Abstract
Bowman-Birk inhibitor concentrate (BBIC), a serine protease inhibitor, has been shown to diminish disuse atrophy of skeletal muscle. Duchenne muscular dystrophy (DMD) results from a loss of dystrophin protein and involves an ongoing inflammatory response, with matrix remodeling and activation of transforming growth factor (TGF)-β1 leading to tissue fibrosis. Inflammatory-mediated increases in extracellular protease activity may drive much of this pathological tissue remodeling. Hence, we evaluated the ability of BBIC, an extracellular serine protease inhibitor, to impact pathology in the mouse model of DMD (mdx mouse). Mdx mice fed 1% BBIC in their diet had increased skeletal muscle mass and tetanic force and improved muscle integrity (less Evans blue dye uptake). Importantly, mdx mice treated with BBIC were less susceptible to contraction-induced injury. Changes consistent with decreased degeneration/regeneration, as well as reduced TGF-β1 and fibrosis, were observed in the BBIC-treated mdx mice. While Akt signaling was unchanged, myostatin activitation and Smad signaling were reduced. Given that BBIC treatment increases mass and strength, while decreasing fibrosis in skeletal muscles of the mdx mouse, it should be evaluated as a possible therapeutic to slow the progression of disease in human DMD patients.
Keywords: protease inhibitor, Duchenne muscular dystrophy, myostatin, transforming growth factor-β1, proteasome, calpain, myostatin
the bowman-birk inhibitor (BBI) is an 8-kDa, soy-derived serine protease inhibitor containing chymotrypsin and trypsin inhibition sites. BBI concentrate (BBIC) has been shown to inhibit disuse atrophy in mouse skeletal muscle (2, 30) as well as free radical injury associated with disuse (2). Disuse atrophy involves activation of protein degradation machinery, primarily via upregulation of what is known as the atrogene program (12, 21, 40).
Duchenne muscular dystrophy (DMD) is an X-linked, childhood-onset genetic disorder caused by the absence of the protein dystrophin (33). This disease is marked by progressive muscle dysfunction, wheel chair confinement, muscle wasting, and death (9). Conventional treatment is centered around corticosteroids, which slow disease progression but have significant side effects (28). The search for additional therapeutics is thus critical, especially developing therapeutics that can slow the disease without significant toxicities.
The absence of the protein, dystrophin, in DMD results in skeletal muscle being more susceptible to contraction-induced injury (32), creating a state of ongoing degeneration/regeneration (8) that is accompanied by chronic inflammation and progressive fibrosis (7). In particular, dystrophin-deficient muscles in mice, dogs, and humans display significant increases in mast cell content, which likely contributes to necrosis and fibrosis (12).
BBIC has previously been demonstrated to inhibit neutrophilic cathepsin, elastase, and chymase found in mast cells, as well as chymotrypsin and trypsin (20, 44, 47). Both neutrophil elastase and mast cell chymase are involved in activation of latent TGF-β1 and fibrosis and likely contribute to the dystrophic pathology (1, 15, 22, 27, 48). Previous studies have identified the potential of protease inhibition in ameliorating the dystrophic phenotype. A mast cell stabilizer, cromlyn, increased muscle strength in exercised mdx mice while a trypsin-like serine protease inhibitor, camostat mesilate, reduced plasma creatine kinase levels and moderately improved muscle quality in mdx mice (16, 35).
Therefore, we examined the effects of BBIC treatment in mdx mice. Further, we assessed the signaling pathways associated with muscle remodeling and fibrosis to determine whether BBIC would allow more successful muscle regeneration with less fibrosis. BBIC is orally bioavailable (5, 31) and has not shown any serious toxicities in animals or in humans (26). Given its ease of administration and lack of toxicity, a demonstration of beneficial effects of BBIC treatment in mdx mice would create the impetus to examine the potential therapeutic impact of BBIC in human DMD patients.
METHODS
Animal Treatments
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania. BBIC was purified as previously described (19, 46) by Central Soya (Ft. Wayne, IN) using a procedure to maintain chymotrypsin inhibitor activity while reducing trypsin inhibitory activity. One-month-old male mdx mice were divided into two groups with one group receiving AIN-93G food pellets (with corn oil; Bioserv, Frenchtown, NJ) containing 0% BBIC and the other receiving AIN-93G mixed with 1% BBIC. The selected dose was based on previous data that showed in vivo efficacy in other animal models (19) and the attenuation of muscle atrophy (2, 30). The mice were treated for 12 wk such that the average daily consumption of 3–5 g of food provided ∼3.6–6.0 chymotrypsin inhibitory (CI) units/day. In addition, companion experiments were performed using C57Bl/6 mice to test the effects of BBIC on normal skeletal muscle. Beginning at 10 wk of age, mice were fed a diet containing 1% BBIC or control food for 6 wk.
At the end of the study, the animals were anesthetized and weighed, blood was taken via a cardiac puncture, and muscles were dissected and removed [tibialis anterior (TA), quadriceps, and diaphragm]. The muscles were then frozen immediately in melting isopentane for histological analysis or directly into liquid nitrogen for biochemical analysis. One extensor digitorum longus (EDL) muscle was prepared for mechanical muscle force measurements, as previously described (3).
