Abstract
Endothelial cell (EC) barrier dysfunction results in increased vascular permeability, a perturbation observed in inflammatory states, tumor angiogenesis, atherosclerosis, and both sepsis and acute lung injury. Therefore, agents that enhance EC barrier integrity have important therapeutic implications. We observed that binding of high-molecular-weight hyaluronan (HMW-HA) to its cognate receptor CD44 within caveolin-enriched microdomains (CEM) enhances human pulmonary EC barrier function. Immunocytochemical analysis indicated that HMW-HA promotes redistribution of a significant population of CEM to areas of cell-cell contact. Quantitative proteomic analysis of CEM isolated from human EC demonstrated HMW-HA-mediated recruitment of cytoskeletal regulatory proteins (annexin A2, protein S100-A10, and filamin A/B). Inhibition of CEM formation [caveolin-1 small interfering RNA (siRNA) and cholesterol depletion] or silencing (siRNA) of CD44, annexin A2, protein S100-A10, or filamin A/B expression abolished HMW-HA-induced actin cytoskeletal reorganization and EC barrier enhancement. To confirm our in vitro results in an in vivo model of inflammatory lung injury with vascular hyperpermeability, we observed that the protective effects of HMW-HA on LPS-induced pulmonary vascular leakiness were blocked in caveolin-1 knockout mice. Furthermore, targeted inhibition of CD44 expression in the mouse pulmonary vasculature significantly reduced HMW-HA-mediated protection from LPS-induced hyperpermeability. These data suggest that HMW-HA, via CD44-mediated CEM signaling events, represents a potentially useful therapeutic agent for syndromes of increased vascular permeability.
Keywords: CD44, vascular permeability, acute lung injury
endothelial cell (EC) barrier dysfunction results in increased vascular permeability, a cardinal feature of inflammation and an essential component of tumor metastasis, angiogenesis, atherosclerosis, and acute lung injury (ALI). There is an important need to find agents that preserve or enhance vascular integrity. We recently demonstrated that the extracellular matrix component high-molecular-weight hyaluronan (HMW-HA) can regulate EC barrier function (51, 53). The potential in vivo therapeutic utility and underlying mechanisms by which HMW-HA regulates vascular integrity are examined in the present study.
Hyaluronan (HA) is a major glycosaminoglycan component of the extracellular matrix of many tissues. Structurally, HA is composed of repeating disaccharide units of d-glucuronic acid and N-acetylglucosamine that exists as a ∼1 × 106-Da random coil structure in vivo (referred to as HMW-HA) (44, 57). Proinflammatory cytokines (TNFα and IL-1β) and LPS induce HA production in EC in vitro (28), and increased HA levels are observed in bronchoalveolar lavage (BAL) fluid from patients with inflammatory lung disorders such as pulmonary fibrosis, ALI, and chronic obstructive pulmonary disease (2, 11, 32, 55). Increased low-molecular-weight fragments of HA (LMW-HA), produced by degradation of HMW-HA by hyaluronidase enzymes and/or reactive oxygen species, are observed in the lungs in experimental models of cigarette smoking, pulmonary hypertension, sepsis/ventilation, and asthma (7, 21, 29, 35). Intratracheal administration of nebulized HMW-HA has been used to prevent injury in experimental emphysema (8), and intravenous administration of HMW-HA protects from sepsis- and intratracheal LPS-induced lung injury (25, 53). HA binds to the hyaladherin family of transmembrane glycoproteins (including CD44), which are expressed in a variety of cells, including EC (47, 48). HA and CD44 regulate IL-2-induced vascular injury syndrome in mouse lung (31, 39).
We and others previously demonstrated HA/CD44 signaling in specialized plasma membrane domains, called caveolin-enriched microdomains (CEM), in EC (34, 47, 51, 53). CEM, also termed detergent-resistant membranes or caveolae, have been implicated in a variety of cellular functions, including potocytosis, cholesterol and Ca2+ regulation, and signal transduction (27, 42, 43, 47). Deletion of caveolin-1 expression in mice inhibits CEM (caveolae) formation in EC and promotes lung pathology (13, 17, 24, 30, 49, 52, 59).
