Abstract
Insulin-like growth factor I (IGF-I) is a potent myogenic factor that plays a critical role in muscle regeneration and muscle hypertrophy. The purpose of this study was to evaluate the effect of IGF-I overexpression on the recovery of muscle size and function during reloading/reambulation after a period of cast immobilization in predominantly fast twitch muscles. In addition, we investigated concomitant molecular responses in IGF-I receptor and binding proteins (BPs). Recombinant adeno-associated virus vector for IGF-I (rAAV-IGF-IA) was injected into the anterior compartment of one of the hindlimbs of young (3 wk) C57BL6 female mice. At 20 wk of age, both hindlimbs were cast immobilized in a shortened position for 2 wk to unload the tibialis anterior (TA) and extensor longus digitorum (EDL) muscles. The TA and EDL muscles were removed bilaterally after 2 wk of cast immobilization and after 1 and 3 wk of free cage reambulation. Increases in IGF-I mRNA and protein levels with IGF-I overexpression were associated with significant increases in muscle wet weight, fiber size, and tetanic force, although overexpression did not protect against cast immobilization-induced muscle atrophy. After 1 wk of reambulation, evidence of enhanced muscle regeneration was noted in IGF-I-overexpressing muscles with an increased prevalence of central nuclei, embryonic myosin, and Pax7 positive fibers. We also observed larger relative gains in muscle size (wet weight and fiber area), but not force, during the 3-wk reambulation period in hindlimb muscles overexpressing IGF-I compared with contralateral control legs. Changes in IGFBP-5 mRNA expression during cast immobilization and reambulation paralleled those of IGF-I, whereas IGFBP-3 expression changed inversely to IGFBP-5.
Keywords: atrophy, hypertrophy
skeletal muscle is a highly plastic tissue, and its physiological responses are dependent upon the amount of mechanical loading the tissue experiences. A number of studies have found that unloading or reduced muscle activity (i.e., prolonged bed rest, surgery, cast immobilization, microgravity) induces significant muscle atrophy and weakness (26, 29, 35, 63, 65). The consequences of these negative muscular changes are far reaching and include development of functional limitations and impairment, decreased motor control and overall fitness, and long-term disability. Although skeletal muscle has an inherent capacity to recover from atrophy, the maintenance and recovery of muscle function after disuse can be slow and, in many cases, inefficient and incomplete (50, 63). Thus, the advancement of therapeutic approaches to reduce the loss of muscle function and promote muscle recovery following disuse is important.
Regardless of the cause of atrophy, effective muscle regeneration or postnatal muscle growth appears to be dependent on myogenic factors such as insulin-like growth factor I (IGF-I). IGF-I is believed to modulate muscle size via autocrine and paracrine signals by directly stimulating muscle protein production in myofibers and the activation of satellite cells (27). Expression of IGF-I in skeletal muscle has been linked with muscle cell differentiation as well as myofiber hypertrophy in transgenic mouse lines (20, 49). In glucocorticoid and nerve crush-induced muscle atrophy models, targeted IGF-I transgene expression has been shown to reverse muscle wasting (52, 57, 68), yet in models of disuse atrophy, overexpression of IGF-I has not been shown to significantly attenuate muscle atrophy (21). Whether IGF-I overexpression may accelerate the recovery of skeletal muscle with mechanical reloading following a period of disuse is less understood. Furthermore, less information is available regarding the adaptive responses in fast-twitch muscles, such as the extensor digitorum longus (EDL).
IGF-I bioactivity is mediated via IGF-I receptors (IGF-IR) and further regulated by a family of IGF-binding proteins (IGFBPs). Mice lacking the IGF-IR exhibit marked muscle hypoplasia at birth (25). Among seven known IGFBPs, IGFBP-4 and IGFBP-5 have been shown to play an important role in skeletal muscle adaptations (6, 27), whereas IGFBP-3 may modulate the free circulating IGF-I (55). In vitro studies have shown that the biological activity and stability of IGFs are influenced both positively and negatively by the abundance and composition of the IGFBPs present. However, evidence from in vivo studies indicate a more complicated picture, and changes in the expression of IGFBPs in skeletal muscle have been reported to depend on age, muscle, and mechanical load. Spangenburg et al. (61) found no significant decreases in IGFBP-4 and no changes in IGFBP-5 mRNA expression after 10 days of recovery from hindlimb immobilization in the soleus muscle of the 4-mo-old rat. In contrast, Awede et al. (6) reported that overloading induced a doubling of IGFBP-4 and significant downregulation of IGFBP-5 mRNA levels in mouse soleus muscle. Although IGF-IR and IGFBPs are believed to primarily mediate IGF-I action, their mechanism of action seems to be vastly different.
The purpose of this study was to evaluate the effect of viral-mediated IGF-I overexpression on muscle regeneration and the recovery of muscle size and function during reambulation after a period of cast immobilization in predominantly fast-twitch muscles. In addition, we investigated concomitant molecular responses in IGF-IR and binding proteins.
MATERIALS AND METHODS
Animals and viral injection.
This study was conducted with approval from the Institutional Animal Care and Use Committee of the University of Florida. Forty-eight female C57BL6 mice (3 wk of age) were studied. A recombinant AAV plasmid (pSUB201) was constructed using the entire rat IGF-IA cDNA, a Myosin Light Chain (MLC) 1/3 promoter and enhancer, and SV40 polyadenylation sequence, as described previously (10, 11). A recombinant AAV serotype 1 (rAAV-2/cap1; AAV-2 genomes pseudopackaged into AAV-1 capsids) was prepared by the University of Pennsylvania Vector Core, following published procedures (43). Reverse transcription PCR analyses have shown persistent, local expression of the myosin light chain-driven IGF-I mRNA up to 9 mo postinjection (10). Under isoflurane anesthesia, the anterior compartment of one hindlimb was injected with 5 × 1010 viral particles in 85 μl of phosphate-buffered saline (PBS). This method of delivery targeted the tibialis anterior (TA) and EDL muscles, allowing the entire muscles' surfaces to be bathed in virus solution. The contralateral limb was injected with the same volume of sterile PBS. Once these mice were recovered from anesthesia, they were sent back to the animal facilities for future studies.
Four months after injection, viral-injected mice were randomly assigned to one of two groups [one-half for protein synthesis measurements (n = 24), one-half for other measurements (n = 24)]. Each of these groups had four subgroups (n = 6 each condition): 1) noncasted, 2) 2 wk of cast immobilization (2 wk Immob), 3) 1 wk of free cage reambulation (1 wk Reamb), and 4) 3 wk of free cage reambulation (3 wk Reamb), as described below. The animals were housed in an accredited animal facility room controlled for temperature (22 ± 1°C), humidity (50 ± 0%), and light (12:12-h light-dark cycle). Mice in the noncasted group remained in their cages and experienced only normal in-cage activity.
