Abstract
The airways contain a dense subepithelial microvascular plexus that is involved in the supply and clearance of substances to and from the airway wall. We set out to test the hypothesis that airway smooth muscle reactivity to bronchoconstricting agents may be dependent on airway mucosal blood flow. Immunohistochemical staining identified vasoconstrictor and vasodilator nerve fibers associated with subepithelial blood vessels in the guinea pig airways. Intravital microscopy of the tracheal mucosal microvasculature in anesthetized guinea pigs revealed that blockade of α-adrenergic receptors increased baseline arteriole diameter by ∼40%, whereas the α-adrenergic receptor agonist phenylephrine produced a modest (5%) vasoconstriction in excess of the baseline tone. In subsequent in vivo experiments, tracheal contractions evoked by topically applied histamine were significantly reduced (P < 0.05) and enhanced by α-adrenergic receptor blockade and activation, respectively. α-Adrenergic ligands produced similar significant (P < 0.05) effects on airway smooth muscle contractions evoked by topically administered capsaicin, intravenously administered neurokinin A, inhaled histamine, and topically administered antigen in sensitized animals. These responses were independent of any direct effect of α-adrenergic ligands on the airway smooth muscle tone. The data suggest that changes in blood flow in the vessels supplying the airways regulate the reactivity of the underlying airway smooth muscle to locally released and exogenously administered agents by regulating their clearance. We speculate that changes in mucosal vascular function or changes in neuronal regulation of the airway vasculature may contribute to airways responsiveness in disease.
Keywords: tracheobronchial vasculature, bronchoconstriction, intravital microscopy, particle clearance
the airway vasculature consists of a dense subepithelial capillary network, a deeper plexus of capacitance vessels, and in some species an adventitial microvascular plexus (49, 50). The regulation of these vascular beds has been studied in detail, and both neural and nonneural mechanisms play significant roles in determining the level of airway vascular tone (11, 16, 18, 27, 28, 39, 48). Catecholamines (acting via α-adrenergic receptors) and neuropeptide Y both constrict vascular smooth muscle and reduce perfusion secondary to sympathetic nerve activation. On the other hand, activation of parasympathetic nerves evokes vasodilatation secondary to the release of acetylcholine or nitric oxide and vasoactive intestinal peptide. In some species neuropeptide-expressing sensory nerves can also mediate vasodilatation via axon reflexes. Locally produced and circulating agents, including those released from the airway epithelium or activated inflammatory cells, also regulate vascular tone and thus airway perfusion.
The function of airway vasculature is undoubtedly critical for the supply of nutrients to the airways and in the regulation of airway wall volume, temperature, and the clearance of insoluble and soluble particles (16, 23, 24, 43, 44, 49, 50). In sheep, for example, a reduction in bronchial blood flow significantly increases airway retention of mucosally deposited soluble particles and reduces the clearance of insoluble particles by mucociliary transport (42, 43). This interdependence of airway blood flow and the clearance of substances might be important in determining the potency of bronchoactive agents, particularly for inhaled drugs or locally produced mediators that must first traverse the mucosal microvasculature before having any actions on the airway smooth muscle. There is some support for this notion. For example, reduced bronchial vascular perfusion in sheep and dogs prolongs recovery from methacholine and histamine-evoked bronchoconstriction, whereas the vasoconstrictor vasopressin prolongs antigen-evoked bronchospasm (10, 23, 24, 44). These observations may be of particular importance in airways diseases where a variety of endogenous mediators and exogenous substances (including therapeutic agents) have significant effects on normal vascular function in the airways. In the present study we therefore set out to directly test the hypothesis that airway microvascular tone is an important determinant of airway smooth muscle responsiveness to exogenously administered and endogenously derived bronchoconstricting agents.
METHODS
All of the experiments described below were conducted on male albino Hartley strain guinea pigs (250–350 g) under approved institutional animal ethics committee protocols.
Whole mount immunohistochemistry.
Whole mount immunohistochemistry was performed as previously described (32). Guinea pigs (n = 3) were deeply anesthetized with 100 mg/kg pentobarbital sodium and transcardially perfused with 10 mM heparinized PBS containing 0.1% procaine. Tracheae were removed, cleaned of excess connective tissue, opened longitudinally along the ventral surface, and pinned to a piece of cork board. The epithelium was gently removed by rubbing with a cotton swab, and the tracheas were dehydrated through ascending concentrations of ethanol (30 min per step) and cleared in xylene (20 min). Tracheas were then rehydrated back through descending concentrations of ethanol, washed in PBS, and fixed in 4% paraformaldehyde (4°C) for 2 h. After fixation, tracheas were washed thoroughly in distilled water and then pinned to the bottom of a petri dish containing Sylgard. Whole mounts were rinsed in 0.01 M PBS and covered in blocking solution (10% goat serum, 1% BSA, 0.5% Tween 20 in PBS) for 1 h. Tissues were then incubated overnight at 37°C with a rabbit polyclonal antibody to tyrosine hydroxylase (1:800, Chemicon, Temecula, CA) and a rat monoclonal antibody to substance P (1:200, Chemicon, Temecula, CA). Following several PBS washes, labeled whole mounts were incubated (1 h, room temperature) with rhodamine goat anti-rabbit and fluorescein goat anti-rat IgG (1:200 dilution, Molecular Probes, Eugene, OR), rinsed in PBS, coverslipped, and viewed using an Olympus BX60 epifluorescent microscope. Images were captured digitally using a Pixera Penguin 600CL cooled CCD digital camera and imported into Adobe Photoshop for preparation of representative montages.
Intravital visualization of submucosal arterioles in guinea pig trachea.
Guinea pig tracheal submucosal blood vessels were visualized in real time using an intravital microscopy technique modified from previous studies described in anesthetized rats and dogs (7, 8, 18, 29). Male Hartley guinea pigs (200–300 g, Hilltop, Scottdale, PA) were anesthetized with urethane (1–1.5 g/kg ip) and positioned supine on a heated pad. The extrathoracic trachea was exposed via a midline incision on the ventral surface of the neck, and the tissue and muscle overlying the trachea was held apart with surgical retractors. The caudalmost portion of the trachea was cannulated, and the animals were mechanically ventilated (60 breaths/min, 6 ml/kg, 2–3 cmH2O of positive end-expiratory pressure) following paralysis (2 mg/kg succinylcholine chloride sc). The ventral tracheal wall was then carefully cut longitudinally from the cartilage ring immediately rostral to the ventilation cannula to the caudal end of the larynx. Several sutures were bridged from the cut cartilage rings to the retractor, thereby opening the lumen of the trachea and exposing the mucosa overlying the trachealis muscle. The abdominal aorta and vena cava were cannulated to monitor arterial blood pressure and for intravenous drug injection, respectively.
