Abstract
The mechanisms by which heat stress impairs the control of blood pressure leading to compromised orthostatic tolerance are not thoroughly understood. A possible mechanism may be an attenuated blood pressure response to a given increase in sympathetic activity. This study tested the hypothesis that whole body heating attenuates the blood pressure response to a non-baroreflex-mediated sympathoexcitatory stimulus. Ten healthy subjects were instrumented for the measurement of integrated muscle sympathetic nerve activity (MSNA), mean arterial blood pressure (MAP), heart rate, sweat rate, and forearm skin blood flow. Subjects were exposed to a cold pressor test (CPT) by immersing a hand in an ice water slurry for 3 min while otherwise normothermic and while heat stressed (i.e., increase core temperature ∼0.7°C via water-perfused suit). Mean responses from the final minute of the CPT were evaluated. In both thermal conditions CPT induced significant increases in MSNA and MAP without altering heart rate. Although the increase in MSNA to the CPT was similar between thermal conditions (normothermia: Δ14.0 ± 2.6; heat stress: Δ19.1 ± 2.6 bursts/min; P = 0.09), the accompanying increase in MAP was attenuated when subjects were heat stressed (normothermia: Δ25.6 ± 2.3, heat stress: Δ13.4 ± 3.0 mmHg; P < 0.001). The results demonstrate that heat stress can attenuate the pressor response to a sympathoexcitatory stimulus.
Keywords: hyperthermia, autonomic nervous system, cardiovascular, blood pressure regulation, cold pressor test
the control of arterial blood pressure is severely compromised in heat-stressed humans, best depicted by profound reductions in orthostatic tolerance (1, 20, 36). The mechanism(s) responsible for this occurrence are not entirely understood. We and others have shown that whole body heating does not impair arterial baroreflex control of heart rate (5, 8, 37, 38) or muscle sympathetic nerve activity (MSNA) (8, 16). In fact, the prevailing data suggest that heat stress heightens MSNA responsiveness to baroreceptor unloading, even when controlling for the greater hypotensive challenge that occurs in the heat-stressed human (9, 16). Despite this, the change in arterial blood pressure to carotid baroreceptor perturbations is impaired in the heat-stressed human (5), suggestive of impaired baroreflex control of arterial blood pressure. The apparent disassociation between normal, and perhaps even elevated, baroreceptor control of heart rate and MSNA compared with impaired arterial blood pressure responses to carotid baroreceptor perturbations during heat stress is perplexing.
To counter hypotension associated with an orthostatic challenge, the sympathetic nervous system is greatly activated via baroreflexes, vestibular inputs, etc. (26). Sympathetically mediated vasoconstriction in muscular, renal, and splanchnic beds are the predominate mechanism for defending against decreases in arterial pressure during orthostatic or hemorrhagic challenges. If the effectiveness of this pathway is reduced, the control of arterial blood pressure could be compromised, even with heightened sympathetic activity. For example, Levine et al. (19) raised the possibility that a disassociation between increases in sympathetic activity and vascular resistance could be a mechanism for orthostatic intolerance after microgravity exposure. A similar mechanism may contribute to compromised blood pressure control in heat-stressed humans. Although untested, it may be that a given increase in sympathetic neural activity induces less of an increase in arterial blood pressure when the individual is heat stressed. In support of this hypothesis, the elevation in arterial blood pressure and vascular resistance to constant and bolus infusions of adrenergic agonists are impaired in heat-stressed anesthetized (17, 21) and conscious (22) rats. In humans, both whole body heating and local surface heating attenuate cutaneous α-adrenergic vasoconstrictor responsiveness (34), whereas the elevation in arterial blood pressure to systemic infusions of the α1-adrenergic agonist phenylephrine are impaired during whole body heat stress (11).
