Abstract
Chronic hypoxia causes remodeling and alters contractile responses in both pulmonary arteries and pulmonary veins. Although pulmonary arteries have been studied extensively in these disorders, the mechanisms by which pulmonary veins respond to hypoxia and whether these responses contribute to chronic hypoxic pulmonary hypertension remain poorly understood. In pulmonary arterial smooth muscle, we have previously demonstrated that influx of Ca2+ through store-operated calcium channels (SOCC) thought to be composed of transient receptor potential (TRP) proteins is likely to play an important role in development of chronic hypoxic pulmonary hypertension. To determine whether this mechanism could also be operative in pulmonary venous smooth muscle, we measured intracellular Ca2+ concentration ([Ca2+]i) by fura-2 fluorescence microscopy in primary cultures of pulmonary venous smooth muscle cells (PVSMC) isolated from rat distal pulmonary veins. In cells perfused with Ca2+-free media containing cyclopiazonic acid (10 μM) and nifedipine (5 μM) to deplete sarcoplasmic reticulum Ca2+ stores and block voltage-dependent Ca2+ channels, restoration of extracellular Ca2+ (2.5 mM) caused marked increases in [Ca2+]i, whereas MnCl2 (200 μM) quenched fura-2 fluorescence, indicating store-operated Ca2+ entry (SOCE). SKF-96365 and NiCl2, antagonists of SOCC, blocked SOCE at concentrations that did not alter Ca2+ responses to 60 mM KCl. Of the seven known canonical TRP (TRPC1–7) and six vanilloid-related TRP channels (TRPV1–6), real-time PCR revealed mRNA expression of TRPC1 > TRPC6 > TRPC4 > TRPC2 ≈ TRPC5 > TRPC3, TRPV2 > TRPV4 > TRPV1 in distal PVSMC, and TRPC1 > TRPC6 > TRPC3 > TRPC4 ≈ TRPC5, TRPV2 ≈ TRPV4 > TRPV1 in rat distal pulmonary vein (PV) smooth muscle. Western blotting confirmed protein expression of TRPC1, TRPC6, TRPV2, and TRPV4 in both PVSMC and PV. Our results suggest that SOCE through Ca2+ channels composed of TRP proteins may contribute to Ca2+ signaling in rat distal PV smooth muscle.
Keywords: calcium signaling, canonical transient receptor potential, intracellular calcium concentration
the vasomotive activity of the pulmonary venous system plays important roles in regulating both distention and recruitment of blood flow from alveolar wall capillaries, and thus facilitate the ventilation-perfusion matching in the lung. Studies in a variety of species indicate pulmonary veins (PV) exhibit constriction in response to a number of vasoconstrictor stimuli, such as endothelin (2, 3, 5, 26, 34), platelet-activating factor (5, 26), thromboxane (10, 25), and hypoxia (8, 24, 28, 35, 49). During prolonged hypoxic exposure, vasoconstriction and structural alterations occur not only in pulmonary arteries (PA) but also in PV, each contributing a significant portion to total pulmonary vascular resistance (5, 24, 25, 26, 49). Despite the functional importance of both PA and PV in regulating pulmonary circulation being recognized, less attention has been paid to the PV side in searching for the molecular mechanism of pulmonary hypertension.
In vascular smooth muscle, increases in [Ca2+]i is an essential signal for vasoconstriction, as well as for myocyte proliferation (27). A rise in [Ca2+]i can be caused by either release of Ca2+ from internal storage sites, such as sarcoplasmic reticulum (SR), and/or Ca2+ influx from an extracellular source. L-type voltage-dependent Ca2+ channels (VDCC), receptor-operated Ca2+ channels (ROCC), and store-operated Ca2+ channels (SOCC) constitute the major Ca2+ influx pathways (11, 30). VDCC act as the main Ca2+ channel in the vascular smooth muscle cell membrane, activated by membrane depolarization, and can be blocked by Ca2+ channel blockers such as nifedipine (11, 21, 22). ROCC are present in many types of smooth muscle cells. They are typically activated by inositol lipid signaling, one of the most widespread signal transduction cascades, which involves G protein-activated phospholipase C and two second messenger diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). SOCC mediates store-operated Ca2+ entry (SOCE) and thus plays a critical role to refill Ca2+ in SR and to maintain Ca2+ homeostasis. Activation of this pathway can be independent of IP3 production, since various procedures that deplete internal stores, i.e., using thapsigargin or cyclopiazonic acid (CPA), are able to stimulate Ca2+ entry across the plasma membrane without affecting the intracellular IP3 level (6, 9).
The molecular components of SOCC have not been completely understood. A growing body of evidence suggests that it is composed of the mammalian homologs of canonical transient receptor potential (TRPC) proteins (19, 20). So far, seven members of TRPC, termed TRPC1–7, have been identified in mammals (20). Presence of various patterns of TRPC isoforms among studies has been reported in PA (16). We recently demonstrated the predominant expression of TRPC1, TRPC4, and TRPC6 in rat PA smooth muscle and PASMC (16, 38, 39). Vanilloid-related transient receptor potential (TRPV) proteins, which are composed of six members (TRPV1–6), constitute another subfamily of TRP channels. Recent studies have revealed that TRPV channels, i.e., TRPV1, TRPV6, may be involved in mediating SOCE under certain conditions (21, 40). Expression of some of the TRPV proteins has been demonstrated in systemic and pulmonary arterial smooth muscle and smooth muscle cells (40, 46). The expression pattern and role of TRPC and TRPV in PV are unknown.
We recently found that in rat distal pulmonary arterial smooth muscle, SOCE plays roles in hypoxic pulmonary vasoconstriction (42), as well as in the development of chronic hypoxic pulmonary hypertension (39). The general goal of this study is to determine whether these mechanisms could also be operative in PV. As an initial step, the present study was designed to define if SOCE occurs in pulmonary venous smooth muscle cells (PVSMC).
METHODS
PVSMC isolation and culture.