The resting length (L0) was set by adjusting muscle length until obtaining maximal twitch tension. Maximal tetanic force was measured by stimulating the muscles with a 120-Hz, 500-ms pulse at supramaximal voltage. Protection against mechanical injury was evaluated by monitoring force decline following a series of five eccentric tetanic contractions. These measurements were performed by initiating a 500-ms isometric tetanic contraction immediately followed by a 200-ms stimulation while stretching the muscle by 10% L0 at a rate of 0.5L0/s. After testing, the muscle was blotted, weighed, and then rapidly frozen in melting isopentane and stored at −80°C for subsequent histological analysis.
Protease Activity
The chymotrypsin-like activity of the proteasome was determined by the method of Vigouroux et al. (43), with modifications. A frozen section of TA was weighed, pulverized on dry ice and homogenized in a 1:10 wt/vol ratio in a 50 mM Tris·HCl buffer (pH 8.0) containing 1.0 mM EDTA, 1.0 mM EGTA, 1.0 mM DTT, 2.0 mM ATP, 10% glycerol, and Complete Protease Inhibitor Cocktail (Roche Diagnostic, Basel, Switzerland). The homogenates were centrifuged for 1 h at 100,000 g at 4°C. Protein concentration of the supernatant was determined using the Bradford reaction (Bio-Rad, Hercules, CA) with BSA as a standard. Homogenate was diluted with assay buffer [50 mM Tris·HCl (pH 7.5), 40.0 mM KCl, 5.0 mM MgCl2, 2.0 mM ATP, 1.0 mM DTT, 10.0 μg BSA] to normalize protein concentration. In our experimental conditions, components were mixed in a 1:1:2 ratio such that 50 μl homogenate, 50 μl substrate, and 100 μl assay buffer [BBIC, BBI (Sigma), or epoximicin (Sigma)] were in each well. Homogenate contained 10 μg of protein. Samples were preincubated with BBIC (100 μg), BBI (100 μg), or, for assay fidelity, epoximicin (100 μM) for 10 min at 37°C before adding substrate.
The substrate Suc-LLVY-MAC was dissolved in assay buffer and diluted to a final concentration of 100 μM. After adding the substrate, the samples were incubated for 1 h at 37°C and then final fluorescence levels measured [excitation (Eex) = 340 nm and emission (Eem) = 465 nm (GENios Pro, Tecan, Austria)].
Calpain activity was determined using 30–50 mg of frozen muscle following homogenization in 10 vol of buffer (100 mM Tris, pH 7.5, 100 mM KCl, 10 mM mercaptoethanol, 0.1 mM EDTA, 1.0 mM PMSF) (39). In accordance with Thompson et al. (42), 0.75 μg protein in 25 μl was added to each microplate well and further diluted with 75 μl dilution buffer containing 20 mM Tris·HCl (pH 7.5), 1 mM EDTA, 100 mM KCl, and 0.1% mercaptoethanol. The reaction was initiated by adding 100 μl of BODIPY-FL-casein (10 μg/ml in dilution buffer with 10 mM Ca2+). Measurements were made using a Tecan fluorometer (GENios Pro, Tecan, Austria) with 485-nm excitation and 535-nm emission beginning immediately after addition of reaction buffer and every 5 or 10 min thereafter. One control was added to account for non-protease substrate degradation (dilution buffer + BODIPY-casein with no sample) while a second control accounted for non-calpain-dependent proteolysis of the substrate (25 μl of 100 mM EDTA, 50 μl of dilution buffer, 25 μl of sample, and 100 μl of BODIPY-casein). Slope was calculated using the linear portion of calpain activity plotted over time. Calpain (cal) activity is calculated as follows: FUcal = FUsample − (FUCa blank + FUEDTA blank)/2, where FU refers to fluorescent units.
Serum Creatine Kinase Activity
Serum was analyzed for creatine kinase (CK) levels in mdx and BBIC-treated mice (Diagnostic Chemicals Limited, Oxford, CT).
Immunoblotting
A portion of the frozen TA muscle was pulverized on dry ice and solubilized at a 1:10 mass/vol ratio in cell lysis buffer (20 mM Tris, 137 mM NaCl, 25 mM B-glycerophosphate, 2 mM sodium pyrophosphate, 2 mM EDTA, 1 mM sodium orthovanadate, 1% Triton X-100, 10% glycerol, 1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 mM benzamidine). The resulting homogenate was centrifuged at 13,000 rpm on at 4°C for 10 min, and the protein concentration of the supernatant was determined. The lysate was diluted to 1.0 mg/ml in Laemmli buffer.
Ten micrograms of protein was loaded into each well on a 4–20% gradient precast gel (Lonza, Valair, Switzerland),separated by electrophoresis and transferred using the I-blot system (23 V, 6 min; Invitrogen, Carlsbad, CA). Membranes were blocked with TBS containing 0.01% Tween-20 and 5% powdered milk for 1 h and incubated overnight at 4°C with one of the following antibodies: rabbit polyclonals against p38, phospho-p38, ERK, phospho-ERK, Smad 2/3, or Akt (Cell Signaling, Boston MA); phospho-Smad 2/3, GDF-8 C-terminus (LabVision), TGF-β1 (abcam), TIMP-1 (Sigma), IκBα (Santa Cruz); mouse monoclonal anti-actin (Neomarkers); mouse polyclonal anti-GDF-8 Propeptide (R and D Systems), or rabbit monoclonal anti-p-Akt (Ser473; Cell Signaling). Following incubation, the secondary antibodies, horseradish peroxidase (HRP) anti-rabbit or HRP anti-mouse (Amersham, Buckinghamshire, UK), were applied at a 1:2,000 dilution. The proteins were visualized with ECL (Pierce) using exposure to film (Eastman-Kodak, Rochester, NY), and band intensities were quantified using a Kodak image station and software. Appropriate loading controls (actin) were run for each separation and were similar between groups and samples.