To identify whether heat stress affects the relationship between sympathetic neural responses and corresponding changes in arterial blood pressure, a perturbation must be applied that changes arterial blood pressure primarily via increases in vascular resistance, with little to no effect on cardiac output, since blood pressure is the product of cardiac output and systemic vascular resistance. One such perturbation is the cold pressor test (CPT). The CPT induces large increases in MSNA via nonbaroreflex mechanisms (12, 18, 31), as well as vasoconstrictions in the splanchnic (3, 4, 14) and renal (2, 24) vascular beds. Conversely, neither the CPT (12) nor orthostatic stress (10, 32, 35) increases skin sympathetic nerve activity. Given that heart rate during the later period of the CPT is not different from pre-CPT baseline (31), coupled with no changes in stroke volume and thus cardiac output during the CPT (33), the accompanying changes in blood pressure are entirely due to increases in vascular resistance. Therefore, using the CPT as the stimulus, we tested the hypothesis that heat stress impairs the elevation in arterial blood pressure to a non-baroreflex-mediated sympathoexcitatory stimulus.
METHODS
Subjects.
Ten healthy volunteers (7 men, 3 women) participated in this study. The average age was (mean ± SD) 34 ± 7 yr, and all were of normal height (173 ± 11 cm) and weight (75 ± 12 kg). The volunteers were normotensive (supine blood pressures <140/90 mmHg), were not taking medications, and had no known cardiovascular diseases. The volunteers refrained from caffeine, alcohol, and intensive exercise 24 h before the study. This study was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital of Dallas, and a written informed consent was obtained from each volunteer.
Measurements.
Internal temperature (Tcore) was obtained from intestinal temperature via a telemetric temperature pill (HQ, Palmetto, FL) that was swallowed by each volunteer on arrival at the laboratory, resulting in the pill being in the individual for at least 1.5 h before normothermic baseline data collection. Mean skin temperature (Tsk) was obtained from the weighted electrical average of six thermocouples attached to the skin (29). Each volunteer was dressed in a tube-lined suit that permitted the control of Tsk by changing the temperature of the water perfusing the suit. Skin blood flow (SkBF) was indexed from the dorsal forearm, using laser-Doppler flowmetry (Perimed, North Royalton, OH), of the contralateral arm relative to the one used for the CPT. Forearm sweat rate was measured adjacent to this area via capacitance hygrometry (Vaisala, Woburn, MA) using the ventilated capsule method (7). This area of forearm skin was not covered by the water-perfused suit.
Beat-by-beat arterial blood pressure was recorded from a finger (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands) with resting values verified by auscultation of the brachial artery (SunTech, Medical Instruments, Raleigh, NC). Heart rate was monitored from the electrocardiogram interfaced with a cardiotachometer (1,000-Hz sampling rate; CWE, Ardmore, PA). Respiratory frequency was monitored using piezoelectric pneumography. Multifiber recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The signal was amplified, filtered with a bandwidth of 500–5,000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). The recording electrode was adjusted until a site was found in which sympathetic bursts were clearly identified using previously established criteria (30).
Protocols.
Studies were conducted in a temperature-controlled room (25 ± 1°C). While normothermic, after the volunteers rested quietly in the supine position for 5 min during which baseline thermal and hemodynamic data were obtained, a CPT was performed by immersing a hand to the wrist in an ice water slurry for 3 min. The ice water slurry was stirred throughout the CPT. Subjects were instructed to remain relaxed, breathe normally, and avoid Valsalva-like maneuvers during hand immersion. After normothermic data collection was complete, Tsk was increased to ∼38°C by perfusing the tube-lined suit with 46°C water until an increase in Tcore of ∼ 0.7°C was achieved. The temperature of the water perfusing the suit was then slightly reduced to attenuate further increases in Tcore. Under this condition, 2 min of baseline thermal and hemodynamic variables was again obtained for the pre-CPT heat stress baseline; thereafter, the 3-min CPT was repeated.
Data analysis.
Data were sampled at 200 Hz through a commercial data-acquisition system (Biopac System, Santa Barbara, CA). MSNA bursts were first identified in real time by visual inspection of data plotted on the chart recorder, coupled with the burst sound from the audio-amplifier. These bursts were further evaluated via a computer software program that identified bursts based on fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (6, 8). Integrated MSNA in both thermal conditions was normalized by assigning a value of 100 to the mean amplitude of the largest 10% of the bursts during the 5-min normothermic baseline period (15). Normalization of the MSNA signal was performed to reduce variability between volunteers attributed to factors including needle placement and signal amplification. Total MSNA was identified from burst areas of the integrated neurogram. Mean arterial blood pressure (MAP) and heart rate were obtained from the arterial blood pressure waveform and the electrocardiogram, respectively. Cutaneous vascular conductance (CVC) was indexed by dividing laser-Doppler flux values by MAP.
