Abstract
The physiological mechanisms that preserve pancreatic β-cell mass (BCM) are not fully understood. Although the regulation of islet function by the autonomic nervous system (ANS) is well established, its potential roles in BCM homeostasis and compensatory growth have not been adequately explored. The parasympathetic vagal branch of the ANS serves to facilitate gastrointestinal function, metabolism, and pancreatic islet regulation of glucose homeostasis, including insulin secretion. Given the functional importance of the vagus nerve and its branches to the liver, gut, and pancreas in control of digestion, motility, feeding behavior, and glucose metabolism, it may also play a role in BCM regulation. We have begun to examine the potential roles of the parasympathetic nervous system in short-term BCM maintenance by performing a selective bilateral celiac branch-vagus nerve transection (CVX) in normal Sprague-Dawley rats. CVX resulted in no detectable effects on basic metabolic parameters or food intake through 1 wk postsurgery. Although there were no differences in BCM or apoptosis in this 1-wk time frame, β-cell proliferation was reduced 50% in the CVX rats, correlating with a marked reduction in activated protein kinase B/Akt. Unexpectedly, acinar proliferation was increased 50% in these rats. These data suggest that the ANS, via the vagus nerve, contributes to the regulation of BCM maintenance at the level of cell proliferation and may also mediate the drive for enhanced growth under physiological conditions when insulin requirements have increased. Furthermore, the disparate effects of CVX on β-cell and acinar cells suggest that the endocrine and exocrine pancreas respond to different neural signals in regard to mass homeostasis.
Keywords: β-cell mass, celiac vagotomy
the pancreatic β-cell has a tremendous capacity to functionally compensate in response to physiological and pathophysiological changes in tissue insulin demands. A key feature of this adaptive response is the ability of the β-cell mass (BCM) to be dynamically regulated. For instance, BCM is normally maintained proportionally to body size. It increases during obesity and pregnancy (5, 14, 20) and regresses postpartum (42) to adjust to physiological insulin requirements. The steady-state BCM is determined by new cell recruitment by hyperplasia of existing cells and neogenesis from pancreatic epithelial progenitors, apoptotic death and clearance, and hypertrophy (5). The relative importance of each of these different processes is largely unknown and depends on the model under study; nonetheless, several recent reports suggest that proliferation from preexisting β-cells predominates in mice (10, 11, 29, 30).
The interplay of nutrients, growth factors, and hormones is known to impact β-cell growth; however, the influence of the nervous system on β-cell growth processes has been understudied. There are a handful of reports highlighting the importance of the vagus efferents in β-cell proliferation. A transient decrease in β-cell proliferation was observed in ob/ob mice, but not in control mice, following a radical subdiaphragmatic vagotomy, independent of food intake, that translated into reduced BCM only after several months (13). The temporary nature of this proliferation effect might have resulted from adaptive changes in the enteric nervous system after vagal damage (31). In addition, alterations of islet blood flow due to changes in vascular tone, regulated by the parasympathetic efferents (19), may contribute to these observed effects on β-cell replication. Whereas the innervation of islets during fetal development is intimately associated with islet morphogenesis (7), the potential impact of the nervous system modulation of BCM is highlighted by a developmental study by Nekrep et al. (34) demonstrating that signals from the pancreatic neural crest derivatives, forming the definitive ganglia and associated nerves, influence the proliferation capacity of fetal β-cells.
Whether or not β-cell proliferation and other growth parameters may be controlled directly by vagal stimulation is unknown. Since, to our knowledge, there are no published reports of neural control over BCM maintenance in “normal” animals, we have studied the role of the parasympathetic branch of the autonomic nervous system (ANS) innervating the pancreas by selective vagal denervation that significantly impacts the steady-state replication dynamics of β-cells after 1 wk without affecting basic metabolic parameters.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (200 g) were obtained from Harlan and housed in the University of Vermont (UVM) Animal Facility for ≥5 days before being used. The guidelines set forth by the UVM Institutional Animal Care and Use Committee, which approved our experiments, were strictly adhered to in these studies.
Celiac vagotomy surgery.
Using isoflurane anesthesia and sterile technique, an ∼3-cm ventral midline incision was made to expose the esophagus, and then the anterior and posterior branches of the vagus ∼1 cm below the diaphragm were sited and exposed by blunt dissection. Arising from each trunk is a branch of the celiac vagus nerve that courses to the pancreas (43). These nerves were either transected [designated celiac vagotomy (CVX); n = 11] or simply exposed (sham surgery; n = 12) using the criteria of Dixon et al. (9), and then the animal was sutured and subjected to standard postoperative care, including warming, eye ointment, and buprenorphene analgesia. Age-matched, untouched rats (n = 7) served as additional controls for comparison.