Cast immobilization.
Casting of both hindlimbs was performed under isoflurane anesthesia with hip and knee joints fixed at ∼160° and ∼180°, respectively, as described previously (29). Briefly, to unload the TA and EDL muscles, the ankle was fixed at 60°. The plaster of Paris cast encompassed both hindlimbs and the caudal fourth of the body (superior to the wings of the ilium). The animals were free to move using their forelimbs and they ate and drank ad libitum. The mice were monitored on a daily basis for chewed plaster, abrasions, venous occlusion, and problems with ambulation. In addition, the mice were checked daily for fecal clearance. Following 2 wk of cast immobilization, 12 mice were euthanized. The remaining 24 mice were allowed to freely reambulate in their cages for either 1 or 3 wk following cast immobilization.
In vitro force development.
In vitro force mechanics were performed on both hindlimb muscles, as described previously (10). Briefly, EDL muscles were immersed horizontally in Ringer's solution at 25°C and equilibrated with 95% O2-5% CO2, pH = 7.4. The distal tendon was tied to a nonmovable post and the proximal tendon attached to a servomotor (Aurora Scientific, Aurora, ON, Canada). Muscle length was adjusted to the optimal length (L0) at which maximal twitch force was reached. EDL maximal tetanic forces were determined using 120-Hz, 500-ms supramaximal electrical pulses. Stimulation was delivered via two parallel platinum electrodes that were positioned along the length of the muscle. Immediately following force mechanics, muscle wet weights were measured, EDL muscles for histology measurements were pinned at resting length and cooled in liquid nitrogen melted with isopentane, and TA muscles for other measurements were snap-frozen in liquid nitrogen. Muscles and serum were stored at −80°C.
RNA isolation and assessment of mRNA abundance for real-time quantitative PCR.
Total RNA from frozen TA muscle samples was isolated with TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA). Briefly, ∼30 mg of muscle was cut with 1.0 ml of TRIzol Reagent using an RNase-free razor blade homogenizer. Each muscle sample was centrifuged, incubated, and precipitated. After isolation, RNA was dissolved in DEPC-treated water and stored at −80°C. The resulting RNA was quantified by optical density at 260 nm.
One microgram of total RNA was reverse transcribed to cDNA with the SuperScript III First-Strand Synthesis for RT-PCR kit (Invitrogen Life Technologies) and a mix of oligo(dT) (100 ng/reaction) and random primers (200 ng/reaction) in a 20-μl total reaction volume according to the manufacturer's instructions. Following RT, samples were stored at −80°C for PCR reactions.
Real-time PCR was performed using the protocols and detection systems of ABI Prism 7900 Sequence Detection System (Applied Biosystems). The reaction components for a single 20-μl reaction contained 10 μl of TaqMan Universal PCR Master Mix, 1 μl of primers mix, and 9 μl of RNase-free water. Primer sequences were selected from the NCBI database and purchased from Applied Biosystems [IGF-I: Rn00710306_m1; IGF-IR: Mm00802831_m1; IGF-BP3: Mm00515156_m1; IGF-BP4: Mm00494922_m1; IGF-BP5: Mm00516037_m1; and 18S ribosomal RNA (18S rRNA): 18S-4319413E]. Each assay contains primer and specific intron-spanning FAM or VIC-labeled (18S ribosomal RNA) TaqMan-MGB probes. The standard curve was calculated automatically via software by plotting the threshold cycle (CT) values against each standard of known concentration and calculation of the linear regression line of this curve. The “−ΔΔCT method” (45) was used to calculate the relative expression ratio (2−ΔΔCT) based on the change in threshold values (CT). The normalization of the target genes with an endogenous standard was done via the reference gene (18S ribosomal RNA) expression. 18S expression was not different at any time point. All samples and standards were run in duplicate or quadruplicate.
Determination of IGF-I protein concentration.
Frozen TA muscles were rinsed with PBS to remove excess blood, homogenized in 1 ml of 1× PBS using FastPrep Homogenizer and Isolation System (Thermo Fisher Scientific, Franklin, MA), and stored overnight at −20°C. The homogenates and serum were then centrifuged for 5 min at 5,000 g. The supernatants and serum were utilized for measurements of total IGF-I in a commercially available ELISA kit specific for rodent IGF-I (R & D Systems, Minneapolis, MN). IGF-I concentration was calculated on the basis of a standard curve generated from recombinant mouse IGF-I. This kit detects total rodent IGF-I, and the measurements are not affected by the presence of IGF-I binding proteins or IGF-II (12). This kit has been validated for the determination of rat IGF-I at 30–3,000 pg/ml with an intra-assay precision of ∼4.3% and an interassay precision of ∼6.0% (12). All samples were measured on a microplate reader at 450 nm in duplicate.
Presence of muscle fiber regeneration and morphological analyses.
Frozen cross-sections (10 μm) from the midbelly of the EDL muscle were subjected to hematoxylin and eosin staining to assess the proportion of central nuclei in the muscle fibers. Immunohistochemistry was used to determine the muscle fiber size and assess the distribution of embryonic myosin and paired box transcription factor 7 (Pax7). Sections were incubated with rabbit anti-laminin (Neomarker; Labvision, Fremont, CA) and mouse anti-embryonic myosin heavy chain antibodies (4°C overnight; DSHB, Iowa City, IA), followed by incubation with rhodamine-conjugated anti-rabbit IgG (Nordic Immunological Laboratories) and FITC-conjugated anti-mouse IgG (Nordic Immunological Laboratories). A mouse blocking kit (BMK-2202, Mouse on Mouse; Vector Laboratories) was used for staining of Pax7. Pax7 staining has previously been shown to correspond well to satellite cell content (66). Primary antibody mouse anti-Pax7 (R & D Systems) and secondary antibody Alexa 488 (Invitrogen Molecular Probes) were used. These slides were mounted with Vectashield mounting medium with 4,6-diamidino-2-phenylindole (Vector Laboratories). Image acquisition was performed on a Leitz DMR microscope with a digital camera (Leica Microsystems, Solms, Germany). The National Institutes of Health Image J program (version 1.62) was used to analyze the data. The pixel settings used for conversion of pixels to micrometer were 1.50 pixels, 1 μm2 for a ×10 objective. The average positive number per 100 muscle fibers was quantified from a sample of 150–250 fibers on the randomly selected slides.
Mixed muscle protein synthesis rate measurements.
EDL muscle protein synthesis rate was quantified using the phenylalanine (Phe) flooding dose method, as described previously (n = 24) (9, 47). An intravenous (iv) infusion line was placed in the jugular vein. At time = 0, a bolus of Phe was injected into the iv line (25 mg/100 g body wt; 40% of the Phe is l-[13C]Phe) (Cambridge Isotope Laboratories, Andover, MA) and flushed into the animal with normal saline. Ten minutes after infusion, each mouse was euthanized. The EDL muscle was dissected and immediately frozen in liquid nitrogen.