Statistical analysis.
Mean values for the normothermic and heat stress periods (pre-CPT), and the last minute of the CPT in both thermal conditions, were used in the analysis of the CPT responses. Statistical analyses were performed using commercially available software (SigmaStat 3.5, SPSS). The effects of the whole body heating (normothermic vs. heat stress) on the responses to CPT (pre-CPT vs. CPT) were evaluated via a two-way repeated-measures ANOVA, followed by multiple comparison post hoc (Tukey) analyses where appropriate. Furthermore, the change in the response to the CPT (i.e., the delta responses to the CPT from the pre-CPT period) was evaluated between thermal conditions via a paired t-test. All values are reported as means ± SE. P values of <0.05 were considered statistically significant.
RESULTS
Appropriate increases in Tsk, Tcore, SkBF, and sweat rate indicate that the volunteers were adequately heat stressed (Table 1). Whole body heating increased resting heart rate and MSNA, while MAP was not significantly changed (Table 1).
Table 1.
Thermal and hemodynamic responses before the cold pressor test
| Normothermia | Heat Stress | P | |
|---|---|---|---|
| Internal temperature, °C | 37.0 ± 0.1 | 37.7 ± 0.1* | <0.001 |
| Mean skin temperature, °C | 34.5 ± 0.2 | 38.0 ± 0.2* | <0.001 |
| Sweat rate, mg·cm−2·min−1 | 0.53 ± 0.09* | <0.001 | |
| SkBF, % of baseline | 100 | 504 ± 71* | <0.001 |
| SBP, mmHg | 120 ± 4 | 126 ± 4 | 0.14 |
| DBP, mmHg | 75 ± 2 | 66 ± 2* | 0.01 |
| MAP, mmHg | 91 ± 3 | 86 ± 2 | 0.14 |
| Heart rate, beats/min | 61 ± 3 | 85 ± 4* | <0.001 |
| MSNA, bursts/min | 17 ± 2 | 30 ± 3* | <0.001 |
| MSNA, units/min | 275 ± 29 | 436 ± 31* | <0.001 |
| Respiratory rate, cycles/min | 16.2 ± 1.0 | 16.1 ± 1.3 | 0.91 |
Data are mean baseline values before the cold pressor test (CPT) in both thermal conditions. Mean arterial blood pressure (MAP) was calculated as two-thirds diastolic blood pressure (DBP) plus one-third systolic blood pressure (SBP), measured by auscultation of the brachial artery. Sweat rate is reported as a change in normothermic baseline. Skin blood flow (SkBF) was expressed as percentage of normothermic baseline. MSNA: muscle sympathetic nerve activity.
Significantly different from normothermia (P < 0.01).
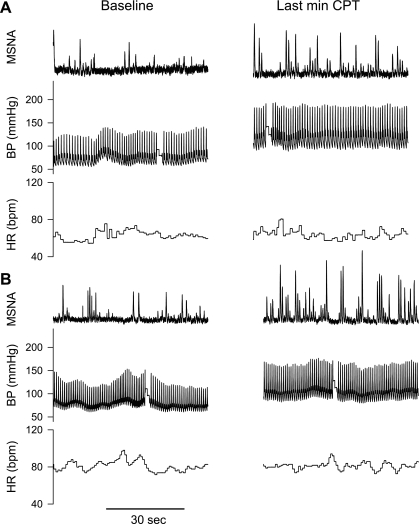
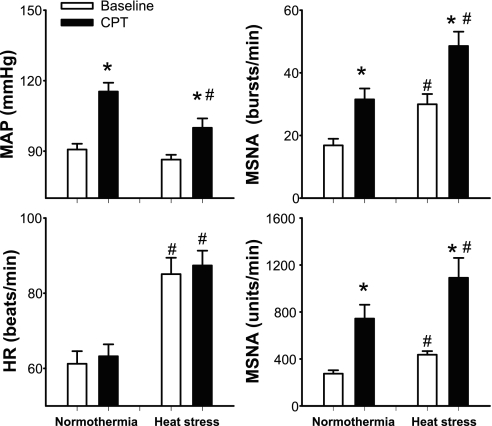
Neither Tsk nor Tcore significantly changed during CPT (Table 2), regardless of the thermal condition. Representative recordings of MSNA, blood pressure, and heart rate during CPT in normothermic and heat-stress conditions are shown in Fig. 1. In both thermal conditions the CPT caused significant increases in MSNA and MAP (Fig. 2), while heart rates were not different from the respective pre-CPT baselines (P = 0.37 for normothermia; P = 0.35 for heat stress). After whole body heating, the increase in MAP during the CPT was ∼50% less than that in the normothermic conditions (normothermia: Δ26 ± 2; heat stress: Δ13 ± 3 mmHg; P < 0.001). Interestingly, the CPT-induced increase in MSNA burst frequency (bursts/min) was similar between thermal conditions (normothermia: Δ14.0 ± 2.6; heat stress: Δ19.1 ± 2.6 bursts/min; P = 0.09), while the increase in MSNA total activity was greater during heat stress (normothermia: Δ458 ± 107; heat stress: Δ655 ± 146 units/min, P = 0.02). Finally, although CPT did not significantly change skin blood flow in either thermal conditions, during heat stress CVC decreased, while only a tendency for a reduction in this variable was observed while subjects were normothermic (Table 2).
