Abstract
Sleep is dependent upon prior brain activities, e.g., after prolonged wakefulness sleep rebound occurs. These effects are mediated, in part, by humoral sleep regulatory substances such as cytokines. However, the property of wakefulness activity that initiates production and release of such substances and thereby provides a signal for indexing prior waking activity is unknown. We propose that extracellular ATP, released during neuro- and gliotransmission and acting via purine type 2 (P2) receptors, is such a signal. ATP induces cytokine release from glia. Cytokines in turn affect sleep. We show here that a P2 receptor agonist, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP), increased non-rapid eye movement sleep (NREMS) and electroencephalographic (EEG) delta power while two different P2 receptor antagonists, acting by different inhibitory mechanisms, reduced spontaneous NREMS in rats. Rat P2X7 receptor protein varied in the somatosensory cortex with time of day, and P2X7 mRNA was altered by interleukin-1 treatment, by sleep deprivation, and with time of day in the hypothalamus and somatosensory cortex. Mice lacking functional P2X7 receptors had attenuated NREMS and EEG delta power responses to sleep deprivation but not to interleukin-1 treatment compared with wild-type mice. Data are consistent with the hypothesis that extracellular ATP, released as a consequence of cell activity and acting via P2 receptors to release cytokines and other sleep regulatory substances, provides a mechanism by which the brain could monitor prior activity and translate it into sleep.
Keywords: cytokine, interleukin-1, sleep deprivation
it has been known for 100 years that waking activity induces the accumulation of sleep regulatory substances (SRSs) in the brain (23, 31). Such substances enhance or inhibit sleepiness and sleep. Several SRSs, including brain cytokines such as interleukin-1β (IL1) and tumor necrosis factor-α (TNF), are well-characterized for their sleep regulatory roles in health and disease (reviewed in Refs. 29, 27). For instance, injection of IL1 or TNF into humans (thereby mimicking their endogenous accumulation during sleep loss) induces symptoms associated with sleep loss such as sleepiness, fatigue, excess sleep, cognitive dysfunction, and enhanced sensitivity to kindling and pain stimuli (reviewed in Ref. 29). Although SRSs provide a mechanism by which the brain can keep track of prior activity (reviewed in Ref. 36), the literature has failed to characterize what it is about wakefulness that causes enhanced SRS activity. We posit that it is neuro- and gliotransmission-associated release of ATP (29).
ATP is coreleased with neurotransmitters including sites within multiple areas of the brain (reviewed in Refs. 6, 7). ATP is also released from glia cells into the extracellular space (reviewed in Ref. 15). Independent studies demonstrated the ATP-enhanced release of cytokines from immunocytes via purine type 2 (P2) receptors (reviewed in Ref. 14). In the brain, ATP induces IL1 (3, 50), TNF (19), and brain-derived neurotrophic factor (BDNF) (53) release from glia via purine P2 receptors. Extracellular ATP, released from synapses, glia, or immunocytes is important for IL1 processing and release (14, 41). ATP stimulation of microglia via P2X7 receptors (purine P2 receptors) enhances TNF production and the neuroprotective effects of TNF in neuron-glia cocultures exposed to glutamate (44). Since these cytokine gliotransmitters are also putative SRSs we posit that the brain keeps track of prior sleep/wake activity via ATP interaction with purine P2 receptors and the subsequent induction of cytokine release. Conversely, it seems likely that sleep would affect P2 receptor expression and that cytokines may provide a feedback signal to alter P2 receptors. In this report, we show that an ATP agonist promotes non-rapid eye movement sleep (NREMS) while ATP antagonists inhibit NREMS in rats. We also provide evidence that P2X7 receptor expression and protein levels vary in brain with the sleep-wake cycle and are affected by injections of exogenous IL1. Finally, we show that mice lacking the P2X7 receptor have attenuated sleep responses to sleep deprivation but not to IL1. Results are consistent with the hypothesis that release of ATP during neuro- and gliotransmission initiates a series of steps that ultimately provide a mechanism by which the brain tracks its prior state history.
MATERIALS AND METHODS
Agents
The P2 receptor agonist 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium (BzATP) and the P2 receptor antagonist periodate oxidized ATP (OxATP) were purchased from Sigma-Aldrich (St. Louis, MO). The P2 receptor antagonists A438079 and AZ10606120 were purchased from Tocris Bioscience (Ellisville, MO). Murine and human IL1 were purchased from R&D Systems (Minneapolis, MN).
Animals and Surgery
Experimental procedures were approved by the Washington State University Institutional Animal Care and Use Committee and were in compliance with National Institutes of Health guidelines.
Rats.
Male Sprague-Dawley rats (250–300 g) from Taconic Farms (Germantown, NY) were kept on a 12:12-h light-dark cycle (lights on at 0000) at 22 ± 2°C ambient temperature. Surgeries were performed with ketamine-xylazine (87 and 13 mg/kg, respectively) anesthesia. Stainless steel screws for electroencephalographic (EEG) recording were implanted over the left frontal (5 mm anterior and 2 mm lateral from bregma) and right parietal (5 mm posterior and 6 mm lateral from bregma) lobes, and a ground electrode was over the left occipital cortex (11 mm posterior and 4 mm lateral from bregma). The EEG signal used for analyses was that with the least amount of signal artifact. An electromyogram (EMG) electrode was implanted into the nuchal muscles. The leads from the EEG and EMG electrodes were routed to a plastic pedestal (Plastics One, Roanoke, VA). The EEG leads were attached to the skull with dental cement (Patterson Dental Supply, St. Paul, MN). An intracerebroventricular (ICV) guide cannula was implanted (0.8 mm posterior, 1.5 mm lateral from bregma). The patency of the ICV guide cannulas was verified by the ICV injection of 40 ng of angiotensin II (Sigma-Aldrich) in 4 μl of isotonic NaCl (30) 3–4 days after the surgery. This treatment evokes an immediate drinking response. The rats with positive drinking responses were allowed at least 1 wk to recover from the surgery before experiments commenced.
Mice.
Male P2X7 receptor homozygous knockout (P2X7 KO) and wild-type control (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME; strains B6.129P2-P2rx7<tm1Gab>/J and C57BL/6J, respectively). Animals were maintained in sound-attenuated environmental chambers with an ambient temperature of 29 ± 2°C (mouse thermoneutral temperature), a 12:12-h light-dark cycle (lights on 0000, lights out 1200), and free access to food and water. Mice were housed in individual cages with cedar shaving bedding provided. Mouse genotypes were confirmed by Transnetyx (Memphis, TN) from tail tip samples. Mice were 10–19 wk old at the time of experiments.