Mixed muscle protein synthesis rates were determined using the incorporation of l-[13C]Phe into muscle proteins. This required the measurement of l-[13C]Phe in the muscle tissue free amino acid pool (intracellular) and in muscle proteins using mass spectrometry (MS). Frozen muscle samples (10–15 mg) were pulverized in liquid nitrogen and then suspended in cold trichloroacetic acid (10%; 1 ml/10 mg muscle powder). The tissue sample was centrifuged (1,000 g × 15 min at 4°C) and the supernatant removed. The pellet was washed/vortexed two to three times with 1 ml of normal saline and centrifuged (1,000 g × 15 min at 4°C). The supernatant from these washes was pooled with the original trichloroacetic acid. In this pooled supernate, the heptafluorobutyric methyl ester of Phe was formed, and the free intracellular [13C]Phe abundance (Ea) was measured by selected ion monitoring of mass-to-charge ratios 355 and 356, using chemical ionization-negative ion monitoring-quadrupole MS. The pellet containing the mixed structural proteins (including contractile proteins) was hydrolyzed in 1 ml of 6 N HCl for 24 h at 110°C. The hydrolyzed amino acids were separated using cation exchange chromatography and formation of N-acetyl-O-propyl esters. [13C]Phe abundance in the structural proteins (Em) was measured using gas chromatography-combustion-isotope ratio MS. The use of gas chromatography-combustion-isotope ratio MS enables accurate measurement of [13C]Phe enrichment as low as 0.0005 atom% in excess of naturally occurring [13C]Phe (67).
Data analysis.
Data are presented as means ± SE. Using SPSS software (version 16.0), a two-way ANOVA was used to analyze the main effects of injection, loading, or an interaction effect of the two. Paired t-tests were used for comparisons between rAAV-1-injected and contralateral control limbs. Paired t-tests were also used to compare relative changes during immobilization (2-wk immob to noncasted) and reambulation (3-wk reamb to 2-wk immob) between rAAV-1-injected and contralateral control limbs. A one-way analysis of variance was used to compare all variables within the same injection groups. Post hoc testing for ANOVA measurements was performed using a Bonferroni test. A significance level of P < 0.05 was used for all comparisons.
RESULTS
Effects of IGF-I overexpression under normal loading conditions.
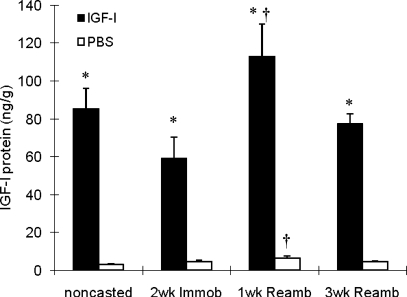
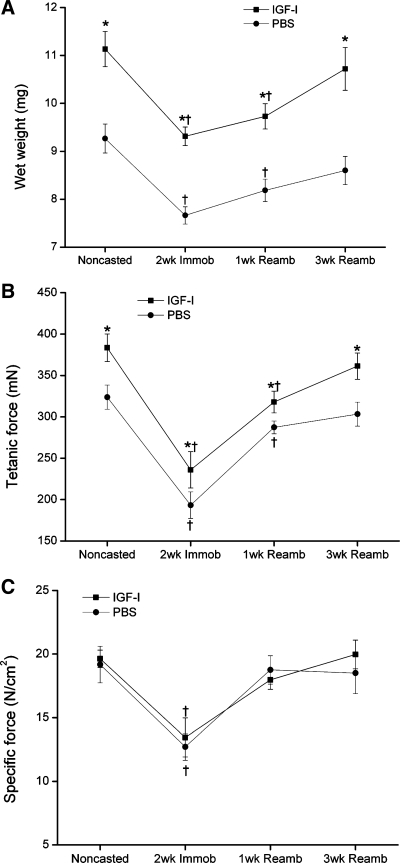
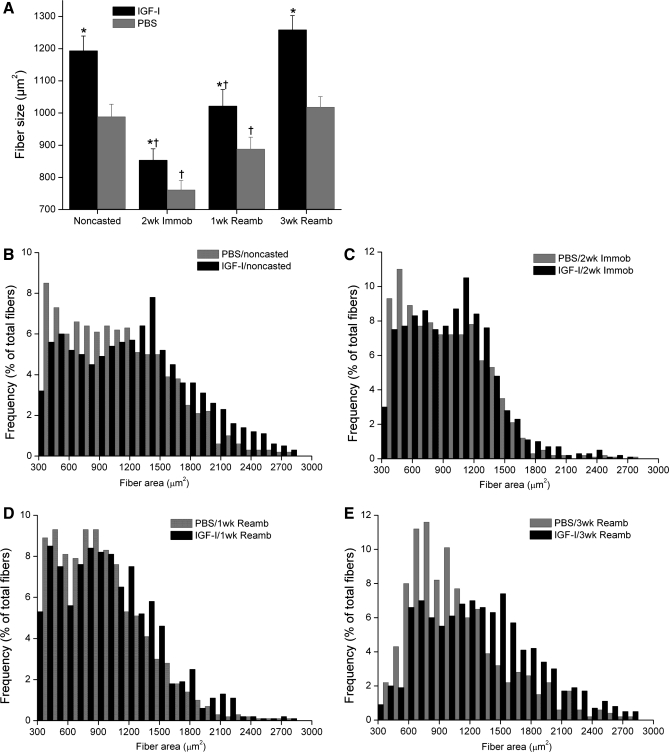
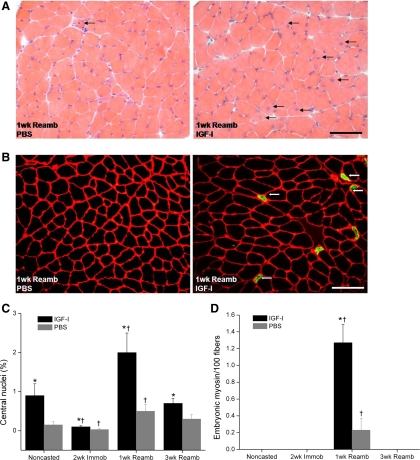
We detected dramatic increases in IGF-I mRNA and protein levels with local rAAV-IGF-IA transfection (185- and 26-fold elevations, respectively, P < 0.05; Table 1 and Fig. 1). Serum IGF-I protein levels did not show any significant difference between rAAV-IGF-IA-injected and control animals (500 ± 20 vs. 510 ± 14 ng/ml). IGF-I overexpression also caused significant increases in EDL muscle wet weight (∼20%, P < 0.05; Fig. 2A), tetanic force (∼18%, P < 0.05; Fig. 2B), and fiber cross-sectional area (CSA; ∼20%, P < 0.05; Fig. 3A). Changes in muscle size were matched by changes in muscle force such that there were no changes in specific force with IGF-I overexpression. Central nuclei were increased sixfold by IGF-I overexpression, indicating enhanced fusion of satellite cells with myofibers (P < 0.05; Fig. 4) (16). Pax7, which is expressed by quiescent, active, and proliferating satellite cells (54), was also increased twofold in the transfected muscles (P < 0.05; Fig. 5).
