Abstract
NF-κB is a transcription factor that controls the gene expression of several proinflammatory proteins. Cell culture and animal studies have implicated increased NF-κB activity in the pathogenesis of insulin resistance and muscle atrophy. However, it is unclear whether insulin-resistant human subjects have abnormal NF-κB activity in muscle. The effect that exercise has on NF-κB activity/signaling also is not clear. We measured NF-κB DNA-binding activity and the mRNA level of putative NF-κB-regulated myokines interleukin (IL)-6 and monocyte chemotactic protein-1 (MCP-1) in muscle samples from T2DM, obese, and lean subjects immediately before, during (40 min), and after (210 min) a bout of moderate-intensity cycle exercise. At baseline, NF-κB activity was elevated 2.1- and 2.7-fold in obese nondiabetic and T2DM subjects, respectively. NF-κB activity was increased significantly at 210 min following exercise in lean (1.9-fold) and obese (2.6-fold) subjects, but NF-κB activity did not change in T2DM. Exercise increased MCP-1 mRNA levels significantly in the three groups, whereas IL-6 gene expression increased significantly only in lean and obese subjects. MCP-1 and IL-6 gene expression peaked at the 40-min exercise time point. We conclude that insulin-resistant subjects have increased basal NF-κB activity in muscle. Acute exercise stimulates NF-κB in muscle from nondiabetic subjects. In T2DM subjects, exercise had no effect on NF-κB activity, which could be explained by the already elevated NF-κB activity at baseline. Exercise-induced MCP-1 and IL-6 gene expression precedes increases in NF-κB activity, suggesting that other factors promote gene expression of these cytokines during exercise.
Keywords: nuclear factor-κB, skeletal muscle, monocyte chemotactic protein-1, interleukin-6
type 2 diabetes mellitus (T2DM) and obesity are insulin-resistant disorders characterized by chronic inflammation (17, 20). Nuclear factor-κB (NF-κB) is a family of transcription factors that control the production of proinflammatory proteins. Data accumulated in recent years suggest that increased NF-κB signaling may be involved in the pathogenesis of insulin resistance (3, 4, 32, 50, 56). Elevated activity of this transcription factor has also been linked to muscle loss (11) and weakness (29), features seen commonly in diabetic subjects (40). In the basal state, NF-κB is localized in the cytoplasm associated with the inhibitory protein inhibitor κB (IκB). Stimuli such as cytokines (19), reactive oxygen species (19), hyperglycemia (19), and free fatty acids (FFAs) (30) activate IκB kinase (IKK), which is a complex of kinases that phosphorylate IκB. IκB phosphorylation by IKK leads to IκB ubiquitination and proteasomal degradation. The IκBs are members of a gene family that includes IκBα, -β, -ϵ, and -γ and Bcl-3 (24). The IκBα and -β isoforms have been the most widely studied, and both can sequester and inhibit NF-κB. Following release from IκB, NF-κB translocates to the nucleus, where it controls gene transcription of inflammatory proteins such as interleukin (IL)-6, monocyte chemotactic protein-1 (MCP-1), IL-1, and interferon-β (19, 38). NF-κB proteins consist of five members, including p65, p50, p52, RelB, and c-Rel. Dimerization of two NF-κB family members is necessary to exert its DNA-binding activity. The predominant activating NF-κB dimer in skeletal muscle is p50/p65 (31). Increased IKK/NF-κB signaling is thought to inhibit insulin signaling through insulin receptor substrate-1 serine phosphorylation by IKK (23) and NF-κB-mediated expression of inflammatory proteins (3). Despite the evidence from cell culture and animal studies indicating that NF-κB may be involved in insulin resistance (34, 56), it is not known whether insulin-resistant subjects have abnormal NF-κB activity in muscle.
Considering the role that NF-κB plays on myocellular differentiation (5) and muscle function (31), it is important to know whether exercise affects NF-κB activity and to understand the physiological significance of such a change. Muscle contraction promotes the generation of reactive oxygen species (43) and increases intracellular calcium levels (8). Because reactive oxygen species (19) and calcium-regulated signaling cascades (27) activate NF-κB, one might expect exercise to increase the activity of this transcription factor. Nonetheless, there have been contradictory findings with respect to the effect of exercise on NF-κB. In rodents, acute treadmill running increases NF-κB activity in muscle (26, 32). In contrast, a study in healthy human subjects reported that resistance exercise reduced NF-κB activity (18). We found that aerobic (cycle) training increased the content of the NF-κB inhibitory protein IκB in human muscle, which would be expected to decrease NF-κB activity (51). Schenk and Horowitz (48) also reported that a single bout of aerobic exercise was sufficient to increase the content of this inhibitory protein in human muscle. Although these data suggest that exercise inhibits NF-κB signaling in humans, the effect of acute aerobic exercise on NF-κB activity in normal glucose-tolerant and insulin-resistant subjects is not known. The goals of this study were to examine whether insulin-resistant subjects have increased NF-κB activity and to determine the effect of acute aerobic exercise on NF-κB activity/signaling in human muscle.
METHODS
Subjects.
