Abstract
Multiple sclerosis (MS) is a progressive neurological disorder that disrupts axonal myelin in the central nervous system. Demyelination produces alterations in saltatory conduction, slowed conduction velocity, and a predisposition to conduction block. An estimated 60–80% of MS patients experience temporary worsening of clinical signs and neurological symptoms with heat exposure. Additionally, MS may produce impaired neural control of autonomic and endocrine functions. This review focuses on five main themes regarding the current understanding of thermoregulatory dysfunction in MS: 1) heat sensitivity; 2) central regulation of body temperature; 3) thermoregulatory effector responses; 4) heat-induced fatigue; and 5) countermeasures to improve or maintain function during thermal stress. Heat sensitivity in MS is related to the detrimental effects of increased temperature on action potential propagation in demyelinated axons, resulting in conduction slowing and/or block, which can be quantitatively characterized using precise measurements of ocular movements. MS lesions can also occur in areas of the brain responsible for the control and regulation of body temperature and thermoregulatory effector responses, resulting in impaired neural control of sudomotor pathways or neural-induced changes in eccrine sweat glands, as evidenced by observations of reduced sweating responses in MS patients. Fatigue during thermal stress is common in MS and results in decreased motor function and increased symptomatology likely due to impairments in central conduction. Although not comprehensive, some evidence exists concerning treatments (cooling, precooling, and pharmacological) for the MS patient to preserve function and decrease symptom worsening during heat stress.
Keywords: demyelination, core temperature, sweating, skin blood flow, fatigue
multiple sclerosis (MS) is a disabling progressive neurological disorder affecting ∼400,000 individuals in the United States. The pathophysiology of MS results in a disruption or loss of axonal myelin in the central nervous system (CNS), leading to the formation of scar tissue (sclerosis). MS is thought to involve a number of autoimmune injury cascades that appear to be dependent on the interaction of complex epigenetic and environmental factors. Immune responses in individuals with MS are skewed toward a proinflammatory state, resulting in inflammation, demyelination, and ultimately loss of axons and disorganization of normal tissue architecture within the CNS (23). Demyelination is associated with corresponding changes in axonal physiology, including a loss of saltatory properties of electrical conduction, reduction in conduction velocity, and a predisposition to conduction block. These pathophysiological mechanisms underlie the myriad of symptoms (Table 1) in individuals with MS and are contingent on the neuroanatomic localization of lesions (23).
Table 1.
Common symptoms of multiple sclerosis
| Most Common Symptoms | Less Common Symptoms |
|---|---|
| Fatigue | Speech disorders |
| Walking (gait), balance, and coordination problems | Swallowing problems |
| Bowel dysfunction | Headache |
| Dizziness and vertigo | Hearing loss |
| Pain | Seizures |
| Emotional changes | Tremor |
| Spasticity | Respiration/breathing problems |
| Numbness | Itching |
| Bladder dysfunction | |
| Vision problems | |
| Sexual dysfunction | |
| Cognitive dysfunction | |
| Depression |
Based on information from National Multiple Sclerosis Society (Ref. 73).
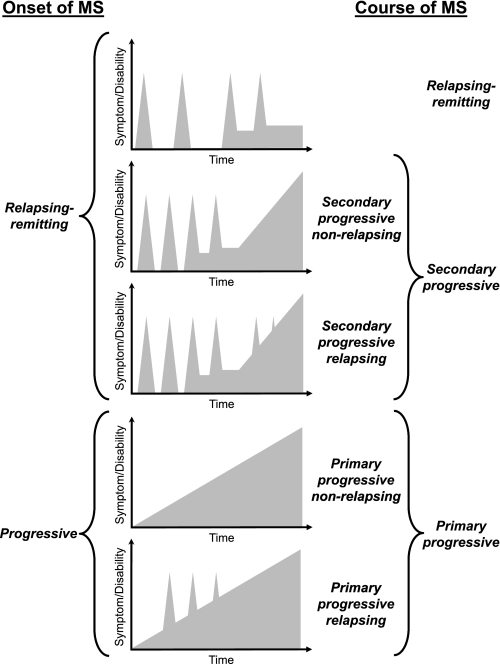
Autonomic dysfunction involving the genitourinary, gastrointestinal, cardiovascular, and thermoregulatory systems is commonly observed in MS (29). In addition to autonomic dysfunction, the majority of MS patients experience transient and temporary worsening of clinical signs and neurological symptoms in response to a number of factors, the most prominent of which are increased ambient or core body temperature and exercise. However, the assessment and understanding of autonomic dysfunction in individuals with MS is problematic due to the variability of early clinical symptoms and the heterogeneity in the clinical course over time (Fig. 1). With these limitations in mind, this review will focus on our current understanding of the thermoregulatory dysfunction in MS while characterizing five main topical themes: 1) heat sensitivity; 2) central regulation of body temperature; 3) thermoregulatory effector responses; 4) heat-induced fatigue; and 5) countermeasures to improve function in MS patients during a thermal stress.
Fig. 1.
Clinical courses of multiple sclerosis (MS). Adapted from Confavreux and Vukusic (11) with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health.
