower in KD-fed mice (n = 9 per group). This lower level of insulin-stimulated whole body glucose disposal was due to decreased heart muscle glucose uptake (B), as well as decreased brown adipose tissue (BAT) glucose uptake (C); there was no difference in muscle [gastrocnemius (D) and quadriceps (QD, E)] or white adipose tissue (WAT, F) glucose uptake (n = 8 per group). Values are means ± SE. *P < 0.05; **P < 0.01 vs. RC.
KD increases hepatic lipid content and impairs insulin signaling.
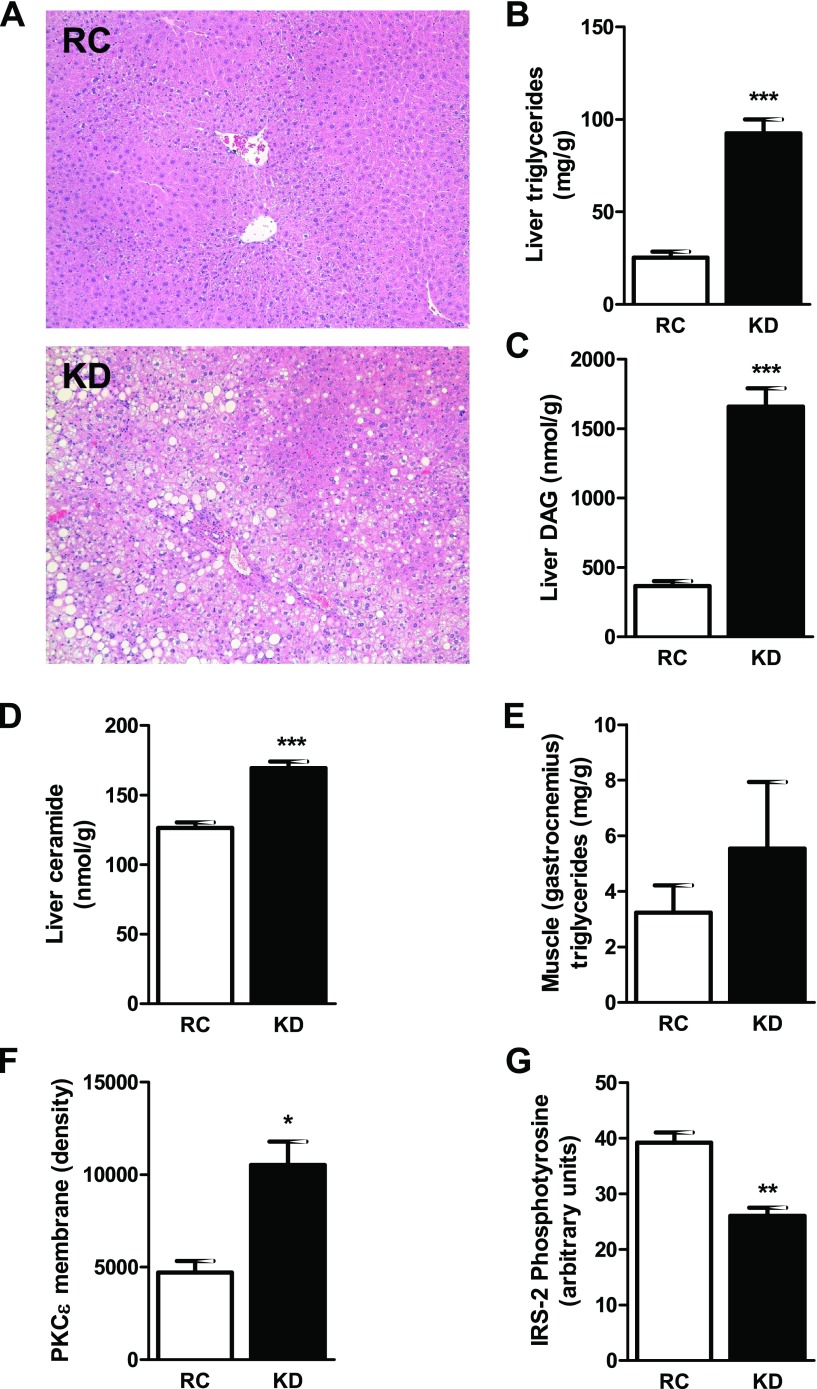
The KD-fed mice developed diet-induced NAFLD with elevated AST and significantly increased ALT plasma concentrations (Table 1). Also, liver microscopy revealed microvesicular lipid infiltration consistent with NAFLD (Fig. 3A) without signs of nonalcoholic steatohepatitis. Liver lipid metabolites, triglycerides (Fig. 3B), DAG (Fig. 3C), and ceramide (Fig. 3D) were significantly increased in the KD-fed mice. This increase in hepatic DAG content was associated with a significant increase in membrane PKCε translocation (Fig. 3F) and a subsequent 34% decrease in insulin-stimulated IRS-2 tyrosine phosphorylation (Fig. 3G). Finally, there was no difference in muscle (gastrocnemius) triglyceride content (Fig. 3E).