Mice were anesthetized with intraperitoneal (IP) injections of ketamine and xylazine (87 and 13 mg/kg, respectively). The animals were provided a stainless steel wire EMG electrode in the nuchal muscle and three gold-plated wire EEG electrodes (Plastics One) over the parietal and frontal cortices as previously described (45, 46). As with rats, the EEG signal with the least amount of movement artifact was used. All electrode leads were routed to a plastic pedestal socket (Plastics One) and fixed to the skull with dental cement. Animals were allowed to recover after surgery for at least 7 days before experimental recordings. During the recovery period, animals were connected to the recording cables and handled daily to habituate them to the experimental conditions.
Recording and Analyses
The EEG and EMG were recorded as previously described (46, 47). Recording cables were connected to the amplifiers via commutators (Plastics One). EEG and EMG data were amplified with polygraph amplifiers (Grass-Telefactor, West Warwick, RI) and then digitized at 128 Hz. EEG signals were filtered below 0.1 Hz and above 40 Hz. EMG signals were filtered below 100 Hz and above 4,000 Hz. Sleep-wake activity was recorded and analyzed for 24 h after each treatment, except in experiment 1, where only 22 h was recorded. Vigilance states were determined visually in 10-s epochs with Sleep Sign for Animal software (Kissei Comtec, Nagano, Japan) and employing previously described criteria (26). Briefly, NREMS was characterized by high-amplitude EEG slow waves in the 0.5- to 4-Hz range, and rapid eye movement sleep (REMS) was characterized by very regular low-amplitude waves with a pronounced theta component (6–9 Hz) and a lack of EMG activity, whereas waking was characterized by less regular low-amplitude EEG waves in a variety of frequencies and EMG activity. Sleep data are presented as minutes spent in NREMS or REMS in 2-h or 12-h time bins.
In addition, the EEG waveforms were analyzed by fast Fourier transformation using two types of analyses. First, delta power (0.5–4 Hz) during NREMS was determined. Within each NREMS epoch, delta wave (0.5–4 Hz) [slow-wave amplitude (SWA)] power was determined. EEG SWA values obtained during control recording periods were averaged for each animal and used as a reference value to normalize data across the 24-h data collection period for both the baseline day and experimental day as previously described (26). Values (μV2) obtained during NREMS for each animal for each 2-h time bin during control or experimental recordings were expressed as a percentage of the reference value. The second type of analysis was power spectrum analysis. On the baseline day, EEG power spectra were calculated in 1.0-Hz bins in the 0.5–20 Hz frequency range for the entire 24-h period. EEG power on the baseline and experimental day was expressed as percentage of total baseline power in 1.0-Hz bins. The time blocks analyzed for power spectra varied between experiments; in most cases, the 0–6 h postinjection period was analyzed. In some cases, the time blocks selected were those within which maximum sleep duration effects occurred.
Experimental Protocols
Experiment 1: effects of BzATP on sleep in rats.
DARK-ONSET INJECTIONS.
Three groups of rats (n = 8 for each group) received 4 μl of pyrogen-free sterile saline and on a separate experimental day one dose of BzATP (0.04, 0.4, or 4.0 nmol; 1 dose/group) ICV during the last 30 min of the light period. EEG and EMG were recorded for 22 h. One rat from the 0.04-nmol BzATP dose was excluded from the EEG SWA and EEG power analysis because of EEG artifacts.
LIGHT-ONSET INJECTIONS.
Another three groups of rats were used in this experiment and were subjected to the same protocol as that used at dark onset except that saline or BzATP [0.04 (n = 9), 0.4 (n = 7), or 4.0 (n = 8) nmol in 4 μl of saline; 1 dose/group] was injected ICV within 30 min of light onset and EEG and EMG were recorded for the next 22 h. One rat from the 0.4-nmol BzATP dose was excluded from the EEG SWA and EEG power analysis because of EEG artifacts. Both light- and dark-onset injections were administered because baseline sleep is substantially lower during the dark period compared with that occurring during the light period.
Experiment 2: effects of P2 receptor antagonists on sleep in rats.
A438079.
Three groups of rats (n = 8 for each group) received ICV injection of saline on the control day and the competitive P2X7 receptor antagonist A438079 (1.0, 10.0, or 1,000.0 nmol in 4 μl) on the following day 30 min before light onset. EEG was recorded for 24 h after both injections. One rat from the 1.0-nmol group was eliminated from data analyses because of technical problems with its headpiece.
AZ10606120.
Two groups of rats (n = 8 for each group) were treated as in the previous experiment except that 24 h after the saline injection an allosteric P2 receptor antagonist, AZ10606120 (1.0 or 10.0 nmol in 4 μl saline), was administered.
OXATP.
The protocols for this experiment were identical as those for experiment 1 except that two doses of the noncompetitive inhibitor OxATP (0.4 or 4.0 nmol; n = 8 for each dose), were injected ICV at light onset.
Experiment 3: diurnal variations of P2X7 receptor mRNA and protein levels and their changes after sleep deprivation and IL1 treatment in rats.
Male Sprague-Dawley rats (280–350 g, from Taconic Farms) were kept on a 12:12-h light-dark cycle at 23°C ambient temperature. Three experiments were performed. In experiment 3a, the diurnal variation of P2X7 receptor mRNA and protein levels was determined at 3-h (mRNA) or 6-h (protein) intervals (n = 6 for each group). In experiment 3b, P2X7 receptor mRNA levels in somatosensory cortex and hypothalamus were determined after 6 h of sleep deprivation by gentle handling starting at light onset (n = 10 for both control and experimental groups). Control animals were allowed to sleep ad libitum. Samples from control and sleep-deprived rats were collected immediately after the end of sleep deprivation (0600). In experiment 3c, P2X7 receptor mRNA levels were determined 2 h and 5 h after ICV injection of IL1 (n = 8 for each group). Rats in this experiment were provided an ICV guide cannula as described for the sleep experiments. Only rats exhibiting positive drinking response to angiotensin II were used. IL1 injections were performed 6 h after light onset 4 days after the surgery. Four groups of rats were used. Two groups received 4 μl of physiological saline, and another two groups were injected with a somnogenic dose (2.5 ng) of human recombinant IL1 (R&D Systems). Somatosensory cortex and hypothalamic samples were harvested 2 h and 5 h after control and IL1 injections and then frozen in liquid nitrogen.
REAL-TIME RT-PCR.
RNA was extracted with TRIzol reagent, and cDNA was prepared and analyzed for IL1, P2X7 receptor, and cyclophilin mRNA levels with semiquantitative real-time RT-PCR as previously described (49). Data analysis of mean values (delta Ct, where Ct is threshold cycle) and one-way ANOVA followed by a post hoc analysis (Tukey test) were used. For P2X7 receptor primers were 5′-CTGCAAGATGTCAAAGGTCAAGAC-3′ and 5′-TGGACCTAGGAACCGCTTCTATC-3′ (152 bp), and IL1 and cyclophilin A primers were those previously reported (49).