Histology and Immunofluorescence
Hematoxylin and eosin (H + E) staining of the frozen muscle sections was performed according to standard techniques. Trichrome staining of the frozen sections staining was accomplished using the Sigma kit (HT15) with slight modifications. For fluorescent laminin staining, frozen sections were incubated with rabbit anti-laminin Ab (Neomarkers; 1:100) in 5% BSA for 1 h at room temperature, labeled with fluorescent secondary antibody (Invitrogen; 1:200 dilution), washed, and mounted using Vectashield containing DAPI (Vector Labs, Burlingame, CA). Microscopy was performed on a Leitz DMR microscope (Leica), and image acquisition and analysis were conducted using a MicroMAX digital camera system (Princeton Instruments) and imaging software (OpenLab, Improvision, Waltham, MA).
Muscle fibrosis in both BBIC-treated and untreated diaphragm muscles was quantified by determining the percentage of nonmuscle area, using hue-saturation-intensity thresholding, in trichrome-stained muscle sections. In each section (n = 3 per group), two to three thresholded regions in the image were analyzed to determine the percent nonmuscle area compared with the total tissue area as a measurement of fibrosis with quantification performed by an independent investigator. Centralized nuclei were determined by analyzing two to four regions of each muscle section (EDL, TA, and diaphragm) from control mdx and BBIC-treated mdx mice (n = 3). The number of muscle fibers with central nuclei was compared with the total number of fibers within the region.
Membrane permeability was investigated using Evans blue dye staining (EBD; 0.2 ml of 0.2% dye ip) injected 16–24 h before the mouse was euthanized. For quantification of membrane damage, EBD infiltration was measured as the ratio of the area of the EBD-positive muscle fibers compared with total muscle area in quadriceps muscles of untreated vs. BBIC-treated mdx mice; quantification was performed by an independent investigator.
Statistics.
Comparisons between mdx mice and mdx mice supplemented with BBIC from 4–12 wk were made using a Student's t-test as were comparisons made between C57 animals and C57 animals supplemented with BBIC from 10–16 wk of age. In vitro proteasome function was assessed using a one-way ANOVA, with Dunnett post hoc test. Data are presented as means ± SE unless otherwise noted. Significance was set at P < 0.05.
RESULTS
Body Mass and Muscle Parameters in C57Bl/6 and mdx Mice
To determine if BBIC would have an effect in normal skeletal muscle, C57Bl/6 mice were fed a diet that contained 1% BBIC. At the end of treatment, it was determined that the addition of BBIC did not affect healthy muscle tissue. In all parameters tested (muscle mass, relative muscle-to-body weight ratio, tetanic force, specific tension, and muscle cross-sectional area), there was no difference between groups (Table 1).
Table 1.
Selected parameters following BBIC feeding in C57Bl/6 mice
| 0% BBIC | 1% BBIC | |
|---|---|---|
| Body mass, g | 30.3 ± 0.7 | 30.2 ± 0.9 |
| EDL mass, mg | 10.9 ± 0.2 | 11.1 ± 0.4 |
| EDL mass/body mass, mg/g | 0.359 ± 0.01 | 0.378 ± 0.01 |
| Twitch force, mN | 123.2 ± 10.7 | 120.6 ± 10.4 |
| Tetanic force, mN | 397 ± 20.4 | 392.1 ± 29.3 |
| Cross-sectional area, mm2 | 1.97 ± 0.04 | 2.02 ± 0.04 |
| Specific tension, mN/cm2 | 20.2 ± 0.8 | 19.4 ± 1.0 |
Data are shown as mean ± SE; n = 6–8 for both groups. C57Bl/6 mice were given either 0% or 1% Bowman-Birk inhibitor concentrate (BBIC) for 6 wk. No significant differences are observed between the 2 groups with the largest percent change (+5.2%) observed in the extensor digitorum longus (EDL)-to-body mass ratio.
When treated dystrophic (mdx) mice were provided food supplemented with 1% BBIC for 12 wk, both morphological and functional improvements were observed. In the treated mdx mice, EDL mass and cross-sectional area were increased compared with control-fed mdx mice (Table 2). Absolute tetanic force was increased by ∼25% in the 1% BBIC-treated mdx mice and the loss of force following a series of five eccentric contractions was reduced by 25% in these mice compared with control-fed mdx mice. Specific tension was similar between the 1% BBIC and control mdx groups suggesting the underlying dystrophic pathology is still present (Table 2). As only dystrophin-deficient mice are affected by BBIC intervention, these results suggest that the mechanism behind the BBIC-dependent functions in mdx muscle is related to a pathological process that becomes exacerbated in the dystrophic skeletal muscle tissue.
Table 2.