Table 2.
Thermal and associated efferent responses to the cold pressor test in normothermic and heat stress conditions
| Normothermia |
Heat Stress |
Main Effect: CPT | Main Effect: Thermal | Interaction | |||
|---|---|---|---|---|---|---|---|
| Baseline | CPT | Baseline | CPT | ||||
| Tcore, °C | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.7 ± 0.1† | 37.8 ± 0.1† | 0.35 | <0.001 | 0.11 |
| Tsk, °C | 34.5 ± 0.2 | 34.5 ± 0.1 | 38.0 ± 0.2† | 37.9 ± 0.2† | 0.70 | <0.001 | 0.63 |
| SR, mg·cm−2·min−1 | 0.53 ± 0.09† | 0.52 ± 0.09† | 0.96 | <0.001 | 0.10 | ||
| SkBF, % | 100 | 94 ± 4 | 504 ± 71† | 506 ± 82† | 0.97 | <0.001 | 0.64 |
| CVC, % | 100 | 72 ± 3 | 500 ± 76† | 427 ± 74*† | <0.001 | <0.001 | 0.02 |
Data are mean baseline values before the CPT (Baseline) and the last minute of the CPT (CPT) in both thermal conditions. SkBF and cutaneous vascular conductance (CVC) are expressed as percentage of the respective normothermic baseline. Tcore, internal temperature; Tsk, mean skin temperature. Sweat rate (SR) is reported as a change from normothermic baseline.
Significant difference from baseline for the indicated thermal condition.
Significant difference from the respective normothermic condition.
Fig. 1.
Representative tracings of muscle sympathetic nerve activity (MSNA), arterial blood pressure (BP), and heart rate (HR) during a cold pressor test (CPT). A: normothermic condition. B: heat stress condition.
Fig. 2.
Mean arterial blood pressure (MAP), HR, and MSNA before (baseline) and during the last minute of the CPT in normothermic and heat stress conditions. *Significantly different from the respective baseline (P < 0.05). #Significantly different from normothermia (P < 0.05).
DISCUSSION
The main finding of this study is that whole body heating attenuates the pressor response to a perturbation that causes similar (or a greater) increases in MSNA. Although the precise mechanism responsible for the disconnect between sympathetic and blood pressure responses during heat stress are unknown, this phenomenon may directly contribute to orthostatic intolerance in heat-stressed individuals, given the importance of increases in vascular resistance for the control of blood pressure during orthostatic stress (19).
In a prior report (7), we showed that fatiguing isometric handgrip exercise while subjects were heat stressed evoked greater increases in MSNA (normothermia vs. heat stress: 16.6 vs. 32.1 bursts/min), heart rate (19.6 vs. 30.5 beats/min), and blood pressure (28.7 vs. 34.8 mmHg) than when subjects were normothermic. Because this stimulus (exercise) evokes increases in heart rate and stroke volume, and thus cardiac output (25, 28), the blood pressure increase was not solely due to increases in vascular resistance. Given those findings, a stimulus was sought that increased arterial blood pressure primarily via increases in vascular resistance (i.e., the CPT) to specifically investigate the effects of heat stress on these responses.