Table 1.
Changes in IGF-I, IGF-IR, and IGFBP mRNA expression in TA muscles (expressed as fold difference compared with the PBS-injected/noncasted TA muscle)
| Noncasted |
2wk Immob |
1wk Reamb |
3wk Reamb |
|||||
|---|---|---|---|---|---|---|---|---|
| Gene | PBS | IGF-I | PBS | IGF-I | PBS | IGF-I | PBS | IGF-I |
| IGF-I | 1.0 ± 0.1 | 185.4 ± 21.6* | 0.4 ± 0.1 | 176.9 ± 9.9* | 2.8 ± 0.9† | 171 ± 48.0* | 1.2 ± 0.2 | 98 ± 24.4*† |
| IGF-IR | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.2† | 1.8 ± 0.03† | 0.5 ± 0.08† | 0.5 ± 0.1† | 0.8 ± 0.07 | 0.8 ± 0.1 |
| IGFBP-3 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.8 ± 0.4† | 2.2 ± 0.3† | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 |
| IGFBP-4 | 1.0 ± 0.1 | 1.4 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.4 | 1.6 ± 0.4 | 1.0 ± 0.1 | 1.2 ± 0.3 |
| IGFBP-5 | 1.0 ± 0.1 | 1.7 ± 0.2* | 0.2 ± 0.01† | 0.3 ± 0.03*† | 2.5 ± 0.4† | 3.3 ± 0.08*† | 1.0 ± 0.1 | 2.2 ± 0.08* |
Values are means ± SE. TA, tibialis anterior; 2 wk Immob, 2 wk of cast immobilization; 1 wk Reamb, 1 wk of free cage reambulation; 3 wk Reamb, 3 wk of free cage reambulation; IGF-IR, IGF-I receptor; IGFBP, IGF-binding protein.
Significant difference within each loading time point between IGF-I-injected and PBS-injected muscles.
Value at this loading time point was significantly different from noncasted group in the same injection group. P < 0.05.
Fig. 1.
Tibialis anterior (TA) IGF-I protein levels. Values are means ± SE. *Significant difference within each loading time point between IGF-I-injected and PBS-injected muscles. †Value at this loading time point was significantly different from noncasted group in the same injection group. An interaction of group (IGF-I injected vs. PBS injected) and time was detected where IGF-I-injected muscles had a significantly higher increase in IGF-I protein (not indicated on figure). P < 0.05. 2 wk Immob, 2 wk of cast immobilization; 1 wk Reamb, 1 wk of free cage reambulation; 3 wk Reamb, 3 wk of free cage reambulation.
Fig. 2.
A: extensor digitorum longus (EDL) wet weights. B: EDL tetanic force. C: EDL specific force. Values are means ± SE. *Significant difference within each loading time point between IGF-I-injected and PBS-injected muscles. †Value at this loading time point was significantly different from noncasted group in the same injection group (P < 0.05).
Fig. 3.
A: EDL fiber cross-sectional area (μm2). Fiber type size distributions as a percentage of total fibers before casting (B), after casting (C), after 1 wk Reamb (D), and after 3 wk Reamb (E). Note the fiber size shift in muscle injected with IGF-I by 3 wk Reamb. Values are means ± SE. *Significant difference within each loading time point between IGF-I-injected and PBS-injected muscles. †Value at this loading time point was significantly different from noncasted group in the same injection group (P < 0.05).
Fig. 4.
Presence of central nuclei (%) in IGF-I-injected and PBS-injected EDL muscles. A: cross-sections of EDL muscle stained with hematoxylin and eosin. Central nuclei (arrows) were most apparent after 1 wk Reamb in IGF-I-injected muscle. B: cross-sections of EDL muscle stained with monoclonal antibody against embryonic myosin isoform (green). Muscle fibers expressing embryonic myosin were most notable after 1 wk Reamb in IGF-I-injected muscle. C: %central nuclei at each time point (means ± SE). D: no. of fibers expressing embryonic myosin per 100 fibers. Values are means ± SE. *Significant difference within each loading time point between IGF-I-injected and PBS-injected muscles. †Value at this loading time point was significantly different from noncasted group in the same injection group. An interaction of group (IGF-I injected vs. PBS injected) and time was detected where IGF-I-injected muscles had a significantly higher increase in central nuclei and embryonic myosin-positive fibers (not indicated on figure). P < 0.05. Bar, 50 μm.
Fig. 5.
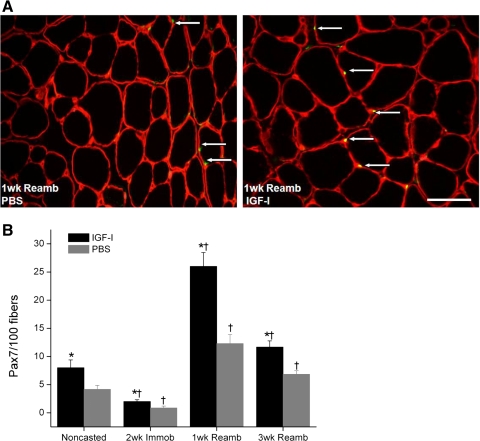
Satellite cell activity in muscle fibers for IGF-I-injected and PBS-injected EDL muscles. A: cross-sections of EDL muscle stained with laminin + 4,6-diamidino-2-phenylindole + paired box transcription factor 7 (Pax7). Pax7-positive fibers (arrows) are most apparent after 1 wk Reamb in IGF-I-injected muscle. B: percentage of Pax7-positive fibers at each time point. Values are means ± SE. *Significant difference within each loading time point between IGF-I injected and PBS injected muscles. †Value at this loading time point was significantly different from noncasted group in the same injection group. An interaction of group (IGF-I injected vs. PBS injected) and time was detected where IGF-I-injected muscles had a significantly higher increase in Pax7-positive fibers (not indicated on figure). P < 0.05. Bar, 25 μm.
IGFBP-5 mRNA expression was significantly higher (∼70%) in the IGF-I-overexpressing TA muscle compared with the contralateral control (P < 0.05) (Table 1). However, no significant changes in IGF-IR, IGFBP-3, or IGFBP-4 mRNA were detected in the transfected adult TA muscles (Table 1).