Selected parameters following 3 mo of BBIC feeding in mdx mice
| mdx, 0% BBIC | mdx, 1% BBIC | %Change | |
|---|---|---|---|
| Body mass, g | 28.9 ± 0.6 | 31.7 ± 1.3 | +9.9% |
| EDL muscle mass, mg | 12.8 ± 0.2 | 16.3 ± 0.8* | +27.3% |
| EDL muscle mass/body mass, mg/g | 0.453 ± 0.01 | 0.512 ± 0.02* | +13.2% |
| Cross-sectional area, mm2 | 2.24 ± 0.04 | 2.86 ± 0.10* | +27.6% |
| Twitch force, mN | 157.2 ± 19.7 | 197.3 ± 18.1 | +25.5% |
| Tetanic force, mN | 406.8 ± 46.2 | 510.5 ± 52.7* | +25.4% |
| Specific tension, mN/cm2 | 18.1 ± 0.6 | 17.9 ± 0.5 | −1.0% |
| Eccentric contraction-induced injury, % force loss | −25.4 ± 2.7 | −20.2 ± 2.7* | +25.7% |
Data are shown as mean ± SE; n = 12 for mdx, 0% BBIC mice and n = 8 for mdx, 1% BBIC mice. mdx animals were fed a diet containing either 0% or 1% BBIC for 3 mo.
Significantly different from mdx, 0% BBIC (P < 0.05).
Histology.
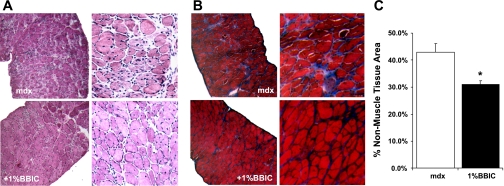
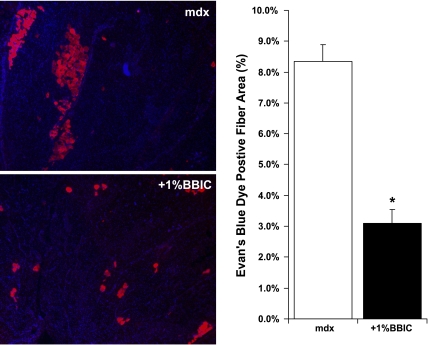
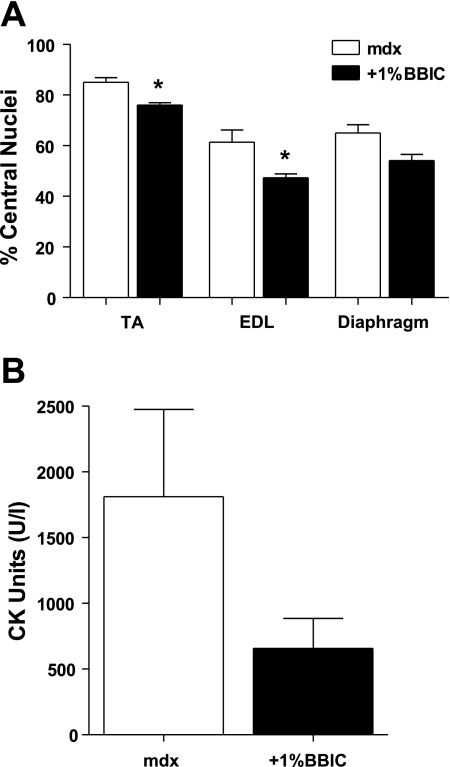
Visualization of muscle fiber cross sections revealed improvements with dietary BBIC supplementation. Specifically, fibrosis was reduced in the diaphragm as is evident following H + E (Fig. 1A) and trichrome staining (Fig. 1B). Quantification of nonmuscle area in the diaphragms (Fig. 1C) shows a significant increase in fibrotic regions of the mdx muscle compared with the BBIC-fed animals (42.8% vs. 31.1%). Further, the presence of EBD-infiltrated muscle fibers was significantly reduced in the quadriceps of the BBIC-treated mdx mice, indicating increased muscle membrane stability (8.3% mdx vs. 3.1%, +1% BBIC; Fig. 2). This finding was supported by an observed change in the number of centrally located nuclei, a common marker of regeneration, in the EDL and TA muscles by 23% and 10%, respectively (P < 0.05), while the number of central nuclei in the diaphragm was 17% lower (P = 0.055; Fig. 3A) in the BBIC-treated mice than the control fed mice. Further, although the mean CK value decreased by nearly threefold (P < 0.1; n = 5) in BBIC-fed mdx mice vs. control-fed mdx mice (Fig. 3B), the change failed to reach statistical significance, at P < 0.05, due to larger than anticipated animal-to animal variability.
Fig. 1.
Representative images of hematoxylin and eosin (H + E)-stained (A) and trichrome-stained (B) diaphragm muscle sections of mdx mice provided standard (top) or 1% Bowman-Birk inhibitor concentrate (BBIC) diet (bottom). C: percent fibrosis was quantified by measuring the nonmuscle area in the trichrome-stained sections relative to the total tissue area. In each section (n = 3 per group), 2–3 regions in the image were used for measurement of fibrosis. In mdx mice, treatment with 1% BBIC attenuated the amount of fibrosis observed in diaphragm muscle (*P < 0.05 vs. control-fed mdx mice).
Fig. 2.
BBIC reduces Evans blue dye (EBD) uptake in the quadriceps muscle of mdx mice. Low-magnification (25×) images of EBD infiltration into the muscle fibers of untreated (top; mdx) and treated (bottom; +1% BBIC) mdx mice, with the nuclei stained with DAPI for visualization. Three months of a 1% BBIC diet reduced the amount of EBD infiltration into muscle fibers of mdx mice compared with control fed animals (*P < 0.05; n = 3 per group).