HEAT SENSITIVITY IN MS
The earliest medical reports of thermal sensitivity in MS are derived from Charles Prosper Ollivier d'Angers who noted in 1824 that a hot bath induced numbness in the right leg and reduced feeling and dexterity in the hands of a patient with MS (56). However, Wilhelm Uhthoff's description of this phenomenon occurring after a hot bath or with exercise in MS patients with a history of optic neuritis has most commonly been cited as the landmark observation of the pathophysiological principle of temperature-induced conduction block in demyelinated axonal segments (Uhthoff's phenomenon) (77). It is estimated that 60–80% of the MS population experience transient and temporary worsening of clinical signs and neurological symptoms as a result of elevated body temperature by immersion in warm water (41–43°C) or exposure to infrared heating lamps (27, 46, 50, 51).
Symptom worsening can result from passive heat exposure, exercise (increase in metabolism), or a combination of heat exposure and increases in metabolism (exercise-heat stress). Both physical (walking, running, driving, writing, etc.) and cognitive (memory retrieval, processing speed, multitasking, etc.) functions can be impaired by heat exposure, greatly impacting overall patient safety as well as the ability of individuals with MS to perform routine activities of daily living, even in mildly affected individuals (57, 59). Symptom worsening has been reported with exercise (78), hot shower (81), and sunbathing (2). Even fluctuations in circadian body temperature from the morning to the afternoon can elicit changes in symptoms (62).
In the past, physicians and health care providers instructed MS patients to minimize their exposure to high ambient temperatures and to avoid exercise or intense physical work in order to avoid symptom worsening. However, lack of exercise often results in deconditioning, reduced functional capabilities, increased risk of injury, and less weight-bearing movement, which has consequences on bone and mineral metabolism (59, 88). Evidence now indicates that exercise is beneficial to individuals with MS by improving fitness and sense of well being, reducing fatigue, and increasing strength and safety of walking and should be incorporated into their overall disease management plan (57).
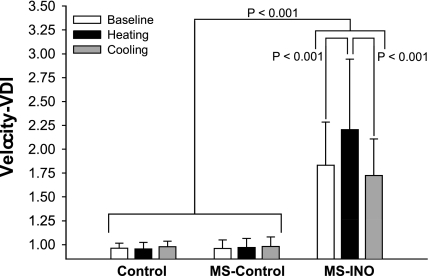
Typically, deficits caused by increases in temperature are reversible by removing heat stressors and allowing subsequent cooling. Davis et al. (15) quantified Uhthoff's phenomenon during indirect whole body heating and its reversibility with the subsequent application of active cooling by objectively measuring horizontal eye movement velocities in a group of MS patients with internuclear ophthalmoparesis, an abnormality characterized by the slowing of the eye moving toward the nose (adduction) during horizontal eye movements. The speed of horizontal eye movements in MS patients with internuclear ophthalmoparesis was slowed from baseline when core body temperature was raised ∼0.8°C with whole body heating and returned to baseline following 1 h of whole body cooling (Fig. 2) (15).
Fig. 2.
Data from healthy controls (Control; n = 8), MS patients without internuclear opthalmoparesis (MS-Control; n = 8), and MS patients diagnosed with internuclear ophthalmoparesis (MS-INO; n = 8) showing ocular function responses [velocity-versional dysconjugacy index (VDI)] during whole body heat stress (increase internal temperature ∼0.8°C) and subsequent whole body cooling (return to normothermic baseline). A significant slowing (P < 0.001) of horizontal eye movements (increasing velocity-VDI) was observed in MS patients with INO during whole body heating. However, ocular function was restored to preheating baseline with subsequent whole body cooling. Data are expressed as means ± SD. Reproduced from Davis et al. (15) with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health.
The precise mechanisms for impaired neural function in demyelinated axons due to increases in temperature are not completely understood but are likely due to a combination of structural and physiological changes within axons in the CNS (75). With demyelination, increases in temperature can affect the propagation of action potentials. Increased temperature may also influence the electrical properties of the nerve fiber by increasing the refractory period (partly mediated by potassium channel activation and sodium channel inactivation), which surpass the action potential-generating processes (sodium channel activation) (32).
Demyelination reduces the axon's safety factor, defined as the ratio of the current available to stimulate a node of Ranvier to the minimal amount of current needed to excite a node of Ranvier (72). In healthy, myelinated axons, the safety factor for saltatory conduction ranges from a factor of 3–7 (i.e., the current available to stimulate the node is 3–7 times greater than the current needed to excite the node) (76). Compared with myelinated axons, demyelinated axons have a much lower safety factor that can approach a critical value (∼1), resulting in failure to generate an action potential across the next node of Ranvier and ultimately culminating in conduction block. Increased temperature further reduces the safety factor, thereby influencing the threshold of current to excite an axon, the rate at which current is generated, and the total amount of current available (13, 14, 66). This combination of demyelination and increased temperature (even as little as 0.5°C) in individuals with MS can cause nerve conduction block (60). The degree of conduction block is a factor of both the magnitude of myelin loss and the time since the demyelination has occurred, suggesting that individuals with more severe cases of MS are at greater risk for developing conduction block (72). In some cases, conduction can be restored to injured axons during periods of disease remission, as some remyelination may occur. However, conduction in these areas of repair is not optimal and is therefore prone to failure (72). Often, areas of axonal injury reach a point in which repair is no longer possible, and the functional deficit becomes permanent.