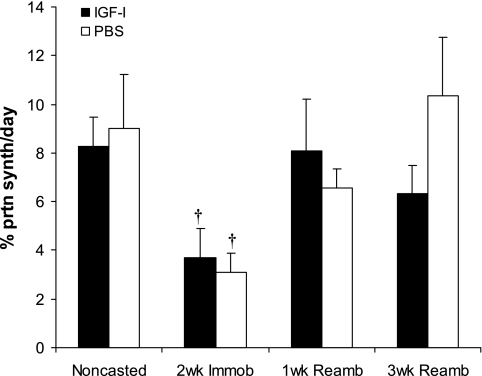
We also did not detect significant changes in protein synthesis rates in 20-wk-old mice with IGF-I overexpression (Fig. 6).
Fig. 6.
EDL protein synthesis (%/day). Values are means ± SE. †Value at this loading time point was significantly different from noncasted group in the same injection group (P < 0.05).
Effects of IGF-I overexpression during immobilization.
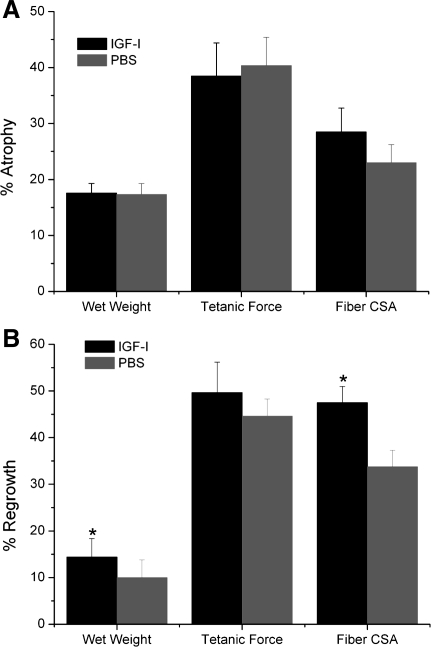
Two weeks of cast immobilization without IGF-I overexpression resulted in significant decreases in EDL muscle wet weight (17%), muscle fiber CSA (23%), tetanic force (40%), specific force (34%), and protein synthesis rate (34%) compared with control muscles (normal loading) (P < 0.05; Figs. 2, 3, and 6).
Immobilization with IGF-I overexpression caused similar decreases in EDL weight, tetanic force, and specific force. However, the relative gains in muscle weight, muscle fiber CSA, and tetanic force that were present before immobilization were preserved with IGF-I overexpression (P < 0.05; Figs. 2, 3, and 7A).
Fig. 7.
%Change in EDL muscle wet weight, tetanic force, and fiber cross-sectional area (CSA). A: normalization of 2 wk Immob values to baseline. B: normalization of 3 wk Reamb values to 2 wk Immob. *Significant difference between IGF-I-injected and PBS-injected groups (P < 0.05).
Cast immobilization increased IGF-IR mRNA and IGFBP-3 mRNA in the TA muscle by ∼60 and 80%, respectively, and decreased IGFBP-5 mRNA by ∼80% (P < 0.05; Table 1). Mostly similar changes were seen in IGF-I-overexpressing vs. PBS-injected TA muscles. Cast immobilization did not change IGF-I mRNA and IGF-I protein levels significantly in either group compared with its baseline at normal weight-bearing conditions (Table. 1 and Fig. 1). We also did not detect significant changes in IGFBP-4 mRNA expression after cast immobilization.
Following 2 wk of cast immobilization, the number of central nuclei (P < 0.05; Fig. 4C) and Pax7-positive fibers (P < 0.05; Fig. 5) was decreased in both injection groups.
Effects of IGF-I overexpression during reambulation.
By 1 wk of reambulation (1wR), specific force had returned to its baseline levels, whereas EDL wet weights and tetanic force remained significantly below baseline until 3 wk of reambulation (3wR) in both IGF-I- and PBS-injected muscles (P < 0.05). However, the absolute tetanic force in the IGF-I-overexpressing EDL muscle at 1wR was almost the same as that of the noncasted PBS-injected muscles. Relative gains in both wet weight and fiber size during 3wR were significantly larger in the IGF-I- vs. PBS-injected EDL muscles (P < 0.05; Fig. 7B). However, the relative gains in force with IGF-I overexpression were not significantly different, although the differences in absolute force between AAV-I-injected and contralateral control limbs appeared slightly larger at the 3wR time point. Protein synthesis returned to baseline levels by 1wR (Fig. 6). IGF-I mRNA increased significantly during the 1st wk of recovery following immobilization and returned to control levels after 3wR in PBS-injected TA muscles. In both IGF-I-overexpressing and PBS-injected TA muscles, IGFBP-3 mRNA and IGF-IR mRNA (which had increased with cast immobilization) returned to baseline after 1wR and 3wR, respectively (Table 1). Also, in both IGF-I-overexpressing and PBS-injected TA muscles, IGFBP-5 mRNA expression (which had decreased during immobilization) increased significantly above baseline after 1wR. After 3wR, IGFBP-5 mRNA returned to baseline in PBS-injected muscle, but it remained elevated in IGF-I-overexpressing muscle (Table 1). No significant differences were detected in IGFBP-4 mRNA expression in the TA muscles of both groups after 1wR or 3wR (Table 1).
IGF-I protein levels were elevated at 1wR in both IGF-I-overexpressing and PBS-injected TA muscles (P < 0.05; Fig. 1) and returned to preimmobilization levels by 3wR. There was also a significantly larger increase in IGF-I protein levels (Fig. 1) at 1wR in the rAAV-IGF-IA injected muscles (loading × injection interaction, P < 0.05).
An increase in markers of regeneration was noted in rAAV-IGF-IA injected muscles compared with PBS-injected muscles after 1wR (a significant interaction effect, P < 0.05). In the PBS-injected EDL muscles, <0.5 and <0.3% of the fibers contained central nuclei and embryonic myosin. In the muscles overexpressing IGF-I, the proportion of centrally nucleated and embryonic myosin fibers increased to 2 and 1.2%, respectively (Fig. 4). The greatest increase in Pax7-positive fibers was also seen after 1wR, with up to a 12-fold increase in the rAAV-IGF-IA-injected muscles following cast immobilization (Fig. 5).