In this study, we utilized several novel techniques, including quantitative proteomic analysis and in vivo models of lung injury utilizing knockout mice and angiotensin I-converting enzyme (ACE) antibody-conjugated liposomal delivery of CD44 small interfering RNA (siRNA), to determine CD44/CEM regulation of HMW-HA-mediated EC barrier function. Increased insights into mechanism(s) by which HMW-HA promotes increased EC barrier function will propel development of novel treatments for disorders characterized by marked vascular barrier disruption.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary microvascular EC (HPMVEC) were obtained from Cambrex (Walkersville, MD) and cultured as previously described (16) in EBM-2 complete medium (Cambrex) at 37°C in a humidified atmosphere of 5% CO2-95% air and used for experimentation at passages 6–10. Unless otherwise specified, reagents were obtained from Sigma (St. Louis, MO). Reagents for SDS-PAGE were purchased from Bio-Rad (Richmond, CA), Immobilon-P transfer membrane and mouse anti-phosphotyrosine antibody from Millipore (Bedford, MA), and gold microelectrodes from Applied Biophysics (Troy, NY). Rabbit anti-von Willebrand factor (vWF) VIII antibody was purchased from Chemicon (Temecula, CA); rat anti-CD44 (IM-7, common domain) antibody from BD Biosciences (San Diego, CA); rabbit anti-caveolin-1 antibody from Cell Signaling Technology (Danvers, MA); mouse anti-annexin II antibody, rabbit anti-protein S100-A10 antibody, mouse anti-filamin A antibody, and goat anti-filamin B antibody from Santa Cruz Biotechnology (Santa Cruz, CA); anti-fibrillarin, anti-cyclooxygenase (COX) IV, anti-lysosomal-associated membrane glycoprotein 2 precursor (LAMP2b), and anti-Golgi reassembly stacking protein (GRASP65) antibodies from Abcam (Cambridge, MA); recombinant human IL-2, goat anti-mouse ACE ectodomain antibody, and goat anti-vascular endothelial (VE)-cadherin antibody from R & D Systems (Minneapolis, MN); mouse anti-β-actin antibody, LPS, and OptiPrep from Sigma; secondary horseradish peroxidase-labeled antibodies from Amersham Biosciences (Piscataway, NJ); and DOTAP and DOPE from Avanti Polar Lipids (Alabaster, AL).
Preparation and quantitation of HMW-HA.
HMW-HA was prepared as described elsewhere (51). Briefly, 500 mg of rooster comb HA (∼1 × 106-Da polymers) (6) were dissolved in distilled water and centrifuged in a 1 × 105-Da molecular-weight-cutoff filter (Ultrafree-MC, Millipore, Bedford, MA), and the flow-through (<1 × 105 Da) was discarded. HMW-HA was quantitated using an ELISA-like competitive binding assay with a known amount of fixed HA and biotinylated HA-binding peptide as the indicator (38). Fluorescein conjugation of HMW-HA was performed according to the procedure described by de Belder and Wik (9).
CEM isolation.
CEM, known as detergent-resistant membranes or lipid rafts, were isolated from HPMVEC as we previously described (50, 51, 53). Triton X-100-insoluble materials were mixed with 0.6 ml of cold 60% OptiPrep and overlaid with 0.6 ml of 40%-20% OptiPrep, the gradients were centrifuged (35,000 rpm) in an SW60 rotor for 12 h at 4°C, and different fractions were collected and analyzed.
Immunoprecipitation and immunoblotting.
Cellular materials from treated or untreated HPMVEC were incubated with immunoprecipitation buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1% Nonidet P-40, 0.4 mM Na3VO4, 40 mM NaF, 50 μM okadaic acid, 0.2 mM phenylmethylsulfonyl fluoride, and a 1:250 dilution of Protease Inhibitor Mixture 3 (Calbiochem)]. The samples were immunoprecipitated with anti-CD44 or anti-dynamin 2 IgG, subjected to SDS-PAGE in 4–15% polyacrylamide gels, transferred onto Immobilon membranes, and developed with specific primary and secondary antibodies. Visualization of immunoreactive bands was achieved using enhanced chemiluminescence (Amersham Biosciences).