Routine metabolic parameters.
Daily (9 AM) body weights, blood glucose (Freestyle; TheraSense), insulin (ELISA; Alpco Diagnostics), and food and water intake were measured. The rats responded well to the treatment with low morbidity.
Glucagon-like peptide-1 assay.
In a distinct group of rats (sham, n = 8; CVX, n = 6), active GLP-1 was measured from plasma obtained at 9 AM under nonfasting conditions using a dipeptidyl peptidase IV inhibitor (1:100; Linco) and an ELISA kit (Alpco Diagnostics).
Intraperitoneal glucose tolerance tests.
In a separate group of rats (n = 7 each group), intraperitoneal glucose tolerance test (IPGTT) was performed on day 7 postsurgery following an overnight fast (∼16 h) at a dosage of 2 g/kg. Blood for plasma insulin concentration was drawn at “0”- and “60”-min time points.
In vitro insulin secretion assay.
In a separate experiment (n = 3/group), islets were isolated using standard procedures, equilibrated for 2 h in Krebs-Ringer bicarbonate buffer with 2.8 mM glucose, segregated into 12-well plates with 10 islets/well, and then subjected to 8.3 or 16.7 mM glucose for 1 h in triplicate. Insulin concentrations in the culture supernatants and acid-ethanol-extracted islets were then measured by ELISA (Alpco Diagnostics). The values of the islet extracts were used to normalize the corresponding values of the secreted insulin.
Tissue processing.
Following 1 wk postsurgery, pancreata were rapidly excised, cleared of fat and lymph nodes, blotted, and weighed before immersion fixing overnight in 4.0% paraformaldehyde in 0.1 mmol/l phosphate buffer at 4°C. After washing in several changes of PBS, tissues were dehydrated and embedded in paraffin.
BCM measurements and analysis of β-cell clusters/small islets.
The proportion of islet β-cell surface area vs. surface area of the whole pancreas was determined planimetrically, as detailed previously (20, 23). To obtain information on potential changes in endocrine cell cluster/islet size dynamics, the relative surface area and number of β-cell clusters and very small islets (>400 μm2, or >23 μm average diameter) were tallied for each animal, using the same sections used for BCM measurements as detailed (20, 23). Image files were analyzed using National Institutes of Health (NIH) Image J and entered into Microsoft Excel for statistical analyses. Significance was determined by Student's t-test, where P < 0.05 was considered significant.
β-Cell proliferation.
At least two slides per adult pancreas were immunohistochemically stained for the nuclear marker Ki-67 and insulin, as detailed previously (20). The Ki-67 antigen is expressed in the nucleus of cells upon cell cycle entry and is thus a useful proliferation marker. The number of Ki-67+ nuclei per 1,000–1,500 islet β-cells was counted for each animal. For verification of the β-cell proliferation trends, the marker PCNA was also used as detailed (22).
Pancreatic acinar cell proliferation.
Using the same Ki-67-stained slides as described above, 12 random, nonoverlapping intermediate power fields consisting primarily of acinar tissue were scored for Ki-67 immunoreactivity. At least 1,100 acinar cell nuclei were counted for each animal.
Analysis of β-cell apoptosis.
For determination of the apoptotic β-cells, a modified terminal deoxynucleotidyl-mediated dUTP nick-end labeling (TUNEL) staining protocol was used (21).
CVX effects on the islet vasculature.
To examine the extent that CVX surgery impacts islet blood flow and may contribute to the β-cell growth deficits, we stained pancreas sections with the biotinylated Lycopersicon sp. lectin (Vector Laboratories) and streptavidin-Cy3 (Jackson ImmunoResearch) and imaged by confocal microscopy (Zeiss LSM 510; UVM Cell Imaging Facility). The surface area of the islet capillaries vs. the islet area in nine islets per pancreas (n = 4/group) was determined using NIH Image J. To assess the sensitivity of this technique, we used pancreata from rats subjected to a 50% (wt/vol) glucose infusion for 4 days (23) that should necessarily increase islet perfusion (19).
Multiple-labeling immunofluorescence and semiquantitative image analysis.