We studied 13 obese [body mass index (BMI) = 32.8 ± 1.1 kg/m2] type 2 diabetic, 10 obese (BMI = 32.6 ± 1.5 kg/m2) nondiabetic, and 11 nonobese (BMI = 25.1 ± 0.7 kg/m2) nondiabetic subjects. Although the mean BMI of the nonobese nondiabetic group is higher than the World Health Organization's definition of normal weight (BMI <25 kg/m2), for the purpose of this study this group will be referred to as “lean.” All subjects were sedentary. Each subject underwent a medical history, physical examination, screening laboratory tests, and a 75-g oral glucose tolerance test (OGTT). Six T2DM subjects took sulfonylureas, which were stopped 2 days before the OGTT, the insulin clamp, and the acute exercise experiments, to avoid hypoglycemia during the fasted state. Seven subjects with T2DM were diet treated. Lean and obese control subjects were normal glucose tolerant. Other than the sulfonylureas, subjects were not taking medication known to affect glucose metabolism. Body weight was stable (±1 kg) for ≥3 mo prior to enrollment. The study was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA), and all subjects gave written voluntary consent.
OGTT.
Plasma glucose levels were measured at baseline and every 15 min for 2 h after the ingestion of 75 g of glucose. Plasma insulin and FFA concentrations were measured at baseline.
Maximum O2 consumption testing.
Within 3–7 days after the OGTT, maximum O2 consumption (V̇o2max) was determined using a cycle ergometer and a Metabolic Measurement System (Sensormedics, Savi Park, CA), as described previously (52).
Measurement of peripheral insulin sensitivity.
Within 3–7 days after V̇o2max testing, subjects returned to the Clinical Research Center (CRC) at 7 AM after an overnight fast to undergo a 180-min euglycemic hyperinsulinemic (160 mU·m2·min−1) clamp study. Insulin-stimulated glucose metabolism (M) was determined on the basis of the mean glucose infusion rate during the last 30 min of the clamp (15).
Acute exercise experiment.
Within 7–10 days after the insulin clamp, subjects came to the CRC at 7 AM after an overnight fast to undergo an acute exercise experiment. Subjects refrained from any exercise, other than habitual walking, for 48 h before arriving at the CRC. Subjects rested for 30 min in the supine position, followed by a basal vastus lateralis muscle biopsy performed under local anesthesia (1% lidocaine) and sterile conditions using a Bergstrom cannula with suction. The muscle was rapidly (within 8–12 s of extraction of the cannula) debrided of adipose and connective tissue and immediately frozen in liquid nitrogen. Subjects then exercised on a cycle ergometer at 70% V̇o2max for a total of 40 min as follows: after 30 min, exercise was stopped, and the subjects briefly (∼30 s) rested on a bed while 1% lidocaine was applied, and exercise was continued for an additional 10 min. After a total of 40 min of exercise, subjects were placed on the bed, and a second muscle biopsy was obtained. After the cessation of exercise, the subjects rested in a bed for 210 min, followed by a third muscle biopsy. Each biopsy site was separated by ≥5 cm.
Laboratory analyses.
Plasma insulin was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), plasma glucose by the glucose oxidase method, using a Beckman Glucose Analyzer, and hemoglobin A1c (Hb A1c) using a DCA2000 Analyzer (Bayer, Tarrytown, NY). Plasma FFA concentration was determined using a colorimetric method (Wako). Blood lactate concentration was measured using a hand-held Accutrend Lactate Analyzer (Roche Diagnostics, Manheim, Germany). Plasma TNFα and IL-6 concentrations were measured using an enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN).
NF-κB activity assay.
For nuclear protein extraction, frozen tissue (∼40–80 mg) was ground in 1 ml of buffer A (10 mM HEPES-KOH, pH 7.9, 1.5 mmol/l MgCl2, 10 mmol/l KCl, and 1 mmol/l dithiothreitol) containing 0.1% NP-40 (Sigma, St. Louis, MO) and protease inhibitors (Boehringer Mannheim, Indianapolis, IN), followed by centrifugation at 15,000 rpm for 5 min at 4°C. The pellet was resuspended in 50 μl of buffer B (20 mmol/l HEPES-KOH, pH 7.9, 25% glycerol, 420 mmol NaCl, 1.5 mmol MgCl2, and 0.2 mmol EDTA) and further incubated on ice for 30 min for high salt extraction. The samples were centrifuged at 15,000 rpm for 30 min at 4°C, and the supernatant was transferred to a prechilled tube. Total protein concentration was measured using the bicinchoninic acid method, following the manufacturer's protocol (Pierce, Rockford, IL). EMSA analysis was performed as described earlier (39). For comparison of basal NF-κB activity between groups, samples from the three groups were distributed in three gels and normalized to an internal control. To assess the effect of exercise, all of the samples from each subject were loaded in one gel, and the exercise and postexercise data were normalized with the preexercise sample. Quantitation of the NF-κB DNA-binding activity was performed by densitometric scanning of the autoradiograms using Imagetool (UTHSCSA).
Western blotting.