Inhibition of protein expression in human EC by siRNA.
HPMVEC were transfected with siRNA against specific mRNA (Santa Cruz Biotechnology), with siPORTamine used as the transfection reagent (Ambion) according to the protocol provided by Ambion. Cells (∼40% confluent) were serum-starved for 1 h and then incubated with 250 nM target siRNA (or scramble siRNA or no siRNA) for 6 h in serum-free medium. The serum-containing medium was added (10% serum final concentration) for 42 h before biochemical experiments and/or functional assays were conducted. Effective silencing of target protein expression was determined with immunoblot analysis of siRNA-transfected EC lysates with use of specific antibodies.
Measurement of EC electrical resistance.
EC were grown to confluence in polycarbonate wells containing evaporated gold microelectrodes, and transendothelial electrical resistance (TER) was measured using an electrical cell substrate impedance-sensing system (Applied Biophysics), as previously described in detail (16). TER values from each microelectrode were pooled at discrete time points and plotted against time as means ± SE.
CD44 cross-linking.
HPMVEC were plated on eight-well glass cover slides and allowed to adhere for 48 h. Cells were then serum-starved for 1 h and incubated with rat anti-CD44 (IM-7) antibody at 10 μg/ml for 2 h. The cells were then washed briefly in PBS, pH 7.4, and the secondary anti-rat IgG (1 μg/ml; Sigma-Aldrich) was added for CD44 antibody cross-linking (36). The cells were incubated for 1 h before fixation in 4% paraformaldehyde (Electron Microscopy Services). Cells were then immunostained for CD44 using a directly labeled mouse anti-CD44-Alexa 488 conjugate (Cell Signaling Technology). Cells were mounted using ProLong antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI).
Immunocytochemistry.
HPMVEC were grown to confluency on glass coverslips and serum-starved for 1 h prior to addition of 100 nM HMW-HA. EC were then washed in PBS, pH 7.4, fixed with 4% paraformaldehyde, and probed with anti-VE-cadherin antibody or anti-caveolin-1 antibody and secondary fluorescent antibodies (Alexa Fluor, Molecular Probes, Invitrogen, Carlsbad, CA). EC were then incubated with ProLong Gold antifade reagent with DAPI (Invitrogen) and analyzed using a Nikon Eclipse TE 300 microscope.
Protein digest, iTRAQ labeling, and strong cation-exchange fractionation.
Control and HMW-HA-treated (100 nM, 5 min) CEM were further purified to enrich plasma membrane-associated proteins by wheat germ agglutinin affinity, as previously described (58). The resulting proteins were dissolved in 1% SDS and 100 mM triethylammonium bicarbonate (TEAB) and diluted five times with 100 mM TEAB. The samples (50 μg each) were reduced, alkylated, and digested with trypsin with a protein-to-enzyme ratio of 20:1 at 37°C overnight. Each digest was concentrated to 15 μl, and 15 μl of 1 M TEAB were added. The isobaric tagging for relative and absolute quantitation (iTRAQ) reagent was dissolved in 70 μl of ethanol and added to the digest. The mixture was incubated at room temperature for 1 h. Control and HMW-HA-treated CEM proteins labeled with different iTRAQ reagents were mixed and dried to a volume of 50 μl. The combined-peptide mixture was fractionated by strong cation-exchange (SCX) chromatography on an Agilent 1100 HPLC system using a PolySULFOETHYL A column (4.6 × 100 mm, 5 μm, 300 Å, PolyLC). The sample was dissolved in 1 ml of SCX loading buffer [25% (vol/vol) acetonitrile and 10 mM KH2PO4, pH 3] and loaded and washed isocratically for 30 min at 50 μl/min. Peptides were eluted with a linear gradient of 0–500 mM KCl [25% (vol/vol) acetonitrile and 10 mM KH2PO4, pH 3] over 30 min at a flow rate of 50 μl/min. The absorbance at 214 nm was monitored, and 15 fractions were collected along the gradient, as we previously described (20).