Animal experiment protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. The isolation and culture of PVSMC was adapted from a procedure as we previously described for PASMC (38). Briefly, proximal (2nd generation) and distal (≥4th generations) PV relative to atrium were dissected from lungs of male Wistar rats (ages 8–10 wk). The thin layer of adventitia was carefully stripped off with fine forceps, and the endothelium was wiped off using a cotton swab. The isolated PV smooth muscle layer was sequentially incubated for 20 min in ice-cold physiological salt solution (PSS), 20 min in reduced-Ca2+ PSS (20 μM CaCl2) at room temperature, and 20–23 min in reduced-Ca2+ PSS containing collagenase, papain, BSA, and dithiothreitol at 37°C to disperse PVSMC into a single-cell suspension. The cells were washed twice with PSS, resuspended in Smooth Muscle Growth Medium-2 (Clonetics, Walkersville, MD) containing 5% serum, plated onto 25-mm coverslips, and cultured for 4–6 days in a humidified atmosphere of 5% CO2-95% air at 37°C. Cells were growth arrested in Smooth Muscle Basal Medium (Clonetics) with 0.3% serum for 12–24 h before measurements.
α-Smooth muscle actin immunocytochemistry of PVSMC.
Immunostaining of the cultured cells was carried out as we previously described for PASMC (38). Briefly, cells were fixed with ethanol and incubated with mouse monoclonal antibody for α-smooth muscle actin (α-actin; Sigma, St. Louis, MO). Bound antibodies were probed with Cy3-linked goat anti-mouse antibody (Jackson Labs, West Grove, PA). Nuclei of cells were stained with YO-PRO-1 dimeric cyanine dye (Molecular Probes, Eugene, OR). Primary antibody was omitted in control-staining cells. The stained cell coverslips were mounted on slides with FluoroGuard Antifade (Bio-Rad, Hercules, CA). Cells were examined under a Zeiss LSM-510 inverted laser-scanning confocal microscope with a Zeiss Plan-Neofluor ×40 oil-immersion objective (Atlanta, GA).
RNA extraction and real-time quantitative PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) for distal PV tissue and using RNeasy kit (Qiagen, Valencia, CA) for PVSMC as previously described (16). DNA contamination in RNA preparations was removed by on-column DNase I digestion (Qiagen). mRNA was reverse transcribed by standard reaction using iScript cDNA synthesis kit (BioRad). cDNA was quantified with real-time quantitative PCR (qPCR) in an iCyclerIQ machine (BioRad) using QuantiTect SYBR Green PCR Master Mix (Qiagen). The qPCR reaction mixture of 25 μl contained 400 nM forward and reverse primers and cDNA template from 6.25 ng of RNA. Primers specifically designed for rat TRPC1–7, TRPV1–6, and β-actin are listed in Table 1. The program of real-time qPCR consisted of three steps including a hot start at 95°C for 15 min, 40 cycles with each containing 94°C for 15 s, 57.5°C for 20 s, and 72°C for 20 s, and melting curves performed at 95°C for 1 min, 55°C for 1 min, and 80 repeats of increment of 0.5°C. Detection threshold cycle (CT) values were generated by iCyclerIQ software. Relative concentration of each transcript was calculated using the Phaffl method (16, 23). Data were expressed as a ratio of TRPC or TRPV to β-actin in the same sample. Specificity of the PCR products was confirmed by the melting curve, agarose gel electrophoresis, and DNA sequencing.
Table 1.
Real-time PCR primers for rat TRPCs, TRPVs, and β-actin
| Gene | Accession No. | Primer Sequence, Left/Right | Product Size, bp | Location in Sequence | Positive Control |
|---|---|---|---|---|---|
| TRPC1 | NM_053558 | 5′-AGCCTCTTGACAAACGAGGA-3′ | 146 | 797–942 | Brain |
| 5′-ACCTGACATCTGTCCGAACC-3′ | |||||
| TRPC2 | NM_022638 | 5′-TTGCTGGGAGAGTCTCTGGT-3′ | 139 | 1235–1393 | Brain |
| 5′-CCTCGATCCACACCTCCTTA-3′ | |||||
| TRPC3 | NM_021771 | 5′-GAGATCTGGAATCGGTGGAA-3′ | 127 | 608–754 | Brain |
| 5′-AAAAGCTGCTGTTGGCAGTT-3′ | |||||
| TRPC4 | NM_053434 | 5′-GACACGGAGTTCCAGAGAGC-3′ | 142 | 771–912 | Brain |
| 5′-GTTGGGCTGAGCAACAAACT-3′ | |||||
| TRPC5 | NM_080898 | 5′-CCGCAAGGAGGTGGTAGG-3′ | 121 | 643–784 | Brain |
| 5′-TGTGATGTCTGGTGTGAACTC-3′ | |||||
| TRPC6 | NM_053559 | 5′-TACTGGTGTGCTCCTTGCAG-3′ | 141 | 1267–1407 | Brain |
| 5′-GAGCTTGGTGCCTTCAAATC-3′ | |||||
| TRPC7 | XM_225159 | 5′-AACGAGACCTTCACAGACTAC-3′ | 114 | 1270–1383 | Brain |
| 5′-GCATTCAGACCAGATCATTCC-3′ | |||||
| TRPV1 | NM_031982 | 5′-GCGAGTTCAAAGACCCAGAG-3′ | 79 | 517–595 | Brain |
| 5′-GTGTCATTCTGCCCATTGTG-3′ | |||||
| TRPV2 | NM_017207 | 5′-GCTGGCTGAACCTGCTTTAC-3′ | 138 | 1865–2002 | Brain |
| 5′-CTACAGCAAAGCCGAAAAGG-3′ | |||||
| TRPV3 | NM_001025757 | 5′-AGTGCCTCTCTGGCAACTGT-3′ | 122 | 322–443 | Small intestine |
| 5′-CTGCCTCTGTTCTTCCTTGG-3′ | |||||
| TRPV4 | NM_023970 | 5′-AGCAACCTGGAGACTGTGCT-3′ | 143 | 1171–1313 | Brain |
| 5′-TTGAACTTGCGAGACAGGTG-3′ | |||||
| TRPV5 | NM_053787 | 5′-GTCCTTTCTGGAGCTTGTGC-3′ | 75 | 1014–1088 | Kidney |
| 5′-TCCTTCACAGGGGTTTGTTC-3′ | |||||
| TRPV6 | NM_053686 | 5′-CTGGTTTTTGAGCCCATGAC-3′ | 93 | 605–697 | Brain |
| 5′-AGCACGGACCAAGTTCACAT-3′ | |||||
| β-actin | NM_031144 | 5′-ACGGTCAGGTCATCACTATC-3′ | 90 | 812–901 | Brain |
| 5′-TGCCACAGGATTCCATACC-3′ |
Western blotting.