Muscle samples were homogenized in ice-cold lysis buffer (containing 20 mmol/l Tris, pH 7.5, 10 mmol/l sodium pyrophosphate, 100 mmol/l sodium fluoride, 2 mmol/l sodium orthovanadate, 5 mmol/l EDTA, pH 8.0, 1% Nonidet P-40, 1 mmol/l PMSF, 3 mmol/l benzamidine, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). Homogenates were rotated for 1 h at 4°C and then centrifuged at 14,000 g for 10 min at 4°C. The supernatants were collected and protein concentrations measured by the Bradford assay. Lysate proteins were separated by 8% SDS-PAGE and transferred to nitrocellulose membranes. After blocking in Tris-buffered saline with 5% nonfat dry milk, the membranes were incubated overnight at 4°C with the primary antibody against human IκBα (Cell Signaling Technology, Beverly, MA), IκBβ (Santa Cruz Biotechnology, Santa Cruz, CA), NF-κB p50 (Cell Signaling Technology), and NF-κB p65 (Upstate, Lake Placid, NY). Bound antibodies were detected with anti-rabbit immunoglobulin-horseradish peroxidase-linked whole antibody using enhanced chemiluminescent reagents (PerkinElmer, Boston, MA). The membranes were exposed to film, and band intensity was quantified using Imagetool (UTHSCSA). Basal and exercise-stimulated samples from one lean, one obese, and one T2DM subject were loaded on one gel, and an internal control sample was utilized in all gels to normalize the data.
IL-6, MCP-1 (CCL2), and cellular-FLICE inhibitory protein mRNA levels.
Total RNA was extracted with Trizol solution (Sigma) and purified with RNeasy and DNase I treatment (Qiagen, Chatsworth, CA). An Agilent Bioanalyzer was used to check RNA quality. Muscle expression of IL-6 and MCP-1 (CCL2) was determined using one-step quantitative real-time PCR (qRT-PCR) from total RNA. qRT-PCR was performed on an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan One Step RT-PCR Master Mix reagents. MCP-1 expression was determined using TaqMan gene assay Hs00234140_m1. IL-6 expression was determined using the following primers/probe: forward primer, 5-GGTACATCCTCGACGGCATCT-3; reverse primer, 5-GTGCCTCTTTGCTGCTTTCAC-3; probe, 5-TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT-3. Cellular-FLICE inhibitory protein (c-FLIP) mRNA levels (7) were determined using the following primers/probe: forward primer, 5-AGCTTTGTGTGTGTCCTGGTGA-3; reverse primer, 5-GCCCTGAGTGAGTCTGATCCA-3; probe, 5-CGAGGAGGCTCCCAGAGTGTGTATGG-3. Expression data were normalized by dividing the amount of the gene of interest by the amount of RN18S gene used as the internal control.
Statistical analysis.
Data are expressed as means ± SE. Comparison of baseline data between groups was done using ANOVA. The effect of exercise on the different groups was analyzed using ANOVA with repeated measures followed by the Tukey test. SigmaStat software was used for statistical analysis.
RESULTS
Subject characteristics.
There were no significant differences in age between the T2DM, obese, and lean groups (Table 1). The T2DM and obese groups had higher BMI than the lean group. Subjects with T2DM had higher fasting plasma glucose, Hb A1c, and FFA concentrations compared with obese and lean subjects. T2DM subjects had elevated plasma insulin levels. Plasma IL-6 concentrations were elevated in T2DM subjects and tended (P = 0.1) to be higher in the obese vs. lean subjects. T2DM and obese subjects were more insulin resistant than the lean subjects based on the lower M value during the clamp. T2DM subjects had lower V̇o2max than lean and obese individuals.
Table 1.
Clinical and laboratory characteristics
| Lean (n = 11) | Obese (n = 10) | T2DM (n = 13) | |
|---|---|---|---|
| Sex (no. of males/females) | 8/3 | 3/7 | 4/9 |
| Age, yr | 46.2 ± 3.2 | 41.7 ± 2.9 | 47.9 ± 3.3 |
| Body mass index, kg/m2 | 25.1 ± 0.7 | 32.6 ± 1.5* | 32.8 ± 1.1* |
| Plasma glucose, mg/dl | 91 ± 3 | 88 ± 3 | 179 ± 20*† |
| Hemoglobin A1c, % | 5.3 ± 0.1 | 5.1 ± 0.2 | 8.3 ± 0.7*† |
| Insulin, μU/ml | 4.4 ± 0.8 | 12.3 ± 6.2 | 11.6 ± 1.8* |
| FFA, μmol/l | 464 ± 60 | 550 ± 60 | 857 ± 40*† |
| IL-6, pg/ml | 1.7 ± 0.2 | 2.8 ± 0.6 | 2.9 ± 0.4* |
| TNFα, pg/ml | 1.0 ± 0.4 | 1.8 ± 0.6 | 1.8 ± 0.4 |
| M value, mg·kg−1·min−1 | 11.1 ± 0.6 | 9.0 ± 0.8* | 6.1 ± 0.9*† |
| V̇o2max, mg·kg−1·min−1 | 21.9 ± 1.5 | 19.1 ± 1.1 | 16.0 ± 1.0*† |
| Workload, W | 84.6 ± 5.7 | 86.6 ± 7.3 | 74.4 ± 5.8 |
Data are means ± SE. T2DM, type 2 diabetes mellitus; FFA, free fatty acids; M, insulin-stimulated glucose metabolism; V̇o2max, maximal oxygen uptake.
P < 0.05 vs. lean;
P < 0.05 vs. obese.
Effect of exercise on glucose and lactate concentrations.