CENTRAL REGULATION OF BODY TEMPERATURE IN MS
Compounding temperature-related nerve conduction problems, individuals with MS may have impaired neural control of autonomic and endocrine functions (30). Thermosensitive neurons of the preoptic anterior hypothalamus process afferent thermal information (central and peripheral) to integrate and direct autonomic thermoeffector responses (6, 63). Areas of the sympathetic nervous system (hypothalamic area and interomediolateral columns of the spinal cord) that are responsible for controlling thermoregulatory function are susceptible to disease-related pathology in individuals with MS (1).
Hypothermia has been documented in a small number of MS patients (18, 42, 44, 74, 83, 87) with core temperatures ranging from 30 to 35°C. Hypothermia can be profound but tolerated in MS patients. Lesions within the CNS, specifically the hypothalamus, may impair the homeostatic control of body temperature in individuals with MS and allow for atypical fluctuations in body temperature (45). Hypothermia may be more common than reported and be indicative of more severe disease (87). Fluctuations in body temperature can be problematic for MS patients when infections have been masked due to the absence of a typical fever response, resulting in serious medical conditions.
HEAT-INDUCED FATIGUE IN MS
Fatigue is a frequent and sometimes debilitating symptom in MS, present in nearly 70% of MS patients (22, 39). Fatigue, evidenced by reports of decreased motor function and increased symptomatology, worsens during thermal stress in individuals with MS. Heat stress has also been shown to induce muscle fatigue in healthy individuals (35, 48, 55). Due to its relative importance, increased fatigue associated with thermal stress in MS will be briefly discussed in this review. A recent review by Marino (47) provides a more detailed discussion of fatigue associated with thermal stress in MS.
Physiological fatigue is defined as the failure to maintain an expected work output (5). Patients with MS experience decreased maximal motor unit firing (61), alterations in motor unit recruitment (52), and excitation-contraction coupling (69) likely contributing to this physiological fatigue. However, these mechanisms do not fully explain fatigue in MS (malaise or lack of energy). It is generally accepted that centrally mediated fatigue, an inability to sustain central drive to spinal motoneurons (24), plays a greater role than peripheral factors in MS fatigue (43). It has been hypothesized that heat-related fatigue is also a form of central fatigue (55). Using transcranial magnetic stimulation, a noninvasive technique used to measure conduction properties of the corticospinal tract and excitability of the motor cortex, White et al. (84) demonstrated that fatigue induced by raising core temperature by 0.8°C via indirect whole body heating in MS patients produces a corresponding decrease in central motor conduction time and cortical excitability. These results most likely occurred as a result of slowed or blocked conduction in demyelinated lesions in the CNS (28, 31) associated with alterations in central activation (58, 70).
THERMOREGULATORY EFFECTOR RESPONSES IN MS
Increases in skin blood flow and sweating are the primary heat dissipation mechanisms in humans. Without these dissipation mechanisms, internal temperature would reach the upper “safe” limit within 10 min of moderate exercise (36). Based on the severe consequences that may occur with elevated body temperature in MS, control of skin blood flow and sweating are crucial for patient safety (81).
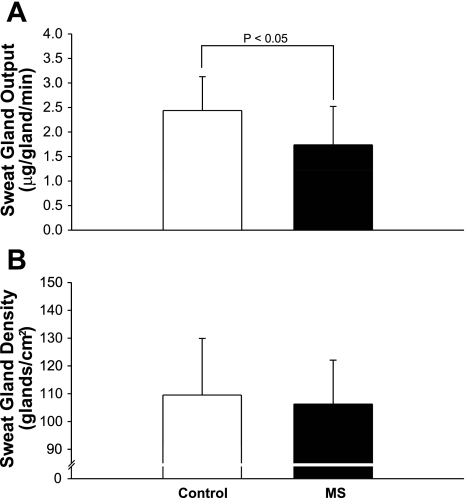
Lesions affecting conduction within the CNS of MS patients can potentially impair thermoregulatory effector responses to eccrine sweat glands. This is evidenced by previous studies that report reduced sweating responses in MS patients (10, 53, 79). In the aforementioned studies, abnormal sweating responses were identified by using quinizarin powder placed on the skin of individuals followed by exposure to a heat stress. Quinizarin powder, normally gray in color, changes to a deep blue when exposed to sweat. The intensity of the change in color provides a visual estimate of sweating (19, 41). However, this technique cannot quantitatively identify differences in sweating or determine whether diminished sweating is due to a decreased number of active sweat glands, altered innervation of the glands, and/or reduced output from activated glands. Using a more quantitative approach, Davis et al. (17) transdermally delivered a cholinergic agonist (pilocarpine) to activate eccrine sweat glands independent of the CNS. Diminished sweat function was identified in individuals with MS, and this reduction was not caused by reduced sweat gland recruitment but was due to reduced sweat gland output per gland (Fig. 3). Even though sweating was induced locally by an agonist, it is possible that CNS impairments or even neuronal loss within the descending sudomotor pathways due to the disease process contributed to the observations of diminished sweat function in these patients (1, 79). Impaired sweat function appears to occur more frequently in MS patients with more severe cases of disease (10).