Samples of PV tissue or PVSMC were homogenized in Laemmli sample buffer containing 62.5 mM Tris·HCl (pH 6.8), 2% SDS, 10% glycerol, 5% protease inhibitor cocktail, 1 mM EDTA, and 200 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride. Protein concentration in the homogenates was quantified using BCA protein assay (Pierce, Rockford, IL). Tissue or cell homogenates were added with dithiothreitol to 150 mM, heated at 95°C for 3 min, and resolved by 10% SDS-PAGE. Separated proteins were transferred onto polyvinylidene difluoride membranes (pore size 0.45 μM, BioRad) and blotted with rabbit polyclonal antibodies against TRPC1 (Sigma), TRPC6, TRPV2, TRPV4, or mouse monoclonal antibody specific for α-actin (Sigma). Bound antibodies were probed with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (KPL, Gaithersburg, MD) and detected using an enhanced chemiluminescence system (ECL; GE Healthcare, Piscataway, NJ).
Measurement of intracellular Ca+ concentration.
As previously described (38), coverslips with PVSMC were incubated with 5 μM fura-2 AM (Invitrogen) for 60 min at 37°C with 5% CO2-95% air, mounted in a closed polycarbonate chamber, and clamped in a heated aluminum platform (PH-2; Warner Instrument, Hamden, CT) on the stage of a Nikon TSE 100 Ellipse inverted microscope (Nikon, Melville, NY). The chamber was perfused at 0.5–1 ml/min with Krebs ringer bicarbonate (KRB) solution equilibrated with 16% O2-5% CO2 at 38°C in heated reservoirs. The perfusate was led via stainless steel tubing and a manifold to an inline heat exchanger (SF-28, Warner Instrument) to rewarm the perfusate just before it entered the cell chamber. The temperature of the heat exchanger and chamber platform was controlled by a dual-channel heater controller (TC-344B, Warner Instrument). Cells were perfused for 10–15 min to remove the extracellular dye, and intracellular Ca2+ concentration ([Ca2+]i) was determined at 12- to 30-s intervals from the ratio of fura-2 fluorescence emitted at 510 nm after excitation at 340 nm to that after excitation at 380 nm (F340/F380) measured in 20–30 cells using a xenon arc lamp, interference filters, electronic shutter, ×20 fluorescence objective, and a cooled charge-coupled device imaging camera. Data were collected online with InCyte software (Intracellular Imaging, Cincinnati, OH). [Ca2+]i was estimated from the calibration curve of F340/F380 measured in standard solutions with [Ca2+] of 0–1,350 nM. For depolarizing cells to assess VDCC, perfusate was supplemented with 60 mM KCl, and NaCl was decreased to 62.7 mM.
Assessment of SOCE.
We assessed SOCE in two ways, Ca2+ restoration and Mn2+ quenching, as previously described (38). Briefly, PVSMC were perfused for 10–15 min with Ca2+-free KRB solution containing 5 μM nifedipine, 10 μM CPA, and 1 mM EGTA. We first measured [Ca2+]i at 0.5-min intervals before and after restoration of extracellular [Ca2+] to 2.5 mM. SOCE were evaluated from the increase in [Ca2+]i caused by restoration of extracellular [Ca2+]. Second, we monitored fura-2 fluorescence excited at 360 nm at 0.5-min intervals before and after addition of MnCl2 (200 μM) to the cell perfusate. Mn2+ competes with Ca2+ to enter the cell; however, it reduces fura-2 fluorescence on binding to the dye. Fluorescence excited at 360 nm is the same for Ca2+-bound and Ca2+-free fura-2; therefore, changes in fluorescence can be assumed to be caused by Mn2+ alone. SOCE was evaluated from the rate at which fura-2 fluorescence was quenched by Mn2+.
Drugs and materials.
Unless otherwise specified, all reagents were obtained from Sigma. TRPC6, TRPV2, and TRPV4 antibodies were purchased from Alomone Laboratories (Jerusalem, Israel). Fura-2 AM (Invitrogen) was prepared on the day of the experiment as a 2.5 mM stock solution in pluronic dimethyl sulfoxide (20% DMSO). Stock solutions of SKF-96365, NiCl2, and mibefradil were made in water at 10, 500, and 30 mM, respectively. Stock solutions of CPA and nifedipine were both made in DMSO at 30 mM.
Statistical analysis.
Data are expressed as means ± SE; n is the number of experiments, which equals the number of animals providing veins or PVSMC. When fura-2 fluorescence was measured, the number of cells in each experiment was 20–30, as indicated in results and the legends for Figs. 1–9. Statistical analyses were performed using Student's t-test. Differences were considered significant when P < 0.05.
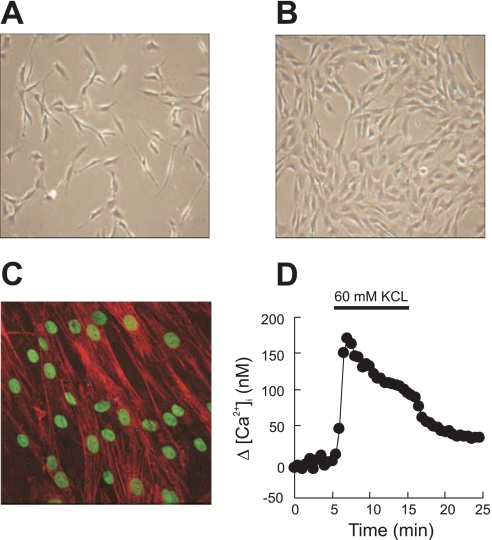
Fig. 1.
