Abstract
Mammalian target of rapamycin complex 1 (mTORC1) signaling is crucial for the regulation of protein synthesis. Most of known mTORC1 regulators have been isolated and characterized using cell culture systems, and the physiological roles of these regulators have not been fully tested in vivo. Previously we demonstrated that the insulin (INS) and amino acid (AA)-induced activation of mTORC1 is developmentally regulated in skeletal muscle (Suryawan A et al. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007). The present study aimed to characterize in more detail the effects of the postprandial rise in INS and AA on the activation and abundance of mTORC1 regulators in muscle and how this is modified by development. Overnight fasted 6- and 26-day-old pigs were studied during 1) euinsulinemic-euglycemic-euaminoacidemic conditions (control), 2) euinsulinemic-euglycemic-hyperaminoacidemic clamps (AA), and 3) hyperinsulinemic-euglycemic-euaminoacidemic clamps (INS). INS, but not AA, enhanced the PRAS40 phosphorylation, and this effect was greater in 6- than in 26-day old pigs. Phospholipase D1 (PLD1) abundance and phosphorylation, and the association of PLD1 with Ras homolog enriched in brain (Rheb), were greater in the younger pigs. Neither INS, AA, nor age altered the abundance of Rheb, vacuolar protein sorting 34 (Vps34), or FK506-binding protein 38 (FKBP38). Although INS and AA had no effect, the abundance of ras-related GTP binding B (RagB) and the association of RagB with Raptor were greater in 6- than in 26-day-old pigs. Neither INS, AA, nor age altered AMPK-induced phosphorylation of Raptor. Our results suggest that the enhanced activation of mTORC1 in muscle of neonatal pigs is in part due to regulation by PRAS40, PLD1, and the Rag GTPases.
Keywords: protein synthesis, insulin signaling, amino acid signaling, PLD1, RagB
during the neonatal period, rapid growth is supported by a high rate of protein synthesis (13). The profound accretion of skeletal muscle protein is in part due to the ability of neonatal muscle to markedly increase protein synthesis in response to feeding, a response that declines rapidly with development (8, 11). The postprandial rise in protein synthesis occurs in virtually all tissues of the body in the neonate but is most pronounced in skeletal muscle (8). Although substantial evidence indicates that insulin and amino acids are the major components responsible for the postprandial increase in protein synthesis in skeletal muscle (9, 10), the mode of action by which these anabolic agents regulate protein synthesis is not completely understood.
While the insulin signaling pathway that regulates protein synthesis is well characterized, amino acid signaling toward mRNA translation is only beginning to be revealed. Although most of the information regarding the signaling mechanisms that mediate protein synthesis has been generated from cell culture studies (31), we have shown that in intact animals, insulin and amino acids share common pathways downstream of protein kinase B (PKB) (42). Unlike insulin signaling, which begins by the activation of the insulin receptor, the starting point of amino acid signaling is largely unknown. However, a common consensus is that amino acids such as leucine initiate signaling downstream of PKB and upstream of mammalian target of rapamycin (mTOR) (32).
mTOR is a master kinase that is activated by both insulin and amino acids and is an essential component of the signaling pathway that regulates protein synthesis (33, 50). mTOR exists in two distinct protein complexes: mTORC1, which consists of mTOR, Raptor, and GβL and is rapamycin sensitive; and mTORC2, which consists of mTOR, Rictor, GβL, and mSin1 and is rapamycin insensitive (34). There are four major inputs that regulate the activation of mTOR: nutrients (amino acids), growth factors (insulin), cellular energy levels (AMP:ATP), and stress (hypoxia) (1, 7, 44). The mechanism by which mTOR is activated by insulin has been well studied (Fig. 1). There are three possible mechanisms: 1) insulin-activated PKB directly phosphorylates mTOR on the Ser-2448 residue, resulting in the activation of mTORC1 (28); 2) insulin-activated PKB phosphorylates TSC2, resulting in inactivation of the TCS1/TCS2 complex, followed by mTORC1 activation; and 3) insulin-activated PKB phosphorylates PRAS40, causing its detachment from mTORC1 and activation of mTORC1. However, the molecular mechanisms that regulate amino acid-induced mTORC1 activation remain unclear. Studies have identified several potential amino acid signaling components that may serve as regulators of mTOR activation (Fig. 1).