DISCUSSION
Local delivery of recombinant AAV1-IGF-IA resulted in increased expression of IGF-I mRNA and a dramatic increase in IGF-I protein levels as well as an ∼20% increase in EDL muscle mass (wet weights), fiber CSA, and tetanic force. The relative gains in muscle size and strength with IGF-I overexpression observed under normal loading conditions were maintained during cast immobilization and 3 wk of reloading/reambulation, yet IGF-I overexpression did not protect against cast immobilization-induced muscle atrophy. After 1 wk of reambulation, evidence of enhanced muscle regeneration was observed in muscles with elevated IGF-I levels, with an increased prevalence of central nuclei, embryonic myosin, and Pax7-positive fibers. In addition, slightly larger relative gains in muscle size (wet weights and fiber CSA) were noted in the IGF-I-overexpressing muscles during 3 wk of reambulation. Changes in IGFBP-5 mRNA expression during cast immobilization/reambulation paralleled those of IGF-I, whereas IGFBP-3 mRNA expression changed inversely to IGFBP-5. At 3 wk of reambulation, all binding protein expression levels reached normal values, except for IGFBP-5 mRNA, which remained elevated in the rAAV-IGF-IA-injected but not the PBS-injected muscles. To our knowledge, this is the first study to evaluate the effect of viral-mediated IGF-I gene transfer on IGF-I, IGF-I receptor, and binding protein expression during and following cast immobilization. Furthermore, although much previous work has focused on the role of IGF-I overexpression with disuse (21, 26), less work has focused on mechanical reloading after a period of disuse.
In the present study, adult fast-twitch hindlimb muscles injected with rAAV1 resulted in the overexpression of IGF-I at a young age (3 wk) and demonstrated a 26-fold increase in IGF-I protein levels and an ∼20% increase in muscle mass and force production under normal loading conditions. These results are consistent with previous studies using viral delivery of the IGF-I construct as well as transgenic animal models (10, 20, 49). Using a similar rAAV vector delivery system (with MLC1/3 promoter), Barton-Davis et al. (10) found a 15% increase in muscle mass and a 14% increase in force production in young adult mice (10). A 27% increase in EDL muscle force was reported in older adult mice compared with uninjected old muscles (10). The hypertrophic effects of IGF-I have been particularly evident in dystrophic mice models such as mdx mice, a naturally occurring mouse model of Duchenne muscular dystrophy. Transgenic IGF/mdx mice (transgenic mdx mice overexpressing mIGF-I) have been reported to display a 40% increase in muscle mass and similar increases in force production (13). The hypertrophic effect of exogenous IGF-I treatment has also been demonstrated previously following the direct infusion of recombinant IGF-1 protein (4).
The potential to preserve skeletal muscle mass and ameliorate muscle wasting with IGF-I has also been reported in several models of skeletal muscle atrophy (52, 57). Schakman et al. (57) found that local overexpression of IGF-I protein by gene transfer attenuated skeletal muscle atrophy induced by glucocorticoids. Rabinovsky et al. (52) used a transgenic IGF-I mouse model to demonstrate that IGF-I intensified muscle regeneration after denervation by accelerating the myogenic differentiation pathway. In contrast, using transgenic mice overexpressing IGF-I, Criswell et al. (21) reported that enhancing IGF-I levels did not prevent atrophy of the hindlimb muscles during hindlimb suspension. Similarly, in our study, we found that IGF-I overexpression did not attenuate the rate of atrophy. IGF-I-overexpressing muscles were larger and produced more force compared with PBS-injected muscles both before and after 2 wk of cast immobilization, with similar relative differences between the injected and contralateral muscles. Our results, using a different disuse model and mode of IGF-I delivery, further affirm that IGF-I overexpression does not prevent or attenuate atrophy in healthy muscles during unloading (21).
Although much previous work has focused on the effect of IGF-I on muscle atrophy during hindlimb suspension, less work has focused on muscle recovery in response to mechanical reloading after a period of disuse. Lee et al. (43) have examined the combined effect of IGF-I overexpression and resistance training in rats. They reported that the combination of resistance training and viral expression of IGF-I induced greater hypertrophy than either treatment alone. In the present study, we found an increase in several markers of muscle regeneration, including the number of fibers expressing embryonic myosin, muscle fibers with central nuclei, and Pax 7-positive nuclei at 1 wk of reambulation. In addition, rAAV-IGF-IA-injected muscles demonstrated an increased prevalence of large muscle fibers (>1,400 μm) at 3 wk of reambulation. Furthermore, during 3 wk of reambulation, small but significant larger relative gains in muscle size (wet weights and fiber area) were found in rAAV-IGF-IA-injected muscles compared with PBS-injected ones. Although there appeared to be a trend toward increased muscle force by 3 wk of reambulation, differences did not reach significance.
Although IGF-I overexpression had a positive effect on muscle regeneration and muscle growth after cast immobilization, the gains were more modest than expected, especially given the dramatic increases in IGF-I levels. Although it is well demonstrated that IGF-I plays a critical role in muscle growth and development (12, 25, 44), whether or not IGF-I is the primary physiological regulator of muscle mass during muscle adaptation with increased mechanical loading is still not clear (28). Experiments blocking the IGF-I receptor during functional overload have still found muscle hypertrophy (60, 62), suggesting that IGF-I may not be required to activate Akt-mTOR-mediated muscle hypertrophy. Alternatively, since the EDL muscle is not a primary antigravity muscle in murine animals (23, 51), it is possible that the limited amount of (re)loading experienced by the EDL may not have invoked as large of a myogenic response. It is also possible that other confounding factors, such as the role of binding proteins, may have interfered with the positive effects of IGF-I overexpression. One of these potential confounders is IGFBP-5, which is the major IGFBP secreted by skeletal muscle. In this study, IGFBP-5 mRNA was significantly higher after 3 wk of reambulation in the IGF-I-overexpressed TA muscles, which could have blocked muscle differentiation by inhibiting IGF-I actions (48, 56). Such inhibitory effect of IGFBP-5 could be attributed to competition for IGF-I with the IGF-I receptor (22).
Another explanation for the discrepancy between profound increases in IGF-I levels, our indices of muscle regeneration and the magnitude of the growth response, could relate to satellite cell responses. Adult muscle growth is thought to be dependent on muscle stem cells (satellite cells), which are small mononucleated cells located between the basal lamina of the muscle and the sarcolemma of myofibers. Jennische and Hansson (38) showed that IGF-I immunoreactivity was detected in the cytoplasm of myoblasts and myotubes and in satellite cells during muscle regeneration. Compared with other markers, Pax7 has been identified as a satellite cell-specific marker, and its function in satellite cell specification, survival, proliferation, and self-renewal is consistent with its expression in all satellite cells (54). In the present study, IGF-I overexpression increased the number of central nuclei fourfold and Pax7-positive nuclei twofold during early reloading, thereby suggesting that IGF-I overexpression enhanced muscle regeneration. Although satellite cells appear to be critical for the regeneration of muscle tissue after injury (59), increased satellite cell content has been demonstrated without an increase in myonuclear number with resistance training (39). Furthermore, muscle hypertrophy induced by activation of Akt, a critical component of the IGF-I/phosphatidylinositol 3-kinase downstream signaling pathway, may not be accompanied by satellite cell activation and new myonuclei incorporation (17). It is possible that in our study satellite cells were activated with mechanical reloading (increased expression of Pax7 and central nuclei with IGF-I overexpression) but then exited the cell cycle and became quiescent again because existing myonuclei were able to support the moderate increase in the cytoplasmic area (39). We should also point out that the number of Pax7-positive fibers observed in the EDL muscle during reambulation is relatively small compared with the numbers reported in antigravity slow-twitch muscles. Gibson and Schultz (30) showed that the absolute number of satellite cells varies with age and fiber type, with the slow-twitch soleus muscle displaying two- to fivefold more satellite cells than the fast-twitch EDL muscle. Similarly, a higher percentage of satellite cells are found in slow-twitch muscle fibers compared with fast-twitch muscle fibers within the same muscle (19, 31, 58). Therefore, it is possible that the effect of IGF-I overexpression during reloading is more pronounced in slow-twitch antigravity muscles such as the soleus muscle compared with the fast-twitch EDL muscle studied herein (7, 8, 18, 46, 65).