Fig. 3.
Data from control and MS patients showing decreased sweat gland output per gland (P < 0.05) compared with matched, healthy controls following iontophoresis of pilocarpine, a cholinergic agent (A). No differences were observed in the number of sweat glands recruited between healthy controls and MS patients (B). Data are expressed as means ± SD. Modified from Davis et al. (17).
Quantification of sweat function during heat stress has produced conflicting findings. Saari and colleagues (65) have reported impaired sweating responses in the lower limbs of MS patients compared with healthy controls during a 15-min passive heat stress of the upper torso. Alternatively, no sweating impairments were reported in a group of MS patients with optic neuritis exposed to the same passive heating paradigm (64). In both of the aforementioned studies, the magnitude of the heat stress was minimal (increase in core temperatures of 0.1°C), and thus it is uncertain whether thermoregulatory effector responses were even engaged. Davis et al. (16) have documented sweating and cutaneous blood flow responses in individuals with MS when internal temperature was increased ∼1.0°C. Whole body heating was performed by placing MS patients in a tube-lined suit through which 48°C water was perfused to increase skin temperature, leading to the increase in internal temperature. The suit covered the entire body surface area except the head, hands, feet, and regions of skin blood flow and sweating assessment (dorsal forearm). Because the suit was not in contact with the regions of skin where skin blood flow and sweat rate were assessed, responses from these areas were not affected by local heating but rather were due to reflex-induced neural modulation in response to changing internal body temperature. Sweating responses to heat stress were significantly lower in individuals with MS compared with healthy controls and may have been due to impairments in neural control of sudomotor pathways or neural-induced changes in eccrine sweat glands. Interestingly, larger increases in cutaneous vasodilation to whole body heating were observed in MS patients compared with healthy controls and suggest neural control of skin blood flow is intact and may compensate for impairments in sweating in an attempt to adequately dissipate heat.
To address the potential for decreases in sweating function being due to detraining or deadaption, Davis et al. (17) trained seven MS patients for 15 wk to improve their sweat function. Aerobic exercise training alters central gain to initiate sweating at a lower body temperature (33, 71) and also increases maximal sweating responses (12, 90). Despite 15 wk of training, this group of MS patients improved neither sweat gland recruitment nor sweat output per gland. Taken together, these data indicate that MS affects thermoregulatory effector responses and that adaptive thermoregulatory responses to exercise training typically observed in healthy individuals are not seen in MS patients, suggesting impaired CNS control in MS patients.
COUNTERMEASURES TO IMPROVE FUNCTION IN MS PATIENTS DURING THERMAL STRESS
To reduce the potentially detrimental effects of heat sensitivity, several treatment strategies have been employed to allow individuals with MS to participate in activities of daily living, including exercise. Simple behavioral strategies are used to minimize heat exposure, such as performing work or exercise outside during the early morning or late evening when temperatures are cooler. A small number of studies have reported potential benefits using cooling strategies that are convenient methods available to most MS patients such as cold showers, applying ice packs, the use of regional cooling devices, and drinking cold beverages (3, 7, 26, 67, 80).
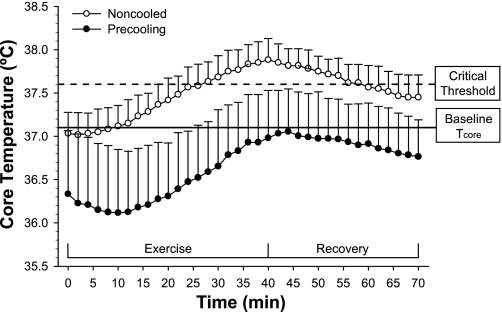
Precooling (cooling before heat exposure) presents another practical and strategic treatment option for minimizing the consequences of heat stress in MS patients. Immersing the lower limbs in cool water (i.e., cold tap water in a bath tub) creates negative heat storage before thermal stress or the initiation of exercise. White et al. (86) demonstrated that water immersion precooling (cooling the lower limbs in 20°C water for 30 min before physical activity) was effective in preventing gains in core temperature during physical work and may minimize heat-induced conduction difficulties in MS patients (Fig. 4). Precooling allows the lower limbs to effectively serve as heat “sinks” in order to blunt internal temperature increases and decrease reliance on eccrine sweating, which may be compromised in MS patients (89). Precooling allows heat-sensitive individuals with MS to perform exercise with greater physical comfort and fewer side effects (86). The heat load induced via increases in metabolism during exercise appears to be reduced most effectively by cooling the greatest body mass while minimizing thermogenic responses (i.e., shivering) (85). This cooling (or temperature-blunting effect) can last for several hours, depending on the intensity of the activities performed by the individual with MS.
Fig. 4.