Fig. 3.
BBIC improves phenotypic properties of mdx mice. A: the percentage of muscle fibers containing centralized nuclei from the extensor digitorum longus (EDL), diaphragm, and tibialis anterior (TA). Treatment with 1% BBIC in the feed significantly reduced the number of centrally located nuclei in the EDL and the TA muscles (lowered by 23% and 10%, respectively); the number of central nuclei fell by 17% in the diaphragm muscle (P = 0.055). *P < 0.05 (2–4 regions per section, n = 3 per group). B: mean serum creatine kinase (CK) levels changed by nearly 3-fold following BBIC treatment (P < 0.10; n = 5).
Modulation of Smad signaling by BBIC.
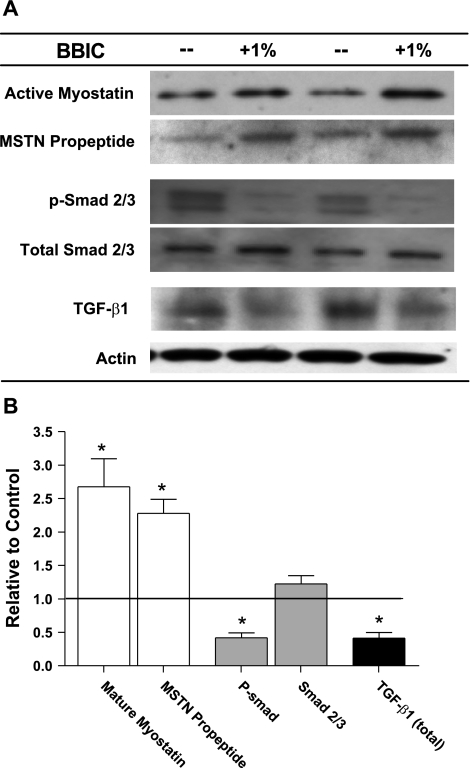
Given the observed muscle mass increase, the possible indirect impact of BBIC on myostatin activation was examined. Analysis of the muscle samples indicated that the expression of both mature myostatin (COOH-terminal antibody) and the NH2-terminal myostatin propeptide were similarly and significantly elevated in the BBIC-treated samples. This suggests the ratio of active myostatin to latent, propeptide-myostatin complex remains constant following BBIC treatment despite increased expression (Fig. 4). Downstream myostatin signaling involves activation of Smad2/3, so the level of Smad phosphorylation was determined in control and BBIC-treated muscles. BBIC treatment reduced phospho-Smad 2/3 levels while maintaining similar total Smad 2/3 expression, suggesting that overall flux through this pathway is reduced.
Fig. 4.
Complex changes in the myostatin pathway of BBIC-treated and untreated mdx mice. A: representative immunoblots of protein expression. B: relative change graph of mature myostatin, myostatin (MSTN) propeptide, phospho-Smad 2/3 (p-Smad 2/3), Smad 2/3, and transforming growth factor (TGF)-β1. *P < 0.05 vs. control-fed mdx.
As TGF-β1 can bind to the same type I receptor (TβRI/ Alk5) as myostatin and also can signal via the Smad pathway, the level of TGF-β1 in the control and treated muscles was measured. Interestingly, TGF-β1 was reduced by ∼50% following treatment with 1% BBIC (Fig. 4). This suggests the reduced Smad activity is primarily due to the decline in TGF-β1 expression. Given the decreased Smad signaling, the apparent accumulation of myostatin in treated animals suggests that there is reduced myostatin activation and subsequent depletion from the extracellular matrix of the muscle. These data could help to explain the muscle hypertrophy as well as the reduction in fibrosis in BBIC-treated mice.
BBIC Does Not Affect Growth Pathways in mdx Mice
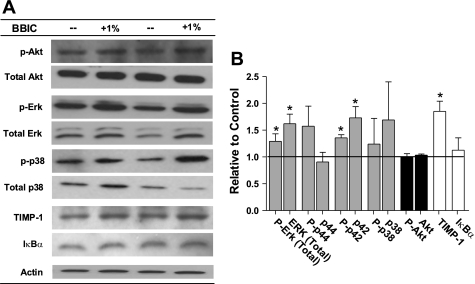
As BBIC has been shown to inhibit a number of proteases, and therefore the downstream effectors, we also investigated possible changes in alternative growth signaling pathways. MAPK protein p42 along with its phosphorylated form were increased, while phospho- and total MAPK protein p44, and total and phospho-p38 remained unchanged (Fig. 5). Further, Akt and phospho-Akt were found to be similar between groups. An inhibitor of metalloproteinase activity, TIMP-1, was significantly elevated, while the NF-κB pathway member IκBα remained unchanged (Fig. 5).
Fig. 5.
Changes in MAPKs, Akt, and TIMP-1 in mdx mice given control or 1% BBIC containing diet. A: representative immunoblots of activity and expression. p-Akt, phospho-Akt; p-Erk, phospho-ERK; p-p38, phospho-p38. B: relative change graph of MAPKs, Akt, and TIMP-1 to show growth signaling changes and endogenous regulation to suppress increased metalloproteinase activity in mdx (n = 4). *P < 0.05 vs. control-fed mdx.