Representative phase-contrast microscopy images (objective ×20) of rat distal pulmonary venous smooth muscle cells (PVSMC) in 2–3 days of culture (A) and 4–6 days of culture (B). C: α-actin fluorescent immunostaining in red, with nuclei counterstained in green in PVSMC cultured for 4–6 days (objective ×40). D: representative traces of time course of [Ca2+]i responses to KCl (60 mM) in rat distal PVSMC.
RESULTS
Characteristics of rat distal PVSMC in primary culture.
In phase-contrast imaging, cells cultured for 2–6 days from distal PV appeared spindle-shaped with elongated fibers extended from a larger central area (Fig. 1, A and B). Immunostaining for α-actin showed specific red staining of actin fiber bundles in >98% of cells with green staining for nuclei (Fig. 1C). All cells in the observed fields exhibited increased [Ca2+]i in response to 60 mM KCl, indicating the presence of VDCC (Fig. 1D). These characteristics confirmed that the cells in the primary culture were smooth muscle cells.
SOCE in rat distal PVSMC.
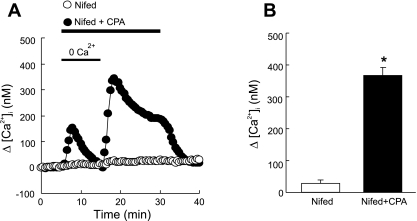
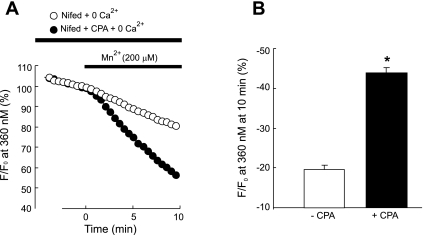
We assessed SOCE by measuring both [Ca2+]i response to extracellular Ca2+ restoration and Mn2+ quenching of fura-2 fluorescence after store depletion with CPA. As seen in Fig. 2A, in the presence of nifedipine, a specific L-type VDCC blockade, perfusion of PVSMC with Ca2+-free KRB solution containing CPA caused an initial small transient increase in [Ca2+]i, which returned to baseline after 5–10 min, indicating calcium release from SR; subsequent restoration of extracellular Ca2+ to 2.5 mM caused a second large increase of [Ca2+]i that went up quickly to a peak of [Ca2+]i = 365 ± 70 nM (Fig. 2, A and B, n = 8, P < 0.0001 vs. the value in the absence of CPA), followed by a lower plateau with final [Ca2+]i = 187 ± 62 nM (n = 8) before it returned to baseline by perfusion of cells with KRB solution. When CPA was omitted in the perfusate, these changes in [Ca2+]i did not happen (Fig. 2, A and B). In the Mn2+ quenching study, perfusion of PVSMC for 10 min with Ca2+-free KRB solution containing Mn2+ resulted in 45 ± 4.5% (n = 7) decrease in fura-2 fluorescence in the presence of nifedipine and CPA, whereas only 20 ± 2.6% decrease in the absence of CPA (n = 5; P < 0.0001 vs. the value in the absence of CPA) (Fig. 3, A and B). These data indicate the occurrence of SOCE after store depletion with CPA in PVSMC. Mibefradil, a selective T-type Ca2+ channel antagonist, did not affect SOCE assessed either by Ca2+ restoration or Mn2+ quenching (data not shown), suggesting Ca2+ entry under these assessments was not mediated by T-type Ca2+ channels.
Fig. 2.
A: time course of [Ca2+]i change (Δ[Ca2+]i) before and after restoration of extracellular [Ca2+] to 2.5 mM in distal PVSMC perfused with Ca2+-free Krebs ringer bicarbonate (KRB) solution containing 10 μM CPA, 1 mM EGTA, and 5 μM nifedipine (Nifed+CPA; n = 8 experiments in 239 cells) and in control cells, which did not receive CPA but were otherwise treated similarly (Nifed; n = 6 experiments in 168 cells). B: average peak change in [Ca2+]i during restoration of extracellular [Ca2+] between 15 and 30 min in cells shown in A. *P < 0.0001 vs. cells absent of CPA.
Fig. 3.
A: time course of fura-2 fluorescence at 360 nm normalized to values at time 0 before and after administration of MnCl2 (200 μM) to distal PVSMC perfused with Ca2+-free KRB solution containing 10 μM CPA, 1 mM EGTA, and 5 μM nifedipine (+CPA; n = 7 experiments in 177 cells) and in control cells, which did not receive CPA but were otherwise treated similarly (−CPA; n = 5 experiments in 137 cells). B: average decrease in fura-2 fluorescence at 10 min after administration of MnCl2 to cells shown in A. *P < 0.0001 vs. cells absent of CPA.
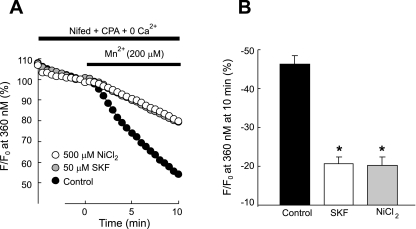
SOCC antagonists, i.e., SKF-96365 and Ni2+, have been shown to block SOCE in various cell types including smooth muscle cells such as PASMC (15, 17, 21, 38, 39); therefore, we evaluated their effects on SOCE assessment to confirm the presence of SOCE in PVSMC. As shown in Fig. 4, although SKF-96365 assessed at 1, 10, and 50 μM or NiCl2 at 50, 200, and 500 μM caused marked attenuation to Ca2+ release by CPA (P < 0.01 compared with control at 189 ± 14.0 nM), the attenuated levels (125 ± 10.3 nM, 106 ± 5.0 nM, and 92 ± 6.5 nM for SKF-96365 at 1, 10, and 50 μM; 128 ± 7.7 nM, 104 ± 8.7 nM, and 101 ± 5.4 nM for NiCl2 at 50, 200, and 500 μM) were not different among different doses (Fig. 4, A–C); however, both SKF-96365 and NiCl2 decreased Ca2+ entry in response to extracellular Ca2+ restoration in a dose-dependent manner, with maximum decrease of the peak of [Ca2+]i response from 338 ± 22.7 nM (n = 8) in untreated control cells to an average of 70 ± 16.5 nM in cells perfused with 50 μM SKF-96365 (n = 5; P < 0.0001 vs. control), and 82 ± 13.8 nM in cells with 500 μM NiCl2 (n = 6; P < 0.0001 vs. control) (Fig. 4, A, B, and D), indicating that SKF-96365 and NiCl2 acted, at least in part, by directly blocking Ca2+ entry. Similarly, in SOCE measured by Mn2+ quenching of fura-2 fluorescence, administration of Mn2+ for 10 min in the perfusate with 50 μM SKF-96365 or 500 μM NiCl2 decreased fura-2 fluorescence by 21 ± 3.3% (n = 5; P < 0.0001 vs. control) and 20 ± 4.1% (n = 5, P < 0.0001 vs. control), respectively; these values were significantly less than the decrease observed in control cells with vehicle (46 ± 5.0%, n = 5) (Fig. 5), confirming that SKF-96365 and NiCl2 blocked Ca2+ entry.