Core body temperatures responses during 30 min of aerobic exercise and subsequent recovery following a noncooled trial (open circles) and a precooled trial (closed circles). Core body temperature during the precooled trial remained below baseline values during exercise and recovery whereas core temperature during the noncooled trial exceeded a critical threshold (0.5°C) for potential increases in symptom worsening. Data are expressed as means ± SD. Modified from White et al. (86) with permission from Sage Publication Ltd., UK.
Investigations have examined the use of cooling garments (microclimate cooling) to combat heat-induced worsening of symptoms in MS patients during daily activities or during exercise (4, 9, 20, 37, 38, 40, 49, 68). Typically these garments come in two designs based on heat exchange properties. Active heat exchange garments are cooled by circulating liquid throughout the garment through a tubing network (21, 82). Passive heat exchange garments have ice or gel packs that are inserted into the garment to provide the cooling effect (75). Cooling garments have demonstrated improvements in neurological function (motor performance and visual acuity) as well as perceived subjective benefits (feeling less fatigued) in thermally sensitive MS patients (4, 9, 20, 37, 40, 68). A number of factors influence the ability of these garments to provide effective cooling: 1) garment fit, 2) location of cooling elements within the garment, 3) cooling temperature and whether the cooling process is continuous or intermittent, 4) body size and shape, and 5) control and regulation of skin blood flow of the skin being cooled (8, 54, 75, 91). Although microclimate cooling has been shown to be effective in reducing heat stress in MS, some caution must be considered because cooling garments may increase metabolic rate and arterial blood pressure, and decrease mechanical efficiency for patients with disabilities during the performance of physical work due to cooling equipment weight or restrictions in joint mobility. The cost of these garments also my limit accessibility and availability to some individuals with MS.
In addition to the previously described precooling and cooling strategies, pharmacological strategies have also been employed to preserve axonal conduction in MS patients. Pharmaceuticals, such as potassium channel blockers, have been prescribed by physicians to treat heat sensitivity in MS patients. 4-Aminopyridine, a potassium channel blocker, has been shown to increase the conduction of action potentials in demyelinated axons by inhibiting potassium channels (34). This drug has recently been shown to improve walking speed in MS patients (25). The Federal Drug Administration recently granted approval for the use of this drug in all forms of MS to improve or preserve mobility. Anecdotal evidence suggests this drug may limit the worsening of MS symptoms during heat exposure or exercise. Investigations are currently under way to determine if pharmaceutical interventions, such as 4-aminopyridine, can protectively modulate axonal channels and improve neural function in individuals with MS during thermal stress. Preliminary pilot data (unpublished observation) indicate that 4-aminopyridine provides some resistance to temperature-induced slowing in horizontal eye movements previously observed in MS patients with internuclear ophthalmoparesis (15).
Perspectives and Summary
Heat stress presents a significant problem to the individual with MS. Despite recent insights into thermoregulatory dysfunction in MS, many questions remain unanswered. Disease involvement in thermoregulatory centers within the CNS, specifically the hypothalamus, likely impairs thermoregulatory function. Quantification of MS involvement in gray matter in the CNS, specifically within the thermoregulatory centers of the hypothalamus, is needed and now may be possible with continuing advances in magnetic resonance imaging techniques. Recent evidence indicates that increased temperature alters conduction characteristics within the CNS of MS patients. It is unclear whether impaired conduction within the CNS with MS alters the processing of afferent information or whether there is an alteration in neural communication between higher brain centers. Progress in functional imaging techniques has the potential to provide information on activation patterns within thermoregulatory centers of the CNS in MS. Further research is also need to determine if the homeostatic control of body temperature is impaired in MS, resulting in individuals with MS operating in lower ranges of core temperature compared with controls. Evidence indicates that thermoeffector responses are impaired in MS. Heat dissipation mechanisms, specifically sweat function, in individuals with MS are reduced compared with healthy controls. It is unclear whether these impairments are related to impairments in neural control or peripheral alterations due to altered neural innervation or a combination of both. Hydration status is another area that warrants further investigation in MS. Patients often voluntarily restrict fluid intake to ease MS related bladder problems (i.e., bladder urgency, frequency of urination, bladder leakage), which could lead to decreases in plasma volume impacting thermoregulatory mechanisms, specifically sweating. Cooling techniques, including precooling, have been shown to be effective in minimizing the consequences of heat stress in MS patients. Advances in pharmacological therapies have demonstrated potential in limiting symptom worsening during heat exposure and warrant further investigation.
GRANTS
The presented work conducted by the authors was funded by grants from the National Multiple Sclerosis Society (RG2922B1/1, PP0887, PP1040, RG4043A1/1) and the National Institutes of Health (R15AR050435).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We express gratitude to the following collaborators who assisted with the authors' studies contained herein: Craig Crandall, PhD, Teresa Frohman, PA, Jack Petajan MD, PhD, and Jamie Vener, PhD.