Fig. 1.
Recent concepts of the insulin and amino acid signaling pathways leading to the activation of mammalian target of rapamycin complex 1 (mTORC1)-induced protein synthesis. IRS1, insulin receptor substrate 1; PI3K, phosphoinositide-3 kinase; PKB, protein kinase B; TSC1/2, tuberous sclerosis complex 1/2; Rheb, Ras homolog enriched in brain; PLD1, phospholipase D1; FKBP38, FK506-binding protein 38; RagA–D, ras-related GTP binding A–D; mVps34, mammalian vacuolar protein sorting 34; 4EBP1, eukaryotic initiation factor 4E binding protein 1; S6K1, S6 kinase 1.
Ras homolog enriched in brain (Rheb) is a key effector upstream of mTORC1 (Fig. 1) and a major regulator of amino acid sensing toward mTORC1 activation (24, 25). Although the exact mechanism by which Rheb regulates mTORC1 activation is unclear, recent studies suggest that FK506-binding protein 38 (FKBP38) and phospholipase D1 (PLD1) are involved in Rheb action (4, 39) Since FKBP38 is an mTORC1 inhibitor that binds to mTORC1, the association of Rheb-GTP to FKBP38 interferes with the ability of FKBP38 to block mTORC1 activation (4). However, several studies also dispute the involvement of FKBP38 as a regulator of mTORC1 (45, 48). A recent in vitro study suggests that PLD1 engages in amino acid-induced mTORC1 activation and Rheb is its upstream regulator (39). According to this observation, Rheb directly binds to PLD1 and stimulates the activity of PLD1. Since PLD1 is an enzyme that generates phosphatidic acid, which has been known to directly activate mTORC1, the role of Rheb in this model is indirect (38). Yet, another model indicates that Rheb directly binds to mTORC1, resulting in mTORC1 activation (39).
Vps34 is a type III phosphoinositide-3 kinase (PI3K; Fig. 1) that has been proposed to participate in the activation of mTORC1 (3, 6, 49). Recent evidence suggests that extracellular amino acids can activate Vps34 via Ca2+, resulting in the activation of mTORC1 (17). Furthermore, association between Vps34 and mTORC1 was detected and seems to be important for mTORC1 activation (3). Another mTORC1 regulator that has emerged recently is the Rag subfamily of GTPases (Rag A–D) (35, 37). The exact mechanism by which these signaling components participate in amino acid-induced mTORC1 activation is unclear; however, the binding between Rag A–D and mTORC1 appears crucial for mTOR activation.
The wealth of information regarding the insulin and amino acid signaling pathways that regulate the activation of mTORC1 leading to mRNA translation has been gained primarily from cancer studies, and while these studies provide crucial information for cancer biology, the results cannot be generalized to all cells or tissue types or to all species. In addition, not all functions of these signaling components can be tested with genetic approaches, and thus their role in vivo under normal physiological conditions is not well understood. Therefore, to better understand the mechanisms involved in the regulation of the mTORC1 pathways in vivo, particularly the role of the postprandial rise in insulin and amino acids in regulating these pathways, in this study we examined the independent effects of amino acids and insulin in the activation of mTORC1 regulators in skeletal muscle of neonatal pigs and how these are modulated by development.
MATERIALS AND METHODS
Animals and housing.