Fig. 4.
Time course effects of different doses of SKF-96365 (A) and NiCl2 (B) on [Ca2+]i change (Δ[Ca2+]i) before and after restoration of extracellular [Ca2+] to 2.5 mM in distal PVSMC perfused with Ca2+-free KRB solution containing 10 μM CPA, 1 mM EGTA, and 5 μM nifedipine. Average peak change in Δ[Ca2+]i before (C, between 0 and 15 min) and after (D, between 15 and 30 min) restoration of extracellular [Ca2+] in cells exposed to SKF-96365 (1, 10, and 50 μM; C), NiCl2 (50, 200, and 500 μM; D), or vehicle control. *Significant difference from respective control (P < 0.05). **P < 0.05 vs. value at 1 μM SKF-96365 or 50 μM NiCl2. ***P < 0.05 vs. value at 10 μM SKF-96365 or 200 μM NiCl2.
Fig. 5.
A: effect of 50 μM SKF-96365 and 500 μM NiCl2 on time course of fura-2 fluorescence at 360 nm normalized to values at time 0 before and after administration of MnCl2 (200 μM) to distal PVSMC perfused with Ca2+-free KRB solution containing 10 μM CPA, 1 mM EGTA, and 5 μM nifedipine. B: average change in fura-2 fluorescence at 360 nm in cells exposed to SKF-96365 (n = 5 experiments in 120 cells), NiCl2 (n = 5 experiments in 118 cells), or vehicle control (n = 5 experiments in 117 cells) at 10 min after administration of MnCl2. *Significant difference from control (P < 0.0001).
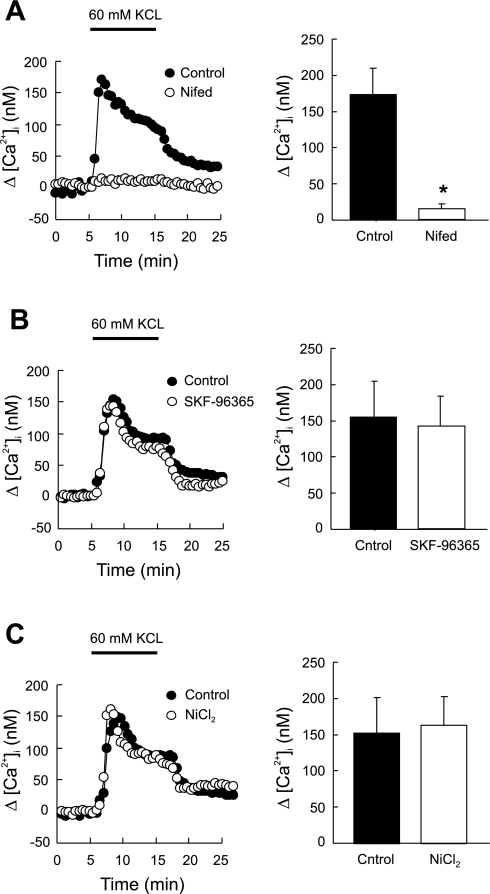
The specificity of 50 μM SKF-96365 or 500 μM NiCl2 on SOCE was verified by testing their alteration on [Ca2+]i response to depolarization with 60 mM KCl. As shown in Fig. 6, A and B, none of them caused significant change in this response (n = 6 each). In contrast, the [Ca2+]i response to KCl was abolished by nifedipine (n = 5; P < 0.0001 vs. control) (Fig. 6C).
Fig. 6.
Effect of nifedipine (5 μM) (A; n = 5 experiments in 145 cells), SKF-96365 (50 μM) (B; n = 6 experiments in 165 cells), and NiCl2 (500 μM) (C; n = 6 experiments in 170 cells) on [Ca2+]i responses to KCl (60 mM) in rat distal PVSMC. *P < 0.0001 vs. control.
TRPC and TRPV expression in distal PVSMC and PV.
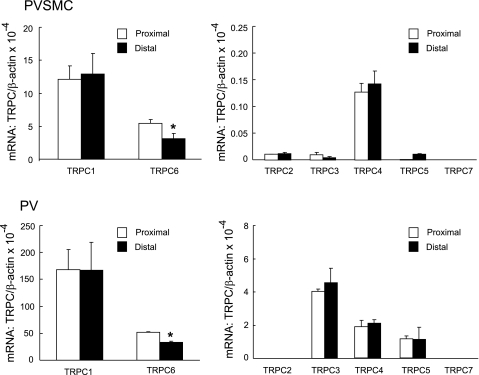
To determine the molecular identity of SOCC responsible for SOCE in distal PVSMC and PV, we screened the expression of TRPC and TRPV transcripts using real-time PCR. Of the seven known isoforms of TRPC, we were able to detect mRNA expression of TRPC1, TRPC2, TRPC3, TRPC4, TRPC5, and TRPC6 with the abundance rank order of TRPC1 > TRPC6 > TRPC4 > TRPC2 ≈ TRPC5 > TRPC3 in distal PVSMC; relative to TRPC1, the expression abundance of TRPC2, TRPC3, TRPC4, TRPC5, and TRPC6 were 9.5 × 10−4, 3.0 × 10−4, 1.1 × 10−2, 8.2 × 10−4, and 2.4 × 10−1, respectively (Fig. 7). Similarly, in PV tissue, we detected TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 mRNA; the rank order of them was TRPC1 > TRPC6 > TRPC3 > TRPC4 ≈ TRPC5 (Fig. 7). TRPC2 was minimally detected in PVSMC, but not in PV. TRPC7 was not found in either distal PVSMC or PV.