WESTERN BLOTS.
Protein was isolated from somatosensory cortical samples taken from the opposite side of the brain as the samples analyzed for mRNA. Four groups sampled every 6 h at 0300, 0900, 1500, and 2100 were used. The tissues were homogenized in RIPA lysis buffer with protease inhibitor mixture and 1% phosphatase inhibitor Cocktail I and II (both from Sigma-Aldrich). After centrifugation the supernatant (20 μg) was subjected to 4–20% SDS-PAGE gel (Bio-Rad, Hercules, CA), transferred to nitrocellulose membranes, and then incubated overnight with a rabbit polyclonal antibody against rat P2X7 receptor (1:15,000, Alomone Labs, Jerusalem, Israel) and 1:40,000 dilution of monoclonal mouse antibody β-actin (Sigma-Aldrich). Immunostaining was performed with ECL blot detection (GE Healthcare Bio-Sciences, Piscataway, NJ). The signals from the protein bands were scanned and analyzed with Image J quantification software. The protein levels were normalized with their corresponding β-actin signal, and the results were expressed as percentage of P2X7 receptor protein-to-β-actin ratio.
Experiment 4: sleep in P2X7 receptor KO mice.
Spontaneous sleep-wake activity of P2X7 KO and WT mice (n = 8 for each genotype) was recorded over a 24-h period beginning from dark onset. On the next day, mice were sleep deprived for the 6 h preceding dark onset (0600–1200) by the gentle handling method. When mice were visually determined to be resting, for example, by exhibiting nesting behavior, stillness, or eye closure, they were gently prohibited from sleeping with a soft artist's paintbrush, usually by rearranging the bedding material or, if necessary, softly touching the animal. Starting immediately after deprivation EEG and EMG were recorded for 24 h.
IL-1β TREATMENT.
The same groups of KO and WT mice used in the sleep deprivation experiment were given 0.1 ml of sterile saline IP on the control day and 400 ng of carrier-free recombinant mouse IL1 (R&D Systems) in 0.1 ml of sterile saline 5 min before dark onset (1200). EEG and EMG were recorded for 24 h after both injections.
Statistics
Two- or three-way repeated-measures ANOVA was performed on sleep and power spectra data (factors: time, treatment, and genotype). Where ANOVA indicated a significant effect paired, or independent when appropriate, Student's t-tests were used for post hoc analyses. In experiments 1, 2, and 4, time spent in sleep (NREMS and REMS) and EEG SWA data were analyzed in 2-h time blocks comparing the baseline day and the experimental days in each group. For the P2X7 receptor antagonist experiments, 12-h blocks of time spent in REMS or NREMS were analyzed with Student's paired t-tests. Animals in the first 6 h after the BzATP injections exhibited significant increases in NREMS; therefore, power spectrum for NREMS was analyzed for the first 6-h time block after the injections. Spectral data from the same time block were analyzed after P2 antagonist administration. In addition, after OxATP animals exhibited significant decreases in NREMS during postinjection hours 3–4; therefore, the power spectrum for NREMS was analyzed for this time block and for a time block when NREMS was not affected (22–24 h). Two-way ANOVA (repeated factor: treatment, independent factor frequency) was used to analyze the power spectrum data during NREMS. P < 0.05 was considered to be significant.
RESULTS
Experiment 1
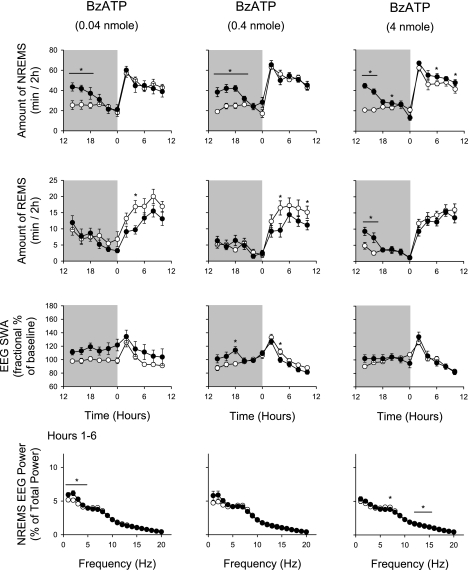
Dark-onset administration of all three doses of BzATP increased duration of NREMS during the first 6–8 h after injection (Fig. 1 and Table 1). After the 0.04-nmol dose, the rats had ∼51 min of extra NREMS during the first 6 h following injections. During the daylight hours, 12–22 h after injection (0000–1000), the two lower doses of BzATP tested failed to alter duration of NREMS compared with control values. The highest dose, 4.0 nmol BzATP, slightly but significantly increased duration of NREMS during this period (243.9 ± 13.4 min on control day vs. 274.4 ± 9.3 min on experimental day). The lower two doses of BzATP had no effect on REMS during the first 12 h after injection but significantly decreased duration of REMS in postinjection hours 12–22 (Fig. 1; 0000–1000). The 4-nmol dose of BzATP increased duration of REMS by ∼10 min for the first 4 h after injection (1200–1600) but had no significant effects on duration of REMS during the rest of the recording period.
Fig. 1.
Nighttime (shaded area) injection of 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) enhanced non-rapid eye movement sleep (NREMS). Rats received 1 of 3 doses of BzATP as indicated at dark onset. All 3 doses enhanced duration of NREMS during the initial 6–8 h after injection. The highest dose also enhanced duration of NREMS in the subsequent light period. BzATP inhibited duration of rapid eye movement sleep (REMS) during the daylight period occurring 12 h after the injection after the lower 2 doses. After the highest dose, REMS was enhanced during the first 4 h after injection. BzATP induced enhancement of electroencephalographic slow-wave activity (EEG SWA). BzATP at the lowest dose enhanced EEG delta power; this effect was absent after the higher doses. EEG power values were obtained during the first 6 h after injection. ○, Control values; ●, values obtained after BzATP injections. *Significant differences between control and test days (P < 0.05, paired t-test).
Table 1.