Two cross-bred (Landrace × Yorkshire × Duroc × Hampshire) pregnant sows (Agriculture Headquarters, Texas Dept. of Criminal Justice, Huntsville, TX) were housed in lactation crates in individual, environmentally controlled rooms 2 wk before farrowing. Sows were fed a commercial diet (no. 5084; PMI Feeds, Richmond, IN) and provided water ad libitum. After farrowing, piglets remained with the sow but were not allowed access to the sow's diet. Piglets were anesthetized for sterile catheter insertion into a jugular vein and carotid artery 3 days before study. Piglets from four litters weighing 1.87 ± 0.32 kg and 5.21 ± 0.79 kg were studied at 6 and 26 days of age, respectively, following a 12-h fast. The protocol, previously described in Suryawan et al. (43), was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Treatments and infusion.
Pigs within each litter were randomly assigned to one of three treatment groups (n = 4–6 per age group per treatment group): 1) euinsulinemic-euglycemic-euaminoacidemic conditions (control), 2) euinsulinemic-euglycemic-hyperaminoacidemic clamp (AA), and 3) hyperinsulinemic-euglycemic-euaminoacidemic clamp (INS), as previously described (43). Briefly, blood samples were obtained and immediately analyzed for glucose (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH) and total branched-chain amino acids (BCAA; rapid enzymatic kinetic assay) to establish the basal concentration of blood glucose and plasma BCAA to be used during the clamp procedures. To initiate the clamps, a primed, constant (12 ml/h) infusion of insulin (Eli Lilly, Indianapolis, IN) at 0 or 100 ng·kg−0.66·min−1 was given to achieve plasma insulin concentrations of ∼3 (fasted insulin) or ∼30 μU/ml (fed insulin level). To clamp glucose and amino acids at fasting levels, venous blood samples were acquired every 5 min and immediately analyzed for glucose and BCAA concentrations. The infusion rate of dextrose (Baxter Healthcare, Deerfield, IL) was adjusted to maintain blood glucose concentrations within ±10% of the basal fasting concentrations. Euaminoacidemia was obtained by adjusting the infusion rate of a balanced amino acid mixture (10) to maintain plasma BCAA within 10% of fasting levels. Hyperaminoacidemia was obtained by infusion of a balanced amino acid mixture to raise plasma BCAA concentrations by twofold the fasting level to reproduce the level of amino acids present in the fed state. Blood samples also were taken at intervals for later determination of circulating insulin concentrations.
Tissue protein synthesis in vivo.
Fractional rates of protein synthesis were measured with a flooding dose of l-[4-3H]phenylalanine (Amersham Biosciences, Piscataway, NJ) injected 30 min before the end of the infusion (43). Protein synthesis (Ks, expressed as % protein synthesized in a day) was calculated as: Ks (%/day) = [(Sb/Sa) × (1,440/t)] × 100, where Sb is the specific radioactivity of the protein-bound phenylalanine, Sa is the specific radioactivity of the tissue free phenylalanine for the labeling period determined from the value of the animal at the time of tissue collection and corrected by the linear regression of the blood-specific radioactivity of the animal against time, and t is the time of labeling in minutes. Pigs were killed at 120 min, and longissimus dorsi muscle samples were collected and immediately frozen in liquid nitrogen and stored at −70°C until they were analyzed.
Muscle homogenates.
Frozen longissimus dorsi muscle samples were homogenized and centrifuged at 10,000 g for 10 min at 4°C (43). The protein concentration was determined in the supernatant by the Bradford method (5). Supernatants were diluted in sample buffer and stored at −70°C until analysis.
Protein immunoblot analysis.
Equal amounts (50 μg) of extracted protein were electrophoretically separated in polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA), which was incubated with appropriate primary antibodies followed by appropriate secondary antibodies as previously described (43). Blots were developed using an enhanced chemiluminescence kit (Amersham), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP, Upland, CA). The phosphorylation of each signaling protein was normalized by dividing its values with the values of its protein abundance in the samples. Primary antibodies that were used in the immunoblotting were mTOR (total and Ser-2448; Cell Signaling Technology, Danvers, MA), PRAS40 (total and Thr-246; Cell Signaling Technology), Rheb (Cell Signaling Technology), Raptor (total and Ser-792; Cell Signaling Technology), PLD1 (total, Thr-147 and Ser-561; Cell Signaling Technology), Vps34 (Cell Signaling Technology), FKBP38 (MBL International, Woburn, MA), and Rag B (Abcam, Cambridge, MA).