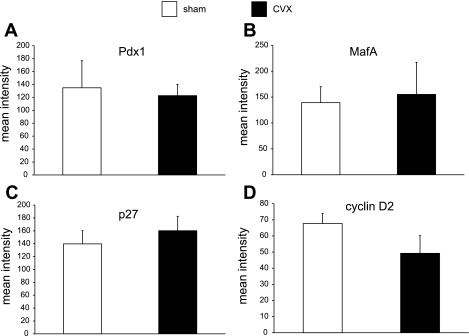
To determine whether the celiac vagus denervation may affect the expression of key β-cell transcription factors that might impact their replicative capacity, a semiquantitative confocal immunofluorescence assessment of nuclear pancreatic/duodenal homeobox-1 (Pdx1; Chemicon) and musculoaponeurotic fibrosarcoma oncogene homolog A (MafA; Bethyl Laboratories) was carried out as detailed previously (20). This allowed comparisons of transcription factor immunoreactivity levels at the cellular and subcellular levels by costaining for insulin to clearly define nuclear and cytoplamsic compartments. Similar nuclear analyses of the β-cell cycle regulators cyclin D2 (Santa Cruz Biotechnology) and p27 (Cell Signaling Technology) were also conducted. To address the effect of CVX on β-cell signaling that could impact growth processes, we measured (cytoplasmic) activated (phospho-)Akt and nuclear phospho-cAMP response element-binding protein (CREB) immunoreactivity. A minimum of 100 islet β-cells was measured for each animal, with four animals per group (CXV and sham) studied. Confocal images for each marker taken under identical conditions and at the same session were analyzed using NIH Image J.
With the exception of Pdx1 and MafA, all of the other markers measured semiquantitatively are also expressed in non-β-cells, thus requiring the sensitivity of multiple-labeling immunofluorescence analysis at the cellular level.
RESULTS
General metabolic parameters.
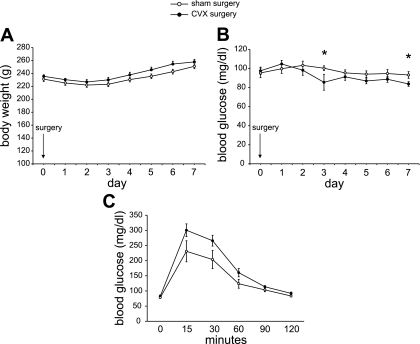
No significant differences in the daily weight gain were observed between the groups for ≤1 wk (Fig. 1A), suggesting that feeding behavior, basic digestive functions, and assimilation were not significantly impaired. Although there were modest reductions in blood glucose concentrations at two time points in the CVX group (Fig. 1B), there were no significant differences in daily food and water intake (not shown). Fed plasma insulin values did not change throughout the 7-day period and were nearly identical (sham, 0.40 + 0.01 ng/ml; CVX, 0.39 + 0.02 ng/ml). Hence, celiac branch vagotomy resulted in no sizeable effects on basic metabolic parameters under steady-state conditions following 1 wk postsurgery.
Fig. 1.
Physiological parameters after 1 wk in celiac vagatomy (CVX) rats are largely unchanged. A: no significant differences in body weight were observed in CVX vs. sham-operated rats. B: blood glucose values were nearly identical in the 2 groups, except for a slight drop (*P < 0.05) in the CVX rats at 3 and 7 days postsurgery; n = 10 (sham controls), n = 11 (CVX). Daily food and water intake were identical between the groups (not shown). C: intraperitoneal glucose tolerance test in a separate cohort of rats (n = 7/group) revealed a moderate trend in reduced glucose tolerance in CVX rats, but the values were not significant.
Glucose tolerance and in vitro insulin secretion.
In a separate cohort of rats 7 days postsurgery, following an overnight fast, IPGTT revealed a modest reduction in glucose tolerance in the CVX group (Fig. 1C). Although there were no significant differences in insulin secretion, “time 0” plasma insulin concentrations in the CVX rats tended to be reduced (0.09 ± 0.02 vs. 0.22 ± 0.07 ng/ml, P = 0.09). At 60 min post-glucose stimulation, plasma insulin concentrations were similar between the groups (CVX/sham, 0.51 ± 0.09 vs. 0.55 ± 0.10 ng/ml). To address whether insulin secretion might be perturbed in vitro in isolated islets of CVX vs. sham rats (n = 3/group), an insulin secretion study revealed no significant differences between the groups (1.63 ± 0.20- vs. 1.88 ± 0.67-fold over basal levels, respectively). Hence, except for a potential subtle defect under fasting conditions, β-cell function appears normal in CVX rats at 1 wk postsurgery.
Plasma GLP-1 levels.
Since it has been reported that the vagus nerve mediates, in part, control over GLP-1 secretion (40), we measured plasma GLP-1 in another group of rats 1 wk after surgery. Whereas (active) GLP-1 was not detected in fasted rats in any group, no differences were found in GLP-1 concentrations between CXV and sham rats in the fed state (1.82 ± 0.57 vs. 1.75 ± 0.44 pmol/l, respectively).
β-Cell morphometry.