It is widely recognized that the CPT increases sympathetic activity and blood pressure in normothermic individuals (31). The elevation in blood pressure is primarily caused by increases in vascular resistance given that cardiac output does not change during the later period of the CPT (31, 33), and the increase in MSNA during the CPT is correlated with the increase in MAP (31). Given this unique characteristic of the CPT, it is an ideal perturbation to evaluate the effects of heat stress on vascular resistance-derived increases in arterial blood pressure. To that end, the primary findings of the present study are that the elevation in MAP to the CPT while subjects were heat stressed was significantly attenuated despite comparable, or perhaps elevated, increases in MSNA, relative to when the subjects were normothermic.
An important concern with the present study is whether the CPT stimulus was equivalent between normothermic and heat stress trials. Although while heat stressed the skin temperature of the hand immersed in the stirred ice slurry is likely higher during the initial period of the CPT, it is expected that such differences would be minimal to nonexistent during the last minute of the CPT between thermal conditions. Thus the noxious stimulus that is primarily responsible for the sympathoexcitatory response (12, 18) is expected to be identical regardless of the subjects' thermal condition. But perhaps the strongest evidence that the CPT afferent stimulus was not attenuated by the heat stress was similar (when expressed as burst rate), or greater (when expressed as total activity), increases in MSNA during the CPT while heat stressed. Although the mechanism(s) for the greater MSNA total activity response is unknown, it is unlikely that an attenuated blood pressure response to the CPT during whole body heating was due to attenuated afferent or efferent neural activation.
The mechanism(s) responsible for the observed attenuated presser response is not known but can be speculated on. One possibility is that heat stress impairs the transduction of the neural signal to postjunctional events that is responsible for increases in vascular resistance. In rats, the elevation in blood pressure to α-adrenergic agonists was attenuated when they were hyperthermic (17, 21, 22). However, in those studies attenuated vasoconstrictor responses were observed only after internal temperature was ≥41°C, which is in contrast to the moderate increase in internal temperature (i.e. ∼0.7°C) in the present study. In humans, the vasoconstrictor responses to local administration of norepinephrine in forearm skin were significantly attenuated during both local heating and whole body heating, the latter via a water-perfused suit (34). Furthermore, the blood pressure elevation to systemic infusions of the α1-agonist phenylephrine was attenuated in heat-stressed humans (11). In that study (11), it could not be identified whether the attenuated blood pressure response was solely due to attenuated cutaneous vasoconstriction (34) or whether heat stress attenuated vasoconstrictor responses in other beds (i.e., splanchnic and muscle vascular beds). Moreover, the effects of heat stress in possibly altering cardiac output responses secondary to phenylephrine-induced elevations in arterial blood pressure could not be accounted for in that study (11).
The CPT increases MSNA, along with splanchnic (3, 4, 14) and renal (2, 24) vasoconstriction, while not changing skin sympathetic nerve activity (12). Consistent with the hypothesis of generalized sympatholytic properties of heat stress, the present data raise the possibility that postsynaptic α-adrenergic mediated vasoconstriction in muscle, and perhaps other vascular beds, may be impaired in heat-stressed humans. Impaired vasoconstrictor responsiveness in these vascular beds will likely attenuate the elevation in vascular resistance while individuals are heat stressed with the potential of compromising blood pressure control.
Skin blood flow increased ∼5-fold due to whole body heating. During heat stress, upwards to 50% of cardiac output is directed toward the skin (27). This, coupled with an absence of an increase in skin sympathetic nerve activity to the CPT (12), raises other possibilities for the observed attenuated increases in arterial blood pressure during the CPT. That is, with such a large fraction of systemic vascular conductance in the cutaneous bed during heat stress (27), the effectiveness of increases in vascular resistance from noncutaneous beds would be minimized relative to when subjects are normothermic. Put another way, with so much of cardiac output going to the skin, increases in a noncutaneous vascular resistance to the CPT may not be sufficient to increase systemic arterial blood pressure to the same extent relative to that which occurs when subjects are normothermic and cutaneous vascular conductance is very low.