Protease activity.
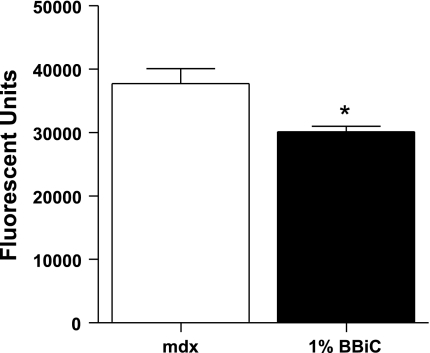
To assess if the increased stability of the muscle membranes, indicated by decreased EBD uptake and reduced central nucleation, results in decreased activation of calpain, we measured calpain protease activity in treated and untreated muscles. As shown in Fig. 6, calpain activity was reduced ∼25% in treated animals compared with control fed animals.
Fig. 6.
Calpain activity was significantly reduced in animals fed a diet containing 1% BBIC compared with a control diet (n = 3–7 per group). *P < 0.05 vs. control-fed mdx.
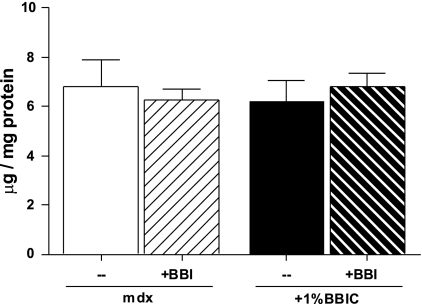
We have previously shown that BBIC does not directly inhibit proteasome activation (30). In the present study, chymotrypsin-like activity of the proteasome was found to be similar between BBIC-treated and control animals, again indicating that BBIC does not appear to directly influence proteasome activity (Fig. 7). To further clarify whether BBIC inhibits chymotrypsin-like activity, 100 μg BBIC (Fig. 7) or 100 μg of purified Bowman-Birk inhibitor (data not shown) was added directly to the assay buffer containing isolated proteasomes from mdx muscles. In both cases, chymotrypsin-like activity was not attenuated, confirming that BBIC does not directly inhibit proteasome activity, even in dystrophic skeletal muscle.
Fig. 7.
Proteasome activity was not changed in mdx animals following 3 mo of a BBIC (+1% BBIC, —) diet compared with control diet (mdx, —) (n = 3–8 per group). Also, addition of exogenous BBIC or purified BBI (+BBI) to the assay did not affect proteasome activity, suggesting this inhibitor does not directly inhibit proteasome activity.
DISCUSSION
We demonstrate that oral administration of a concentrate of the Bowman-Birk inhibitor (BBIC) improves muscle function and decreases pathology in the mouse model of Duchenne muscular dystrophy (mdx mouse). BBIC-treated mdx mice exhibited increased EDL mass, tetanic force, and fiber cross-sectional area and improved resistance to contraction-induced injury compared with untreated muscles. Further, markers generally associated with progressive muscle damage in the mdx mouse, such as fibrosis, loss of membrane integrity (EBD penetration), and centralized nuclei (indicating decreased ongoing regeneration), were all reduced following BBIC treatment. These results are the first to demonstrate that there is an observable therapeutic effect in mdx animals treated with this serine protease inhibitor.
The addition of 1% BBIC to the diet reduced fibrosis, central nucleation, and EBD infiltration compared with a control diet. Further, the addition of 1% BBIC to the diet resulted in a threefold change in the mean serum CK levels (P < 0.10). Increased serum CK is a hallmark of dystrophin deficiency (32). These observations, coupled with improved resistance to contraction-induced injury, suggest that BBIC-treated animals are experiencing less ongoing degeneration. This is significant because contraction-induced injury is widely believed to be a major contributor to muscle injury in DMD patients (32).
The observed decreases in fibrosis, necrosis (EBD uptake), and decreased ongoing regeneration are all expected consequences of decreased mast cell protease activity via BBIC inhibition. Furthermore, healthy C57 mice fed BBIC did not exhibit any alterations in muscle mass or force production (Table 1). These results suggest that BBIC is working to modulate pathological processes that become activated in mdx tissue but not healthy muscle tissue. One of the major distinctions between normal and dystrophic skeletal muscle is the repeated cycles of degeneration and regeneration (8) and the concomitant inflammation found in dystrophic skeletal muscle (7). BBIC has been shown to exhibit anti-inflammatory as well as antioxidant properties (10). As part of the inflammatory cascade, mast cell numbers are increased in mdx mice and they contain chymase and trypase, two serine proteases (13, 14). The well-known BBIC target, chymase is released from mast cells and has been shown to play a role in mdx pathology (16). We and others have previously shown that chymase is inhibited by BBIC (30, 44), and we also showed that this resulted in a decrease in the activation matrix metalloproteases (MMP) 2/9, which can be activated by chymase (41). Elevated MMP2/9 activities have been reported in dystrophic skeletal muscle (11, 17).