Fig. 3.
KD increases liver lipid metabolites and impairs hepatic insulin signaling. A: histological evidence of nonalcoholic fatty liver disease in KD-fed mice with microvesicular pattern lipid infiltration (hematoxylin and eosin staining, ×100 magnification). B–D: increase in liver lipid metabolites. Triglycerides (B), diacylglycerol (DAG, C), and ceramide (D) were significantly increased in KD-fed mice (n = 6 per group). Consequently, in KD-fed mice, PKCε was significantly translocated to the membrane (F), and insulin receptor substrate-2 (IRS-2) tyrosine phosphorylation was decreased (G) (n = 4–5 per group). Muscle (gastrocnemius) triglyceride content (E) was not different between groups. Values are means ± SE (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.001 vs. RC.
KD prevents weight gain through an increase in energy expenditure.
The KD-fed mice gained significantly less weight than the RC-fed mice but showed a significant increase in the percentage of fat mass, without change in percentage of lean body mass (Table 1), suggesting no increase in muscle proteolysis, despite the extreme composition of the KD. However, consistent with the decrease in body weight, total lean body mass was significantly decreased by 16% in the KD-fed mice, whereas total body fat was similar between groups. The lower weight gain could be attributed to an increase in energy expenditure on a per-mouse basis (Table 1). Although caloric intake was similar, food intake in grams was lower for the KD-fed mice, and this lower food intake was associated with a more than threefold increase in plasma NAPE concentrations (Table 1).
KD modifies hepatic metabolism gene expression.
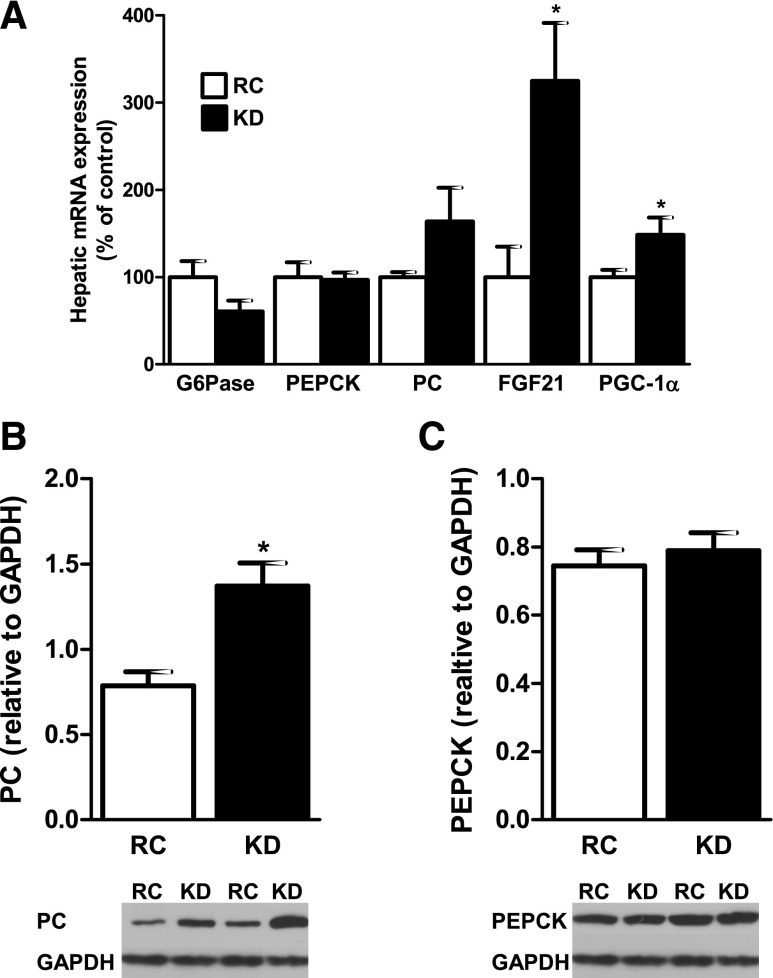
There were no differences in hepatic mRNA expression for glucose-6-phosphatase (G6Pase), PC, and PEPCK in the KD-fed mice (Fig. 4A). Protein expression for PC was increased by 74% in the KD-fed mice (Fig. 4B), but there were no differences in PEPCK protein expression (Fig. 4C). Hepatic mRNA expression of FGF21, a recently discovered hormone that has been implicated in the regulation of long-term energy balance and metabolism (26), was increased by more than threefold in the KD-fed mice (Fig. 4A) and consistent with the 818% increase in plasma FGF21 concentrations (Table 1). Hepatic mRNA expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which is known to be induced by FGF21 (29), was also significantly increased (Fig. 3A).
Fig. 4.
KD modifies gene expression regulating hepatic glucose metabolism. A: liver mRNA expression was not different for glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK), and pyruvate carboxylase (PC), whereas fibroblast growth factor-21 (FGF21) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA were significantly increased (n = 6 per group). PC protein level (B) was significantly increased in KD-fed mice, whereas PEPCK protein level (C) was not different (n = 4–5 per group). Values are means ± SE. *P < 0.05 vs. RC.
DISCUSSION