Fig. 7.
Expression of TRPC mRNA normalized to β-actin in rat proximal and distal PVSMC (top: n = 5) and PV (bottom: n = 5) as determined by real-time quantitative PCR. *P < 0.05 vs. respective proximal cells or tissue.
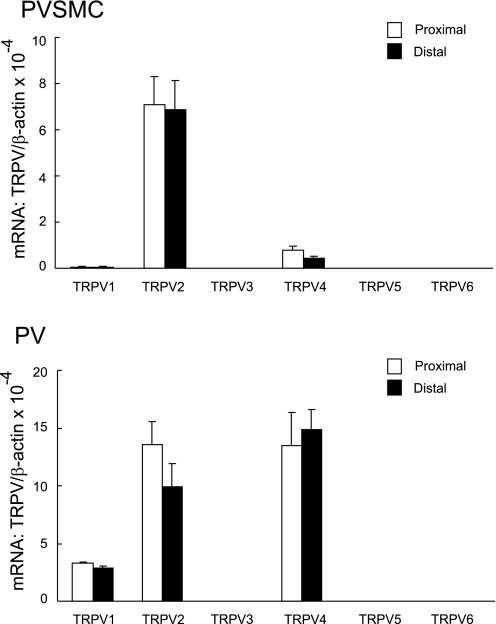
Of the six so far identified subtypes of TRPV (TRPV1–6), we detected the mRNA expression of TRPV1, TRPV2, and TRPV4 in distal PVSMC and PV (Fig. 8). In PVSMC, TRPV2 is 16-fold more abundant than TRPV4, and 110-fold more than TRPV1. In PV tissue, the abundance of TRPV2 and TRPV4 was similar; TRPV1 was three- to fivefold less.
Fig. 8.
Expression of TRPV mRNA normalized to β-actin in rat proximal and distal PVSMC (top: n = 5) and PV (bottom: n = 5) as determined by real-time quantitative PCR.
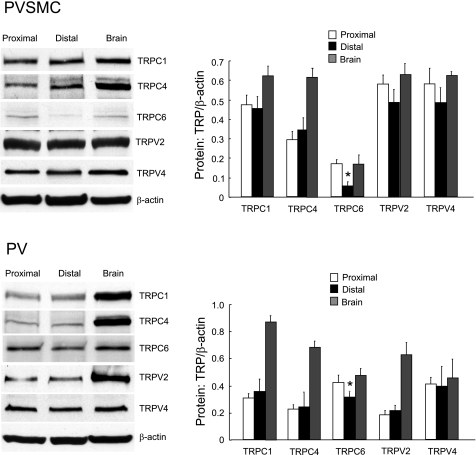
With rat brain as positive control, we demonstrated the protein expression of TRPC1, TRPC6, TRPV2, and TRPV4 by Western blotting, which was found most abundantly expressed among the TRPC and TRPV in both distal PVSMC and PV (Fig. 9).
Fig. 9.
Expression of TRPC and TRPV proteins in rat proximal and distal PVSMC and PV as determined by Western blotting using rat brain as positive control. Data show representative blots for TRPC1, TRPC4, TRPC6, TRPV2, TRPV4, and β-actin in rat distal PVSMC (top: n = 5) and PV (bottom: n = 3). *P < 0.05 vs. respective proximal cells or tissue.
Reduced expression of TRPC6 in distal PVSMC and PV compared with proximal.
As shown in Fig. 7, the mRNA expression profiles and the amount of TRPC and TRPV in proximal and distal PVSMC and PV were similar, with the exception for TRPC5, which was minimally detected in distal PVSMC, but not in proximal cells at all, and for TRPC6, which exhibited reduced expression in distal PV and PVSMC compared with the respective proximal tissue and cells. Western blotting also indicated that the expression of TRPC6 in distal PV and PVSMC is less than proximal; in terms of the expression of TRPC1, TRPV2, and TRPV4, there is no difference between distal and proximal tissue and cells (Fig. 8).
DISCUSSION
The present study demonstrated the occurrence of SOCE and expression of TRPC and TRPV in rat distal PV smooth muscle. Cells isolated and cultured from distal PV were identified to be smooth muscle cells at high purity. To reduce the chance of cardiomyocyte contamination, we chose distal PV to work with, as the main PV that approaches the left atrium was thought to contain cardiac myocytes (18). The cells in the primary culture exhibit the common characteristics of other smooth muscle cells such as PASMC, in terms of the spindle-shaped morphology, the expression of α-actin, and presence of VDCC (47). In this highly pure PVSMC culture, we assessed the presence of SOCE as the increase in [Ca2+]i following restoration of extracellular [Ca2+] and as the decreased rate of Mn2+ quenching, with both blocked by SOCC antagonists Ni2+ and SKF-96365. By screening the expression of various isoforms of TRPC and TRPV, we found TRPC1, TRPC6, TRPV2, and TRPV4 were the predominant ones expressed in PVSMC and PV.