REFERENCES
- 1. Andersen EB, Nordenbo AM. Sympathetic vasoconstrictor responses in multiple sclerosis with thermo-regulatory dysfunction. Clin Auton Res 7: 13–16, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Avis SP, Pryse-Phillips WE. Sudden death in multiple sclerosis associated with sun exposure: a report of two cases. Can J Neurol Sci 22: 305–307, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bassett SW, Lake BM. Use of cold applications in the management of spasticity: report of three cases. Phys Ther Rev 38: 333–334, 1958 [DOI] [PubMed] [Google Scholar]
- 4. Beenakker EA, Oparina TI, Hartgring A, Teelken A, Arutjunyan AV, De Keyser J. Cooling garment treatment in MS: clinical improvement and decrease in leukocyte NO production. Neurology 57: 892–894, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7: 691–699, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Boulant JA. Hypothalamic neurons regulating body temperature. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol Soc., sect. 4, vol. I, chapt. 6, 1996, p. 105–126 [Google Scholar]
- 7. Boynton BL, Garramone PM, Buca JT. Observations on the effects of cool baths for patients with multiple sclerosis. Phys Ther Rev 39: 297–299, 1959 [DOI] [PubMed] [Google Scholar]
- 8. Cadarette BS, Cheuvront SN, Kolka MA, Stephenson LA, Montain SJ, Sawka MN. Intermittent microclimate cooling during exercise-heat stress in US army chemical protective clothing. Ergonomics 49: 209–219, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Capello E, Gardella M, Leandri M, Abbruzzese G, Minatel C, Tartaglione A, Battaglia M, Mancardi GL. Lowering body temperature with a cooling suit as symptomatic treatment for thermosensitive multiple sclerosis patients. Ital J Neurol Sci 16: 533–539, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Cartlidge NE. Autonomic function in multiple sclerosis. Brain 95: 661–664, 1972 [DOI] [PubMed] [Google Scholar]
- 11. Confavreux C, Vukusic S. Natural history of multiple sclerosis: implications for counselling and therapy. Curr Opin Neurol 15: 257–266, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Crandall CG, Shibasaki M, Wilson TE, Cui J, Levine BD. Prolonged head-down tilt exposure reduces maximal cutaneous vasodilator and sweating capacity in humans. J Appl Physiol 94: 2330–2336, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Davis FA. Axonal conduction studies based on some considerations of temperature effects in multiple sclerosis. Electroencephalogr Clin Neurophysiol 28: 281–286, 1970 [DOI] [PubMed] [Google Scholar]
- 14. Davis FA. Pathophysiology of multiple sclerosis and related clinical implications. Mod Treat 7: 890–902, 1970 [PubMed] [Google Scholar]
- 15. Davis SL, Frohman TC, Crandall CG, Brown MJ, Mills DA, Kramer PD, Stuve O, Frohman EM. Modeling Uhthoff's phenomenon in MS patients with internuclear ophthalmoparesis. Neurology 70: 1098–1106, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Davis SL, Korkmas MA, Crandall CG, Frohman EM. Impaired sweating in multiple sclerosis leads to increased reliance on skin blood flow for heat dissipation (Abstract). FASEB J 24: 991, 925, 2010 [Google Scholar]
- 17. Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J Appl Physiol 98: 1740–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Edwards S, Lennox G, Robson K, Whiteley A. Hypothermia due to hypothalamic involvement in multiple sclerosis. J Neurol Neurosurg Psychiatry 61: 419–420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fealey RD, Sato K. Disorders of the eccrine sweat glands and sweating. In: Fitzpatrick's Dermatology in General Medicine, edited by Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Columbus, OH: McGraw-Hill, 2008, p. 215–243 [Google Scholar]
- 20. Flensner G, Lindencrona C. The cooling-suit: a study of ten multiple sclerosis patients' experiences in daily life. J Adv Nurs 29: 1444–1453, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Flouris AD, Cheung SS. Design and control optimization of microclimate liquid cooling systems underneath protective clothing. Ann Biomed Eng 34: 359–372, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil 65: 135–138, 1984 [PubMed] [Google Scholar]
- 23. Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354: 942–955, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol (Lond) 490: 529–536, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 373: 732–738, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Grahn DA, Murray JV, Heller HC. Cooling via one hand improves physical performance in heat-sensitive individuals with multiple sclerosis: a preliminary study. BMC Neurology 8: 14, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guthrie TC. Visual and motor changes in patients with multiple sclerosis; a result of induced changes in environmental temperature. AMA Arch Neurol Psychiatry 65: 437–451, 1951 [DOI] [PubMed] [Google Scholar]
- 28. Guthrie TC, Nelson DA. Influence of temperature changes on multiple sclerosis: critical review of mechanisms and research potential. J Neurol Sci 129: 1–8, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Haensch CA, Jorg J. Autonomic dysfunction in multiple sclerosis. J Neurol 253, Suppl 1: I3–I9, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Huitinga I, De Groot CJ, Van der Valk P, Kamphorst W, Tilders FJ, Swaab DF. Hypothalamic lesions in multiple sclerosis. J Neuropathol Exp Neurol 60: 1208–1218, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Humm AM, Beer S, Kool J, Magistris MR, Kesselring J, Rosler KM. Quantification of Uhthoff's phenomenon in multiple sclerosis: a magnetic stimulation study. Clin Neurophysiol 115: 2493–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Huxley AF. Ion movements during nerve activity. Ann NY Acad Sci 81: 221–246, 1959 [DOI] [PubMed] [Google Scholar]