Exercise decreased the fasting plasma glucose concentration by 15 mg/dl (P < 0.05 vs. baseline) in T2DM subjects but not in lean and obese individuals (Table 2). Exercise increased blood lactate concentrations significantly in the three groups (Table 2).
Table 2.
Effect of exercise on plasma glucose and lactate concentrations
| Lean | Obese | T2DM | |
|---|---|---|---|
| Glucose before exercise, mg/dl | 95 ± 4 | 94 ± 3 | 177 ± 19*† |
| Glucose after exercise, mg/dl | 96 ± 4 | 90 ± 1 | 162 ± 17‡ |
| Lactate before exercise, mmol/l | 1.8 ± 0.1 | 1.9 ± 0.1 | 2.3 ± 0.2 |
| Lactate after exercise, mmol/l | 5.0 ± 0.7‡ | 4.3 ± 0.2‡ | 5.8 ± 0.6‡ |
Data are means ± SE.
P < 0.05 vs. lean;
P < 0.05 vs. obese;
P < 0.05 vs. preexercise within group.
NF-κB activity.
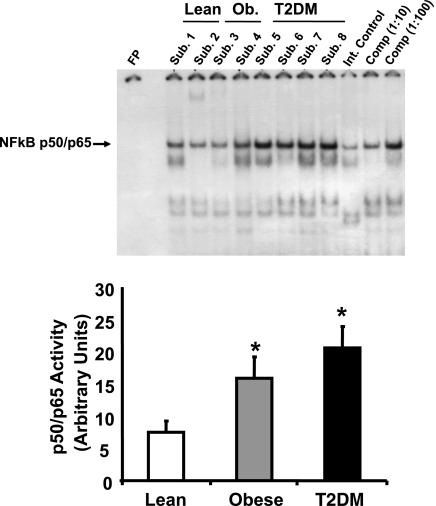
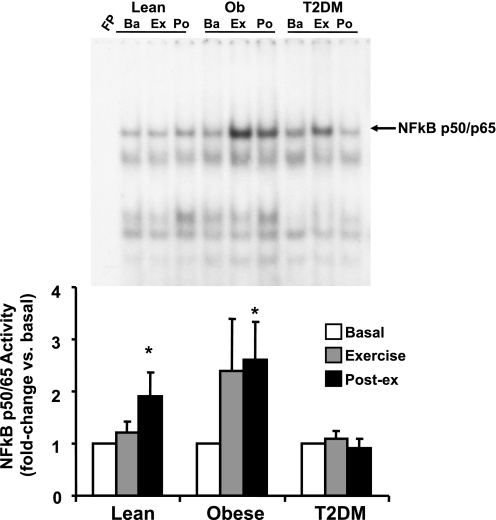
As shown in Fig. 1, baseline p50/p65 NF-κB activity was elevated significantly in obese (2.1-fold, P < 0.05) and T2DM (2.7-fold, P < 0.05) individuals. NF-κB activity did not change in any group after 40 min of exercise (Fig. 2). However, NF-κB activity was increased in the muscle biopsy obtained 210 min postexercise 1.9-fold (P < 0.05) in lean and 2.6-fold (P < 0.05) in obese subjects (Fig. 2). In contrast, NF-κB activity was not increased at 210 min after exercise in T2DM subjects.
Fig. 1.
Basal NF-κB p50/p65 DNA-binding activity in skeletal muscle. NF-κB activity was measured in vastus lateralis muscle by EMSA. Graphic data are means ± SE. Representative blots are shown for 2–4 subjects (Sub). *P < 0.05 vs. lean group. An internal (Int) control (baboon muscle tissue) was utilized to normalize the data. To confirm the identity of the NF-κB p50/p65 band, increasing concentrations of a specific competitive (Comp) inhibitor were also tested (using sample from Sub. 8). Ob, obese; FP, free probe; T2DM, type 2 diabetes mellitus.
Fig. 2.
Effect of acute exercise on NF-κB p50/p65 activity in muscle. Biopsies were done at baseline (Ba), 40 min of exercise (Ex), and 210 min postexercise (Po), and NF-κB activity was measured by EMSA. Graphic data are means ± SE. Representative blots are shown for 1 subject/group. *P < 0.05 vs. basal of respective group. Data were normalized to the preexercise sample within each subject. FP, free probe.
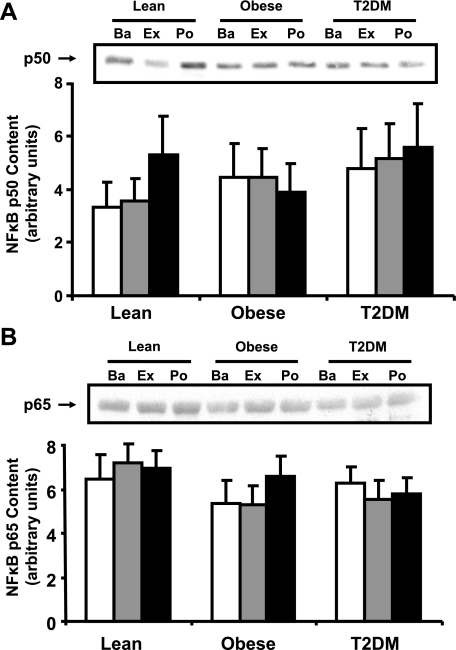
NF-κB p50 and p65 protein content.