The exposed tracheal mucosa was superfused with warmed (37°C), oxygenated Krebs buffer [composition (mM) 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, 11.1 dextrose]. Indomethacin (3 μM) and propranolol (2 μM) were added to the buffer to block the local effects of prostaglandins and to block any β-adrenergic receptor-mediated effects of circulating and neurally released catecholamines on the tracheal segment studied (31). In addition, the buffer contained 1 μM atropine and 10 μM histamine to ensure consistency with our functional studies of airway smooth muscle tone (described below). The buffer was removed from the rostralmost end of the trachea with gentle suction. Animals were also pretreated intravenously with propranolol (1 mg/kg). At the conclusion of each experiment, animals were killed by inhalation of 100% CO2, delivered through the inspiratory port of the ventilator.
To observe the submucosal vascular plexus in the trachea, the preparation was mounted under a reflected light microscope (Olympus, Melville, NY) with a water-immersion objective lens (×40 magnification; working distance: 3.3 mm; depth of field: 1.52 μm) and an eyepiece (×10 magnification). The preparation was transilluminated with a halogen light source. Real-time images were acquired using an Olympus BX50W1 color camera, displayed onto a Sony Trinitron color video monitor (PVM-14N5U; Sony Electronics, San Jose, CA) and recorded on a Sony super-VHS videocassette recorder (Sony SVO-9500 MDP). At the onset of each experiment a single small arteriole was selected for continuous observation. Suitable arterioles were defined as having a vessel diameter between 15 and 80 μm, relatively high blood flow (qualitatively assessed), and clear evidence that the vessel gave rise to further smaller vessels at some point distal to the observation site (and blood flow was in the direction of these smaller vessels). Vessel diameters were analyzed offline using digital calipers. An equilibration period of 10 min was then allowed before initiating any treatments to ensure stability in the vessel under study.
α-Adrenergic-mediated regulation of tracheal vascular tone was determined by assessing both the degree of vasodilatation evoked following the addition of 1 μM phentolamine to the superfusion buffer and, in separate experiments, vasoconstriction evoked by 1 μM phenylephrine (n = 7 each). Maximum attainable dilatation and constriction of each vessel was determined at the end of the experiment by the addition of 0.1 mM papavarine or 1 mM phenylephrine to the superfusion buffer (n = 3 each).
In situ measurements of airway smooth muscle tone in the absence and presence of vasoactive agents.
Airway smooth muscle tone was measured in situ using an anesthetized guinea pig preparation previously described in detail (31). Guinea pigs were anesthetized with 1–1.5 g/kg urethane and prepared for mechanical ventilation, intravenous drug delivery, and monitoring of arterial blood pressure as described above. The tracheal lumen was kept intact, and stainless steel hooks were passed between two cartilage rings (rings 6 and 7 caudal to the larynx) on either side of the trachea, rostral to the tracheal cannula. One hook was sutured to a fixed bar and the other hook was sutured to an isometric force transducer (model FT03C, Grass Instruments, Quincy, MA). The tracheal lumen was perfused with Krebs buffer containing 3 μM indomethacin and 2 μM propranolol, and animals were pretreated (iv) with 1 mg/kg propranolol as described above. Optimal baseline tracheal tension was set (1.5–2 g) and maintained throughout a 20-min equilibration period. In this preparation bronchoactive agents can be studied following topical (tracheal perfusate), inhaled (nebulized into the ventilator circuit), or intravenous delivery methods, and the output can be assessed as changes in tracheal tension and/or pulmonary insufflation pressure (PIP).
In initial experiments we assessed the ability of 1 μM phentolamine to reverse a steady-state contraction of the trachealis evoked by topically applied histamine. As the tracheal smooth muscle in this preparation is in receipt of ongoing cholinergic nerve activity (and hence is partially contracted, see Fig. 1a; see also Ref. 31), we first added atropine (1 μM) to the perfusate to reverse this baseline cholinergic tone. Tracheal tone was then restored by the addition of histamine to the tracheal perfusate (0.3 μM to 1 mM, one concentration per animal, n = 3–4 animals per concentration). Once a steady-state histamine-evoked tracheal contraction was attained, the effect of reducing mucosal vascular tone (and presumably increasing mucosal blood flow) by addition of phentolamine (1 μM) was then quantified. In parallel experiments, the effect of 1 μM phentolamine was also assessed on 1) the level of baseline cholinergic tone (n = 4) and 2) baseline tracheal tension (i.e., the 1.5–2 g of tension initially applied to the trachea) in vagotomized guinea pigs (n = 3). In a limited number of additional experiments the effects of phentolamine were confirmed with 1 μM prazosin.
Fig. 1.
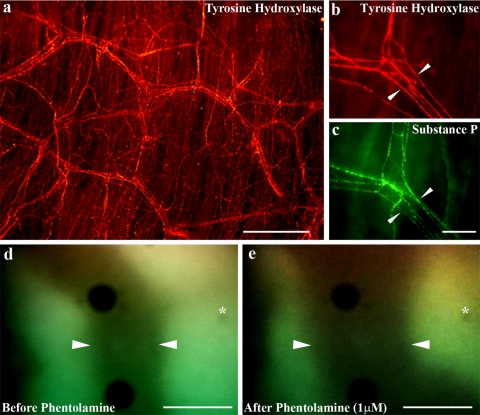
Visualization of tracheal microvasculature. a: Low-power magnification photomicrograph showing an extensive organized subepithelial network of tyrosine hydroxylase immunoreactive nerve fibers (red) in a whole mount preparation of the guinea pig trachea. b: Higher power magnification showing a Y-shaped blood vessel flanked by several tyrosine hydroxylase-positive (red) nerve fibers (arrowheads). This vessel is also flanked by numerous distinct substance P-positive (green) nerve fibers (c, arrowheads). d and e: Representative single subepithelial arteriole indentified in vivo using intravital microscopy (the margins of which are delineated by arrowheads). This vessel is shown before (d) and 10 min after (e) topical application of 1 μM phentolamine. Asterisk shows reference mark (small dark spot) in the field of view which is unchanged in location following phentolamine application. Note the dramatic change in the shape of the dilated blood vessel. Scale bars, 200 μm (in a) and 40 μm (in b–e).