Analysis of protein-protein interaction.
To determine the association between a signaling component with its alleged partners (Rheb-FKBP38, Rheb-PLD1, Vps34-mTOR, and Rag A/B-Raptor), muscle samples were homogenized in CHAPS buffer as previously described (43). Briefly, the CHAPS buffer (40 mM HEPES, pH 7.5, 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM β-glycerolphosphate, 40 mM NaF, 1.5 mM sodium vanadate, 0.3% CHAPS, 0.1 mM PMSF, 1 mM benzamidine, and 1 mM DTT) was used for tissue homogenization. The homogenate was mixed on a platform rocker for 30 min at 4°C and then centrifuged at 1,000 g centrifugation for 3 min (4°C). The protein concentration was determined in the supernatant by Bradford method (5). The supernatant containing 500 μg of protein was combined with 2 μl of anti-Rheb, -mTOR, or -Raptor antibody and mixed on a platform rocker overnight at 4°C. Following the incubation, the immune complexes were isolated with a goat anti-mouse/donkey anti-rabbit BioMag IgG (PerSeptive Diagnostics, Cambridge, MA) bead slurry. The magnetic bead complexes were collected using a magnetic stand, washed twice with CHAPS buffer, and once in CHAPS buffer containing 200 mM instead of 120 mM NaCl and 60 mM instead of 40 mM HEPES. The precipitates were rinsed with 100 μl of 1× SDS sample buffer and then boiled for 5 min and centrifuged to collect the supernatant. The samples were subjected to SDS-PAGE followed by immunoblotting with anti-FKBP38 antibody, anti-PLD1 antibody, anti-Vps34 antibody, and anti-Rag B antibody. The protein-protein complexes were normalized by the appropriate protein abundance in the precipitates.
Statistics.
Two-way ANOVA was used to assess the effect of insulin, amino acids, age, and their interaction on the abundance and activation of signaling components leading to translation initiation. When significant interactions were detected, the value in each treatment group for each age was compared with the control value by use of t-tests. Probability values of P < 0.05 were considered statistically significant. Data are presented as means ± SE.
RESULTS
Signaling components that regulate mTORC1 activation.
In our previous study, we demonstrated that insulin and amino acids independently stimulate mTORC1 activation and these effects are reduced by development (43). In the present study, we concentrated our efforts on the role of signaling components downstream of PKB/Akt that have been purported to regulate mTORC1 activation.
The phosphorylation of PRAS40 (Thr-246) was robustly increased by insulin, but not by amino acids, and the effect of insulin was greater in 6- than in 26-day-old-pigs (Fig. 2A). AMPK-induced phosphorylation of Raptor, a potent inhibitory signal for mTORC1 activation, was unaffected by insulin or amino acids and did not change with age (Fig. 2B).
Fig. 2.
Phosphorylation (p) of PRAS40 at Thr-246 (A) and phosphorylation of Raptor at Ser-792 (B) in longissimus dorsi muscle of 6- and 26-day-old pigs in response to euinsulinemic-euglycemic-euaminoacidemic condition (control; C), euinsulinemic-euglycemic-hyperaminoacidemic clamps (AA), and hyperinsulinemic-euglycemic-euaminoacidemic clamps (INS). Values of the phosphoproteins were normalized for protein abundance in samples. Values are means ± SE; n = 4–6/treatment. T, total. Means with different letters differ at P < 0.05.
To determine the involvement of PLD1 in the activation of mTORC1 activation, we determined PLD1 abundance, phosphorylation, and association with Rheb. Although neither insulin nor amino acids had any effect, we found that the abundance as well as the association of PLD1 with Rheb were greater in 6- than in 26-day-old-pigs (Fig. 3, A and D, respectively). Furthermore, the phosphorylation of PLD1 at Thr-147 and Ser-561 also decreased with development but were unaffected by insulin and amino acids (Fig. 3, B and C, respectively).