Western analysis revealed that the baseline protein content of NF-κB p50 (Fig. 3A) and p65 (Fig. 3B) subunits was similar in muscle from lean, obese, and T2DM subjects. Acute exercise did not affect the protein content of these proteins (Fig. 3, A and B).
Fig. 3.
NF-κB p50 and p65 protein content in muscle. Biopsies were done at Ba, Ex, and Po, and NF-κB p50 (A) and p65 content (B) were measured by Western blotting. Graphic data are means ± SE. Representative blots are shown for 1 subject/group.
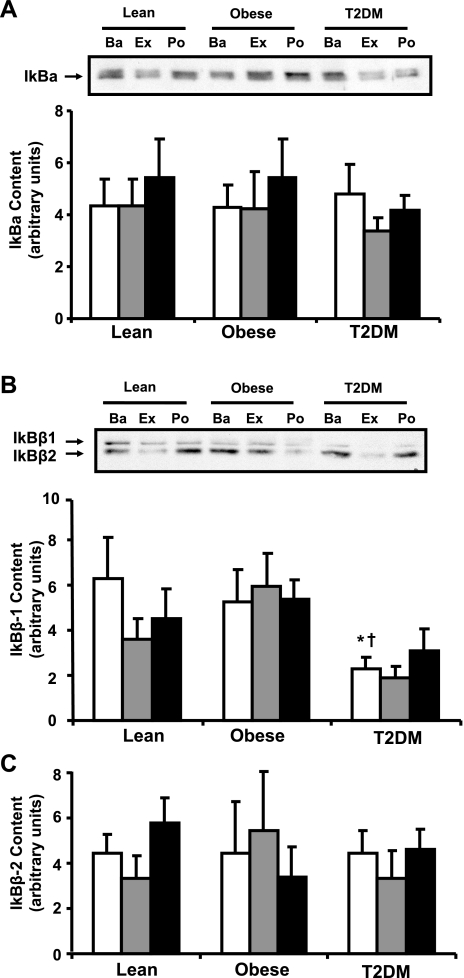
IκBα and IκBβ protein content.
The basal protein content of IκBα was similar in lean, obese, and T2DM groups, and the content of this protein did not change with exercise (Fig. 4A). IκBβ was observed as a doublet band corresponding to the IκBβ mRNA splice variants IκBβ1 and IκBβ2 (Fig. 4, B and C) (25). IκBβ1 content was reduced in muscle from T2DM subjects (40% of lean, P < 0.05, and 33% of obese subjects, P < 0.05; Fig. 4B), whereas IκBβ2 was similar in the three groups (Fig. 4C). The content of IκBβ1 and IκBβ2 did not change with exercise (Fig. 4, B and C).
Fig. 4.
IκB protein content in muscle. Biopsies were done at Ba, Ex, and Po, and IκBα (A), IκBβ1 (B), and IκBβ2 content (C) were measured by Western blotting. Graphic data are means ± SE. Representative blots are shown for 1 subject/group; *P < 0.05 vs. lean at baseline; †P < 0.05 vs. obese at baseline.
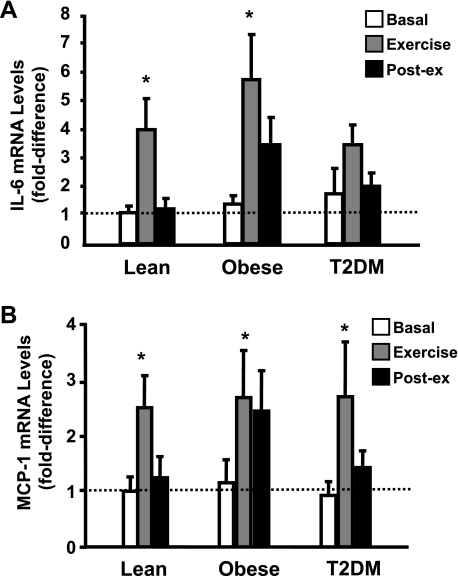
IL-6 gene expression.
After the EMSA and Western blotting experiments, sufficient muscle tissue was available to measure IL-6 and MCP-1 gene expression in seven lean, seven obese, and eight T2DM subjects. Baseline IL-6 gene expression tended to be elevated in the obese and T2DM groups (Fig. 5A), although these differences did not achieve statistical significance (P = not significant). Forty minutes of exercise caused a significant increase in IL-6 mRNA levels in the lean and obese nondiabetic groups (P < 0.05 vs. basal in both groups) and tended to increase IL-6 mRNA levels in diabetic subjects (P = 0.1 vs. basal), although there was not a significant difference in mRNA levels between groups after 40 min of exercise.
Fig. 5.
Effect of acute exercise on IL-6 (A) and monocyte chemotactic protein-1 (MCP-1; B) mRNA expression in muscle. Biopsies were done at baseline, 40 min of exercise, and 210 min postexercise, and IL-6 and MCP-1 mRNA expression were measured by real-time PCR. Data are means ± SE and normalized to lean at baseline. *P < 0.05 vs. basal of respective group.
MCP-1 gene expression.
There were no significant differences in basal MCP-1 levels between groups (Fig. 5B). Exercise significantly increased MCP-1 mRNA levels in lean, obese, and T2DM subjects (P < 0.05, basal vs. 40-min time point in all groups), and there were no differences in exercise-induced MCP-1 expression between groups.