Changes in NREMS, REMS, EEG slow-wave activity, and EEG power during NREMS in postinjection hours 1–6 after BzATP injection
| BzATP |
|||
|---|---|---|---|
| 0.04 nmol | 0.4 nmol | 4 nmol | |
| Dark-onset administration | |||
| NREMS | |||
| Treatment | F(1,7) = 1.63 | F(1,7) = 34.3* | F(1,7) = 35.7* |
| Interaction | F(10,70) = 5.0* | F(10,70) = 4.0* | F(10,70) = 5.2* |
| REMS | |||
| Treatment | F(1,7) = 34.3* | F(1,7) = 5.5* | F(1,7) = 0.0 |
| Interaction | F(10,70) = 4.0* | F(10,70) = 4.1* | F(10,70) = 4.5* |
| EEG SWA | |||
| Treatment | F(1,7) = 5.3 | F(1,6) = 0.2 | F(1,7) = 0.1 |
| Interaction | F(10,70) = 0.3 | F(10,60) = 3.8* | F(10,70) = 3.2 |
| EEG power hours 1-6 | |||
| Treatment | F(1,140) = 2.6 | F(1,120) = 4.2 | F(1,140) = 0.6 |
| Interaction | F(19,140) = 5.5* | F(19,120) = 3.3* | F(19,140) = 2.5* |
| Light-onset administration | |||
| NREMS | |||
| Treatment | F(1,8) = 6.4* | F(1,6) = 29.9* | F(1,7) = 0.2 |
| Interaction | F(10,80) = 2.4* | F(10,60) = 2.2* | F(10,70) = 2.0* |
| REMS | |||
| Treatment | F(1,8) = 2.1 | F(1,6) = 0.3 | F(1,7) = 8.6* |
| Interaction | F(10,80) = 0.9 | F(10,60) = 4.4* | F(10,70) = 3.4* |
| EEG SWA | |||
| Treatment | F(1,8) = 3.5 | F(1,6) = 0.5 | F(1,7) = 6.6* |
| Interaction | F(10,80) = 2.7* | F(10,60) = 13.0* | F(10,70) = 11.9* |
| EEG power hours 1-6 | |||
| Treatment | F(1,8) = 4.7 | F(1,6) = 3.9 | F(1,7) = 105.8* |
| Interaction | F(19,152) = 3.0* | F(19,114) = 13.2* | F(19,133) = 13.2* |
Values are statistical results for changes in non-rapid eye movement sleep (NREMS), rapid eye movement sleep (REMS), electroencephalographic (EEG) slow-wave activity (EEG SWA), and EEG power during NREMS in postinjection hours 1–6 after 3 doses of 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) injected at dark or light onset.
Significant difference between baseline and treatment condition (P < 0.05).
BzATP induced an increase in EEG SWA during NREMS after all three doses, although the effects were statistically significant only after the 0.4-nmol dose (Table 1). This dose also decreased EEG SWA during NREMS during the light period 14–16 h after injection (0200–0400). The BzATP-enhanced EEG delta power was evident in the power spectra after the 0.04-nmol dose (Fig. 1). Power in higher frequencies after the 0.04-nmol dose was not affected by BzATP, nor was power in any frequency significantly altered after the higher two doses of BzATP during the first 6 postinjection hours (1200–1800), although after the higher dose there were small, but significant, increases in the 11–15 Hz band.
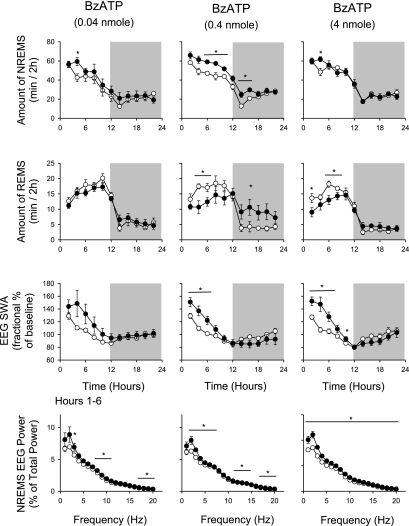
Light-onset administration of BzATP increased duration of NREMS (Fig. 2). After an ∼2-h delay, NREMS increased after all three doses tested (Table 1). The effects were largest after the 0.4-nmol dose: NREMS increased by 62.0 ± 10.6 min after BzATP in the light period (Fig. 2). Increased NREMS persisted into the first 4 h of the dark period (1200–1600). The other two doses tested, 0.04 and 4 nmol, significantly increased duration of NREMS only during postinjection hours 2–4 (Fig. 2). The lower dose of BzATP injected during the daytime had no effect on duration of REMS. In contrast, the higher two doses inhibited REMS during the first 12 h after injection and then promoted duration of REMS during postinjection hours 12–22, although these effects were only significant during postinjection hours 14–16 after the 0.4-nmol dose (Fig. 2).
Fig. 2.
Daytime (light areas) injection of BzATP enhanced duration of rat NREMS, EEG SWA during NREMS, and EEG spectral power. In contrast, duration of REMS was decreased during daylight hours but enhanced in the subsequent dark period. EEG power values were obtained during postinjection hours 1–6. ○, Values from control recordings; ●, values obtained after BzATP injections. *Significant differences between control and test days (P < 0.05, paired t-test).
Light-onset administration of BzATP increased EEG SWA during NREMS (Table 1). These effects were evident after all three doses and were statistically significant after the higher two doses (Fig. 2). EEG power spectrum analyses indicated that delta power was also significantly increased after all three doses during postinjection hours 1–6 (Fig. 2). EEG power in other frequency bands was also increased, although the effects were much smaller (Fig. 2).
Experiment 2
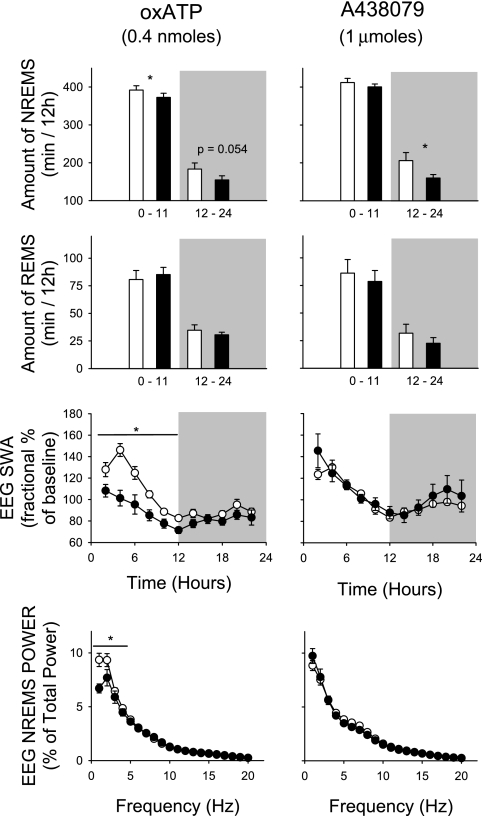
Light-onset administration of the high dose, 1,000 nmol, of A438079 (the competitive inhibitor of P2X7 receptors) suppressed the duration of NREMS (Fig. 3, top right). A slight but nonsignificant decrease in NREMS was observed during the light period, while the decrease was statistically significant during the subsequent dark period. In the first 24 h after the A438079 injection, the rats were awake for an additional 60 min compared with the saline injection day. The two lower doses did not alter NREMS duration or any other sleep parameter (data not shown). Light-onset injection of the high dose of A438079 had little effect on EEG power during the first 6 h after injection. However, it inhibited EEG power during NREMS from 5 to 8 Hz (theta range) during 7 and 8 h after injection (data not shown). Conversely, A438079 treatment elicited a robust increase in the EEG power spectrum during the latter part of the dark period (2200–2300) in the 1–6 Hz range. REMS duration decreased with A438079 administration, but the effect was not significant.