Fig. 3.
PLD1 abundance (A), phosphorylation of PLD1 at Thr-147 (B), phosphorylation of PLD1 at Ser-561 (C), and PLD1-Rheb association (D) in longissimus dorsi muscle of 6- and 26-day-old pigs in response to C, AA, and INS. Values of the phospho-PLD1 were normalized for PLD1 protein abundance in samples. Values for protein complexes were normalized for Rheb content in the immunoprecipitant. Values are means ± SE; n = 4–6/treatment. Means with different letters differ at P < 0.05.
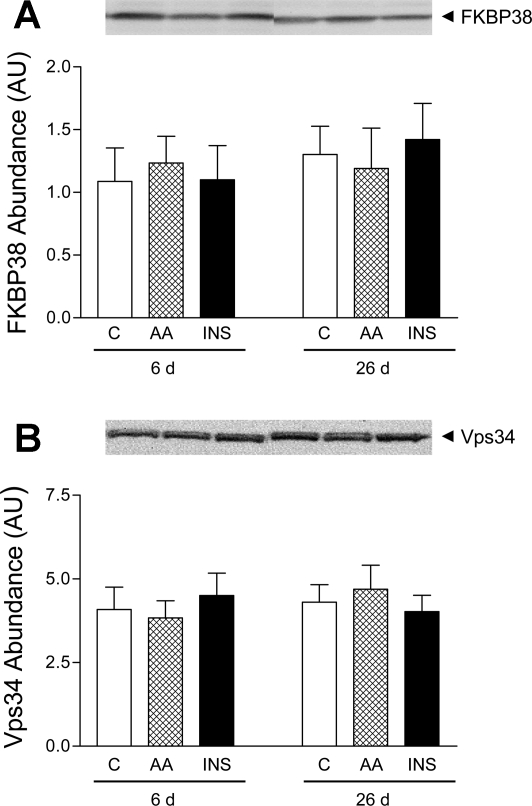
We examined the possible roles of FKBP38 and Vps34 in the activation of mTORC1 in vivo by measuring their abundance and protein-protein interactions (FKBP38-Rheb and Vps34-mTOR). The abundances of both FKBP38 and Vps34 were unaffected by insulin and amino acids and did not change with age (Fig. 4, A and B, respectively). We tried to determine the associations between FKBP38 and Rheb and between Vps34 and mTOR. However, we could not detect any protein-protein interactions of these signaling components.
Fig. 4.
FKBP38 abundance (A) and Vps34 abundance (B) in longissimus dorsi muscle of 6- and 26-day-old pigs in response to C, AA, and INS. Values are means ± SE; n = 4–6/treatment.
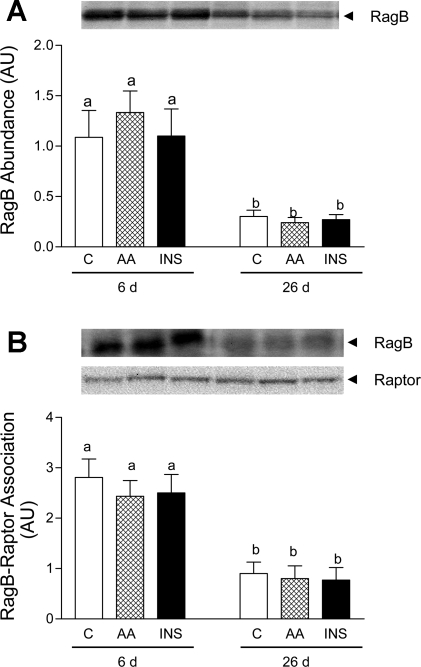
Finally, to examine the possible role of the Rag protein family (A, B, C and D) on mTORC1 activation in vivo, we measured Rag B abundance and the association of RagB with Raptor. Even though we found that neither insulin nor amino acids affected Rag B abundance or RagB-Raptor association, both of these parameters were significantly higher in 6- than in 26-day-old-pigs (Fig. 5, A and B, respectively).