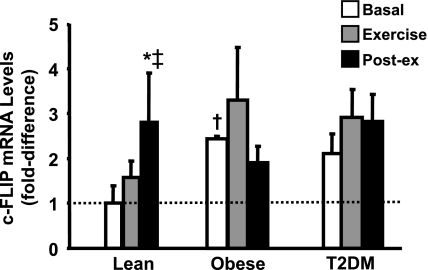
c-FLIP mRNA levels.
c-FLIP is an antiapoptotic factor that helps to prevent muscle loss, and there is evidence from in vitro studies indicating that the expression of c-FLIP in muscle is controlled by NF-κB (7). Consistent with the NF-κB activity assay, we found that basal c-FLIP mRNA levels were increased significantly in the obese subjects and tended to be higher (P = 0.1) in the T2DM individuals (Fig. 6). In addition, exercise also increased c-FLIP expression, although this effect was statistically significant only in the lean subjects. Of note, there was sufficient mRNA to perform c-FLIP measurement in only six lean, four obese, and six diabetic subjects. Therefore, this experiment likely was underpowered to establish clearly whether the ability of exercise to induce c-FLIP expression differs between groups.
Fig. 6.
cellular-FLICE inhibitory protein (c-FLIP) mRNA levels. c-FLIP mRNA was measured before, during, and after exercise. Data are means ± SE and normalized to lean at baseline. *P < 0.05 vs. basal of lean group; ‡P < 0.05 vs. exercise of lean group; †P < 0.05 vs. lean at baseline.
DISCUSSION
The role that the NF-κB pathway plays in the pathogenesis of insulin resistance is controversial. Findings from several studies performed in cell culture systems and insulin-resistant rodents suggest that the NF-κB pathway plays a role in insulin resistance (3, 4, 32, 50, 56). However, results from other studies in which IKK was either knocked down (46) or overexpressed (42) in rodents do not support the concept that the NF-κB pathway negatively affects insulin sensitivity. Using a primary human muscle cell culture system, Austin et al. (4) demonstrated recently that silencing IKK via siRNA prevents TNFα-induced insulin resistance. Furthermore, in vivo experiments carried out in humans have shown that salicylates, which function as inhibitors of IKK (56) and NF-κB (35), improve insulin sensitivity in obese nondiabetic (21) and T2DM (28) subjects. The ability of salicylates to improve peripheral insulin sensitivity in obese nondiabetic and diabetic subjects suggests that the NF-κB pathway is upregulated in these individuals. Nonetheless, NF-κB activity was not measured in these studies. In fact, there is little evidence showing that NF-κB activity is altered in insulin-resistant subjects, particularly in muscle, which is the main tissue responsible for insulin-mediated glucose disposal (14). In this study, we demonstrate that insulin-resistant subjects have increased NF-κB activity in muscle, as determined by EMSA, which is considered the gold standard technique for measuring NF-κB activity.
One limitation of this study is that there were more women than males in the obese and T2DM groups, whereas in the lean group more males were recruited. Friedrichsen et al. (22) reported recently that the protein content of IκBβ (normalized to p65 protein) was increased in muscle from males vs. females. This suggests that IκB-NF-κB signaling could be higher in the females, although direct measurements of NF-κB activity were not performed in that study (22). Although we cannot entirely rule out that differences in sex distribution did not contribute to the alterations in NF-κB activity in the obese and diabetic individuals, we did not observe a difference in NF-κB activity between sexes within each group, or after pooling the males and female subjects from the three groups, suggesting that our findings were not caused by sex differences.
Exercise is a cornerstone in diabetes prevention and management. In addition to its potential role in glucose metabolism, the NF-κB pathway is thought to be involved in several aspects of exercise biology, including maintenance of muscle integrity and muscle strength, and regulation of muscle protein turnover (10, 11, 29). On the basis of previous reports indicating that resistance exercise decreases NF-κB activity in muscle from nondiabetic subjects (18) and that muscle unloading increases NF-κB activity in rodents (18, 29), we predicted that aerobic exercise would also lower NF-κB activity. To the contrary, we found that acute cycle exercise increased NF-κB activity in nondiabetic (lean and obese) subjects. Furthermore, the stimulatory effect of aerobic exercise on NF-κB activity observed in humans is in line with results from prior studies performed in rodents (10, 26, 32). The reason for the discrepancy with the human study by Durham et al. (18), which showed a reduction in NF-κB activity, is likely related to the different modes of exercise utilized, i.e., resistance exercise producing muscle fatigue vs. aerobic (cycle) exercise. The molecular basis for the discordant effects of resistance and aerobic exercise on NF-κB activity is not known. However, these modes of exercise activate different signaling pathways in muscle. For example, some of the metabolic and cellular effects of aerobic exercise are thought to be mediated by stress-activated kinases such as AMP-activated protein kinase (52), atypical protein kinase C (45), and mitogen-activated protein kinases (MAPK) (37), whereas resistance training predominantly stimulates the phosphoinositide (PI) 3-kinase-Akt-mammalian target of rapamycin (9) axis. Whether the contrasting effect of resistance vs. aerobic exercise on NF-κB activity is due to the differential activation of these upstream signaling cascades is yet to be determined.