Based on previous studies showing increases in IGF-I mRNA and protein in response to muscle overload (1, 3), we expected a decrease in IGF-I mRNA during cast immobilization. However, the IGF-I protein level at 2 wk of cast immobilization was equivalent to that measured in the TA muscle under normal loading conditions (control), whereas IGF-I protein levels in both the rAAV-IGF-IA- and PBS-injected limb were increased at 1 wk of reambulation. Our findings are similar to that of Awede et al. (6), who reported that IGF-I mRNA levels were not different from the control values after 8 days of hindlimb suspension and were increased significantly after 8 days of overloading. In other studies, neither 5 wk of unilateral limb suspension in humans (33) nor 4 days of spinal cord isolation in rats (34) resulted in a decrease in IGF-I mRNA. Some studies even indicate an increase in IGF-I mRNA in human muscle during chronic disuse (53) and rat muscle following spinal cord isolation (41).
Although we found significant modulation in IGF-I receptor expression under altered loading conditions (cast immobilization vs. reloading), the impact of IGF-I receptor in mediating changes in muscle function with overexpression of IGF-I is not clear. Studies have demonstrated that IGF-I receptor is essential during early normal development because mice lacking IGF-I receptor exhibit marked muscle hypoplasia and die soon after birth (25). Other investigations have suggested that the in vivo effects of growth hormone on muscle mass and strength are mediated primarily by activation of the IGF-I receptor (40). But more recently, Spangenburg et al. (62) found that increased mechanical load can induce muscle hypertrophy independent of a functioning IGF-I receptor. In the present study, we found that IGF-I receptor mRNA levels were elevated 60–80% after cast immobilization, which was also observed in spinal cord-isolated rats (34) and dropped back to baseline levels with reambulation. If IGF-I receptor plays a role in muscle atrophy or hypertrophy, it is possible that an elevation of IGF-I receptor during atrophy may offer a compensatory mechanism to attenuate muscle atrophy by being more available for IGF-I binding. Also, although there was a large increase in IGF-I protein and mRNA levels in rAAV-IGF-IA-injected muscles at 20 wk of age, we did not detect a significant difference in IGF-I receptor expression between IGF-I- and PBS-injected groups under normal loading conditions. We expect that IGF-I receptor mRNA differences may have been present at an earlier time point after virus injection (13).
The influence of IGF-I-binding proteins on the cellular effects of IGF-I is less clear. IGF-I-binding proteins are known to modulate the action of IGF-I (61). Among the seven known IGFBPs, the predominant IGFBPs in muscle are IGFBP-5 and IGFBP-4 (37), whereas IGFBP-3 is the most abundant IGFBP in serum and regulates the concentration of free circulating IGF-I (6, 24, 27, 32). Although measurements of mRNA have limitations, these findings provide some insights regarding the potential modulating actions of IGFBPs. In the present study, IGFBP-5 mRNA levels were reduced significantly at 2 wk of cast immobilization and increased to above baseline in both rAAV-IGF-IA- and PBS-injected muscles after 1 wk of reambulation. In contrast, IGFBP-4 mRNA expression was not altered significantly at any of the time points. Hypertrophic conditions such as electromyostimulation (15) and overloading (2, 6) have been reported to be associated with increases in IGFBP-4 mRNA, whereas findings in regard to IGFBP-5 have been less consistent (2, 6, 61, 64). IGFBP-5 has been shown to both stimulate and suppress cell survival, proliferation, and differentiation (5, 14, 69). Interestingly, after 3 wk of reambulation, IGFBP-5 mRNA returned to baseline in PBS-injected muscles but remained elevated in rAAV-IGF-IA-injected muscles. In addition, changes in IGFBP-5 mRNA during cast immobilization/reambulation paralleled those of IGF-I, whereas IGFBP-3 expression changed inversely to IGFBP-5. Few studies have investigated changes in IGFBP-3 with unloading, and yet IGFBP-3 is thought to play a critical role toward modulating the availability of IGF-I in blood (6). Since IGFBP-3 is reported to suppress the proliferation of the cultured porcine myogenic cells (36), unloading-induced increase in IGFBP-3 may inhibit the proliferation of muscle satellite cells or IGF-I binding to its receptors. The lack of clarity regarding the regulation of IGFBPs across studies may also be a result of transient increases in mRNA that are extremely sensitive to the timing of the measurements or because of transcription in binding protein levels through yet-unknown mechanisms of regulation.
Despite increases in muscle mass, fiber CSA, and force with rAAV-induced IGF-I overexpression, protein synthesis rates were not found to be elevated in IGF-I-injected limbs at baseline. One possible explanation for this finding is that increases in protein synthesis rates were transient and occurred early after rAAV-IGF-IA injection (42, 65). In the present study, protein synthesis measurements were performed at least 16 wk after injection. Similarly with reambulation, the time points chosen for measurement (1 and 3 wk) may not have been optimal to detect changes in protein synthesis. (42, 65).
In conclusion, local delivery of recombinant rAAV-IGF-IA in fast-twitch, lower-limb muscles resulted in a dramatic increase in IGF-I protein levels, 20% muscle hypertrophy, and a concomitant increase in muscle strength. Relative gains in muscle size and force production observed under normal loading conditions were maintained during cast immobilization/reambulation. Muscles overexpressing IGF-I demonstrated evidence of enhanced muscle regeneration, with an increased prevalence of embryonic myosin-positive fibers, central nuclei, and Pax7-positive nuclei. In addition, rAAV-IGF-IA-injected muscles showed larger gains in muscle size compared with uninjected muscles during 3 wk of reambulation. Alterations in IGFBP-5 mRNA most closely paralleled the changes in IGF-I during cast immobilization and reambulation, whereas IGFBP-3 mRNA expression changed inversely to IGFBP-5 mRNA.