Given large increases in the fraction of cardiac output going to the skin, control of cutaneous vascular conductance becomes more important in the regulation of blood pressure when subjects are heat stressed relative to normothermic. It is interesting to note decreases in cutaneous vascular conductance were observed during the heat stress CPT (see Table 2), although this response was small relative to the capacity by which the cutaneous vasculature could constrict to this perturbation. The mechanism(s) for this reduction in cutaneous vascular conductance, given an absence of an increase in skin sympathetic nerve activity during the CPT (12), remains unclear. Possible explanations for these differing responses include difference in skin sympathetic activity measured to the foot by Fagius et al. (12) relative to forearm skin sympathetic nerve activity, circulating catecholamines induced by the CPT (31), and/or withdrawal of active cutaneous vasodilator activity that may not be detected in the integrated skin sympathetic nerve signal. Moreover, a myogenic response associated with increases in arterial pressure (23) may also contribute to the decrease in cutaneous vascular conductance. Regardless of the mechanism, this reduction in cutaneous vascular conductance alone was insufficient to compensate for otherwise attenuated increases in vascular resistance during the CPT while heat stressed, resulting in less of an increase in arterial blood pressure.
When taken together, either (or both) of the aforementioned mechanisms could contribute to the attenuated pressor response during the CPT while subjects are heat stressed, despite similar or greater increases in MSNA. Regardless of the mechanisms, the present data suggest a “systemic” disassociation between sympathetic activity and the corresponding changes in arterial blood pressure. The balance, or lack thereof, between increases in sympathetic activity and associated increases in systemic vascular resistance may be a major determinant in the etiology of compromised blood pressure control during heat stress.
Limitations to the interpretation of the data.
The CPT was first performed when subjects were normothermic and this was followed by a repeated CPT after whole body heating. This approach raises the possibility of an order effect, despite the second CPT being performed 40–60 min after the first. Although intra-individual variability in responses to repeated CPT were identified (13), the same study also showed that group mean responses were quite stable across the repeated tests. Importantly, if there was an order effect in the present study, then one would expect this to be reflected in the MSNA responses, which were similar (when expressed as burst rate) or greater increases (when expressed as total activity) during the second CPT. Thus, it is unlikely that attenuated increases in mean blood pressure during the heat stress was related to the performing sequential CPTs.
Given that the CPT also induces splanchnic (3, 4, 14) and renal (2, 24) vasoconstriction in normothermic individuals, it is likely that vasoconstriction in these vascular beds also occurred during the CPT while heat stressed. However, it remains unknown whether the observed MSNA responses are reflective of renal and/or splanchnic neural responses under the imposed conditions, or whether vasoconstrictor responsiveness to a given neural signal is attenuated in these regions by whole body heat stress.
While it is clear that cardiac output does not increase throughout a CPT while subjects are normothermic, cardiac output responses to this perturbation while subjects are heat stressed remains unknown. Given the absence of a significant increase in heart rate during the heat stress CPT (Fig. 2), coupled with prior findings of no change in stroke volume in normothermic subjects during this perturbation, we presume that cardiac output likewise did not increase during the heat stress CPT. However, this variable was not evaluated, thus leaving open the possibility that some of the increase in arterial blood pressure during the heat stress CPT may have a cardiac output component. That said, if cardiac output increased during the heat stress CPT, such would provide even further evidence of an attenuated increase in systemic vascular resistance to this perturbation since the elevation in arterial blood pressure was attenuated despite a possible contributing effect of cardiac output.
In conclusion, the present data show that heat stress attenuates the increase in arterial blood pressure in response to a non-baroreflex-mediated sympathoexcitatory stimulus (i.e., the CPT), that is primarily vascular resistance dependent. The result suggests that heat stress can cause an apparent disassociation between MSNA and the corresponding blood pressure response to a vasoconstrictor stimulus. Attenuated responsiveness to the sympathetic stimulus will compromise blood pressure control, which is commonly observed in heat-stressed individuals.
GRANTS
The project was supported in part by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072 and American Heart Association Grant 0635245N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present addresses: M. Shibasaki, Dept. of Environmental Health, Nara Women's University, Nara, Japan; D. A. Low, Neurovascular and Autonomic Unit, St. Mary's Hospital Faculty of Medicine, Imperial College of London, London, United Kingdom; D. M. Keller, Dept. of Kinesiology, Univ. of Texas at Arlington, Arlington, TX; and S. L. Davis, Dept. of Applied Physiology and Wellness, Southern Methodist Univ., Dallas, TX.