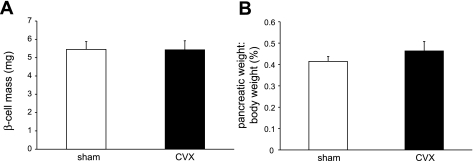
Histomorphometric analyses to measure BCM revealed no differences between the groups (Fig. 2A), nor were there changes in the prevalence of β-cell clusters or islet size (data not shown) at 1 wk postsurgery. Sham-operated rats exhibited BCM values that were similar to untouched control rats (not shown). Since vagotomy could have a negative trophic effect on the digestive organs, we calculated the ratio of the pancreatic weight to body weight. Again, no differences were found (Fig. 2B). Furthermore, analysis of apoptotic β-cells using a fluorescent TUNEL technique revealed no differences between the groups (not shown).
Fig. 2.
β-Cell mass is unchanged after 1 wk in CVX rats. A: β-cell mass was the same in both groups. B: no differences in the pancreatic weights were observed between the groups.
β-Cell proliferation is reduced in CVX rats.
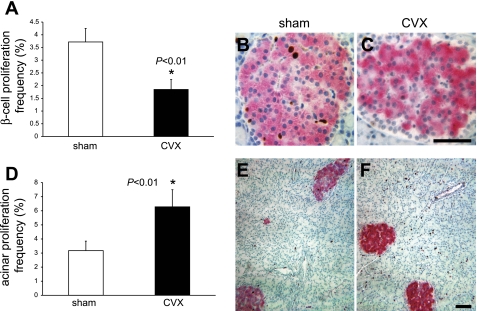
Although BCM is not affected 1 wk postsurgery, when the frequency of Ki-67+ β-cells was measured (∼1,500 β-cells/animal counted), a significant (∼50%) decrease in the number of replicating β-cells in the CVX rats was detected (Fig. 3, A–C). Importantly, the β-cell proliferation frequency of the sham surgery group was identical to that of age-matched, untouched control rats (not shown). Unexpectedly in CVX rats, acinar cell proliferation increased twofold (Fig. 3, D–F). The marked reduction in β-cell proliferation secondary to denervation is in agreement with a report by Kiba et al. (27), although their study employed a more radical bilateral subdiaphragmatic vagal transection following ventromedial hypothalamus (VMH) lesioning in rats. Hence, the ANS may leverage tighter control over steady-state BCM than previously thought.
Fig. 3.
β-Cell proliferation is reduced in CVX rats. A: 1 wk following the CVX surgery, the β-cell proliferation frequency was reduced to 50% of the sham values, as determined by Ki-67 and insulin immunostaining. Age-matched, untouched control rats exhibited β-cell proliferation values identical to the sham controls (data not shown). B: representative fields of a sham control rat islet. C: CVX rat islet demonstrating proliferating cells. Scale bar, 20 μm. D: acinar cell proliferation increased 2-fold in the CVX group. E: representative low-power fields of a sham control pancreas. F: CVX rat pancreas showing increased Ki-67 staining in acinar cells of the latter. Scale bar, 20 μm. Brown, Ki-67 immunoreactivity; magenta, insulin; blue, nuclei.
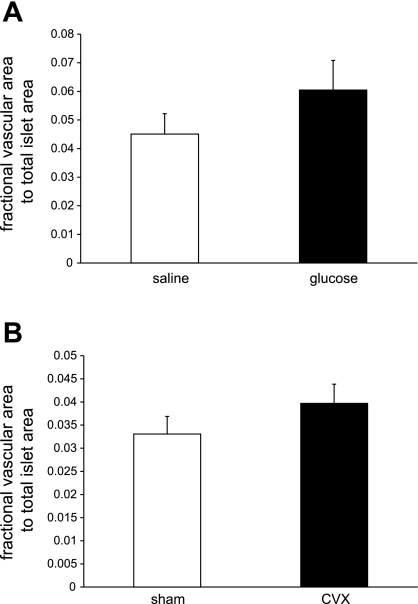
Changes in islet blood flow might explain altered β-cell proliferation. Using lectin fluorescence histochemistry, we devised an assay to estimate changes in islet vascular area. Whereas rats infused chronically with 50% glucose exhibited increased β-cell proliferation (23) concomitant with a trend in increased islet vascular area (Fig. 4A), no reduction in islet vascular area was detected between the CVX and sham-operated groups (Fig. 4B). Hence, these reductions in β-cell proliferation appear to not be the result of a significantly altered blood flow within the 1-wk time frame.
Fig. 4.
Islet vasculature area does not change within 1 wk following CVX surgery. Whereas a tendency toward islet vascular area increases upon chronic glucose infusion (A), no decrease in vascular area was observed in the CVX vs. the sham-operated rats (B), suggesting that islet perfusion differences are unlikely to account for the observed β-cell proliferation decrease.