Fig. 3.
Both P2X7 receptor antagonists, injected at light onset (filled bars, ●), suppressed NREMS compared with saline injections (open bars, ○). oxidized ATP (OxATP) attenuated SWA during the first 12 h at the 1–4 Hz frequencies. Although EEG SWA was not significantly affected by A438079 during the first 6 h. With select 2-h time bins A438079 induced a biphasic effect, initially suppressing but then augmenting the EEG power spectra at different frequencies (see text for details, data not shown). Two smaller doses of A438079 were also tested, 1 nmol and 10 nmol; neither dose affected any of the parameters measured (*P < 0.05 between control and test days).
Light-onset administration of 10 nmol of the allosteric P2X7 receptor inhibitor AZ10606120 appeared to suppress NREMS and REMS duration in the dark period (data not shown), but these effects were not statistically significant (P = 0.086). Moreover, there were no AZ10606120-induced differences in the power spectra at any frequency across the 24-h postinjection times.
Light-onset administration of the irreversible P2X7 receptor antagonist OxATP inhibited duration of NREMS during the light period and decreased duration of NREMS in the subsequent dark period, although the latter effect was not significant (Fig. 3, top left). For the 24-h postinjection period these rats were awake ∼50 min more than control rats. Light-onset injections of OxATP inhibited EEG SWA during NREMS during the first 12-h postinjection period (Fig. 3, 0000–1200). During the first 6 h after injection EEG power was greater in the 1–5 Hz frequency range after OxATP treatment (Fig. 3, bottom left).
Experiment 3
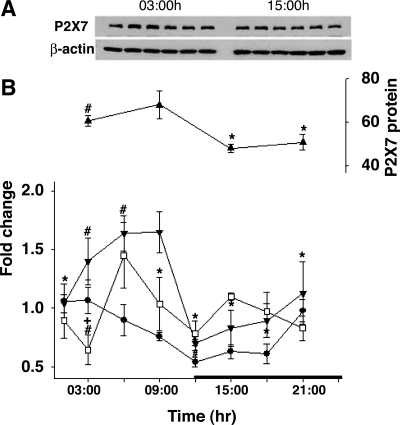
There was a diurnal variation in the levels of P2X7 receptor mRNA in the hypothalamus and the somatosensory cortex (Fig. 4). In the somatosensory cortex, higher levels of both P2X7 receptor mRNA and protein occurred during daylight hours and lower levels occurred at night (Fig. 4). These changes paralleled changes in IL1 mRNA, although the changes in P2X7 receptor levels were more pronounced.
Fig. 4.
Diurnal variation in the relative amount of interleukin-1β (IL1) mRNA and P2X7 receptor mRNA and protein in the hypothalamus and somatosensory cortex. A: Western blot analyses of somatosensory cortex samples showed variation of P2X7 receptor protein levels at 1500 (3 h after light onset) or 0300 (3 h after dark onset). No differences in time of day were observed in the standard, β-actin. B: time course of P2X7 receptor mRNA and protein (▴) and IL1 mRNA (●). The levels of P2X7 receptor protein (somatosensory cortex) and mRNA [(hypothalamus (□) and somatosensory cortex (▼)] were greater during the light period than during the dark period (black horizontal bar indicates dark period). Expression of protein levels was normalized to β-actin levels. *Statistical significance (P < 0.05) from values obtained at 2100; +significant differences from dark onset; #significant differences from values obtained 12 h before.
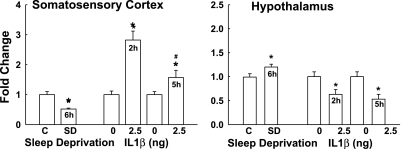
In the somatosensory cortex after 6 h of sleep deprivation, P2X7 receptor mRNA levels were reduced to about half of those occurring spontaneously at the time samples were harvested (0300) (Fig. 5, left). In contrast, in the hypothalamus sleep deprivation induced a slight but significant increase in P2X7 receptor mRNA.
Fig. 5.
Expression of P2X7 receptor mRNA in the somatosensory cortex and hypothalamus after 6 h of sleep deprivation (SD) or 2 h and 5 h after intracerebroventricular injection of 2.5 ng of IL1. Left: SD significantly decreased the levels of P2X7 receptor mRNA in the somatosensory cortex, and IL1 enhanced P2X7 mRNA levels both 2 and 5 h after the injection. Right: in the hypothalamus SD enhanced P2X7 mRNA levels, and IL1 significantly decreased P2X7 receptor mRNA. *Significant difference from corresponding control (C).
Similarly, the effects of IL1 on P2X7 receptor expression were opposite in the somatosensory cortex and hypothalamus. ICV injection of a somnogenic IL1 dose enhanced levels of P2X7 receptor mRNA 2 and 5 h after injection in the somatosensory cortex (Fig. 5, left). In contrast, in the hypothalamus this dose of IL1 decreased P2X7 receptor mRNA levels at both time points.