In subsequent (unpaired) experiments we determined the effect of 1 μM phentolamine and 1 μM phenylephrine (n = 5–7 for each experiment) on tracheal contractions evoked by 1) capsaicin (10 μM) applied to the tracheal perfusate, 2) ovalbumin (1 nM to 10 mM) applied to the tracheal perfusate in antigen-sensitized animals (see below for antigen sensitization protocol), and 3) intravenously administered neurokinin A (NKA; 0.5–5 nmol/kg). We also assessed the effect of adding either phentolamine (1 μM) or phenylephrine (1 μM) to the tracheal perfusate on the ability of intravenous administration of the histamine H1 receptor antagonist pyrilamine (0.003–0.1 mg/kg) to reverse a steady-state tracheal contraction evoked by tracheal superfusion of 10 μM histamine. Finally, we assessed the effect of nebulized phentolamine (30 μM) or phenylephrine (30 μM) on increases in PIP evoked by nebulized histamine (0.01–10 mg/ml).
In all experiments, the magnitude of tracheal contractions was expressed as a percentage of the maximum contraction evoked by adding 300 mM barium chloride (BaCl2) to the tracheal perfusate at the end of the experiment. Changes in PIP were expressed as a percentage change relative to the baseline PIP.
In vitro studies.
For comparison with the results from in vivo experiments (in which the airway circulation is intact and functioning), parallel in vitro experiments were conducted using tracheal tissues harvested from guinea pigs (therefore devoid of vascular perfusion). As previously described (25), tracheal strips (2 rings each) were suspended with 1.5 g of passive tension in 10-ml organ baths. Tissues were equilibrated for 1 h by washing four times with warmed (37°C), oxygenated Krebs bicarbonate buffer containing 3 μM indomethacin and 2 μM propranolol. Contractions were measured isometrically (Grass FT03) and displayed on a Grass model 7D polygraph. Experiments assessed the effect of vehicle, phentolamine (1 μM), and phenylephrine (1 μM) on 1) histamine (10 nM–1 mM)-evoked tracheal contractions and 2) the time course of tracheal contractions evoked by 10 μM capsaicin. In a limited number of additional experiments the effects of phentolamine were confirmed with 1 μM prazosin.
Antigen sensitization.
Conscious animals were treated on days 1, 3, and 5 with an intraperitoneal injection of ovalbumin (10 mg/kg in 0.5 ml in sterile saline). Three to four weeks following the final injection, animals were anesthetized and used as described above for localized (tracheal) challenges with ovalbumin.
Data analysis.
All data are presented as means ± SE. Differences between group means were assessed using ANOVA on Statview for Macintosh (Berkeley, CA). P values of <0.05 were considered significant. When significant variation between groups was detected, treatment group means were compared using Scheffé's F-test for unplanned comparisons. Occasionally (<2% of experiments), preparations exhibited little or no contraction to any stimulus, including BaCl2. Such preparations were excluded from subsequent statistical analyses.
Drugs.
Atropine sulfate, barium chloride, heparin sulfate, indomethacin, histamine, urethane (ethyl carbamate), succinylcholine chloride, capsaicin, pyrilamine, phentolamine hydrochloride, d,l-propranolol hydrochloride, phenylephrine, prazosin, papaverine, and ovalbumin were purchased from Sigma (St. Louis, MO). Stock solutions of all drugs added to the tracheal perfusate in vivo or to organ baths in vitro were made in distilled water except capsaicin, which was dissolved in absolute ethanol. Drugs administered intravenously, subcutaneously, or as an aerosol were dissolved in saline.
RESULTS
Localization of vasomotor nerves in the guinea pig submucosal vascular plexus.
Immunohistochemical staining of guinea pig tracheal whole mounts revealed a dense subepithelial plexus of tyrosine hydroxylase-expressing nerve fibers (Fig. 1a). On close inspection it was readily apparent that the majority of these fibers flanked blood vessels of the subepithelial vascular plexus (Fig. 1b). A second deeper group of tyrosine hydroxylase-positive fibers was also seen within the tracheal smooth muscle (not shown). Immunohistochemical staining for the vasodilatory sensory neuropeptide substance P also revealed nerve fibers associated with the subepithelial vasculature (Fig. 1c). Dual staining for substance P and tyrosine hydroxylase showed that vessels were often flanked by both tyrosine hydroxylase-positive and substance P-positive fibers (Fig. 1, b and c).
Regulation of subepithelial vascular tone.
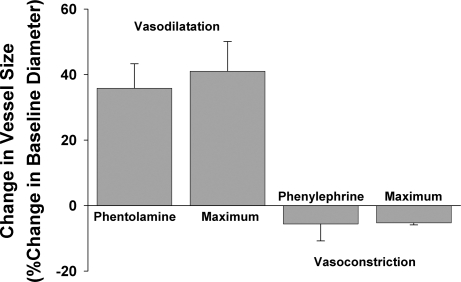
The average baseline diameter of the subepithelial arterioles studied using intravital microscopy was 36.8 ± 3.5 μm (range 15–60 μm, n = 14). There was no significant difference between the baseline diameter of the vessels used for constriction and dilatation studies (n = 7 each). Addition of 1 μM phentolamine to the superfusion buffer evoked a large and immediate increase in the diameter of each of the 7 vessels studied (Fig. 1, d and e, and Fig. 2). The maximum dilatation evoked by phentolamine (∼40% increase in diameter over baseline) peaked within 10 min and then remained unchanged for up to a further 20 min of observation (in the continued presence of phentolamine). Phentolamine-evoked dilatation approximated the maximum attainable response evoked by papavarine (Fig. 2). In two additional experiments, a similar degree of dilatation was evoked by the sensory neuropeptide substance P (1 nM, data not shown). Conversely, addition of 1 μM phenylephrine to the tracheal perfusate of seven separate preparations only produced a moderate decrease (∼5%) in baseline vessel diameter (Fig. 2). This response also showed a rapid onset and appeared to be stable once attained in the continued presence of phenylephrine. Increasing the concentration of phenylephrine to 1 mM produced no further constriction (Fig. 2). In five experiments, 10 μM histamine evoked variable effects on baseline vascular tone, producing overall a small vasodilatory response (10.1 ± 6.9% increase in mean vessel diameter). None of the drugs administered directly to the tracheal perfusate had any measurable effects on heart rate or systemic blood pressure.
Fig. 2.
Mean changes in tracheal subepithelial arteriole diameter in vivo following topical application of 1 μM phentolamine or 1 μM phenylephrine. Data represent means ± SE of 3–7 individual experiments. Maximum responses were defined in the presence of 0.1 mM papavarine (for vasodilatation) and 1 mM phenylephrine (for vasoconstriction). See text for further details.
Effects of phentolamine and phenylephrine on tracheal contractions evoked by histamine: in vivo and in vitro experiments.