Low-carbohydrate, high-fat diets are among the most popular dietary regimens for the treatment of obesity. Although these diets have been shown to be effective in promoting weight loss, the effects of these diets on hepatic lipid and glucose metabolism are unclear. Recent studies have suggested that KD may improve insulin sensitivity (8, 30, 37), despite inducing hepatic steatosis in mice (2, 25). The present study is the first, to our knowledge, to demonstrate that KD induce severe hepatic insulin resistance in mice, as reflected by a significant impairment of insulin suppression of hepatic glucose production during a hyperinsulinemic-euglycemic clamp. In this case, hepatic insulin resistance could be attributed to a more than threefold increase in hepatic DAG content, resulting in PKCε activation and, in turn, decreased insulin signaling at the level of IRS-2 tyrosine phosphorylation (33, 34), although a potential role of triglycerides and/or ceramide in this process cannot be excluded. The insulin-resistant state was also due to a decrease in insulin-stimulated whole body glucose disposal, which was, at least partly, due to decreased lean body mass of the KD-fed mice and also to peripheral insulin resistance secondary to a decrease in insulin-stimulated heart muscle and BAT glucose uptake. These findings are consistent with a recent study showing that, in a ketotic environment, the heart reduces its ketolytic capacity, thus improving fatty acid oxidation (42). Also, ketones have previously been shown to decrease glucose uptake in cultured cardiomyocytes (38). While it is theoretically possible that the weight loss per se contributed to the development of hepatic insulin resistance, most studies have found that weight loss improves insulin sensitivity in humans (41) and mice (36). Moreover, in the present study, KD feeding resulted mostly in the prevention of weight gain, rather than major reductions in body weight (average weight loss of 0.7 g over 5 wk).
Notably, the KD resulted in a 50% reduction in plasma insulin concentrations, despite the presence of severe hepatic insulin resistance. Interestingly, the KD also resulted in a 23% reduction in plasma glucagon concentrations, which might be secondary to the more than ninefold increase in plasma FGF21 (26) and may have contributed to the reduction in fasting plasma glucose and insulin concentrations. Indeed, FGF21 has been shown to inhibit glucagon secretion from rat pancreatic α-cells (26). The lack of fasting hyperinsulinemia or hyperglycemia in KD-fed mice led to HOMA-IR and QUICKI scores that would have suggested increased whole body insulin sensitivity and demonstrated how these methods can be very misleading for assessing whole body insulin sensitivity under certain conditions. The finding that the KD-fed mice are insulin-resistant may seem inconsistent with previously published data (2, 25). However, in these studies, insulin sensitivity was assessed by intraperitoneal glucose tolerance tests; one of these studies (2) reported improvement in QUICKI in KD-fed wild-type mice compared with RC-fed wild-type mice but no correlation with the insulin sensitivity.