To begin to examine the underlying mechanisms involved in the reduction in β-cell proliferation frequency upon CVX surgery, we studied the expression of key proteins involved in β-cell function, maturation, proliferation, and growth. Since the principal β-cell factor Pdx1 has been ascribed a role in regulating proliferation (29) as well as maintaining β-cell function (33), we investigated the relative levels of nuclear β-cell Pdx1 immunoreactivity between the groups (n = 4/group) counting >100 islet β-cells/animal. No differences were observed between the groups (Fig. 5A), nor were there differences in MafA immunoreactivity (Fig. 5B), a factor regulating β-cell functional maturation (25).
Fig. 5.
Subcellular semiquantitative analyses of key β-cell proteins involved in β-cell function and proliferation. Semiquantitative confocal analysis of the β-cell nuclear signal showed no differences in the key factors pancreatic/duodenal homeobox-1 (Pdx1; A) and MafA (B) between the CVX and control groups or in the cell cycle inhibitor p27 (C). D: a trend in the reduction of nuclear cyclin D2, the principal cyclin D isoform in β-cells, was observed.
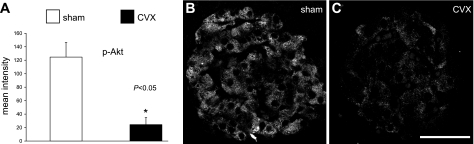
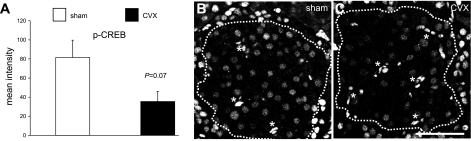
Several studies, including our own, have established the importance of the insulin-signaling and glucoincretin pathways in β-cell growth processes (20, 22, 23, 37). Akt kinase in particular has been ascribed a central role in β-cell proliferation through activating cell cycle progression through cyclin D2 (2) and its inhibition of the cell cycle inhibitor p27 (45). Whereas β-cell nuclear levels of the cell cycle inhibitor p27 did not significantly change following CVX (Fig. 5C), the key cell cycle progression protein cyclin D2 did show a trend in reduced nuclear levels (P = 0.21) (Fig. 5D). However, upon investigating the activity of β-cell Akt following CVX, a striking 80% reduction was observed (Fig. 6). Since glucoincretin receptor pathways are likely integrated with the insulin-signaling cascade at the level of Irs2 transcription via the nuclear factor CREB (24), we also examined activated nuclear CREB levels in celiac vagus transectioned rats. Accordingly, β-cell-activated (phospho-)CREB levels were decreased ∼57% in the CVX group (Fig. 7), although the values only approached significance (P = 0.07). Other regulatory cascades, including those possibly related to muscarinic G protein-coupled receptor pathways, may underlie these effects.
Fig. 6.
Subcellular analyses of activated β-cell Akt involved in β-cell growth and survival. A: semiquantitative analyses of the β-cell cytoplasmic signal for phospho (Ser473)-Akt reveals an 80% reduction in CVX rats. representative fields of phospho-Akt signal in islets from sham (B) and CVX rats (C). Principal signal is limited to β-cell cytoplasm. Scale bar, 50 μm.
Fig. 7.
Subcellular analyses of activated cAMP response element-binding protein (CREB). A: semiquantitative analyses of the β-cell nuclear signal for phospho-CREB (p-CREB) shows a strong trend toward reduced immunoreactivity among β-cells of CVX rats. Typical sections from sham (B) and CVX (C) rats demonstrate decreased nuclear p-CREB levels in CVX rats. β-Cell area is outlined. *Bright bodies are autofluorescing erythrocytes. Scale bar, 50 μm.
DISCUSSION
The role of the nervous system in the control of metabolism and pancreatic function has been well documented (reviewed in Refs. 3, 4, 6, 18, 28, and 47). The parasympathetic-vagal branch of the ANS acts to facilitate gastrointestinal function and insulin secretion. Given the functional relevance of the vagus nerve and its branches to the liver, gut, visceral adipose tissue, and pancreas in control of digestion, motility, feeding behavior, and energy metabolism, the nervous system plays an important role the maintenance of glucose homeostasis. As such, it may also regulate BCM.
Here, we have demonstrated in normal rats that severing the specific vagal trunks that innervate the pancreas results in a significant reduction in β-cell proliferation after 1 wk, with no detectable impairment of feeding or assimilation or increased morbidity but with only minor effects on glucose homeostasis. Although BCM was not reduced in this time frame, additional studies are warranted to determine whether the CVX surgery may lead to significantly impaired glucose tolerance and decreased BCM at longer postsurgery intervals or perhaps that β-cell hypoproliferation may be transient, similar to that reported by Edvell and Lindstrom (13) in ob/ob mice upon subdiaphragmatic vagotomy.