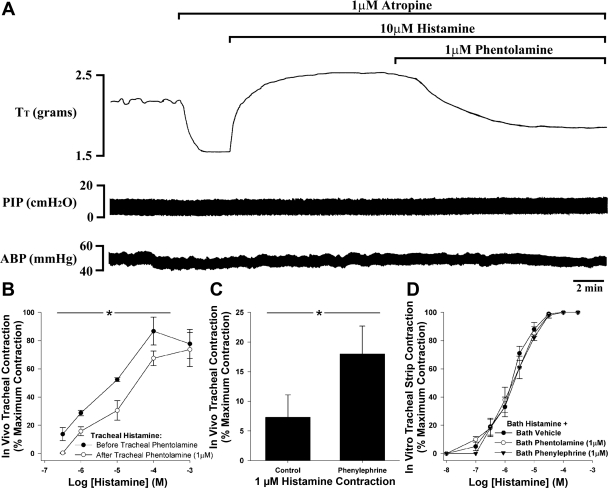
In anesthetized guinea pigs with the vagi intact, the addition of 1 μM atropine to the tracheal perfusate evoked a rapid fall in tracheal tension, indicative of ongoing cholinergic nerve activity in this preparation (30; Fig. 3A). Baseline tracheal cholinergic tone in 24 in vivo preparations averaged 35.9 ± 2.9% of the maximum attainable contraction. Addition of histamine (0.3 μM–1 mM; one concentration per animal) to the perfusate after baseline cholinergic tone was reversed with atropine evoked a dose-related and sustained increase in tracheal tone (Fig. 3, A and B). The subsequent addition of 1 μM phentolamine to the tracheal perfusate, once the contraction to a given dose of histamine was stable, produced an immediate reversal of the histamine contraction in all preparations (Fig. 3, A and B). For example, 10 μM histamine (as shown in Fig. 3A) added to the tracheal perfusate in the absence of phentolamine evoked half-maximal contractions of the trachea (52.3 ± 1.2% of the maximum contraction, n = 3), which were subsequently reversed by 42.1 ± 10.6% on the addition of phentolamine to the tracheal buffer (see Fig. 3B for mean data). In four additional experiments the α1-adrenergic antagonist prazosin similarly evoked a 24.2 ± 7.1% reversal of the contractions evoked by 10 μM histamine. Tracheal application of phentolamine was, however, without effect on baseline cholinergic tone (cholinergic tone was 40.7 ± 4.0 and 37.4 ± 5.0% of the maximum attainable contraction in the absence and presence of phentolamine, respectively), assessed in four animals with intact vagi and in the absence of atropine in the perfusate. Furthermore, in vagotomized animals with atropine in the perfusate (to ensure no vagal or cholinergic tone), phentolamine produced no change in resting tracheal tension, indicating that there is no ongoing α-adrenergic tone in the guinea pig trachea (baseline passive tension was set at 1,866.7 ± 28.9 mg before phentolamine and equaled 1,860.0 ± 36.1 mg after addition of phentolamine to the tracheal perfusate).
Fig. 3.
Effects of phentolamine and phenylephrine on histamine-evoked contractions of the guinea pig trachea in vivo and in vitro. A: representative trace showing in vivo recordings of tracheal tone (Tt), pulmonary insufflation pressure (PIP), and arterial blood pressure (ABP) in a urethane-anesthetized, artificially ventilated guinea pig. Addition of atropine to the tracheal perfusate evokes a rapid and profound reversal of ongoing baseline cholinergic (neural) tone in this preparation. Subsequent addition of histamine (10 μM) restores tone, which is then partially reversed on addition of phentolamine (1 μM) to the perfusate. B: mean data showing the reversal of histamine (0.3 μM–1 mM)-evoked tracheal contractions by 1 μM phentolamine in vivo. C: mean data showing the potentiation of a histamine (1 μM) contraction by 1 μM phenylephrine in vivo. D: mean data showing the lack of effect of phentolamine and phenylephrine on histamine-evoked contractions of guinea pig tracheal strips in vitro. In additional experiments the α-adrenergic antagonist prazosin also significantly reversed the magnitude of histamine-evoked contractions in vivo but was without effect in vitro (see data in text). Each data point represents the mean ± SE of 3–8 experiments. *P < 0.05, significantly different from corresponding control data point.
In separate series of unpaired experiments, cumulative administration of histamine (1, 10, and 100 μM) to the tracheal perfusate (following atropine pretreatment) similarly evoked a dose-dependent increase in tracheal tone. In the presence of 1 μM phenylephrine the magnitude of the histamine contractions was moderately larger compared with vehicle-treated animals, although this effect of phenylephrine was most prominent at the lower concentrations of histamine employed (Fig. 3C). For example, contractions evoked by 1 μM histamine were 7.3 ± 3.8 and 18.9 ± 4.7% of the maximum in vehicle and phenylephrine treated animals, respectively (P < 0.05). The effects of phenylephrine were negligible at histamine concentrations of 10 and 100 μM.
In vitro, bath application of histamine similarly evoked a dose-dependent contraction of isolated tracheal rings (EC50 = 2.39 ± 0.53 μM). Neither phentolamine nor phenylephrine had any effect on baseline tracheal tension in vitro, and neither drug altered the potency of histamine in these preparations (EC50s = 2.26 ± 0.82 and 2.23 ± 0.52 μM, respectively, n = 8; Fig. 3D). Comparable results were obtained in additional experiments employing 1 μM prazosin (histamine EC50 = 1.47 ± 0.14 μM in the presence of prazosin, n = 4).
Effects of phenylephrine on tracheal contractions evoked by capsaicin: in vivo and in vitro experiments.
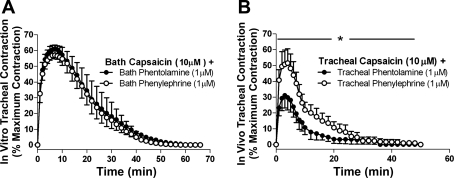
Capsaicin added to either the tracheal perfusate in vivo or the bath containing isolated tracheal rings in vitro evoked an increase in tracheal tone that was almost abolished by pretreatment with the neurokinin (NK) 1 and NK2 receptor antagonists 0.3 μM CP-99994 and SR-48968, respectively. For example, in vitro capsaicin-evoked contractions were 49.3 ± 6.8 and 3.9 ± 2.1% of the maximum contraction in vehicle and CP-99994/SR-48968 treated tissues, respectively. Contractions evoked by capsaicin tended to be larger in magnitude and longer in duration in the in vitro experiments compared with in vivo (see Fig. 4). Although capsaicin-evoked contractions in vitro in the presence of either phentolamine or phenylephrine were indistinguishable (Fig. 4A), both the magnitude and duration of the contraction in vivo in the presence of phenylephrine and phentolamine were quite distinct. Indeed, capsaicin-induced responses in vivo were significantly greater in the presence of phenylephrine (P < 0.05, significant increase in the total area under the curve). This effect of phenylephrine resulted in capsaicin contractions that approximated the responses observed in vitro (Fig. 4B). Interestingly, phentolamine did not alter the magnitude or duration of capsaicin responses in vivo compared with a vehicle pretreatment (the peak magnitude of contraction was 33.5 ± 3.5 and 40.6 ± 9.0% maximum contraction in vehicle- and phentolamine-treated animals, respectively, and the duration of contraction was 25.5 ± 7.5 and 27.6 ± 8.3 min in control and phentolamine-treated animals, respectively).