Experiment 4
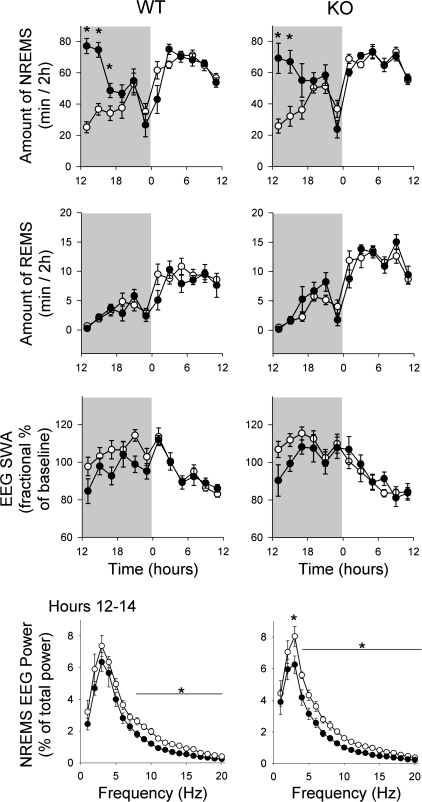
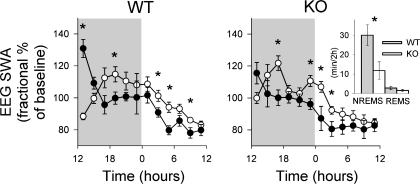
Spontaneous NREMS, REMS, and EEG SWA in P2X7 receptor KO mice did not differ from those observed in WT mice (compare baseline data in Fig. 7). Both strains of mice showed the typical diurnal variations in sleep parameters, with more NREMS and REMS during the light period and less during the dark period. Six hours of sleep deprivation induced different sleep responses in WT and KO mice. WT mice showed significantly larger increases in NREMS duration after sleep deprivation compared with P2X7 receptor KO mice; during the first 2 h after sleep deprivation WT mice had 30 min of additional NREMS while P2X7 receptor KO mice only had 11 min of additional NREMS above that observed during baseline recordings (Fig. 6, inset; P < 0.02, t-test). Although both strains increased duration of REMS in response to sleep deprivation, REMS duration was not significantly higher in either mouse strain. Both strains responded to sleep deprivation with changes in EEG. The initial increase in EEG SWA values during NREMS was greater in WT compared with P2X7 receptor KO mice during the first 2 h after sleep deprivation (P < 0.03, t-test). These initial sleep deprivation-induced increases were followed by decreases in EEG SWA below baseline values that extended for the next 10 h of recording in both strains of mice, as previously described by Jhaveri et al. (24) (Fig. 6).
Fig. 7.
IL1 enhances duration of NREMS and inhibits EEG SWA during NREMS after intraperitoneal injections in P2X7 receptor KO and WT mice. ●, Data from IL1-treated mice; ○, values from saline-treated control mice. Unlike intracerebroventricular or intravenous injections in rats, intraperitoneal injections of IL1 reduced EEG power as previously reported (37). The responses to IL1 were similar in both genotypes (WT, left; KO, right), suggesting that IL1's influence on sleep results from a step downstream from the ATP-P2 receptor interaction. *P < 0.05 difference between baseline and test days.
Fig. 6.
Sleep responses induced by sleep deprivation were attenuated in P2X7 receptor knockout (KO) mice compared with wild type (WT) control mice. Mice were deprived of sleep from 0600 to 1200 and then allowed to sleep ad libitum. WT mice exhibited typical NREMS rebound (right, inset) and EEG SWA enhancements (left) during the first 2 h after deprivation as previously reported (24). In contrast, P2X7 receptor KO mice had attenuated NREMS and EEG SWA responses during the same period. Both strains had reduced EEG SWA for postdeprivation hours 6–22 (1800–1000) as previously reported (24). *P < 0.05 difference between WT and KO mice for NREMS and EEG SWA. ○, Baseline values; ●, values after sleep deprivation.
Changes in sleep-wake activity after IL1 injections were similar in the two genotypes (Fig. 7). In both strains, NREMS duration significantly increased to roughly the same extent compared with IP saline injections [2-way ANOVA, treatment effect first 12 h WT: F(1,14) = 30.17, P = 0.001; KO: F(1,14) = 7.13, P = 0.018]. The duration of REMS was not altered by IL1. IL1 depressed EEG SWA during NREMS in both genotypes, consistent with the previously reported effect of IL-1 on EEG SWA in mice (37). There was a reduction in EEG power across the spectrum during the first 2 h after injection [Fig. 7; 2-way ANOVA treatment effect WT: F(1,14) = 6.37, P = 0.024; KO: F(1,14) = 35.69, P = 0.0001]. In the subsequent daytime hours, 12 h after injection, EEG SWA during NREMS and the EEG power spectra returned to control values in both strains.
DISCUSSION
The results implicate extracellular ATP and P2 receptors in sleep regulation. We demonstrate that an ATP agonist increases sleep, ATP antagonists inhibit spontaneous sleep, and expression of the P2X7 receptor varies with sleep propensity and, if removed, reduces sleep deprivation-induced sleep responses. Previously extracellular ATP had been posited to be involved in sleep regulation (29), and our preliminary report (28) supported this hypothesis. Furthermore, the expression patterns of P2X receptors in zebra fish and ectonucleotidases in zebra fish and rats suggested a role for extracellular ATP in sleep regulation (1, 2). Collectively, the present and prior reports suggest an involvement of extracellular ATP-P2 receptors in sleep regulation.
There are substantial data, primarily pharmacological, in support of a role for adenosine in sleep regulation (reviewed in Ref. 4), suggesting the possibility that the somnogenic actions of BzATP result from its release of adenosine. Thus ICV administration of adenosine is reported to enhance duration of NREMS in rats within a limited dose range (39). However, when we administered the most effective dose, 100 nmol adenosine ICV, to rats in preliminary studies there was no effect on sleep. These results suggest that the sleep-promoting actions of BzATP do not result from its catabolism to adenosine. It seems likely that extracellular adenosine's involvement in sleep regulation is dependent upon its continuous production, some of which would come from extracellular ATP.
The inhibition of NREMS by the P2 antagonists provides evidence that extracellular ATP via P2 receptors is involved in spontaneous sleep. That the P2X7 KO mice had normal spontaneous sleep is likely explained by either developmental compensation or that the multiple P2X and P2Y receptors have redundancy of action. Regardless, P2 agonists and antagonists lack specificity for individual P2 receptors (6, 7); thus the contribution of antagonist inhibition of P2 receptors, in addition to P2X7 receptors, to their sleep inhibitory actions cannot be ruled out.
Halassa et al. (16) described a role for astrocytes in sleep regulation; ATP is released from glia as a consequence of Ca2+ activation. Halassa and colleagues (16) interpreted their results as being the consequence of reduced extracellular adenosine derived from extracellular ATP. Their data are consistent with the attenuated EEG delta power responses to sleep deprivation observed in conditional KO mice lacking the purine type I A1a receptor. However, the typical enhancements in duration of NREMS after sleep loss are not affected by either the conditional removal of the A1a receptor (5) or inhibition of gliotransmission (16). Furthermore, in A1a KO mice (43), or in Drosophila lacking adenosine receptors (55), sleep responses to sleep loss are normal. These results stand in contrast to the present data, where the P2X7 KO mice had deficits in both duration of NREMS and EEG delta power during recovery sleep following sleep deprivation. Furthermore, if P2X7 KO mice are given IL1, which is downstream from the ATP-P2 receptor interaction, they have responses indistinguishable from WT control mice.