- 33. Ichinose TK, Inoue Y, Hirata M, Shamsuddin AK, Kondo N. Enhanced heat loss responses induced by short-term endurance training in exercising women. Exp Physiol 94: 90–102, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Judge SI, Bever CTJ. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther 111: 224–259, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Kay D, Marino FE, Cannon J, St. Clair Gibson A, Lambert MI, Noakes TD. Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur J Appl Physiol 84: 115–121, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Kenney WL, Johnson JM. Control of skin blood flow during exercise. Med Sci Sports Exerc 24: 303–312, 1992 [PubMed] [Google Scholar]
- 37. Kinnman J, Andersson T, Andersson G. Effect of cooling suit treatment in patients with multiple sclerosis evaluated by evoked potentials. Scand J Rehabil Med 32: 16–19, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Kinnman J, Andersson U, Wetterquist L, Kinnman Y, Andersson U. Cooling suit for multiple sclerosis: functional improvement in daily living? Scand J Rehabil Med 32: 20–24, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol 45: 435–437, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Ku YT, Montgomery LD, Lee HC, Luna B, Webbon BW. Physiologic and functional responses of MS patients to body cooling. Am J Phys Med Rehabil 79: 427–434, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Kuno Y. Human Perspiration. Springfield, IL: Charles Thomas, 1956 [Google Scholar]
- 42. Lammens M, Lissoir F, Carton H. Hypothermia in three patients with multiple sclerosis. Clin Neurol Neurosurg 91: 117–121, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Latash M, Kalugina E, Nicholas J, Orpett C, Stefoski D, Davis F. Myogenic and central neurogenic factors in fatigue in multiple sclerosis. Mult Scler 1: 236–241, 1996 [PubMed] [Google Scholar]
- 44. Linker RA, Mohr A, Cepek L, Gold R, Prange H. Core hypothermia in multiple sclerosis: case report with magnetic resonance imaging localization of a thalamic lesion. Mult Scler 12: 112–115, 2006 [DOI] [PubMed] [Google Scholar]
- 45. MacKenzie MA. Pathophysiology and clinical implications of human poikilothermia. Ann NY Acad Sci 813: 738–740, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Malhotra AS, Goren H. The hot bath test in the diagnosis of multiple sclerosis. JAMA 246: 1113–1114, 1981 [PubMed] [Google Scholar]
- 47. Marino FE. Heat reactions in multiple sclerosis: an overlooked paradigm in the study of comparative fatigue. Int J Hyperthermia 25: 34–40, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Martin PG, Marino FE, Rattey J, Kay D, Cannon J. Reduced voluntary activation of human skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp Physiol 90: 225–236, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Meyer-Heim A, Rothmaier M, Weder M, Kool J, Schenk P, Kesselring J. Advanced lightweight cooling-garment technology: functional improvements in thermosensitive patients with multiple sclerosis. Mult Scler 13: 232–237, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Nelson DA, Jeffreys WH, McDowell DF. Effects of induced hyperthermia on some neurological diseases. AMA Arch Neurol Psychiatry 79: 31–39, 1958 [DOI] [PubMed] [Google Scholar]
- 51. Nelson DA, McDowell DF. The effects of induced hyperthermia on patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 22: 113–116, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ng AV, Miller RG, Gelinas D, Kent-Braun JA. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 29: 843–852, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Noronha MJ, Vas CJ, Aziz H. Autonomic dysfunction (sweating responses) in multiple sclerosis. J Neurol Neurosurg Psychiatry 31: 19–22, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nunneley SA, Maldonado RJ. Head and/or torso cooling during simulated cockpit heat stress. Aviat Space Environ Med 54: 496–499, 1983 [PubMed] [Google Scholar]
- 55. Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91: 1055–1060, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Ollivier CP. De la moelle epiniere et de ses maladies. Paris: Crevot, 1824 [Google Scholar]
- 57. Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 39: 432–441, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Petajan JH, White AT. Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin Neurophysiol 111: 2188–2195, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Med 27: 179–191, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Rasminsky M. The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol 28: 287–292, 1973 [DOI] [PubMed] [Google Scholar]
- 61. Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve 15: 1123–1132, 1992 [DOI] [PubMed] [Google Scholar]