LC-MS analysis.
Each SCX fraction was dried and dissolved in 0.1% formic acid. The resulting fractions were analyzed on Qstar Pulsar (Applied Biosystems-MDS Sciex) interfaced with an HPLC system (model 1100, Agilent), as we previously described. Peptides were separated on a reverse-phase column packed with 10 cm of C18 beads (360 × 75 μm, 5 μm, 120 Å, YMC ODS-AQ, Waters, Milford, MA) with an emitter tip (New Objectives, Woburn, MA) attached. The HPLC gradient was 5–40% B for 60 min (A: 0.1% formic acid; B: 90% acetonitrile in 0.1% formic acid), and the flow rate was 300 nl/min. Survey scans were acquired from mass-to-charge ratio of 400–1,200 with up to three precursors selected for MS/MS using a dynamic exclusion of 45 s. A rolling collision energy was used to promote fragmentation, and the collision energy range was ∼20% higher than that used for unlabeled peptides due to iTRAQ tags (20).
MS data analysis.
The MS/MS spectra were extracted and searched against the SwissPro-Unipro database using ProteinPilot software (Applied Biosystems) with the Paragon method and the following parameters: Homo sapiens as species, trypsin as enzyme (1 missed cleavage allowed), cysteine static modification with methylmethanethiosulfate, and iTRAQ (peptide labeled at the NH2 terminus and lysine) as sample type, as we previously described. Mass tolerance was set to 0.15 atomic mass unit for precursor and 0.1 atomic mass unit for fragment ions. The raw peptide identification results from the Paragon algorithm (Applied Biosystems) searches were further processed using the Pro Group algorithm (Applied Biosystems) within the ProteinPilot software before they were displayed. The Pro Group algorithm uses the peptide identification results to determine the minimal set of proteins that can be reported for a given protein confidence threshold. The protein confidence threshold cutoff for this study is total ProtScore of 2.0 (unused) with at least one peptide with 99% confidence. The mean, standard deviation, and P values to estimate statistical significance of the protein changes were calculated by Pro Group. For ProteinPilot software, the hypothesis being tested is as follows: “The actual protein ratio is 1, and the observed protein ratio is different from 1 by chance.” Proteins identified with changes (ratio >1.2 or <0.8) that were consistent between two independent experiments starting from cell cultures were manually validated and quantified. Peak areas for each of the signature ions (114, 115, 116, and 117) were obtained and corrected according to the manufacturer's instructions to account for isotopic overlap, and only those signature ions with intensities <1,500 counts were used for quantitation (20).
ACE antibody-conjugated liposomal delivery of CD44 siRNA.
Liposomes were prepared utilizing a 1:1 molar ratio of DOTAP to DOPE dissolved in chloroform to a concentration of 10 mg/ml. The solvent was evaporated in a water bath set at 50°C under nitrogen. The resulting dry lipid film was immediately suspended in 100 μl of PBS (pH 7.4, final concentration 20 mg/ml). The cationic lipid dispersion was combined with siSTABLE CD44 siRNA (1 μg per 10 μg lipid) in a glass container. The liposome-siRNA mixture was sonicated in a water bath sonicator (Fisher Scientific, Itasca, IL) to clarity. For the ACE antibody, primary amines were blocked with sulfo-NHS acetate in PBS (pH 7.4) and incubated for 1 h at room temperature. The solution was then filtered with a 30-kDa filter (Ultrafree-MC) and adjusted to a final concentration of 0.2 mg/ml. The modified ACE antibody was cross-linked to liposomes containing CD44 siRNA by covalent linking of the carboxyl groups on ACE antibody with the amine groups on liposomes utilizing 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloric acid reagent (Pierce, Rockford, IL). Labeled liposomes were purified by dialysis in a Slide-A-Lyzer (20-kDa cutoff; Pierce) against a 1,000-fold excess volume of sterile PBS (pH 7.4) overnight. Sterile ACE-conjugated liposomes containing CD44 siRNA (100 μl) were injected into the internal jugular vein of C57B6/6J mice. Animals were treated for 5 days with siRNA (10 mg/kg).