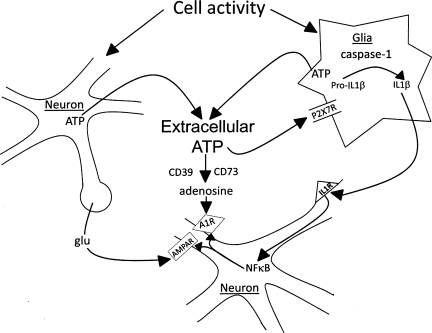
The conversion of extracellular ATP associated with neuro- and gliotransmission to adenosine is regulated enzymatically in the brain (9) (Fig. 8). Ectonucleoside triphosphate diphosphohydrolase 1 (also called CD39) catalyzes ATP and ADP to 5′-adenosine monophosphate (5′-AMP) (40). Ecto-5′-nucleotidase (also called CD73) catalyzes 5′-AMP to adenosine (9). If extracellular adenosine is involved in sleep, it most likely is derived from extracellular, rather than intracellular, ATP. For instance, mice lacking the ability to convert extracellular ATP to adenosine (CD73 KO) have more spontaneous sleep than WT control mice (Zielinski et al., unpublished observations). Cell metabolism, whether neuronal or glial, does not seem a likely source for extracellular adenosine because for every degree of increase in temperature there is an ∼13% increase metabolism (22). However, fever per se does not increase sleep (e.g., see Refs. 25, 26, 51). Such considerations are consistent with the premise that extracellular ATP-modulated K+ channels (P2X receptors) mediate cortical network response state (12).
Fig. 8.
Extracellular ATP involvement in sleep regulation. ATP is released into the extracellular space as a consequence of cell activity during neuro- or gliotransmission. Extracellular ATP then activates P2 receptors (R), e.g., P2X7, that in turn are involved in IL1 processing and release as well as release of other sleep regulatory substances such as tumor necrosis factor and brain-derived neurotrophic factor (not shown). IL1, in turn, activates nuclear factor-κB (NF-κB), leading to changes in receptor trafficking. This changes the cell's long-term sensitivity to neurotransmitters such as glutamate (glu) and to neuromodulators such as adenosine. Extracellular ATP is also catabolized to adenosine via the actions of ectonucleotidases such as CD39 and CD73; this action is faster than the ATP-P2-induced changes in transcription and translation. Both actions of ATP are likely involved in sleep regulation. AMPA, amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid.
Some of the released extracellular ATP acts on glia P2 receptors and causes the release of IL1, TNF, and BDNF as well as additional ATP (see Fig. 8 and Ref. 29). The IL1 precursor pro-IL1 is processed by caspase-1, and this is triggered by ATP activation of P2X7 receptors (14, 41). TNF and IL1 released from glia act on adjacent neurons, leading to the activation of nuclear factor-κB (NF-κB). NF-κB promotes transcription of receptor mRNAs such as the adenosine A1a receptor and the glutamate AMPA receptor-gluR1 mRNAs (29). Translation of those mRNAs into their respective proteins and the subsequent receptor membrane expression would change the sensitivity of the postsynaptic neuron. This is a prototypical scaling effect since the expression of postsynaptic receptors is modulated by the activity of the presynaptic neuron (i.e., the amount of released ATP). Thus the sensitivity of the postsynaptic neuron is scaled to the prior use of the synapse. Indeed, TNF and BDNF are the two molecules most firmly linked to synaptic scaling (52). We view these ATP-P2-cytokine interactions as key sleep regulatory events (29). Mechanistically, the transient increase in extracellular ATP consequent to neuro- and gliotransmission, by interacting with nearby P2 receptors (10), causes the release of IL1, TNF, and BDNF. Such a mechanism offers the possibility for the translation of prior cellular activity into local levels of SRSs (29). SRSs, in turn, by a slow process (gene transcription/translation), alter electrical properties of nearby neurons by altering their own production and that of receptor populations. The SRSs by a fast process (diffusion over short distances) also directly interact with receptors on neurons to alter electrical properties (see, e.g., Refs. 13, 42). Furthermore, some ATP is converted to extracellular adenosine that in turn acts on P1 receptors to influence sleep on a more rapid timescale. These events are posited to happen within local networks (10), and the collective electrical changes result in a shift in input-output relationships within the networks that originally exhibited the enhanced activity, i.e., a state shift. The present results are consistent with this hypothesis.
Sleep is dependent upon the nature of activity during wakefulness, as illustrated in the sleep and learning, sleep and exercise, and sleep and infectious disease literatures. Nevertheless, the specific aspects of our hypothesis for the translation of activity into sleep are thus far tested only indirectly. For instance, we showed that IL1 and TNF expression in neurons is dependent on prior neuronal activity. From that work we are aware that different patterns of waking stimulation resulted in slightly different patterns of cytokine expression in the cortex (8). Such results suggest that SRS expression/release and subsequent SRS-induced sleep are stimulation pattern dependent. Indeed, ATP release is disproportionally greater during high-frequency stimulation (11, 54). IL1 and TNF are also upregulated in brain after excessive stimulation as occurs during kindling (38, 56), during sleep deprivation (18, 21, 33, 48), after whisker stimulation in the somatosensory cortex (17), or during brain inflammation (32). The present demonstration of sleep- and IL1-dependent expressions of P2X7 receptor mRNA suggests that sleep mechanisms feed back to influence brain expression of P2X7 receptors. Our results are consistent with the prior report that IL1 transiently enhances P2X7 receptor mRNA and protein in astrocytes (35). Such findings are also consistent with diurnal variations in P2X7 receptor mRNA and protein reported here to the extent that sleep propensity covaries with expression. How the differential P2 receptor expressions observed in the somatosensory cortex versus the hypothalamus are integrated into sleep regulation remains unknown.
The well-known wake-promoting actions of caffeine are often thought to result from the antagonist actions of caffeine on P1 receptors. However, caffeine inhibits sleep in Drosophila by inhibiting cAMP phosphodiesterase activity independently of adenosine receptors (55). Caffeine also inhibits TNF production via activation of the cAMP/protein kinase A pathway (20); TNF is a well-characterized SRS (29). Finally, ATP-induced currents in PC12 neurons in vitro are also dependent on cAMP-dependent protein kinase (34). Such findings reinforce the posited role for extracellular ATP in sleep regulation and suggest that the paradigm that caffeine promotes wakefulness via adenosine antagonism is incomplete.