- 62. Romani A, Bergamaschi R, Versino M, Zilioli A, Callieco R, Cosi V. Circadian and hypothermia-induced effects on visual and auditory evoked potentials in multiple sclerosis. Clin Neurophysiol 111: 1602–1606, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Saari A, Tolonen U, Paakko E, Suominen K, Jauhiainen J, Sotaniemi KA, Myllyla VV. Sudomotor dysfunction in patients with optic neuritis. Clin Auton Res 20:199–204, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Saari A, Tolonen U, Paakko E, Suominen K, Jauhiainen J, Sotaniemi KA, Myllyla VV. Sweating impairment in patients with multiple sclerosis. Acta Neurol Scand 120: 358–363, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Schauf CL, Davis FA. Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry 37: 152–161, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scherokman BJ, Selhorst JB, Waybright EA, Jabbari B, Bryan GE, Maitland CG. Improved optic nerve conduction with ingestion of ice water. Ann Neurol 17: 418–419, 1985 [DOI] [PubMed] [Google Scholar]
- 68. Schwid SR, Petrie MD, Murray R, Leitch J, Bowen J, Alquist A, Pelligrino R, Roberts A, Harper-Bennie J, Milan MD, Guisado R, Luna B, Montgomery L, Lamparter R, Ku YT, Lee H, Goldwater D, Cutter G, Webbon B. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology 60: 1955–1960, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Sharma KR, Kent-Braun JA, Mynhier MA, Weiner MW, Miller RG. Evidence of an abnormal intramuscular component of fatigue in multiple sclerosis. Muscle Nerve 18: 1403–1411, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain 120: 299–315, 1997 [DOI] [PubMed] [Google Scholar]
- 71. Shibasaki M, Wilson TE, Cui J, Levine BD, Crandall CG. Exercise throughout 6 degrees head-down tilt bed rest preserves thermoregulatory responses. J Appl Physiol 95: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Smith KJ, McDonald WI. The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci 354: 1649–1673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. National Multiple Sclerosis Society Symptoms of MS. http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/symptoms/indexaspx [3 June, 2010]. [Google Scholar]
- 74. Sullivan F, Hutchinson M, Bahandeka S, Moore RE. Chronic hypothermia in multiple sclerosis. J Neurol Neurosurg Psychiatry 50: 813–815, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Syndulko K, Jafari M, Woldanski A, Baumhefner RW, Tourtellotte WW. Effects of temperature in multiple sclerosis: a review of literature. J Neurol Rehabil 10: 23–34, 1996 [Google Scholar]
- 76. Tasaki I. Properties of myelinated fibres in frog sciatic nerve and in spinal cord as examined by microelectrodes. Jpn J Physiol 3: 73, 1953 [DOI] [PubMed] [Google Scholar]
- 77. Uhthoff W. Untersuchungen uber die bei der multiplen Herdsklerose vorkommenden Augenstorungen. Arch Psychiatr Nervenkr 20: 55, 1889 [Google Scholar]
- 78. van Diemen HA, van Dongen MM, Dammers JW, Polman CH. Increased visual impairment after exercise (Uhthoff's phenomenon) in multiple sclerosis: therapeutic possibilities. Eur Neurol 32: 231–234, 1992 [DOI] [PubMed] [Google Scholar]
- 79. Vas CJ. Sexual impotence and some autonomic disturbances in men with multiple sclerosis. Acta Neurol Scand 45: 166–182, 1969 [DOI] [PubMed] [Google Scholar]
- 80. Watson CW. Effect of lowering of body temperature on the symptoms and signs of multiple sclerosis. N Engl J Med 261: 1253–1259, 1959 [DOI] [PubMed] [Google Scholar]
- 81. Waxman SG, Geschwind N. Major morbidity related to hyperthermia in multiple sclerosis. Ann Neurol 13: 348, 1983 [DOI] [PubMed] [Google Scholar]
- 82. Webb P, Troutman SJ, Jr, Annis JF. Automatic cooling in water cooled space suits. Aerospace Med 41: 269–277, 1970 [PubMed] [Google Scholar]
- 83. Weiss N, Hasboun D, Demeret S, Fontaine B, Bolgert F, Lyon-Caen O, Chabas D. Paroxysmal hypothermia as a clinical feature of multiple sclerosis. Neurology 72: 193–195, 2009 [DOI] [PubMed] [Google Scholar]
- 84. White AT, Davis SL, Vener JM, Wendt L. Effect of increased core temperature on cortical excitability and fatigue in multiple sclerosis patients (Abstract). Med Sci Sports Exerc 40: S300, 2008 [Google Scholar]
- 85. White AT, Davis SL, Wilson TE. Metabolic, thermoregulatory, and perceptual responses during exercise after lower vs. whole body precooling. J Appl Physiol 94: 1039–1044, 2003 [DOI] [PubMed] [Google Scholar]
- 86. White AT, Wilson TE, Davis SL, Petajan JH. Effect of precooling on physical performance in multiple sclerosis. Mult Scler 6: 176–180, 2000 [DOI] [PubMed] [Google Scholar]
- 87. White KD, Scoones DJ, Newman PK. Hypothermia in multiple sclerosis. J Neurol Neurosurg Psychiatry 61: 369–375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med 34: 1077–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 89. Wilson TE, Johnson SC, Petajan JH, Davis SL, Gappmaier E, Luetkemeier MJ, White AT. Thermal regulatory responses to submaximal cycling following lower-body cooling in humans. Eur J Appl Physiol 88: 67–75, 2002 [DOI] [PubMed] [Google Scholar]
- 90. Wilson TE, Monahan KD, Fogelman A, Kearney ML, Sauder CL, Ray CA. Aerobic training improves in vivo cholinergic responsiveness but not sensitivity of eccrine sweat glands. J Invest Dermatol 130: 2328–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Young AJ, Sawka MN, Epstein Y, Decristofano B, Pandolf KB. Cooling different body surfaces during upper and lower body exercise. J Appl Physiol 63: 1218–1223, 1987 [DOI] [PubMed] [Google Scholar]