Fig. 5.
RagB abundance (A) and RagB-Raptor association (B) in longissimus dorsi muscle of 6- and 26-day-old pigs in response to C, AA, and INS. Values for protein complexes were normalized for Raptor content in the immunoprecipitant. Values are means ± SE; n = 4–6/treatment. Means with different letters differ at P < 0.05.
DISCUSSION
Feeding is one of the multiple factors that regulates the growth of skeletal muscle in neonates (9). In previous studies (12, 41), we showed that feeding stimulates skeletal muscle protein synthesis through the activation of signaling pathways leading to mRNA translation and this effect decreases with development. Recently, we shown that the fractional rate of protein synthesis in the longissimus dorsi, a skeletal muscle that primarily contains fast-twitch glycolytic fibers, was independently stimulated by insulin and amino acids and these effects were significantly greater in 6- than in 26-day-old-pigs (43). Furthermore, mTOR phosphorylation (Ser-2448), an indicator of mTORC1 activation and an important positive regulator of protein synthesis, was independently activated by insulin and amino acids and this activation was reduced with development. However, the signaling components that regulate mTORC1 in vivo have not been elucidated.
The master kinase, mTOR, plays a crucial role in physiological processes including cell growth (2). Scientists have studied signaling factors that regulate the activation of mTORC1 for some time using simple and reliable methods such as in vitro and cell culture systems. However, these methods inherently possess a number of limitations including unphysiological conditions that probably are not feasible in vivo and the use of immortal cell lines. Furthermore, only some of the signaling factors have been tested genetically. Thus, to more fully understand the regulation of mTORC1 signaling, it is important to study intact animals under normal physiological conditions. In this study, we report novel findings regarding signaling factors that regulate the activation of mTORC1 in skeletal muscle of neonatal pigs, and how these factors are affected by insulin, amino acids, and age.
PRAS40 has been identified as a potent negative regulator of mTORC1 when it binds to the complex, and in response to insulin, PRAS40 dissociates from mTORC1 (14, 29, 46). The interaction of PRAS40 with mTORC1 is induced under conditions that inhibit mTORC1 activation such as nutrient deprivation (14). Furthermore, mTOR and PKB can phosphorylate PRAS40 at Ser-221 and Thr-246, respectively, causing the dissociation of PRAS40 from the complex and the activation of mTORC1 (47). In this study, we found that insulin, but not amino acids, enhanced PRAS40 phosphorylation at Thr-246 (a PKB phosphorylation site), and this effect was reduced with development. Interestingly, a recent study by Sanchez-Canedo et al. (36) revealed that leucine induces the phosphorylation of PRAS40 at this site in a PDK-1-dependent manner in mature heart, thus undermining the notion that Thr-246 is an exclusive target for PKB.
Genetic studies in the mouse demonstrated that Raptor plays an essential role in the activation of mTORC1 (16). Although the exact mechanism by which Raptor regulates mTORC1 activation is not fully understood, the consensus is that it has to bind to mTORC1 to activate this kinase (19, 51). In support of this, we (40) and others (30) have found that the rapamycin-induced reduction in mTORC1 activation results in the dissociation of Raptor from the mTORC1 complex. Recently, Raptor was reported to be phosphorylated by AMPK at Ser-792 causing the inhibition of mTORC1 (18). In our present study, we found that Raptor phosphorylation (Ser-792) was not affected by insulin or amino acid stimulation and did not change with age. This is consistent with our previous finding that AMPK activation is not affected by feeding status (20, 40).