GRANTS
This study was supported by National Institutes of Health Grants R01-HD-042955 and R01-HL-78670. Muscle protein synthesis analyses were conducted by the Washington University Biomedical Mass Spectrometry Research Resource (RR-000954, DK-020579, and DK-056341).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev 26: 31–60, 1998 [PubMed] [Google Scholar]
- 2.Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283: C1182–C1195, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81: 2509–2516, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem 267: 22467–22472, 1992 [PubMed] [Google Scholar]
- 6.Awede B, Thissen J, Gailly P, Lebacq J. Regulation of IGF-I, IGFBP-4 and IGFBP-5 gene expression by loading in mouse skeletal muscle. FEBS Lett 461: 263–267, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 90: 345–357, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Baldwin KM, Herrick RE, McCue SA. Substrate oxidation capacity in rodent skeletal muscle: effects of exposure to zero gravity. J Appl Physiol 75: 2466–2470, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Bark TH, McNurlan MA, Lang CH, Garlick PJ. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol Endocrinol Metab 275: E118–E123, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167: 301–305, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 100: 1778–1784, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157: 137–148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab 278: E967–E976, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94: 2255–2262, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development 109: 943–952, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23: 3896–3905, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol 76: 1764–1773, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Criswell DS, Booth FW, DeMayo F, Schwartz RJ, Gordon SE, Fiorotto ML. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol Endocrinol Metab 275: E373–E379, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol 167: 344–351, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Eng CM, Smallwood LH, Rainiero MP, Lahey M, Ward SR, Lieber RL. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol 211: 2336–2345, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ewton DZ, Coolican SA, Mohan S, Chernausek SD, Florini JR. Modulation of insulin-like growth factor actions in L6A1 myoblasts by insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5: a dual role for IGFBP-5. J Cell Physiol 177: 47–57, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Fernandez AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest 109: 347–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitts RH, Riley DR, Widrick JJ. Physiology of a Microgravity Environment Invited Review: Microgravity and skeletal muscle. J Appl Physiol 89: 823–839, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Flueck M, Goldspink G. Counterpoint: IGF is not the major physiological regulator of muscle mass. J Appl Physiol 108: 1821–1823, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle Nerve 32: 672–674, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6: 574–580, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202: 329–337, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Haddad F, Adams GR. Selected contribution: acute cellular and molecular responses to resistance exercise. J Appl Physiol 93: 394–403, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Haddad F, Baldwin KM, Tesch PA. Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 98: 46–52, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. II. Molecular markers of protein deficits. J Appl Physiol 95: 791–802, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Hakkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Hakkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53: B415–B423, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Hembree JR, Pampusch MS, Yang F, Causey JL, Hathaway MR, Dayton WR. Cultured porcine myogenic cells produce insulin-like growth factor binding protein-3 (IGFBP-3) and transforming growth factor beta-1 stimulates IGFBP-3 production. J Anim Sci 74: 1530–1540, 1996 [DOI] [PubMed] [Google Scholar]
- 37.James PL, Jones SB, Busby WH, Jr, Clemmons DR, Rotwein P. A highly conserved insulin-like growth factor-binding protein (IGFBP-5) is expressed during myoblast differentiation. J Biol Chem 268: 22305–22312, 1993 [PubMed] [Google Scholar]
- 38.Jennische E, Hansson HA. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol Scand 130: 327–332, 1987 [DOI] [PubMed] [Google Scholar]
- 39.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology 146: 1772–1779, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Roy RR, Kim JA, Zhong H, Haddad F, Baldwin KM, Edgerton VR. Gene expression during inactivity-induced muscle atrophy: effects of brief bouts of a forceful contraction countermeasure. J Appl Physiol 105: 1246–1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289: E969–E980, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol 96: 1097–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75: 59–72, 1993 [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 46.McDonald KS, Delp MD, Fitts RH. Fatigability and blood flow in the rat gastrocnemius-plantaris-soleus after hindlimb suspension. J Appl Physiol 73: 1135–1140, 1992 [DOI] [PubMed] [Google Scholar]
- 47.McNurlan MA, Essén P, Thorell A, Calder AG, Anderson SE, Ljungqvist O, Sandgren A, Grant I, Tjäder I, Ballmer PE, Wernerman J, Garlick PJ. Response of protein synthesis in human skeletal muscle to insulin: an investigation with l-[2H5]phenylalanine. Am J Physiol Endocrinol Metab 267: E102–E108, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee A, Wilson EM, Rotwein P. Insulin-like growth factor (IGF) binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions. Mol Endocrinol 22: 206–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Nilsson G, Nyberg P, Ekdahl C, Eneroth M. Performance after surgical treatment of patients with ankle fractures—14-month follow-up. Physiother Res Int 8: 69–82, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Pregelj P, Sketelj J. Effect of mechanical load on acetylcholinesterase mRNA levels in the slow soleus muscle of the rat. Pflugers Arch 440: R112–R114, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, Shenaq SM, Schwartz RJ. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J 17: 53–55, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E. Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve 24: 893–899, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435: 948–953, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Rosendal L, Langberg H, Flyvbjerg A, Frystyk J, Orskov H, Kjaer M. Physical capacity influences the response of insulin-like growth factor and its binding proteins to training. J Appl Physiol 93: 1669–1675, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, Carter EJ, Cobb LJ, Eisemann JE, Pell JM. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci USA 101: 4314–4319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, Ketelslegers JM, Thissen JP. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 146: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23: 617–626, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev 20: 1692–1708, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Spangenburg EE. Changes in muscle mass with mechanical load: possible cellular mechanisms. Appl Physiol Nutr Metab 34: 328–335, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Spangenburg EE, Abraha T, Childs TE, Pattison JS, Booth FW. Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am J Physiol Endocrinol Metab 284: E340–E350, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Spangenburg EE, Leroith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586: 283–291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens JE, Walter GA, Okereke E, Scarborough MT, Esterhai JL, George SZ, Kelley MJ, Tillman SM, Gibbs JD, Elliott MA, Frimel TN, Gibbs CP, Vandenborne K. Muscle adaptations with immobilization and rehabilitation after ankle fracture. Med Sci Sports Exerc 36: 1695–1701, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551: 33–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1–12, 1990 [DOI] [PubMed] [Google Scholar]
- 66.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/combustion isotope ratio mass spectrometry. Biol Mass Spectrom 21: 486–490, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin HN, Chai JK, Yu YM, Shen CA, Wu YQ, Yao YM, Liu H, Liang LM, Tompkins RG, Sheng ZY. Regulation of signaling pathways downstream of IGF-I/insulin by androgen in skeletal muscle of glucocorticoid-treated rats. J Trauma 66: 1083–1090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin P, Xu Q, Duan C. Paradoxical actions of endogenous and exogenous insulin-like growth factor-binding protein-5 revealed by RNA interference analysis. J Biol Chem 279: 32660–32666, 2004 [DOI] [PubMed] [Google Scholar]