The occurrence of SOCE in PVSMC was demonstrated via the following evidence through three approaches. First, immediately after exposure of PVSMC to Ca2+-free perfusate containing nifedipine and CPA, a small transient rise in [Ca2+]i was observed, indicating Ca2+ release from SR; subsequently, restoring extracellular Ca2+ caused a bigger and sustained increase in [Ca2+]i, reflecting Ca2+ influx. These responses were dependent on store depletion, as they did not occur in PVSMC exposed to Ca2+-free perfusate containing nifedipine but not CPA. Second, to further confirm that depletion of SR Ca2+ stores caused Ca2+ entry, we also determined the rate of Mn2+ quenching on fura-2 fluorescence in PVSMC. Mn2+ is a Ca2+ surrogate; it reduces fura-2 fluorescence upon binding to the dye. The intensity of fluorescence excited at 360 nm is the same (isosbestic) for Ca2+-bound and Ca2+-free fura-2; therefore, changes in fluorescence induced by Mn2+ can be assumed to be due to Mn2+ alone. Before exposure of PVSMC to Mn2+, there was a slow decline in fura-2 fluorescence, a phenomenon that was similar as observed in PASMC, thought to be due to photo bleaching of the dye (38); however, Mn2+ caused a marked increase of this decline in cells exposed to CPA, suggesting enhanced entry through SOCC. In cells not exposed to CPA, Mn2+ only increased quenching of fura-2 fluorescence slightly, suggesting the presence of Ca2+ entry pathways other than SOCC or L-type VDCC. In these assessments, nifedipine was included in the perfusate to block the activity of VDCC, and CPA, a sarcoplasmic-endoplasmic Ca2+-ATPase (SERCA) pump inhibitor, was used to deplete intracellular stores; the single dose of nifedipine at 5 μM and CPA at 10 μM was chosen because they have been proven to be effective in blocking VDCC or depleting SR Ca2+ in various cell types including smooth muscle cells (38). In addition, considering the previous observation that Ca2+ influx through SOCC proteins (i.e., TRPC6) could contribute to membrane depolarization, which may subsequently activate low voltage-activated Ca2+ channels (i.e., T-type Ca2+ channels), mibefradil was included in the perfusate, and we found that SOCE in PVSMC assessed either by Ca2+ restoration or Mn2+ quenching were not influenced by T-type Ca2+ channels. Last, SKF-96365 and Ni2+ are commonly used SOCC antagonists and have been used as a pharmacological tool to identify SOCE in various smooth muscle cell preparations or vascular tissues from different species (7, 14, 21, 38, 41). Therefore, here we determined their effects in PVSMC to confirm the presence of SOCE. Indeed, exposure to SKF-96365 (50 μM) or Ni2+ (500 μM) blocked [Ca2+]i responses to Ca2+ restoration (>75% of reduction) and the rate of Mn2+ quenching to fura-2 fluorescence (>50% of reduction); however, neither of them altered the increase in [Ca2+]i elicited by depolarization with 60 mM KCl, indicating their inhibitory effect on Ca2+ influx was unlikely due to blocking VDCC. Unexpectedly, under the tested doses, SKF-96365 and Ni2+ also caused a significant reduction of the transient Ca2+ release initiated by CPA. Hence, the reduced Ca2+ influx caused by SKF-96365 or Ni2+ could be due to direct inhibition on Ca2+ entry through SOCC and incomplete Ca2+ store depletion. In fact, divergent effects of SKF-96365 on Ca2+ entry and release have been observed in various cell types (14). The mechanisms of how SKF-96365 or Ni2+ inhibited Ca2+ release in PVSMC need to be further identified. Together, these results provide strong evidence indicating the presence of SOCE in rat distal PVSMC. Our results are consistent with a previous report that SOCE exists in PVSMC using thapsigargin (TG) to inhibit SERCA pump activity. Shimizu et al. (29) observed a transient increase in [Ca2+]i caused by TG and a rapid peak increase in [Ca2+]i, followed by a sustained increase in [Ca2+]i after restoring extracellular Ca2+ in canine PVSMC.
Besides the functional demonstration of the presence of SOCE in distal PVSMC, we furthermore found that, of the known isoforms of TRPC, TRPC1 and TRPC6 were the ones predominantly expressed in both distal PVSMC and distal PV smooth muscle. Although we were able to detect the mRNA of TRPC3, TRPC4, TRPC5, and/or TRPC2 in PVSMC and PV, their expression was far less (<100-fold in PVSMC and <35-fold in PV) compared with TRPC1. Regardless of their expression level, all of these TRPC channel proteins have been shown to contribute to SOCE under certain conditions (22). For example, overexpression of TRPC1 mediated by adenovirus vectors enhanced SOCE in rat PA rings (13). In contrast, RNA interference for TRPC1 resulted in attenuated SOCE in mouse osteoblasts (1). Tiruppathi et al. (33) reported that mouse microvascular endothelial cells lacking TRPC4 produced a similar initial increase of intracellular Ca2+ secondary to store depletion, but drastically reduced Ca2+ influx induced by agonists such as thrombin compared with cells from TRPC4 wild-type mice. Conversely, increased expression of TRPC4 was stimulated by agonist, i.e., ATP was associated with enhanced SOCE in human PASMC (48). As for TRPC6, although it has been identified as a putative receptor-operated channel mediating store-independent Ca2+ currents (9), increased expression of TRPC6 was later on also found to be associated with enhanced SOCE in rat PASMC (47). Thus, it has been assumed that SOCC are likely composed of multiple TRPC isoforms, which associate into complex to form heteromeric channels (12). The dominant expression of TRPC1 and TRPC6 suggests they likely play a major role in eliciting SOCE in PV smooth muscle.
The understanding of TRPV proteins as components of SOCC is far more elusive than TRPC. Among the six members of TRPV, mRNA of TRPV1, TRPV2, and TRPV4 were found expressed in PVSMC and PV, whereas TRPV3, TRPV5, and TRPV6 were not. A few studies have suggested that TRPV1 may be involved in SOCE through function as Ca2+ release channels in SR (36, 37, 44). Chronic hypoxia increases TRPV1 expression in association with increased SOCE in human PASMC (46). TRPV2 or TRPV4 are found to be activated by very diverse stimuli, including heat, cell swelling, and mechanical stress in different cell types (23). So far, no evidence indicates if TRPV2 or TRPV4 plays a role in SOCE. Among the various TRPV members, the predominant expression of TRPV2 and TRPV4 in PVSMC suggests they may be involved in thermosensing, osmosensing, mechanosensing, and/or basal Ca2+ homeostasis in these cells.