PLD1 has been shown to activate mTORC1 through the generation of phosphatidic acid (38, 39). A recent study in HEK 293 cells found that PLD1 was involved in the amino acid-induced activation of mTORC1 (39). A mechanism proposed for the activation of mTORC1 by PLD1 involves the binding of Rheb to PLD1 (39). The phosphorylation of PLD1 at Thr-147 and Ser-561 is partly responsible for the activation of this enzyme (23). Kim et al. (23) demonstrated that mutation at Thr-147 and Ser-561 residues significantly impaired the activation of PLD1. They further showed that protein kinase Cα (PKCα) is responsible for the phosphorylation of these residues (23). Insulin can activate PKC isoforms including PKCα (15); however, whether amino acids can do similar tasks is unknown. In our laboratory, we have not determined the effect of insulin, amino acids, or development on the abundance and the activation of PKC. In the present study, we found that the abundance of PLD, the formation of the PLD1-Rheb complex, and the phosphorylation of PLD1 on Thr-147 and Ser-561 were significantly higher in 6- than in 26-day-old pigs. The greater PLD1 phosphorylation and abundance of the PLD1-Rheb complex in 6- than in 26-day-old were positively associated with the developmental change in the activation of mTORC1. This suggests that younger pigs have a higher PLD activation compared with their older counterparts. However, further studies are needed to evaluate the effects of insulin and amino acids on the activation of PLD1 in whole animals.
Another proposed mechanism by which Rheb regulates the activation of mTORC1 is by binding to FKBP38, a potent mTORC1 inhibitor (4, 26). Rheb association with FKBP38 is thought to allow the removal of this inhibitor from mTORC1 (4). However, this model was challenged by several investigators who found that FKBP38 is not involved in mTORC1 activation (45, 48). In our present study, we found no developmental regulation of FKBP38 abundance and we were unable to detect an interaction of FKBP38 with Rheb. Thus our data support the notion that FKBP38 does not play an important role in mTORC1 activation, although further in vivo studies are warranted.
Several reports suggest an important role of Vps34, a type III PI 3-kinase, in mediating amino acid-induced activation of mTORC1 (3, 6, 49). It was demonstrated that in order for Vps34 to activate mTORC1, it binds to the complex in a Ca2+-dependent mechanism (17). In the present study, the Vps34 protein abundance was unaffected by age and an interaction of Vps34 with mTOR complex was not detectable. To this point, we are not aware of any in vivo finding of a Vsp34-mTOR association. Interestingly, a genetic study using the fruitfly (Drosophila) demonstrated that deletion of the Vps34 gene has no effect on dTORC1 activation (21). It was speculated that Vps34 participates in amino acid signaling to TORC1 only in vertebrate and mammalian cells (3, 49). A recent study in fasting rats showed that an exercise-induced increase in intramuscular leucine was associated with an increase in Vps34 activity and the activation of mTORC1 (27). The possible role of Vps34 in the amino acid-induced mTORC1 activation after feeding requires further study.
Recently, two separate investigators identified the Rag subfamily of GTPases (Rag A, B, C, and D) as new components in the amino acid-dependent pathway that regulates mTORC1 activation (22, 35). Although the exact mechanism is unclear, Sancak et al. (35) proposed that amino acids, by promoting Rag A/B GTP charging, enhance Rag interaction with Raptor and the recruitment of mTORC1 to the (rab-7-containing) membrane compartment that contains the mTORC1 proximal activator, Rheb, and that this process is required for mTORC1 activation. Our results show for the first time that RagB abundance and its association with Raptor are greater in 6-day-old pigs compared with older pigs. The absence of an amino acid effect on RagB-Raptor association may be due to the timing of the harvest of the skeletal muscle during the in vivo clamp procedure.
Perspectives
Our study presented herein is likely to be one of only a handful of studies on the regulation of mTORC1 activation using whole animals. Of the several mTORC1 regulators we studied, PRAS40, Rheb, PLD1, and Rag proteins were found to be significant players for mTORC1 activation in skeletal muscle of neonatal pigs. The physiological conditions of this study do not support the previous in vitro reports of an effect of amino acids on mTORC1 activation by FKBP38 and Vps34. Interestingly, our previous study (40) also showed that while S6K1 and 4EBP1 phosphorylation, indicators of mTORC1 activation, were increased by amino acids, AMPK and TSC1/2 were unaffected. Because the exact mechanism by which anabolic factors, such as amino acids, regulate mTORC1 activation is not fully understood, further in vivo studies are warranted.