Animal preparation and treatment.
Male CD44 knockout, caveolin-1 knockout, or C57BL/6J wild-type mice (8–10 wk old; Jackson Laboratories, Bar Harbor, ME) pretreated with or without ACE antibody-conjugated CD44 siRNA-containing liposomes (see above) were anesthetized with intraperitoneal ketamine (150 mg/kg) and acetylpromazine (15 mg/kg). LPS (2.5 mg/kg) or saline (control) was intratracheally instilled. After 4 h, HMW-HA (1.5 mg/kg) or saline control was injected intravenously via neck incision to expose the internal jugular vein, as we previously described (53). The animals were allowed to recover for 24 h after treatment before BAL protein analysis and/or lung immunohistochemistry. All animal procedures were approved by the Institutional Animal and Care and Use Committee of the University of Chicago.
Murine lung immunohistochemistry.
For characterization of protein expression in mouse lung vascular EC, lungs from C57BL/6J control (untreated) mice were fixed in formalin, 5-μm paraffin sections were obtained and hydrated, and epitope retrieval was performed (Target Retrieval Solution, pH 6.0, DakoCytomation, Carpinteria, CA). The sections were histologically evaluated by FITC-conjugated anti-CD44 antibody or anti-factor VIII (vWF) antibody and secondary fluorescent antibody [Alexa Fluor 610 (for vWF) and 350 (for CD44); Molecular Probes, Invitrogen]. Negative controls for immunohistochemical analysis were performed by the method described above, but without primary antibody. Immunofluorescent-stained sections were photographed (×100) using a Leica Axioscope (Bannockburn, IL).
Determination of BAL protein concentrations.
BAL was performed by an intratracheal injection of 1 ml of Hanks' balanced salt solution followed by gentle aspiration. The recovered fluid was processed for protein concentration (bicinchoninic acid protein assay kit; Pierce), as previously described (53).
Statistical analysis.
Student's t-test was used to compare the means of data from two or more different experimental groups. Results are expressed as means ± SD.
RESULTS
Role of CD44 and caveolin-1 in HMW-HA regulation of human pulmonary EC barrier function.
To determine the role(s) of CD44 and caveolin-1 on HMW-HA-mediated EC barrier function, we initially sought to characterize in vitro isolated CEM materials. Consistent with previous published data (51), CEM isolated from human EC (4°C Triton X-100-insoluble material localized to the 20% OptiPrep layer upon discontinuous gradient centrifugation) contains the CEM marker caveolin-1 but excludes other subcellular markers (Fig. 1A). The standard form of CD44 (CD44s) localizes to CEM (Fig. 1B). As CEM formation is dependent on cholesterol, we observe that localization of caveolin-1 and CD44s to the 20% OptiPrep fraction is abolished with cholesterol depletion by methyl-β-cyclodextrin (MβCD). Furthermore, silencing (siRNA) of CD44 or caveolin-1 (but not annexin A11) expression inhibits HMW-HA binding to human EC (Fig. 1, C and D).
Fig. 1.