We examined the expression of key principal nuclear factors that may underlie the reduction in β-cell proliferation following CVX surgery. Although the chief β-cell transcription factors that regulate functional maturity and glucose sensitivity are unchanged, we reasoned that two important β-cell proteins that are crucial for cell cycle progression in mice, cyclin D2 (2, 30) and p27 (17, 45), were likely to be altered in rat β-cell nuclei. We have previously reported increased β-cell nuclear cyclin D2 in a mouse pancreas regeneration model that correlated with transiently increased proliferation (37). Whereas no change in nuclear p27 immunoreactivity, a cell cycle inhibitor, was observed in the CVX rats, cyclin D2, a positive cell cycle regulator (30), exhibited only a trend in decreased levels in the CVX rats. Nonetheless, the relative activity of Akt, a central mediator in β-cell growth pathways, was reduced 80% in the CVX rats compared with sham-operated controls, suggesting that parasympathetic innervation to β-cells may be required for sustaining proliferation capacity and, by inference, steady-state β-cell mass. Furthermore, a reduction in β-cell CREB activation points to a relative loss of cAMP/protein kinase A signaling associated with potentially impaired glucoincretin receptor pathways. Future studies are warranted to resolve how these central β-cell growth and survival pathways are altered upon selective vagotomy.
Although we cannot definitively rule out the possibility that islet blood flow may have partially contributed to these β-cell effects, we failed to detect any changes in islet vascular area in CVX rats, suggesting that blood flow changes are likely to be modest within the 1-wk postsurgical period. Since vagal interruption to the pancreas would necessarily decrease cholinergic stimulation to the β-cells, the observed changes in steady-state β-cell proliferation might be a direct result of loss of parasympathetic drive. It has been demonstrated that infusion of rats with the acetylcholine receptor (AChR) agonist carbachol causes preferential β-cell proliferation similar to that of VMH-lesioned animals (48). Hence, the β-cell replication decrease upon selective pancreatic vagotomy in the current study may be mediated through β-cell muscarinic receptors. Because the principal β-cell muscarinic receptor subtype is M3R (12), and mice with a β-cell specific M3R-null mutation exhibit a profound insulin secretory defect, but their BCM is not detectably altered (15), it is unclear whether this isoform may play a direct role in β-cell growth. In a range of tissues, including the gut (44), reproductive organs (1, 32), and brain (39), signaling through both neural and nonneural muscarinic AChRs has been shown to mediate proliferation. Future studies are warranted to resolve whether parasympathetic activity involving AChR activation may lead to β-cell replication through M3R, or perhaps other β-cell muscarinic receptor subtypes, or be regulated indirectly, such as from glucoincretin pathways. Since normal GLP-1 release from intestinal “L” cells is dependent on an intact vagus nerve as part of a “neuroendocrine loop” involving enteroendocrine glucose-dependent insulinotropic peptide and neural gastrin-releasing peptide (40), GLP-1 may play a role in the observed effects of CVX on β-cell proliferation. Although we did not detect differences in plasma GLP-1 levels between CXV and control rats under steady-state “fed” conditions, more comprehensive fasting/refeeding studies will be necessary to definitively determine whether it may have a role in the reduction of β-cell proliferation.
Few details are known on the central nervous system (CNS) centers that may regulate β-cell growth through autonomic nerves. The influence of the sympathetic nervous system on β-cell growth has been addressed and may involve β-adrenergic receptor pathways. For instance, Das et al. (8) have found that 60–70% partial pancreatectomy in rats resulted in enhanced numbers of β2-adrenergic receptors in the cerebrum, hypothalamus, and brainstem as well as in the pancreas. Importantly, the upregulation of these receptors was temporally correlated with a transient DNA synthesis in islets. These investigators also mapped the centers of the brainstem that are activated upon pancreatic regeneration following partial pancreatectomy (38).