Fig. 4.
Effects of phentolamine and phenylephrine on capsaicin-evoked contractions of the guinea pig trachea in vivo and in vitro. Time courses of capsaicin evoked tracheal contractions in vitro (A) and in vivo (B) in the presence of either phentolamine or phenylephrine. The mean peak (maximum) capsaicin-evoked contraction (irrespective of the time at which it occurred) was significantly greater (P < 0.05) in vitro compared with in vivo in the presence of phentolamine (60.5 ± 7.3% maximum contraction in vitro vs. 31.5 ± 3.5% maximum contraction in vivo). However, in the presence of phenylephrine, peak in vitro and in vivo contractile responses were comparable (61.4 ± 4.9 vs. 51.4 ± 8.9% maximum contraction, respectively). Data represent means ± SE of 4–7 experiments. *P < 0.05, significant difference between the area under the curves.
Effects of phentolamine and phenylephrine on responses evoked by tracheal ovalbumin and inhaled histamine in anesthetized guinea pigs.
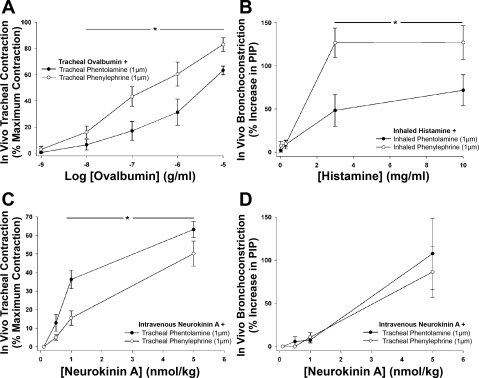
Tracheal administration of ovalbumin in actively sensitized guinea pigs evoked a dose-dependent increase in tracheal tone. The magnitude of the contraction evoked by each concentration of ovalbumin was higher in animals treated (via the tracheal perfusate) with phenylephrine compared with animals receiving phentolamine (estimated ED50s were 1.55 ± 0.77 and 0.06 ± 0.02 μg/ml of ovalbumin in the presence of phentolamine and phenylephrine, respectively; n = 7 P < 0.05; Fig. 5A). Similarly, in vagotomized guinea pigs, histamine when inhaled (nebulized) into the lower airways dose-dependently increased PIP (Fig. 5B). The magnitude of the PIP response was significantly larger when 30 μM phenylephrine was included in the nebulized solution compared with 30 μM phentolamine [the estimated dose of histamine required to evoke a 25% increase in PIP (PD25) was 2.27 ± 1.32 and 0.51 ± 0.09 mg/ml in the presence of phentolamine and phenylephrine, respectively P < 0.05; Fig. 5B].
Fig. 5.
Effects of phentolamine and phenylephrine on increases in tracheal tone and pulmonary insufflation pressure (PIP) in vivo evoked by antigen, inhaled histamine, and intravenous neurokinin A. A: tracheal superfusion with ovalbumin in actively sensitized guinea pigs evoked a concentration-dependent increase in tracheal tone that was larger in the presence of phenylephrine. B: inhaled histamine evoked a dose-dependent increase in PIP that was potentiated in the presence of phenylephrine. Intravenously administered neurokinin A (NKA) evoked a dose-dependent increase in tracheal tension (C) and PIP (D). NKA-evoked tracheal contractions, but not PIP responses, were potentiated when phentolamine was present in the tracheal perfusate compared with phenylephrine. See text for description of the methods. Each data point represents mean ± SE of 4–7 experiments. *P < 0.05, significant difference between responses in the presence of phentolamine and phenylephrine.
Effects of phentolamine and phenylephrine on responses evoked by intravenously administered agents in anesthetized guinea pigs.
Intravenous administration of NKA evoked a dose-dependent increase in tracheal tone and PIP. The magnitude of the NKA-evoked tracheal contraction was significantly larger in animals treated with phentolamine in the tracheal perfusate compared with phenylephrine (NKA ED50s were 1.76 ± 0.45 and at least 8.16 ± 2.42 nmol/kg for phentolamine- and phenylephrine-treated preparations, respectively; P < 0.05; N.B., the exact ED50 in the presence of phenylephrine could not be calculated as in one preparation the peak tracheal contraction to NKA was only 23% of the maximum attainable by BaCl2; Fig. 5C). As expected, tracheal treatment with either phentolamine or phenylephrine did not alter the PIP response to intravenous NKA (NKA PD25s were 2.43 ± 0.80 and 2.35 ± 0.62 nmol/kg, respectively; Fig. 5D). In a similar series of experiments, the ability of intravenous administration of the H1 receptor antagonist pyrilamine (3 μg/kg) to reverse a stable contraction of the trachea evoked by administering 10 μM histamine to the tracheal perfusate was significantly different between preparations containing phentolamine and phenylephrine in the tracheal perfusate (46.6 ± 13.0 and 21.1 ± 7.8% reversal of the histamine contraction in phentolamine- and phenylephrine-treated animals, respectively; n = 5 each; P < 0.05). Consistent with the data described above, the histamine contractions tended to be smaller in the animals treated with phentolamine compared with phenylephrine (71.2 ± 13.0 and 86.6 ± 6.6% maximum, respectively).
DISCUSSION
α-Adrenergic regulation of airway vascular tone.
Airway vascular smooth muscle tone is subject to modulation by a variety of chemical mediators, including neurally released and circulating catecholamines (7, 11, 16, 18, 27, 28, 39, 48). Some level of tracheobronchial vascular tone has been described in the airways of a variety of species, including humans (4, 18, 27, 28, 30, 37, 38, 46). In our in vivo intravital microscopy experiments, blockade of α-adrenergic receptors produced near maximum attainable vasodilatation, whereas the α-adrenergic receptor agonist phenylephrine produced a level of vasoconstriction that was only modestly in excess of the baseline tone. This would suggest, first that, under the conditions of our anesthetized guinea pig preparation, there exists a level of baseline tone in the tracheal subepithelial vasculature that nearly approximates the maximal tone attainable in these vessels. The data also suggest that under these conditions, the baseline tone is derived primarily by α-adrenergic-mediated mechanisms, presumably (at least in part) via α1-adrenergic receptors given that prazosin mimicked the effects of phentolamine. We did not determine the source of the catecholamines mediating these contractions (neuronal vs. hormonal), but their neuronal dependence seems likely, given the dense adrenergic (tyrosine hydroxylase-immunoreactive) innervation these vessels receive and our previous studies describing sympathetic adrenergic innervation of the airways (14, 22, 35, present study).