The stimulation of NF-κB by exercise seems counterintuitive, since the NF-κB pathway has been associated with insulin resistance, whereas physical activity typically improves insulin sensitivity. However, in this study we examined the effect of a single bout of acute exercise, whereas chronic exercise (i.e., physical training) may evoke a different response. Indeed, we reported previously that IκB protein content increased (an indirect indication of lower IKK activity) in response to 8 wk of aerobic training (51). A possible explanation for the differential effect of acute vs. chronic exercises is their effect on oxidative stress, a well-known activator of this pathway. Acute muscle contraction has a reactive oxygen species-generating effect, whereas chronic exercise reduces oxidative stress (10). Training also reduces the content of lipid metabolites such as diacylglycerol (17), an effect that also is expected to downregulate the NF-κB axis (19, 30). To test this, we are currently examining the effect that exercise training has on NF-κB activity, as measured by EMSA, in normal glucose-tolerant and insulin-resistant individuals.
In contrast to nondiabetic subjects, acute exercise did not increase NF-κB activity in the T2DM group. Since diabetic subjects had the highest basal NF-κB activity of the three groups, it is possible that exercise was not able to increase NF-κB activity because it was maximally stimulated at baseline. Consistent with this notion, the NF-κB pathway is subject to negative feedback loops. Autoregulatory mechanisms include IKK autophosphorylation at serine sites within the COOH-terminal region (16) and NF-κB-mediated gene transcription of NF-κB inhibitory enzymes such as CYLD (36) and IκBα (6, 53), although the normal IκBα content observed in the diabetic subjects argues against an inhibitory effect by this enzyme. Hyperglycemia stimulates the NF-κB pathway through the generation of reactive oxygen species (19), and acute exercise caused a decrease in plasma glucose concentrations in the diabetic subjects. Although we did not measure levels of reactive oxygen species in this study, the reduction in plasma glucose concentration during exercise in the diabetic subjects could have blunted the activation of NF-κB with exercise. Another potential explanation for the lack of NF-κB stimulation by exercise in T2DM subjects could be related to the exercise intensity utilized in this study. The diabetic subjects had a lower baseline V̇o2max. Therefore, they tended to exercise at a lower absolute workload than the lean and obese individuals (P = 0.1, T2DM vs. lean; P = 0.1, T2DM vs. obese).
Because the promoter region of the IL-6 gene has an NF-κB binding site (38), we measured IL-6 gene expression as an indicator of NF-κB transcriptional activity in vivo. In a prior study in which we evaluated a different cohort, we found that muscle from obese and T2DM subjects had increased basal IL-6 mRNA levels by about twofold (44). In the present cohort there was only a trend for increased basal IL-6 expression in obese and T2DM subjects, although this study was likely underpowered to detect statistically significant differences in baseline IL-6 mRNA levels because sufficient tissue was not available to perform gene expression experiments in all subjects. Although our data (Ref. 44 and the present study) suggest that baseline IL-6 gene expression is mildly elevated in insulin-resistant subjects, the effect that this cytokine has on muscle insulin sensitivity and glucose metabolism is a matter of debate (12). Some studies suggest that IL-6 induces insulin resistance (33), whereas others suggest that this cytokine works to increase muscle glucose transport/metabolism (1), especially during exercise (41). Since IL-6 gene expression in muscle is highly regulated by NF-κB (43), we predicted that the activation of NF-κB by exercise would precede increases in IL-6 mRNA levels. To the contrary, we found that maximal exercise-induced IL-6 mRNA expression occurred after 40 min, whereas NF-κB activity was not significantly elevated until the 210-min postexercise time point. This suggests that exercise-induced IL-6 expression in skeletal muscle is controlled by other factors, such as p38 MAPK (13).
We found that cycle exercise significantly increased MCP-1 gene expression in skeletal muscle. MCP-1 has been implicated in the induction of an inflammatory response that is important for muscle repair following injury (55). However, the physiological relevance of the increases in MCP-1 expression that occur after aerobic exercise is not clear. Aerobic training increases capillary density in human muscle (2), and MCP-1 promotes blood vessel formation (47). This suggests that MCP-1 could promote the angiogenic response to exercise. In airway smooth muscle cells, MCP-1 gene expression is dependent upon NF-κB (54). Yet in the present study NF-κB activity was not significantly elevated until the 210-min post-exercise time point, whereas maximal MCP-1 gene expression occurred within 40 min of exercise, suggesting that (aerobic) exercise-induced MCP-1 expression is not mediated via NF-κB.
In the classical NF-κB pathway, activation of the p50/p65 dimer involves IKK phosphorylation of IκB, leading to its degradation by the proteasome. In the present study, the content of IκBα, IκBβ1, and IκBβ2 did not change significantly with exercise despite the activation of NF-κB. Although unexpected, this finding is in line with the study by Ho et al. (26), who demonstrated that acute treadmill running activates NF-κB in rat muscle without changes in IκB content. These observations suggest that NF-κB can be activated through as-yet-undefined mechanisms.
In summary, insulin-resistant subjects have elevated NF-κB activity in muscle. A single bout of exercise increased NF-κB activity in nondiabetic subjects. The reason for the lack of NF-κB stimulation by exercise in T2DM is unclear, but it could be explained by the already elevated NF-κB activity at baseline or by the lower absolute workload. In addition, exercise rapidly increased IL-6 and MCP-1 gene expression, although this effect is probably mediated by NF-κB-independent mechanisms.