Although the relative contribution of the parasympathetic branch to β-cell growth potential is not known, it has recently been suggested through studies focused on global and tissue-specific M3R-knockout mice that energy metabolism and the propensity to develop obesity may be dictated by a balance between sympathetic and parasympathetic stimulation at the level of the hypothalamus (reviewed in Ref. 16). Therefore, BCM may respond to the prevailing parasympathetic outflow through the vagus nerves. It is well established that experimental lesions of the VMH lead to hyperphagia, increased β-cell activity, and hyperinsulinemia (reviewed in Ref. 28). Kiba et al. (26) reported that VMH lesioning also results in reflex hyperproliferation of the key organs of digestion and nutrient metabolism, including the gut, liver, and pancreas. Furthermore, they demonstrated that β-cells in particular are affected and that this growth influence is lost upon subdiaphragmatic vagotomy (27). By contrast, our finding that the pancreatic acinar cells exhibit increased proliferation following a selective celiac vagotomy suggests that exocrine-associated vagal efferents may tonically inhibit proliferation under normal steady-state conditions in rats. Since there is accumulating evidence that discrete brain regions, including the hypothalamus and brainstem, mediate compensatory β-cell growth and regeneration (8, 28), the precise roles of the CNS/ANS in normal BCM maintenance and BCM expansion due to increased physiological insulin requirements require further study.
The liver serves as a key metabolic sensor that, in concert with autonomic nerves and brain, may play an important role in adaptive changes in BCM. Recently, Imai et al. (18) demonstrated that experimentally or physiologically enhanced extracellular-regulated kinase (ERK/MAPK) signaling in the liver, mediated through a circuit involving splanchnic afferents, the brain, and the vagus nerve, also impacts BCM via inducing proliferation; however, the nature of how ERK pathway activation leads to enhanced hepatic afferent nerve activity has not yet been determined.
Interestingly, although CVX leads to an approximate 50% decrease in proliferation among β-cells, we detected a concomitant increase in acinar cell proliferation. This was unexpected, because our original assumption was that CVX would similarly affect both endocrine and exocrine cell populations. It has recently been shown that pancreas-specific 3-phosphoinositide-dependent protein kinase 1-knockout mice suffer developmental defects in both exocrine and endocrine cell size, proliferation, and number (46). However, by postnatal day 21, acinar cells have increased compensatory replicative activity with high levels of activated (phospho-Ser473) Akt, but with no corresponding increase in β-cell proliferation or mass. Sangiorgi and Capecchi (41) identified a Bmi1+ self-renewing population of differentiated acinar cells that expand in response to experimentally induced acinar damage, with full recovery of exocrine mass without disturbance of BCM or function. Collectively, these results suggest to us that there exist distinct control mechanisms for mass regulation for the exocrine and endocrine pancreas that respond to different physiological stimuli. This idea is further supported by Nekrep et al. (34), who showed increases in β-cell proliferation and mass in Phox2b-null mice without changes in overall pancreatic mass, indicating β-cell-specific effects. Phox2b encodes a homeobox factor that specifies neural crest-derived neural precursors.
Currently, the nature of the disparity in proliferation status between β-cells and acinar cells is unknown, but we hypothesize that the presence of distinct growth and homeostatic mechanisms may account, in part, for our results. Additionally, since the exocrine pancreas is more directly coupled to gastrointestinal function, there may be signaling from the enteric nervous system and/or enteroendocrine cells that may result in the compensatory acinar proliferation in the CVX rats.
We have established and characterized a useful rodent model to study the parasympathetic-vagus system to elucidate its potential roles in BCM maintenance and physiological adaptation. Additional studies are needed to resolve the molecular mechanisms by which parasympathetic stimulation, presumably via ACh/muscarinic receptors leading to increased [Ca2+]i, impinges on β-cell growth mechanisms such as those regulated by the ERK/MAPK and/or the phosphoinositide 3-kinase/Akt signaling pathways.
GRANTS
These studies were supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-068329) and the Juvenile Diabetes Research Foundation (to T. L. Jetton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Brian Everill for conducting the CVX surgery and for participation in many insightful discussions on this project. We are also grateful to Dr. Everill and Dr. J. Leahy for critically reading the manuscript.