As our primary intent with these experiments was to assess the role of mucosal blood flow in regulating airway smooth muscle reactivity, some of the drug pretreatments we used to optimize our ability to measure tracheal smooth muscle tone likely enhanced resting vascular tone. Thus propranolol was administered systemically and topically to the airways of all animals, leaving postjunctional α-adrenergic constrictor effects unopposed by coincident activation of the dilating β-adrenoceptors. Indomethacin also likely prevented formation of locally produced and vasodilating prostanoids. Regardless of all of the above, the results demonstrate that mucosal vascular blood flow is tonically regulated by neuronally derived and/or hormonal catecholamines. We of course cannot rule out the possibility that anesthesia (necessary for the invasive physiology experiments) modifies baseline vascular tone.
Effect of vasodilatation and vasoconstriction on tracheal smooth muscle reactivity.
We have presented a variety of experimental data consistent with the hypothesis that changes in subepithelial blood flow can regulate airway smooth muscle reactivity. For example, tracheal contractions evoked by topical application of histamine in anesthetized guinea pigs were largely reversed by topically applied phentolamine and modestly potentiated by topical phenylephrine. This resembles the pronounced vasodilatation and modest vasoconstriction that these agents produced in our intravital microscopy studies. The effects of phentolamine and phenylephrine on the magnitude and duration of capsaicin-evoked tracheal contractions in vivo provide additional evidence to support our assertions. Importantly, the effects of phentolamine were confirmed with alternative α-adrenergic antagonists and were shown not to be due to a direct action on the tracheal smooth muscle as phentolamine 1) had no effect on baseline cholinergic tone in the trachea, 2) failed to change baseline tension in the trachea of vagotomized animals [i.e., there is no baseline α-adrenergic tone (30)], and 3) failed to alter either histamine- or capsaicin-evoked contractions of the trachealis in vitro (where the vasculature is not perfused). The lack of effect of phenylephrine on histamine and capsaicin responses in vitro is also consistent with our conclusions. Furthermore, the data cannot be explained by an action of α-adrenergic ligands on any populations of airway nerve terminals as this would have been revealed during in vitro experiments or during experiments addressing baseline cholinergic tone. Propranolol and atropine were routinely included in the tracheal perfusate to negate any possible actions of β-adrenoceptors and muscarinic receptors, and previous studies (2, 13) have shown that prejunctional nerve inhibition is mediated by α2- (and not α1-) adrenoceptors (hence phenylephrine and prazosin would not be expected to have an effect on airway nerves).
Careful inspection of the data from these in vivo and in vitro histamine and capsaicin studies may provide additional supporting evidence for our conclusions. The potentiation of capsaicin-evoked responses by phenylephrine in vivo was more pronounced than the effects of the α-adrenergic agonist on histamine-evoked responses in vivo. Furthermore, the effects of phentolamine were pronounced in the in vivo histamine experiments but were absent compared with vehicle in the capsaicin experiments. This may seem at odds, especially in light of the results from our intravital microscopy studies. However, the reversal in the effectiveness of the two α-adrenergic ligands in these distinct experiments may reflect different vasoactive properties of histamine and capsaicin. For example, tracheal/laryngeal capsaicin (but not histamine) may evoke a reflex-mediated decrease in sympathetic tone in the airways (5, 12, 15, 20). Capsaicin, secondary to the release of substance P and other neuropeptides from capsaicin-sensitive sensory nerves, is also well known to produce neurogenic inflammatory response that include extensive vasodilatation (21, 30, 38) and does so when administered to the tracheal lumen (47). Conceivably, vasodilatation of tracheal vessels in the presence of capsaicin in vivo may increase the vasoconstrictor potential of phenylephrine but might limit any additional vasodilatation by phentolamine, perhaps explaining the more pronounced effects of phenylephrine on capsaicin-evoked contractions. Indeed, our immunohistochemical studies show that the tracheal vasculature receives substance P-immunoreactive innervation [which almost certainly arises from capsaicin-sensitive C-fibers in this species; (26, 40)]. We observed (in 2 experiments) that exogenously applied substance P evoked near-maximal dilatation of tracheal arterioles.
In contrast to the results described here and elsewhere with capsaicin, our data would suggest that histamine is not a particularly effective vasodilator of guinea pig tracheal microvessels in vivo. Our intravital microscopy studies were performed in the presence of 10 μM histamine (to mimic the other physiology studies; see methods) and under these conditions the subepithelial vessels possessed near-maximum tone, which was readily reversible by phentolamine. Furthermore, we demonstrated variable effects of histamine in our preparation, producing on average only a modest degree of vasodilation. Consistent with this, both vasoconstrictor and vasodilator effects of histamine in the airways have been reported (36, 46). For example, Okpako (36) reported that histamine perfused via the pulmonary artery of in vitro guinea pig lung preparations evoked a pressor response that could be converted to a depressor response by pretreatment with a H1 receptor antagonist. In these studies neither the pressor nor the depressor responses were altered by propranolol or atropine, suggesting that different histamine receptor subtypes likely mediate vasoconstriction and vosdilatation in this species. H3 receptor activation may in part underlie vasorelaxant effects of histamine via inhibition of sympathetic mediated vascular tone (19). However, given that phenylephrine produced negligible vasoconstriction in the presence of histamine whereas phentolamine effectively reversed vasomotor tone under the same conditions, it seems unlikely that any significant level of H3-mediated sympathoinhibition was present in our studies.
The effects of phentolamine and phenylephrine on airway smooth muscle reactivity are neither specific for the trachea nor specific for exogenously or topically applied bronchoactive agents. Bronchospasm following inhalation of histamine in the presence of phentolamine was substantially less compared with that in the presence of phenylephrine, suggesting that changes in vascular dynamics in the lower bronchial tree produce effects on smooth muscle reactivity similar to those observed in the trachea. We also noted that intravenously administered NKA produced tracheal contractions that were enhanced in the presence of topically applied phentolamine. Similarly, intravenous administration of the histamine receptor (H1) antagonist pyrilamine was more effective at reversing a stable histamine-evoked tracheal contraction when phentolamine was present in the tracheal perfusate. Thus vasoconstriction and vasodilatation of the airway vasculature not only alter airway smooth muscle reactivity to agents that must first traverse the vasculature (such as topically applied agents) but also regulate reactivity to agents that reach the airways via the vasculature. Finally, our experiments with antigen in sensitized animals confirm previous studies suggesting that the effectiveness of endogenously released bronchoconstrictors (such as the mast derived bronchoactive products responsible for antigen-evoke bronchospasm) is also regulated by vascular dynamics.