GRANTS
Funding for this research was received from National Institutes of Health Grant R01-AR-44474 and USDA/ARS Cooperative Agreement no. 58-6250-6-001.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank J. C. Stubblefield for care of the animals and L. F. Weiser for secretarial assistance.
This work is a publication of the U.S. Dept. of Agriculture, Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does the mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1. Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278: 29655–29660, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care 8: 67–72, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 318: 977–980, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 6. Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25: 6347–6360, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Denne SC, Kalhan SC. Leucine metabolism in human newborns. Am J Physiol Endocrinol Metab 253: E608–E615, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem 282: 24514–24524, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol 20: 6323–6333, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7: 456–465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Jeyapalan AS, Orellana RA, Suryawan A, O'Connor PM, Nguyen HV, Escobar J, Frank JW, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through an. Am J Physiol Endocrinol Metab 293: E595–E603, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181: 655–666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y, Han JM, Park JB, Lee SD, Oh YS, Chung C, Lee TG, Kim JH, Park SK, Yoo JS, Suh PG, Ryu SH. Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites. Biochemistry 38: 10344–10351, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Long X, Lin Y, Ortiz-Vega S, Busch S, Avruch J. The Rheb switch 2 segment is critical for signaling to target of rapamycin complex 1. J Biol Chem 282: 18542–18551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 280: 23433–23436, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ma D, Bai X, Guo S, Jiang Y. The switch I region of Rheb is critical for its interaction with FKBP38. J Biol Chem 283: 25963–25970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol 587: 253–260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mothe-Satney I, Gautier N, Hinault C, Lawrence JC, Jr, Van OE. In rat hepatocytes glucagon increases mammalian target of rapamycin phosphorylation on serine 2448 but antagonizes the phosphorylation of its downstream targets induced by insulin and amino acids. J Biol Chem 279: 42628–42637, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem 282: 20329–20339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9: 359–366, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem 269: 5338–5349, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans 35: 1187–1190, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Proud CG. Cell signaling. mTOR, unleashed. Science 318: 926–927, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6: 729–734, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanchez Canedo C, Demeulder B, Ginion A, Bayascas JR, Balligand JL, Alessi DR, Vanoverschelde JL, Beauloye C, Hue L, Bertrand L. Activation of the cardiac mTOR/p70S6K pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am J Physiol Endocrinol Metab 298: E761–E769, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Shaw RJ. mTOR signaling: RAG GTPases transmit the amino acid signal. Trends Biochem Sci 33: 565–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun Y, Chen J. mTOR signaling: PLD takes center stage. Cell Cycle 7: 3118–3123, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci USA 105: 8286–8291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295: E868–E875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281: E908–E915, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Suryawan A, O'Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134: 24–30, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun 313: 443–446, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Uhlenbrock K, Weiwad M, Wetzker R, Fischer G, Wittinghofer A, Rubio I. Reassessment of the role of FKBP38 in the Rheb/mTORC1 pathway. FEBS Lett 583: 965–970, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Vander HE, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem 283: 15619–15627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem 283: 30482–30492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan Y, Backer JM. Regulation of class III (Vps34) PI3Ks. Biochem Soc Trans 35: 239–241, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Yang X, Yang C, Farberman A, Rideout TC, de Lange CF, France J, Fan MZ. The mTOR-signaling pathway in regulating metabolism and growth. J Anim Sci 86, Suppl 14: :E36–E50, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Yonezawa K, Tokunaga C, Oshiro N, Yoshino K. Raptor, a binding partner of target of rapamycin. Biochem Biophys Res Commun 313: 437–441, 2004 [DOI] [PubMed] [Google Scholar]