REFERENCES
- 1. Allen J, Crossley R. Effect of controlled elevation in body temperature on human tolerance to 1Gz acceleration. J Appl Physiol 33: 418–420, 1972 [DOI] [PubMed] [Google Scholar]
- 2. Boddi M, Sacchi S, Lammel RM, Mohseni R, Serneri GG. Age-related and vasomotor stimuli-induced changes in renal vascular resistance detected by Doppler ultrasound. Am J Hypertens 9: 461–466, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Chaudhuri KR, Thomaides T, Hernandez P, Alam M, Mathias CJ. Noninvasive quantification of superior mesenteric artery blood flow during sympathoneural activation in normal subjects. Clin Auton Res 1: 37–42, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Chaudhuri KR, Thomaides T, Mathias CJ. Abnormality of superior mesenteric artery blood flow responses in human sympathetic failure. J Physiol 457: 477–489, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol 279: H1955–H1962, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576: 625–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui J, Shibasaki M, Davis SL, Low DA, Keller DM, Crandall CG. Whole body heat stress attenuates baroreflex control of muscle sympathetic nerve activity during postexercise muscle ischemia. J Appl Physiol 106: 1125–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 282: R252–R258, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevations in arterial blood pressure are attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 283: R1221–R1226, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fagius J, Karhuvaara S, Sundlof G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand 137: 325–334, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Fasano ML, Sand T, Brubakk AO, Kruszewski P, Bordini C, Sjaastad O. Reproducibility of the cold pressor test: studies in normal subjects. Clin Auton Res 6: 249–253, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Fujimura J, Camilleri M, Low PA, Novak V, Novak P, Opfer-Gehrking TL. Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst 67: 15–23, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol 88: 767–773, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kregel KC, Gisolfi CV. Circulatory responses to vasoconstrictor agents during passive heating in the rat. J Appl Physiol 68: 1220–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454: 359–371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538: 331–340, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lind A, Leithead C, McNicol G. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol 25: 268–276, 1968 [DOI] [PubMed] [Google Scholar]
- 21. Massett MP, Lewis SJ, Kregel KC. Effect of heating on the hemodynamic responses to vasoactive agents. Am J Physiol Regul Integr Comp Physiol 275: R844–R853, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Massett MP, Lewis SJ, Stauss HM, Kregel KC. Vascular reactivity and baroreflex function during hyperthermia in conscious rats. Am J Physiol Regul Integr Comp Physiol 279: R1282–R1289, 2000 [DOI] [PubMed] [Google Scholar]
- 23. McCord GR, Minson CT. Cutaneous vascular responses to isometric handgrip exercise during local heating and hyperthermia. J Appl Physiol 98: 2011–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Middlekauff HR, Nitzsche EU, Hamilton MA, Schelbert HR, Fonarow GC, Moriguchi JD, Hage A, Saleh S, Gibbs GG. Evidence for preserved cardiopulmonary baroreflex control of renal cortical blood flow in humans with advanced heart failure. A positron emission tomography study. Circulation 92: 395–401, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Nobrega AC, Williamson JW, Garcia JA, Mitchell JH. Mechanisms for increasing stroke volume during static exercise with fixed heart rate in humans. J Appl Physiol 83: 712–717, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Ray CA. Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 279: H2399–H2404, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Rowell LB. Thermal stress . In: Human Circulation Regulation during Physical Stress, edited by Rowell LB. New York: Oxford Univ. Press, 1986, p. 174–212 [Google Scholar]
- 28. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 31. Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure in humans. J Physiol 481: 233–241, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vojacek J, Hannan WJ, Muir AL. Ventricular response to dynamic exercise and the cold pressor test. Eur Heart J 3: 212–222, 1982 [DOI] [PubMed] [Google Scholar]
- 34. Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Wilson TE, Cui J, Crandall CG. Mean body temperature does not modulate eccrine sweat rate during upright tilt. J Appl Physiol 98: 1207–1212, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Yamazaki F, Sagawa S, Torii R, Endo Y, Shiraki K. Effects of acute hyperthermia on the carotid baroreflex control of heart rate in humans. Int J Biometeorol 40: 200–205, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. J Appl Physiol 88: 393–400, 2000 [DOI] [PubMed] [Google Scholar]