Analysis of CD44 and caveolin-1 regulation of high-molecular-weight hyaluronan (HMW-HA) binding to human pulmonary endothelial cells (EC). A: EC were grown to confluency and serum-starved for 1 h, and Triton X-100-soluble, Triton X-100-insoluble, and OptiPrep fractions were prepared. The 20% OptiPrep fraction represents the caveolin-enriched microdomain (CEM) fraction. Fractions were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-caveolin-1 (a), anti-fibrillarin (b), anti-cyclooxygenase (COX) IV (c), anti-lysosomal-associated membrane glycoprotein 2 precursor (LAMP2b, d), anti-Golgi reassembly stacking protein 65 (GRASP65, e), or anti-VEGF receptor (anti-VEGFR, f). B: EC were grown to confluency, serum-starved for 1 h, and either left untreated (control) or treated with 100 nM HMW-HA (5 min) or the CEM-abolishing cholesterol-depletion agent methyl-β-cyclodextrin (MβCD, 5 mM) for 1 h prior to 100 nM HMW-HA treatment (5 min). Cellular material was solublized in 4°C 1% Triton X-100, and soluble and insoluble fractions were obtained. Triton X-100-insoluble fraction was overlaid with 60%, 40%, 30%, and 20% OptiPrep and centrifuged at 35,000 rpm in an SW60 rotor for 12 h at 4°C. Triton X-100-soluble material and OptiPrep fractions were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-caveolin-1 (a), anti-CD44s (IM-7, standard domain, b), or anti-VEGF receptor 2 (anti-VEGFR2, c) antibody. The 20% OptiPrep fraction is the CEM fraction. Experiments were performed in triplicate, with highly reproducible findings, and representative data are shown. C: immunoblot analysis of small interfering RNA (siRNA)-treated or untreated human EC. Cellular lysates from untransfected (control, no siRNA), scramble siRNA (siRNA that does not target any known human mRNA), caveolin-1 siRNA, or CD44 siRNA transfection were analyzed using immunoblotting with anti-caveolin-1 (a), anti-CD44 (IM-7, b), or anti-actin (c) antibody. Experiments were performed in triplicate, each with similar results, and representative data are shown. D: quantitation of fluorescein-conjugated HMW-HA binding to scramble siRNA-, annexin A11 siRNA-, CD44 siRNA-, or caveolin-1 siRNA-treated EC. Fluorescein-conjugated HMW-HA (100 nM) was added for 15 min to EC in serum-free medium, cells were washed 3 times in serum-free medium, and fluorescence intensity was quantified. Cells were counted utilizing a hemocytometer.
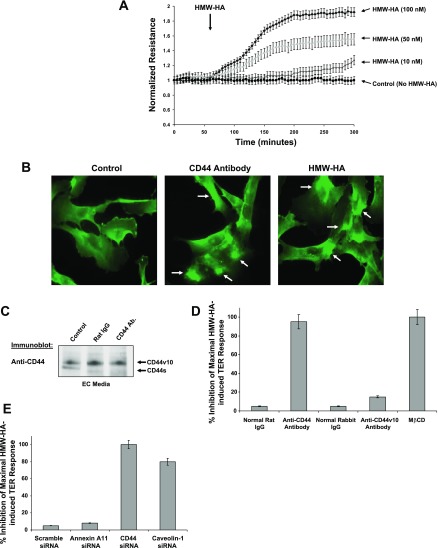
We next examined the functional significance of CD44 and CEM on HMW-HA-mediated human EC barrier function. Consistent with our previous published data (51), we observed that HMW-HA promotes EC barrier enhancement in a dose-dependent manner, reflected by increases in TER (Fig. 2A). Structurally, we observed that HMW-HA causes clustering of CD44 on human EC, similar to cross-linked CD44 antibody treatment (Fig. 2B). However, cross-linked CD44 antibody does not induce EC barrier enhancement, indicating that clustering of CD44 receptors alone is not sufficient for this effect (data not shown). HMW-HA-mediated EC barrier enhancement was attenuated by the pan-CD44 antibody IM-7, which does not cause significant CD44 shedding (Fig. 2C), and cholesterol depletion (MβCD), but not with an antibody specific to CD44v10 (Fig. 2D). Silencing (siRNA) CD44 or caveolin-1 (but not annexin A11) blocked HMW-HA-mediated EC barrier enhancement, further validating the specific regulatory roles of CD44 and CEM (Fig. 2E).
Fig. 2.