An important finding was less weight gain in the KD- than the RC-fed mice because of an increase in energy expenditure. Although caloric intake was similar between groups, food intake in grams was lower in the KD-fed mice, which may be attributable to a more than threefold increase in plasma NAPE concentrations. NAPE is a gut-derived factor secreted into the circulation from the intestine after fat ingestion (18). Intraperitoneal administration of NAPE, to achieve plasma concentrations similar to those we observed in the KD-fed mice, has been shown to decrease food intake in a dose-dependent manner through direct interaction in the central nervous system of mice and rats without inducing taste aversion (18). In contrast, leptin probably did not play an important role in causing decreased food ingestion in KD-fed mice, as a previous study using the same KD reported no difference in plasma leptin concentrations between KD- and RC-fed mice (25). Moreover, in humans, similar diets reduce plasma leptin concentrations (12, 19). The increased energy expenditure observed in the KD-fed mice is consistent with a previous study (25) and is likely due to increased expression of uncoupling protein-1 protein levels in BAT (25). Indeed, it was recently shown that uncoupling protein-1 was the only protein mediating diet-induced adrenergic thermogenesis and that its activity could be a determinant for obesity development, at least in mice (15). However, glucose uptake was lower in BAT of the KD-fed mice, suggesting a predominance of fatty acid oxidation, as KD are known to upregulate fatty acid oxidation (4), although this mechanism remains speculative. These findings, however, highlight the importance of BAT in the development of obesity and regulation of body weight (39). The increased energy expenditure may be induced by FGF21 itself, as injection of recombinant FGF21 increases energy expenditure in diet-induced obese mice (44) and causes thermogenic activation of BAT in neonatal mice (20).
Also, the KD-fed mice had a higher body fat content, although body weight of the KD-fed mice was lower than that of the RC-fed mice, which is in accordance with their deleterious metabolic phenotype. This is probably a consequence of KD composition, leading to an increase in fat deposition in different tissues, such as liver, which, in turn, leads to the development of NAFLD. However, we did not find a decrease in the percentage of lean body mass, suggesting that muscle proteolysis is prevented, despite the extreme composition of this diet. However, total lean body mass was significantly decreased in these mice, consistent with their lower body weight. Finally, we did not find an increase in skeletal muscle triglyceride content, consistent with the fact that we did not observe insulin resistance in skeletal muscle. It is, however, unknown if a prolonged exposure to such a diet would induce skeletal muscle insulin resistance.
Consistent with a recent study (29), elevated FGF21 in KD-fed mice induced PGC-1α expression in liver. The latter plays a crucial role in the adaptive starvation response, leading to an increase in fatty acid oxidation and gluconeogenesis without increasing glycogenolysis (29), although a recent study found that loss of PGC-1α in fasted liver led to increased expression of FGF21 (14). These apparently contradictory findings emphasize the distinct adaptive hepatic metabolism between fasting and starvation, with KD inducing more likely a starvation state.
We found an increase in PC protein level in the KD-fed mice, despite no difference in PC mRNA, suggesting a posttranscriptional regulation of the increased endogenous glucose production we observed during the clamp. However, we found no difference in G6Pase and PEPCK mRNA or protein content. These findings are consistent with a recent report suggesting that increased transcriptional expression of G6Pase and PEPCK does not account for the hepatic insulin resistance and increased gluconeogenesis in patients with type 2 diabetes mellitus (31).