Physiological significance.
The results of the present study suggest that vasodilatation of the airway vasculature results in increased clearance of substances applied topically to the airway mucosa while increasing delivery of blood-borne substances to the airway smooth muscle. This would explain how phentolamine reduced the potency of topically applied bronchoconstrictors and increased the potency of intravenously administered agents. Conversely, vasoconstriction presumably reduces/slows clearance and reduces vascular delivery to the airways. However, this effect of vascular perfusion may not extend to all regulators of airway smooth muscle tone. For example, the lack of effect of α-adrenergic blockade on baseline cholinergic (parasympathetic) tone would suggest that neurotransmitters such as acetylcholine, released directly onto the muscle at the neuromuscular junction, are protected from the effects of alterations in airway blood flow.
Previous studies also support our hypothesis. For example, reducing tracheal or bronchial perfusion/blood flow in sheep prolongs methacholine-evoked bronchospasm and potentiates antigen-evoked bronchospasm while reducing soluble tracer uptake from the airways (10, 44). Conversely, the vasodilator nitroglycerin elevates mucosal blood flow in sheep and reduces antigen-evoked smooth muscle responses (10). The endogenous vasoconstrictor ANG II has also been shown to potentiate aerosol agonist-induced bronchospasm and antigen-evoked airways hyperresponsiveness (effects that are prevented by AT1 receptor antagonists) (34, 45) in animals, and it is tempting to speculate that this is due to the vasoconstrictor actions of ANG II. A comparable intravenous ANG II-mediated enhancement of responsiveness to inhaled methacholine has been noted in asthmatics. Importantly, however, the same researchers showed that ANG II was without effect on responsiveness to the bronchoconstrictor endothelin, an autacoid that also constricts airway vascular smooth muscle (while methacholine relaxes vascular smooth muscle; 6, 33). Similarly, the loop diuretic furosemide reduces airways reactivity to a variety of stimuli in humans (3, 41), and evokes vasodilatation of airway microvessels in vivo and in vitro, but does not relax airway smooth muscle in vitro (8, 9). Interestingly the nonselective adrenergic agonist epinephrine is more potent and efficacious than the β-adrenergic agonist albuterol at relaxing airway smooth muscle and preventing histamine-induced contractions in vitro, whereas in vivo epinephrine (which would evoke both bronchodilatation and vasoconstriction) has no effect on reactivity while albuterol is acutely effective (1).
Contrary to the hypothesis that blood flow is directly related to particle clearance, Hanafi and colleagues (16) reported that the uptake of soluble tracer particles from an isolated perfused tracheal segment in sheep is inversely related to tracheal arterial perfusion. That is, increasing tracheal perfusion (either pharmacologically by injecting vasoactive drugs into the perfusion circuit at a constant artificial perfusion pressure or mechanically by changing artificial perfusion pressure) reduces tracer uptake by the vasculature, whereas reducing tracheal perfusion enhances uptake (16). These findings may seem paradoxical as elevated blood flow might be expected to “wash out” soluble particles from the airway wall. However, the resultant increase in interstitial volume that would accompany an increase in airway blood flow may hinder particle transport in the airway wall and reduce vascular uptake (16, 43, 50). It is difficult to reconcile this opposing observation with our data. Nevertheless, an increased interstitial fluid volume as a result of vasodilatation would conceivably reduce (dilute) the local concentration of a soluble bronchoactive agent in the airway wall, even if the total number of particles was unchanged. This may have an effect that is comparable to increased solute clearance.
Conclusions.
Tracheobronchial blood flow is an important regulator of soluble particle concentration in the airway wall and hence contributes to the functional responsiveness of the airway smooth muscle. This role probably influences responsiveness to inhaled, locally produced and systemically delivered bronchoactive agents but is unlikely to extend to the regulation of neural bronchomotor tone. These data highlight the role of the airway vasculature in contributing to airway reactivity and may have important implications in disease and/or disease therapy. The results also highlight a novel role for sympathetic nerves in regulating airways reactivity.
GRANTS
This research was funded by a grant awarded to B. J. Canning from the National Institutes of Health (HL-58525). S. B. Mazzone is an NHMRC of Australia RD Wight Fellow (Grant no. 454776).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Baldwin DR, Sivardeen Z, Pavord ID, Knox AJ. Comparison of the effects of salbutamol and adrenaline on airway smooth muscle contractility in vitro and on bronchial reactivity in vivo. Thorax 49: 1103–1108, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai TR, Lam R, Prasad FY. Effects of adrenergic agonists and adenosine on cholinergic neurotransmission in human tracheal smooth muscle. Pulm Pharmacol 1: 193–199, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Bianco S, Pieroni MG, Refini RM, Rottoli L, Sestini P. Protective effect of inhaled furosemide on allergen-induced early and late asthmatic reactions. N Engl J Med 321: 1069–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Brieva J, Wanner A. Adrenergic airway vascular smooth muscle responsiveness in healthy and asthmatic subjects. J Appl Physiol 90: 665–669, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Chalmers GW, Millar EA, Little SA, Shepherd MC, Thomson NC. Effect of infused angiotensin II on the bronchoconstrictor activity of inhaled endothelin-1 in asthma. Chest 115: 352–356, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Corboz MR, Ballard ST, Boyette ST, Taylor AE. Distribution of functional adrenergic receptor subtypes in the microcirculation of rat trachea. Am J Respir Crit Care Med 151: 1589–1596, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Corboz MR, Ballard ST, Inglis SK, Taylor AE. Dilatory effect of furosemide on rat tracheal arterioles and venules. Am J Respir Crit Care Med 156: 478–483, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Corboz MR, Ballard ST, Gao H, Benoit JN, Inglis SK, Taylor AE. Differential effects of furosemide on porcine bronchial arterial and airway smooth muscle. J Appl Physiol 89: 1360–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Csete ME, Chediak AD, Abraham WM, Wanner A. Airway blood flow modifies allergic airway smooth muscle contraction. Am Rev Respir Dis 144: 59–63, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Franco-Cereceda A, Matran R, Alving K, Lundberg JM. Sympathetic vascular control of the laryngeo-tracheal, bronchial and pulmonary circulation in the pig: evidence for non-adrenergic mechanisms involving neuropeptide Y. Acta Physiol Scand 155: 193–204, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Gerber U, Polosa C. Some effects of superior laryngeal nerve stimulation on sympathetic preganglionic neuron firing. Can J Physiol Pharmacol 57: 1073–1081, 1979 [DOI] [PubMed] [Google Scholar]