In the PV vasculature, intralobar distal PV constitutes the major site of vasomotor responses; therefore, in this study, we focused on determination of the presence of SOCE in cells from the distal part, with an attempt to investigate the role of SOCE in regulating the vasomotor tone of PV under normal and disease conditions for future study. Interestingly, we also found that, while the expression profiles of TRPC and TRPV were similar in proximal and distal PV and PVSMC, the mRNA and protein expression of TRPC6 in distal PV and PVSMC were drastically less than proximal. The functional significance of this reduction is unknown at this time; however, we think it may (or partially) reflect the molecular basis of the differential contractile and relaxant properties of proximal and distal PV, a fact that has been found in a previous report (4). In addition, it is also noteworthy that our results could be age related given that aging was thought to affect the contractile response of PV in various species including rat according to several lines of previous observations (31, 32, 45).
In summary, we demonstrated the presence of SOCE and defined the expression profiles of TRPC and TRPV in rat distal PV smooth muscle. Our study may serve as a basis for future identification of the patho/physiological function of SOCE and various cation channel proteins in pulmonary venous vasculature.
GRANTS
This work was supported by National Natural Science Foundation of China (30770953), Chinese Central Government Key Research Projects of the 973 grants (2009CB522107), Guangdong Department of Science and Technology, China (2009B050700041, J. Wang), National Heart, Lung, and Blood Institute Grants R01-HL-093020 and HL-079981 (J. Wang), American Lung Association of Maryland (J. Wang), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2008, J. Wang), China. G. Peng was supported by China Scholarship Council (20073021).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol 26: 146–158, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Aharinejad S, Schraufnagel DE, Miksovsky A, Larson EK, Marks SC., Jr Endothelin-1 focally constricts pulmonary veins in rats. J Thorac Cardiovasc Surg 110: 148–156, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Brink C, Gillard V, Roubert P, Mencia-Huerta JM, Chabrier PE, Braquet P, Verley J. Effects and specific binding sites of endothelin in human lung preparations. Pulm Pharmacol 4: 54–59, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Bronquard C, Maupoil V, Arbeille B, Fetissof F, Findlay I, Cosnay P, Freslon JL. Contractile and relaxant properties of rat-isolated pulmonary veins related to localization and histology. Fundam Clin Pharmacol 21: 55–65, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Raj JU. Role of veins in regulation of pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 288: L213–L226, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci 19: 266–269, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Hillier SC, Graham JA, Hanger CC, Godbey PS, Glenny RW, Wagner WW., Jr Hypoxic vasoconstriction in pulmonary arterioles and venules. J Appl Physiol 82: 1084–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Kadowitz PJ, Hyman AL. Comparative effects of thromboxane B2 on the canine and feline pulmonary vascular bed. J Pharmacol Exp Ther 213: 300–305, 1980 [PubMed] [Google Scholar]
- 11.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharm Rev 49: 157–230, 1997 [PubMed] [Google Scholar]
- 12.Kiselyov K, Patterson RL. The integrative function of TRPC channels. Front Biosci 14: 45–58, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287: L962–L969, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Leung YM, Kwan CY. Current perspectives in the pharmacological studies of store-operated Ca2+ entry blockers. Jpn J Pharmacol 81: 253–258, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L870–L880, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Michelakis ED, Weir EK, Wu X, Nsair A, Waite R, Hashimoto K, Puttagunta L, Knaus HG, Archer SL. Potassium channels regulate tone in rat pulmonary veins. Am J Physiol Lung Cell Mol Physiol 280: L1138–L1147, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Minke B. TRP channels and Ca2+ signaling. Cell Calcium 40: 261–275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev 82: 429–472, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 38: 233–252, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Raj JU, Chen P. Micropuncture measurement of microvascular pressures in isolated lamb lungs during hypoxia. Circ Res 59: 398–404, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Raj JU, Hillyard R, Kaapa P, Gropper M, Anderson J. Pulmonary arterial and venous constriction during hypoxia in 3- to 5-wk-old and adult ferrets. J Appl Physiol 69: 2183–2189, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Raj JU, Toga H, Ibe BO, Anderson J. Effects of endothelin, platelet activating factor and thromboxane A2 in ferret lungs. Respir Physiol 88: 129–140, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Remillard CV, Yuan JX. TRP channels, CCE, and the pulmonary vascular smooth muscle. Microcirculation 13: 671–692, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DW, Farhi LE, Russell JA. Prolonged lobar hypoxia in vivo enhances the responsivity of isolated pulmonary veins to hypoxia. Am Rev Respir Dis 145: 640–645, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Ding X, Murray PA. Intravenous anesthetics inhibit capacitative calcium entry in pulmonary venous smooth muscle cells. Anesthesiology 104: 791–797, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schraufnagel DE, Kurtulus MM, Patel TH. Effect of age on the contraction of pulmonary venous sphincters in rats. Am J Respir Crit Care Med 149: 227–231, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Schindler MB, Hislop AA, Haworth SG. Postnatal changes in pulmonary vein responses to endothelin-1 in the normal and chronically hypoxic lung. Am J Physiol Lung Cell Mol Physiol 292: L1273–L1279, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Toga H, Ibe BO, Raj JU. In vitro responses of ovine intrapulmonary arteries and veins to endothelin-1. Am J Physiol Lung Cell Mol Physiol 263: L15–L21, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Tracey WR, Hamilton JT, Craig ID, Paterson NA. Responses of isolated guinea pig pulmonary venules to hypoxia and anoxia. J Appl Physiol 67: 2147–2153, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release activity. Biochem J 371: 341–350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 36: 19–28, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Wang YX, Wang J, Wang C, Liu J, Shi LP, Xu M, Wang C. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol 223: 151–159, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol 117: 566–572, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Wisnoskey BJ, Sinkins WG, Schilling WP. Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem J 372: 517–528, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wongcharoen W, Chen YC, Chen YJ, Chen SY, Yeh HI, Lin CI, Chen SA. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm 4: 1338–1349, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287: C1192–C1201, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Packer CS, Rhoades RA. Pulmonary vein contracts in response to hypoxia. Am J Physiol Lung Cell Mol Physiol 265: L87–L92, 1993 [DOI] [PubMed] [Google Scholar]