In conclusion, the work reported here suggests that extracellular ATP and P2 receptors have a role in sleep regulation and link neuro- and gliotransmission to sleep. Because such actions are also linked to molecules known to be involved in central nervous system connectivity, they suggest that sleep mechanisms cannot be separated from neuronal connectivity.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grants NS-25378 and NS-31453.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Christopher Gabel, Amgen Seattle, for counseling us on purine receptor pharmacology and Dr. Bryan Slinker for providing statistical guidance. We also thank Stewart Bohnet, Marc Urza, Mingxiang Wang, Kathryn Jewett, and Lissette Jimenez for technical help.
Present address of A. De: Dept. of Obstetrics and Gynecology, School of Medicine, University of Missouri-Kansas City, Kansas City, MO 64108.
REFERENCES
- 1. Appelbaum L, Skariah G, Mourrain P, Mignot E. Comparative expression of p2x receptors and ecto-nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174: 66–75, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience 137: 1331–1346, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bianco F, Pravettoni E, Colombi A, Schenk U, Moller T, Matteoli M, Verderia C. Astrocyte-derived ATP induces vesicle shedding and IL-1beta release from microglia. J Immunol 174: 7268–7677, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol 7: 238–245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci 29: 1267–1276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burnstock G. Purinergic signaling—an overview. Novartis Found Symp 276: 26–48, 2006 [PubMed] [Google Scholar]
- 7. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor alpha: activity-dependent expression and promotion of cortical column sleep in rats. Neuroscience 156: 71–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int 52: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem 67: 2180–2187, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci USA 103: 5597–5601, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De A, Krueger JM, Simasko SM. Tumor necrosis factor α increases cytosolic Ca++ responses to AMPA and KCl in primary cultures of rat hippocampal neurons. Brain Res 981: 133–142, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ferrari D, Pizzirani C, Adinolfi E, Lemoli R, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57: 343–346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallett H, Churchill L, Taishi P, De A, Krueger JM. Whisker stimulation increases expression of nerve growth factor and interleukin-1beta immunoreactivity in the rat somatosensory cortex. Brain Res 1333: 48–56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heiser P, Dickhaus B, Opper C, Schreiber W, Clement HW, Hasse C, Hennig J, Krieg JC, Wesemann W. Platelet serotonin and interleukin-1beta after sleep deprivation and recovery sleep in humans. J Neural Transm 104: 1049–1058, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem 75: 965–972, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Horrigan LA, Kelly JP, Connor TJ. Caffeine suppresses TNF alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol 4: 1409–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Hu J, Chen Z, Gorczynski CP, Gorczynski LY, Kai Y, Lee L, Manuel J, Corczynski RM. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFα, and IL-6 levels. Brain Behav Immun 17: 498–504, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci 10: 199–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishimori K. True cause of sleep—a hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakkai Zasshi 23: 423–457, 1909 [Google Scholar]
- 24. Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun 21: 975–987, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johannsen L, Toth LA, Rosenthal RS, Opp MR, Obál F, Jr, Cady AB, Krueger JM. Somnogenic, pyrogenic, and hematologic effects of bacterial peptidoglycan. Am J Physiol Regul Integr Comp Physiol 258: R182–R186, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Kapás L, Bohnet SG, Traynor TR, Majde JA, Szentirmai É, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. J Appl Physiol 105: 1187–1198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med 11: 481–484, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Krueger JM, De A, Taishi P, Wang M, Jimenez L, Urza M, Szentirmai E, Bohnet S. An ATP agonist promotes and an ATP antagonist inhibits sleep (Abstract). Sleep 32: A24, 2009 [Google Scholar]
- 29. Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9: 910–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 276: R1334–R1338, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Legendre R, Pieron H. Des resultats histo-physiologiques de l'injection intra-occipito-atlantoidienne des liquids insomniques. C R Soc Biol (Paris) 68: 1108–1109, 1913 [Google Scholar]
- 32. Leyva-Grado V, Churchill L, Wu M, Williams TJ, Taishi P, Majde JA, Krueger JM. Influenza virus- and cytokine-immunoreactive cells in the murine olfactory pathway and hypothalamus before and after illness onset. J Neuroimmunol 211: 73–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mackiewicz M, Sollars PJ, Ogilvie MD, Pack AI. Modulation of IL-1beta gene expression in the rat CNS during sleep deprivation. Neuroreport 7: 529–533, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Manago Y, Kanahori Y, Shimada A, Sato A, Amano T, Sato-Sano Y, Setsuie R, Sakurai M, Aoki S, Wang YL, Osaka H, Wada K, Noda M. Potentiation of ATP-induced currents due to the activation of P2X receptors by ubiquitin carboxy-terminal hydrolase L1. J Neurochem 92: 1061–1072, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49: 245–258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obál F, Jr, Krueger JM. Biochemical regulation of sleep. Front Biosci 8: 520–550, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Olivadoti MD, Opp MR. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience 153: 338–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res 75: 248–258, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Radulovacki M, Virus RM, Raposa D, Crane RA. A comparison of the dose response effects of pyrimidine ribonucleosides and adenosine on sleep in rats. Psychopharmacology (Berl) 87: 136–140, 1985 [DOI] [PubMed] [Google Scholar]
- 40. Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solle M, Labsi J, Perragaux DG, Stam E, Petrushova N, Killer BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem 276: 125–132, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res 12: 283–290, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 24: 1–7, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci USA 106: 14069–14074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szentirmai E, Kapas L, Sun Y, Smith RG, Krueger JM. Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am J Physiol Regul Integr Comp Physiol 298: R467–R477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Szentirmai É, Yasuda T, Taishi P, Wang W, Churchill L, Bohnet S, Magrath P, Kacsóh B, Jimenez L, Krueger JM. Growth hormone-releasing hormone: cerebral cortical sleep-related EEG actions and expression. Am J Physiol Regul Integr Comp Physiol 293: R922–R930, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Taishi P, Chen Z, Obál F, Jr, Hansen MK, Zhang J, Fang J, Krueger JM. Sleep associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res 18: 793–798, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Taishi P, De A, Alt J, Gardi J, Obal F, Jr, Krueger JM. Interleukin-1β stimulates growth hormone-releasing hormone receptor mRNA expression in the rat hypothalamus in vitro and in vivo. J Neuroendocrinol 16: 113–118, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-induced release and processing of IL-1beta in microglial cells. Crit Rev Immunol 29: 335–345, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun 56: 1785–1791, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verderio C, Bianco F, Blanchard MP, Bergami M, Canossa M, Scarfone E, Matteoll M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biol 35: 187–201, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res 485: 244–250, 1989 [DOI] [PubMed] [Google Scholar]
- 55. Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci 29: 11029–11037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yi PL, Tsai CH, Lin JG, Lee CC, Chang FC. Kindling stimuli delivered at different times in the sleep-wake cycle. Sleep 27: 203–212, 2004 [DOI] [PubMed] [Google Scholar]