Additionally, BBIC treatment proportionately increased muscle mass and tetanic force in the EDL, indicating a functional gain in muscle tissue while the specific tension (force per unit area) was similar between treated and untreated mdx mice. While an overall lessening of pathology could be expected given the known anti-inflammatory and antioxidant actions of BBIC, the increased size of the muscle in the dystrophic skeletal muscle was not expected. A possible explanation for this hypertrophy is based on our earlier observation that there was decreased activation of MMP2/9 in hindlimb-suspended animals fed BBIC, likely due to inhibition of chymase (30). This indirectly could decrease activation of myostatin and TGF-β1, since activation of MMP2/9 has been linked to the activation of TGF-β family members (18). This could account for the observed muscle growth, as it is known that interfering with myostatin signaling in the mdx mouse results in hypertrophy (6). Indeed, a dramatic reduction in downstream Smad 2/3 signaling was observed in BBIC-treated animals compared with control. We also ruled out an indirect effect of BBIC on Akt activity as the mediator of the observed hypertrophy. As shown in Fig. 5, there was no change in the activation of the Akt signaling pathway following BBIC treatment.
An attenuation of Smad signaling implies altered activation and/or expression of one or more of the TGFβ family members that are expressed in skeletal muscle. Of these family members, myostatin is thought to be the primary negative regulator of skeletal muscle mass (24). Intriguingly, the attenuation of Smad signaling by BBIC administration was accompanied by an increase in myostatin expression (Fig. 4). Such a compensatory increase in myostatin production has been previously observed to accompany skeletal muscle hypertrophy resulting from increased levels of the myostatin inhibitory propeptide (24). The increase in expression of mature myostatin protein was matched by similar, or slightly decreased, levels of propeptide, suggesting that there was an elevation in total myostatin content, but not in myostatin activation. This could be due to an overall decrease in myostatin proteolytic activation (23) resulting in more mature myostatin remaining in the muscle ECM, or perhaps due to more myostatin synthesis via a feedback mechanism in an unsuccessful attempt to increase Smad signaling in the face of the inhibitor.
The observed inhibition of Smad 2/3 signaling by BBIC could be via a reduction in myostatin signaling, TGF-β1 signaling, or both. Increased TGF-β1 expression has been associated with fibrosis in the muscles of dystrophic patients (4) while neutralizing antibodies to TGF-β1 reduced fibrosis in the diaphragms of mdx mice (1), suggesting a role for this signaling pathway during disease progression. Following BBIC treatment, a significant reduction in fibrosis was observed. Consistent with that observation is the finding of decreased TGF-β1 levels in BBIC-treated mice (Fig. 4).
The possible indirect effects of BBIC on the activation of intracellular proteolytic pathways, including calpain and proteasome pathways, were also examined. Increased calpain activity has been reported in mdx animals as well as DMD patients (34, 37) as calpain-mediated release of sarcomeric proteins appears to be an essential step during protein degradation via the proteasome (36). Spencer and Mellgren (38) found that calpastatin overexpression in mdx mice improved some markers of dystrophic pathology, primarily in younger animals. Interestingly, decreased levels of calpain activity following BBIC-treatment of mdx mice (Fig. 6) were measured. This is consistent with a slowing of the overall turnover of the dystrophic muscle in response to BBIC and indicates that there is less damage and therefore a reduced calcium influx with administration of the protease inhibitor. However, BBIC treatment does not result in an inhibition of downstream proteasome activity (Fig. 7).
In summary, a diet supplemented with the serine protease inhibitor BBIC increased muscle mass and function in mdx mice compared with mdx mice receiving a control diet. The increased muscle mass was likely due to the observed reduction in Smad signaling, which may have resulted from either decreased myostatin activation or TGF-β1 levels (or a combination of the two). The decreased TGF-β1 levels also likely account for the decreased fibrosis. The decreased levels of TGF-β1 may not only reduce fibrosis by lessening stimulation of fibroblasts but also may induce greater proliferation of satellite cells to stimulate regeneration (25, 29). We also saw evidence of decreased muscle turnover and reductions in activation of calpain, which could contribute to greater muscle mass. None of the benefits of BBIC involved inhibition of proteasome activity or constitutive activation of growth pathways. Additional studies are necessary to delineate a possible impact of BBIC administration on the inflammatory and oxidative stress pathologies seen in dystrophic muscle (45).
The present study suggests oral administration of BBIC shows promise as a possible therapeutic to slow the pathological changes associated with loss of dystrophin in skeletal muscle. If the observed effects in mdx mice are recapitulated in humans, then BBIC treatment could result in prolonged mobility and improved quality of life for Duchenne muscular dystrophy patients.
GRANTS
This work was supported by Wellstone Muscular Dystrophy Cooperative Research Center Grant U54-AR-052646, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants F32-AR-055005-01 and T32-AR-053461, and a Parent Project Muscular Dystrophy (PPMD) postdoctoral fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of C. A. Morris: Orphan and Genetic Diseases Unit, Pfizer, Cambridge MA 02140.
Present address of J. T. Selsby: Iowa State Univ., Dept. of Animal Science, Ames, IA, 50011.