- 13. Grundstom N, Andersson RG, Wikberg JE. Prejunctional alpha 2 adrenoceptors inhibit contraction of tracheal smooth muscle by inhibiting cholinergic neurotransmission. Life Sci 28: 2981–2986, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Haberberger R, Schemann M, Sann H, Kummer W. Innervation pattern of guinea pig pulmonary vasculature depends on vascular diameter. J Appl Physiol 82: 426–434, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Häbler HJ, Boczek-Funcke A, Michaelis M, Jänig W. Responses of distinct types of sympathetic neuron to stimulation of the superior laryngeal nerve in the cat. J Auton Nerv Syst 66: 97–104, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Hanafi Z, Corfield DR, Webber SE, Widdicombe JG. Tracheal blood flow and luminal clearance of 99mTc-DTPA in sheep. J Appl Physiol 73: 1273–1281, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Haxhiu MA, Chavez JC, Pichiule P, Erokwu B, Dreshaj IA. The excitatory amino acid glutamate mediates reflexly increased tracheal blood flow and airway submucosal gland secretion. Brain Res 883: 77–86, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hennessy E, White S, van der Touw T, Quail A, Porges W, Glenfield P. Control of resting bronchial hemodynamics in the awake dog. Am J Physiol Heart Circ Physiol 265: H649–H660, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Hey JA, del Prado M, Egan RW, Kreutner W, Chapman RW. Inhibition of sympathetic hypertensive responses in the guinea-pig by prejunctional histamine H3-receptors. Br J Pharmacol 107: 347–351, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huangfu DH, Guyenet PG. Sympatholytic response to stimulation of superior laryngeal nerve in rats. Am J Physiol Regul Integr Comp Physiol 260: R290–R297, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Ito T, Takubo T, Hussain S, Martin JG. A peptidergic component to vagally induced tracheal vasodilation in the dog. J Appl Physiol 73: 1102–1107, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Kadowitz PJ, Knight DS, Greenberg S, Hyman AL. Structure and function of the adrenergic nerves in the canine pulmonary vascular bed. Methods Find Exp Clin Pharmacol 3: 347–359, 1981 [PubMed] [Google Scholar]
- 23. Kelly L, Kolbe J, Mitzner W, Spannhake EW, Bromberger-Barnea B, Menkes H. Bronchial blood flow affects recovery from constriction in dog lung periphery. J Appl Physiol 60: 1954–1959, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Kelly LJ, Mitzner W, Spannhake EW, Bromberger-Barnea B, Menkes HA. Pulmonary blood flow affects recovery from constriction in dog lung periphery. J Appl Physiol 60: 1554–1560, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Kesler BS, Mazzone SB, Canning BJ. Nitric oxide-dependent modulation of smooth-muscle tone by airway parasympathetic nerves. Am J Respir Crit Care Med 165: 481–488, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Laitinen LA, Laitinen MA, Widdicombe JG. Dose-related effects of pharmacological mediators on tracheal vascular resistance in dogs. Br J Pharmacol 92: 703–709, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laitinen LA, Laitinen MV, Widdicombe JG. Parasympathetic nervous control of tracheal vascular resistance in the dog. J Physiol 385: 135–146, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim LH, Bochner BS, Wagner EM. Leukocyte recruitment in the airways: an intravital microscopic study of rat tracheal microcirculation. Am J Physiol Lung Cell Mol Physiol 282: L959–L967, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Matran R, Alving K, Martling CR, Lacroix JS, Lundberg JM. Effects of neuropeptides and capsaicin on tracheobronchial blood flow of the pig. Acta Physiol Scand 135: 335–342, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci 99: 91–101, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Mazzone SB, McGovern AE. Na+-K+-2Cl− cotransporters and Cl− channels regulate citric acid cough in guinea pigs. J Appl Physiol 101: 635–643, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Millar EA, Nally JE, Thomson NC. Angiotensin II potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J 8: 1838–1841, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Myou S, Fujimura M, Kurashima K, Tachibana H, Watanabe K, Hirose T. Type 1 angiotensin II receptor antagonism reduces antigen-induced airway reactions. Am J Respir Crit Care Med 162: 45–49, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Oh EJ, Mazzone SB, Canning BJ, Weinreich D. Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol 573: 549–564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okpako DT. A dual action of histamine on guinea-pig lung vessels. Br J Pharmacol 45: 311–321, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pereira A, Mendes E, Ferreira T, Wanner A. Effect of inhaled racemic and (R)-albuterol on airway vascular smooth muscle tone in healthy and asthmatic subjects. Lung 181: 201–211, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Pisarri TE, Coleridge JC, Coleridge HM. Capsaicin-induced bronchial vasodilation in dogs: central and peripheral neural mechanisms. J Appl Physiol 74: 259–266, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Quail A, McIlveen S, Bishop R, McLeod D, Gunther R, Davis J, Talken L, Cottee D, Parsons G, White S. Autonomic control of bronchial blood flow and airway dimensions during strenuous exercise in sheep. Pulm Pharmacol Ther 20: 190–199, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496: 521–530, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robuschi M, Pieroni M, Refini M, Bianco S, Rossoni G, Magni F, Berti F. Prevention of antigen-induced early obstructive reaction by inhaled furosemide in (atopic) subjects with asthma and (actively sensitized) guinea pigs. J Allergy Clin Immunol 85: 10–16, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Wagner EM, Foster WM. Importance of airway blood flow on particle clearance from the lung. J Appl Physiol 81: 1878–1883, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Wagner EM, Foster WM. Interdependence of bronchial circulation and clearance of 99mTc-DTPA from the airway surface. J Appl Physiol 90: 1275–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wagner EM, Mitzner WA. Bronchial circulatory reversal of methacholine-induced airway constriction. J Appl Physiol 69: 1220–1224, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Watanabe K, Myou S, Fujimura M, Tachibana H, Kita T, Nakao S. Importance of the angiotensin type 1 receptor in angiotensin II-induced bronchoconstriction and bronchial hyperresponsiveness in the guinea pig. Exp Lung Res 30: 207–221, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Webber SE, Salonen RO, Widdicombe JG. H1- and H2-receptor characterization in the tracheal circulation of sheep. Br J Pharmacol 95: 551–561, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wells UM, Duneclift S, Widdicombe JG. H2O2 increases sheep tracheal blood flow, permeability, and vascular response to luminal capsaicin. J Appl Physiol 82: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Widdicombe JG. Neural control of airway vasculature and edema. Am Rev Respir Dis 143: S18–S21, 1991 [DOI] [PubMed] [Google Scholar]
- 49. Widdicombe J. New perspectives on basic mechanisms in lung disease. 4. Why are the airways so vascular? Thorax 48: 290–295, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Widdicombe J. The tracheobronchial vasculature: a possible role in asthma. Microcirculation 3: 129–141, 1996 [DOI] [PubMed] [Google Scholar]