REFERENCES
- 1.Avellar MC, Lázari MF, Porto CS. Expression and function of G-protein-coupled receptors in the male reproductive tract. An Acad Bras Cienc 81: 321–344, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem 284: 7832–7842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bereiter DA, Rohner-Jeanrenaud F, Berthoud HR, Jeanrenaud B. CNS modulation of pancreatic endocrine function. Multiple modes of expression. Diabetologia 20Suppl: 417–425, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Bloom SR, Edwards AV, Hardy RN. The role of the autonomic nervous system in the control of glucagon, insulin and pancreatic polypeptide release from the pancreas. J Physiol 280: 9–23, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol 24: 297–302, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 431: 405–423, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Burris RE, Hebrok M. Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience 150: 592–602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das VA, Robinson R, Paulose CS. Enhanced β-adrenergic receptors in the brain and pancreas during pancreatic regeneration in weanling rats. Mol Cell Biochem 289: 11–19, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dixon KD, Williams FE, Wiggins RL, Pavelka J, Lucente J, Bellinger LL, Gietzen DW. Differential effects of selective vagotomy and tropisetron in aminoprivic feeding. Am J Physiol Regul Integr Comp Physiol 279: R997–R1009, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Dor Y. beta-Cell proliferation is the major source of new pancreatic beta cells. Nat Clin Pract Endocrinol Metab 2: 242–243, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 53: 1714–1720, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Edvell A, Lindstrom P. Vagotomy in young hyperglycemic mice: effects on syndrome development and islet proliferation. Am J Physiol Endocrinol Metab 274: E1034–E1039, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3: 449–461, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gautam D, Jeon J, Li JH, Han SJ, Hamdan FF, Cui Y, Lu H, Deng C, Gavrilova O, Wess J. Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review. J Recept Signal Transduct Res 28: 93–108, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Georgia S, Bhushan A. p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes 55: 2950–2956, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science 322: 1250–1254, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Jansson L, Hellerström C. Glucose-induced changes in pancreatic islet blood flow mediated by central nervous system. Am J Physiol Endocrinol Metab 251: E644–E647, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54: 2294–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Jetton TL, Liang Y, Cincotta AH. Systemic treatment with sympatholytic dopamine agonists improves aberrant beta-cell hyperplasia and GLUT2, glucokinase, and insulin immunoreactive levels in ob/ob mice. Metabolism 50: 1377–1384, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Jetton TL, Liu YQ, Trotman WE, Nevin PW, Sun XJ, Leahy JL. Enhanced expression of insulin receptor substrate-2 and activation of protein kinase B/Akt in regenerating pancreatic duct epithelium of 60%-partial pancreatectomy rats. Diabetologia 44: 2056–2065, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Jetton TL, Everill B, Lausier J, Roskens V, Habibovic A, LaRock K, Gokin A, Peshavaria M, Leahy JL. Enhanced β-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 294: E679–E687, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev 17: 1575–1580, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneto H, Miyatsuka T, Kawamori D, Yamamoto K, Kato K, Shiraiwa T, Katakami N, Yamasaki Y, Matsuhisa M, Matsuoka TA. PDX-1 and MafA play a crucial role in pancreatic beta-cell differentiation and maintenance of mature beta-cell function. Endocr J 55: 235–252, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kiba T, Tanaka K, Inoue S, Endo O, Takamura Y. Comparison of DNA contents of visceral organs in rats with ventromedial hypothalamic lesions and fed a high fat diet. Neurosci Lett 126: 127–130, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Kiba T, Tanaka K, Numata K, Hoshino M, Misugi K, Inoue S. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology 110: 885–893, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Kiba T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: recent developments. Pancreas 29: e51–e58, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114: 828–836, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 25: 3752–3762, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Owyang C. Musings on the Wanderer: What's New in Our Understanding of Vago-Vagal Reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol 285: G461–G469, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mayerhofer A, Kunz L. A non-neuronal cholinergic system of the ovarian follicle. Ann Anat 187: 521–528, 2005 [DOI] [PubMed] [Google Scholar]
- 33.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia 44: 1203–1214, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Nekrep N, Wang J, Miyatsuka T, German MS. Signals from the neural crest regulate beta-cell mass in the pancreas. Development 135: 2151–2160, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J Clin Invest 116: 775–782, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 281: 1159–1168, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 55: 3289–3298, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Renuka TR, Savitha B, Paulose CS. Muscarinic M1 and M3 receptor binding alterations in pancreas during pancreatic regeneration of young rats. Endocr Res 31: 259–270, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal 7: 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 40: 1687–1694, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA 106: 7101–7106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology 136: 5461–5468, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Inoue S, Nagase H, Takamura Y. Modulation of arginine-induced insulin and glucagon secretion by the hepatic vagus nerve in the rat: effects of celiac vagotomy and administration of atropine. Endocrinology 127: 2017–2023, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Tobin G, Giglio D, Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol 60: 3–21, 2009 [PubMed] [Google Scholar]
- 45.Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11: 175–182, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Westmoreland JJ, Wang Q, Bouzaffour M, Baker SJ, Sosa-Pineda B. Pdk1 activity controls proliferation, survival, and growth of developing pancreatic cells. Dev Biol 334: 285–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods SC, Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev 54: 596–619, 1974 [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura R, Omori H, Somekawa S, Osaka T, Ito R, Inoue S, Endo Y. Continuous carbachol infusion promotes peripheral cell proliferation and mimics vagus hyperactivity in a rat model of hypothalamic obesity. Biomed Res 27: 81–88, 2006 [DOI] [PubMed] [Google Scholar]