REFERENCES
- 1. Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J Neuroimmunol 175: 77–86, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol 102: 956–964, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest 96: 1137–1144, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Billings PC, St Clair WH, Maki PA, Kennedy AR. Distribution of the Bowman Birk protease inhibitor in mice following oral administration. Cancer Lett 62: 191–197, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418–421, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65: 826–834, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord 10: 113–120, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Engel AG, Ozawa E. Dystrophinopathies. In: Myology, edited by Engel A, Franzini-Armstrong C. New York: McGraw-Hill, 2004, p. 961–1025 [Google Scholar]
- 10. Frenkel K, Chrzan K, Ryan CA, Wiesner R, Troll W. Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes. Carcinogenesis 8: 1207–1212, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda S, Ikeda S. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord 8: 54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37: 1974–1984, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Gorospe JR, Tharp M, Demitsu T, Hoffman EP. Dystrophin-deficient myofibers are vulnerable to mast cell granule-induced necrosis. Neuromuscul Disord 4: 325–333, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Gorospe JR, Tharp MD, Hinckley J, Kornegay JN, Hoffman EP. A role for mast cells in the progression of Duchenne muscular dystrophy? Correlations in dystrophin-deficient humans, dogs, and mice. J Neurol Sci 122: 44–56, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve 30: 645–653, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Granchelli JA, Avosso DL, Hudecki MS, Pollina C. Cromolyn increases strength in exercised mdx mice. Res Commun Mol Pathol Pharmacol 91: 287–296, 1996 [PubMed] [Google Scholar]
- 17. Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am J Pathol 170: 633–643, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huet C, Li ZF, Liu HZ, Black RA, Galliano MF, Engvall E. Skeletal muscle cell hypertrophy induced by inhibitors of metalloproteases; myostatin as a potential mediator. Am J Physiol Cell Physiol 281: C1624–C1634, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kennedy AR, Szuhaj BF, Newberne PM, Billings PC. Preparation and production of a cancer chemopreventive agent, Bowman-Birk inhibitor concentrate. Nutr Cancer 19: 281–302, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Larionova NI, Gladysheva IP, Tikhonova TV, Kazanskaia NF. [Inhibition of cathepsin G and elastase from human granulocytes by multiple forms of the Bowman-Birk type of soy inhibitor]. Biokhimiia 58: 1437–1444, 1993 [PubMed] [Google Scholar]
- 21. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, Wang CH, Chung KF, Kuo HP. Neutrophil-derived elastase induces TGF-beta1 secretion in human airway smooth muscle via NF-kappaB pathway. Am J Respir Cell Mol Biol 35: 407–414, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lee SJ. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS One 3: e1628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371–19378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lichtenstein GR, Deren JJ, Katz S, Lewis JD, Kennedy AR, Ware JH. Bowman-Birk inhibitor concentrate: a novel therapeutic agent for patients with active ulcerative colitis. Dig Dis Sci 53: 175–180, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Maffia PC, Zittermann SE, Scimone ML, Tateosian N, Amiano N, Guerrieri D, Lutzky V, Rosso D, Romeo HE, Garcia VE, Issekutz AC, Chuluyan HE. Neutrophil elastase converts human immature dendritic cells into transforming growth factor-beta1-secreting cells and reduces allostimulatory ability. Am J Pathol 171: 928–937, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev CD003725, 2008 [DOI] [PubMed] [Google Scholar]
- 29. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135–1147, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris CA, Morris LD, Kennedy AR, Sweeney HL. Attenuation of skeletal muscle atrophy via protease inhibition. J Appl Physiol 99: 1719–1727, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Oreffo VI, Billings PC, Kennedy AR, Witschi H. Acute effects of the Bowman-Birk protease inhibitor in mice. Toxicology 69: 165–176, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prior TW, Bridgeman SJ. Experience and strategy for the molecular testing of Duchenne muscular dystrophy. J Mol Diagn 7: 317–326, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy PA, Anandavalli TE, Anandaraj MP. Calcium activated neutral proteases (milli- and micro-CANP) and endogenous CANP inhibitor of muscle in Duchenne muscular dystrophy (DMD). Clin Chim Acta 160: 281–288, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Sawada H, Nagahiro K, Kikukawa Y, Ban S, Kakefuda R, Shiomi T, Yokosawa H. Therapeutic effect of camostat mesilate on Duchenne muscular dystrophy in mdx mice. Biol Pharm Bull 26: 1025–1027, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Smith IJ, Dodd SL. Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp Physiol 92: 561–573, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem 270: 10909–10914, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet 11: 2645–2655, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Spencer MJ, Tidball JG. Calpain concentration is elevated although net calcium-dependent proteolysis is suppressed in dystrophin-deficient muscle. Exp Cell Res 203: 107–114, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551: 33–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem 280: 9291–9296, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Thompson VF, Saldana S, Cong J, Goll DE. A BODIPY fluorescent microplate assay for measuring activity of calpains and other proteases. Anal Biochem 279: 170–178, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Vigouroux S, Farout L, Clavel S, Briand Y, Briand M. Increased muscle proteasome activities in rats fed a polyunsaturated fatty acid supplemented diet. Int J Biochem Cell Biol 35: 749–755, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Ware JH, Wan XS, Rubin H, Schechter NM, Kennedy AR. Soybean Bowman-Birk protease inhibitor is a highly effective inhibitor of human mast cell chymase. Arch Biochem Biophys 344: 133–138, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33: 657–662, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Yavelow J, Collins M, Birk Y, Troll W, Kennedy AR. Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress x-ray-induced transformation in vitro. Proc Natl Acad Sci USA 82: 5395–5399, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Kouzuma Y, Miyaji T, Yonekura M. Purification, characterization, and cDNA cloning of a Bowman-Birk type trypsin inhibitor from Apios americana Medikus tubers. Biosci Biotechnol Biochem 72: 171–178, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H, Shang FJ, Niu XL, He YP, Lu XL. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem 310: 159–166, 2008 [DOI] [PubMed] [Google Scholar]