Although FGF21 has been suggested to play a critical role in ketogenesis (3, 4, 22), this effect remains controversial in mice (21) and humans (17), and it is not clear if FGF21 induces ketogenesis or if it is the opposite (11). Moreover, this endocrine factor is elevated in the plasma of insulin-resistant subjects, such as obese and type 2 diabetic patients, and it remains to be determined whether this represents a compensatory mechanism to offset insulin resistance or is a causative factor in the development of insulin resistance (9). The results from this study would suggest that an increase in hepatic expression of FGF21 and an increase in plasma FGF21 concentrations may represent a compensatory mechanism to counteract NAFLD and insulin resistance.
It should be mentioned that the KD used in this study, as well as in other studies in mice (2–4, 25), may differ from KD used in humans. Indeed, this KD contains almost no carbohydrate and the protein proportion is low, whereas KD used in humans usually contain less fat, more protein, and also more carbohydrate. Also, in humans, low-carbohydrate diets usually result in decreased caloric intake (16, 30), which was not observed here and, thus, demonstrates the differences between humans and mice in the metabolic adaptations to such diets. However, these data clearly offer perspectives for research in dietary manipulations aimed at treating obesity.
In conclusion, the present study shows that a high-fat KD causes hepatic insulin resistance in mice, which can be attributed to an increase in hepatic DAG content, leading to PKCε activation and subsequent impaired insulin signaling. Moreover, this study found that a KD increases energy expenditure, which results in weight loss. Given the widespread use of KD in the treatment of obesity and the role of NAFLD and hepatic insulin resistance in promoting type 2 diabetes, these results may have important clinical implications, as obese patients on such diets could lose weight but develop NAFLD and hepatic insulin resistance.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-40936 (G. I. Shulman) and U24 DK-076169 (V. T. Samuel and G. I. Shulman) and a Veterans Affairs Merit Grant (V. T. Samuel). F. R. Jornayvaz was funded by a Naomi Berrie Fellowship Award from Columbia University and grants from the Geneva University Hospital, Switzerland, and Swiss Foundation for Fellowship in Medicine and Biology Grant PASMP3-132563.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mario Kahn, Christopher Carmean, Xiaoxian Ma, and Aida Groszman for expert technical assistance.
REFERENCES
- 1. Atkins RC. Dr. Atkins' New Diet Revolution. New York: Quill, 2002. [Google Scholar]
- 2. Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independent of weight loss. Am J Physiol Endocrinol Metab 297: E1197–E1204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150: 4931–4940, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am J Clin Nutr 73: 554–559, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 7. Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD. Efficacy and safety of low-carbohydrate diets: a systematic review. JAMA 289: 1837–1850, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr 90: 23–32, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32: 1542–1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282: 22678–22688, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 94: 3594–3601, 2009. [DOI] [PubMed] [Google Scholar]
- 12. de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Bellioo D, Conde R. Effects of a low-fat versus a low-carbohydrate diet on adipocytokines in obese adults. Horm Res 67: 296–300, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 16: 400–402, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM. PGC-1α negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erbα axis. Proc Natl Acad Sci USA 106: 22510–22515, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 348: 2082–2090, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab 8: 169–174, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI. N-acylphosphatidylethanolamine, a gut-derived circulating factor induced by fat ingestion, inhibits food intake. Cell 135: 813–824, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayes MR, Miller CK, Ulbrecht JS, Mauger JL, Parker-Klees L, Gutschall MD, Mitchell DC, Smiciklas-Wright H, Covasa M. A carbohydrate-restricted diet alters gut peptides and adiposity signals in men and women with metabolic syndrome. J Nutr 137: 1944–1950, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11: 206–212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150: 4625–4633, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5: 415–425, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr 83: 1055–1061, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292: E1724–E1739, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93: S9–S30, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2: 55–65, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348: 2074–2081, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci USA 106: 12121–12126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55: 2042–2050, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117: 739–745, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 116: 817–824, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi H, Akunuru S, Bierman JC, Hodge KM, Mitchell MC, Foster MT, Seeley RJ, Reizes O. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 17: 1702–1709, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 140: 778–785, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson JL, Coderre L. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab 281: E1205–E1212, 2001. [DOI] [PubMed] [Google Scholar]
- 39. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. [DOI] [PubMed] [Google Scholar]
- 41. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wentz AE, d'Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 285: 24447–24456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med 113: 30–36, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 140: 769–777, 2004. [DOI] [PubMed] [Google Scholar]
- 46. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.