GRANTS
This study was supported by grants from the American Diabetes Association (N. Musi, E. Cersosimo, and R. A. DeFronzo), the National Institutes of Health (AG-030979 and DK-80157 to N. Musi, DK-24092 to R. A. DeFronzo, and CA-112175 to S. Mohan), the San Antonio Nathan Shock Center (N. Musi), the UTHSCSA Executive Research Committee (N. Musi), the South Texas Health Research Center (N. Musi), the US Department of Veterans Affairs (R. A. DeFronzo), the Faculty of Medicine Siriraj Hospital Mahidol University of Thailand (A. Sriwijitkamol), the Endocrine Fellows Foundation (A. Sriwijitkamol), and the Thai Ministry of Public Health and Maharat Nakhon Ratchasima Hospital (P. Tantiwong). S. Ghosh was funded by T32-HL-04776.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank all the volunteers that participated in the study.
REFERENCES
- 1.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270: 677–690, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Austin RL, Rune A, Bouzakri K, Zierath JR, Krook A. siRNA-mediated reduction of inhibitor of nuclear factor-kappaB kinase prevents tumor necrosis factor-alpha-induced insulin resistance in human skeletal muscle. Diabetes 57: 2066–2073, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 180: 787–802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev 7: 2064–2070, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Benayoun B, Baghdiguian S, Lajmanovich A, Bartoli M, Daniele N, Gicquel E, Bourg N, Raynaud F, Pasquier MA, Suel L, Lochmuller H, Lefranc G, Richard I. NF-kappaB-dependent expression of the antiapoptotic factor c-FLIP is regulated by calpain 3, the protein involved in limb-girdle muscular dystrophy type 2A. FASEB J 22: 1521–1529, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80: 1215–1265, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 38: 1950–1957, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor κB activation. J Physiol 586: 3979–3990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 47: 1135–1142, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chan MH, McGee SL, Watt MJ, Hargreaves M, Febbraio MA. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J 18: 1785–1787, 2004 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68: 1468–1474, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durham WJ, Li YP, Gerken E, Farid M, Arbogast S, Wolfe RR, Reid MB. Fatiguing exercise reduces DNA binding activity of NF-κB in skeletal muscle nuclei. J Appl Physiol 97: 1740–1745, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Evans JL, Goldfine JD, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev 19: 99–125, 1991 [PubMed] [Google Scholar]
- 21.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 31: 289–294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrichsen M, Ribel-Madsen R, Wojtaszewski J, Grunnet L, Richter EA, Billestrup N, Ploug T, Vaag A, Poulsen P. Dissociation between skeletal muscle inhibitor-kappaB kinase/nuclear factor-kappaB pathway activity and insulin sensitivity in nondiabetic twins. J Clin Endocrinol Metab 95: 414–421, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277: 48115–48121, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 109: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hirano F, Chung M, Tanaka H, Maruyama N, Makino I, Moore D, Scheidereit C. Alternative splicing variants of IκBβ establish differential NF-κB signal responsiveness in human cells. Mol Cell Biol 18: 2596–2607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho RC, Hirshman MF, Li Y, Cai D, Farmer JR, Aschenbach WG, Witczak CA, Shoelson SE, Goodyear LJ. Regulation of IκB kinase and NF-κB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol 289: C794–C801, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hughes K, Antonsson A, Grundstrøm T. Calmodulin dependence of NFkappaB activation. FEBS Lett 441: 132–136, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109: 1321–1326, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 114: 1504–1511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J 18: 1499–1506, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53: 1060–1067, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265: 956–959, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424: 801–805, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J Appl Physiol 103: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10: 2327–2334, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohan S, Hamuro M, Koyoma K, Sorescu GP, Jo H, Natarajan M. High glucose induced NF-kappaB DNA-binding activity in HAEC is maintained under low shear stress but inhibited under high shear stress: role of nitric oxide. Atherosclerosis 171: 225–234, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med 24: 455–469, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541: 261–271, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polkinghorne E, Lau Q, Cooney GJ, Kraegen EW, Cleasby ME. Local activation of the IκK-NF-κB pathway in muscle does not cause insulin resistance. Am J Physiol Endocrinol Metab 294: E316–E325, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol 73: 1805–1809, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol 560: 909–918, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Röhl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, De Lorenzi R, Krone W, Rajewsky K, Brüning JC. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest 113: 474–481, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96: 34–40, 2000 [PubMed] [Google Scholar]
- 48.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelest E, Kel AE, Göessling E, Wingender E. Prediction of potential C/EBP/NF-kappaB composite elements using matrix-based search methods. In Silico Biol 3: 71–79, 2003 [PubMed] [Google Scholar]
- 50.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes. Diabetes 55: 760–767, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259: 1912–1915, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Sutcliffe AM, Clarke DL, Bradbury DA, Cobett LM, Patel JA, Knox AJ. Transcriptional regulation of monocyte chemotactic protein-1 release by endothelin-1 in human airway smooth muscle cells involves NF-kappaB and AP-1. Br J Pharmacol 157: 436–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of IKK beta. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]