Analysis of CD44 and caveolin-1 regulation of HMW-HA-mediated human EC barrier enhancement. A: EC were plated on gold microelectrodes, serum-starved for 1 h, and treated with PBS, pH 7.4 (control), or 10, 50, or 100 nM HMW-HA. Arrow indicates HMW-HA addition. Transendothelial electrical resistance (TER) trace represents pooled means ± SE from 3 independent experiments. B: HMW-HA induces CD44 receptor clustering. Human pulmonary microvascular EC were plated on 8-well glass cover slides and allowed to adhere for 48 h. Cells were then serum-starved for 1 h and incubated with 100 nM HMW-HA for 15 min and fixed in 4% paraformaldehyde or incubated with rat anti-CD44 (IM-7) antibody at 10 μg/ml for 2 h. Cells were then washed briefly in PBS, pH 7.4, and secondary anti-rat IgG (1 μg/ml; Sigma-Aldrich) was added for CD44 antibody cross-linking (36). Cells were incubated for 1 h before fixation in 4% paraformaldehyde. Cells were then immunostained for CD44 with use of a directly labeled mouse anti-CD44-Alexa 488 conjugate (Cell Signaling Technology). C: anti-CD44 antibody (IM-7) does not induce appreciable CD44 shedding in human EC. Human EC were serum-starved for 1 h prior to the addition of no antibody, rat IgG (1 μg/ml), or rat anti-CD44 (IM-7) antibody (1 μg/ml) for 3 h. EC medium was collected, concentrated, and immunoblotted with anti-CD44 (IM-7) antibody. D: percent inhibition of maximal HMW-HA-induced TER response (as described in A) with addition of normal rat IgG, anti-CD44 antibody, normal rabbit IgG, anti-CD44v10 antibody (10 μg/ml), or 5 mM MβCD. E: percent inhibition of maximal HMW-HA-induced TER response (as described in D) in human EC with scramble, annexin A11, CD44, or caveolin-1 siRNA treatment.
Immunocytochemical study of the role of HMW-HA in CEM dynamics in intact EC.
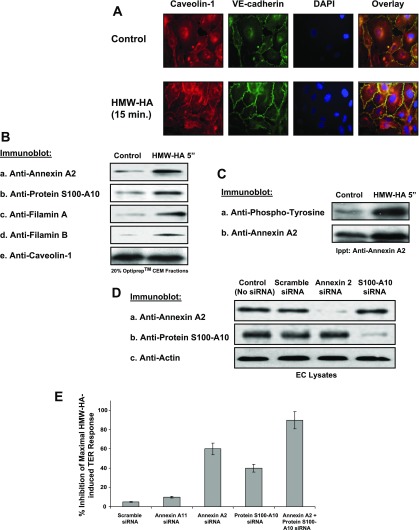
CEM are dynamic structures that can redistribute in EC under certain conditions, including cytokine stimulation, laminar flow, and migration (18, 54). Therefore, we examined the effects of HMW-HA treatment of human EC on CEM dynamics. Our results indicate that human EC challenged with HMW-HA (15 min) induced CEM recruitment to cell-cell junctions (Fig. 3A), where these structures are likely involved in regulation of cell-cell junctional dynamics.
Fig. 3.
Role of HMW-HA-induced recruitment of annexin A2 and protein S100-A10 to human EC CEM. A: HMW-HA induces caveolin-1 redistribution to EC-EC junctions. Human EC were grown to confluency, serum-starved for 1 h, and either left untreated (control) or treated with 100 nM HMW-HA (15 min), fixed in 4% paraformaldehyde, and stained with anti-caveolin-1 antibody, anti-vascular endothelial (VE)-cadherin antibody, or 4′,6-diamidino-2-phenylindole (DAPI). Overlay is a merged image of caveolin-1, VE-cadherin, and DAPI fluorescence, with yellow color indicating colocalization of caveolin-1 and VE-cadherin. B and C: EC were grown to confluency, serum-starved for 1 h, and either left untreated (control) or treated with 100 nM HMW-HA (5 min) and CEM fractions (20% OptiPrep layer). B: CEM fractions were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-annexin A2 (a), anti-protein S100-A10 (b), anti-filamin A (c), anti-filamin B (d), or anti-caveolin-1 (e) antibody. Experiments were performed in triplicate, with highly reproducible findings, and representative data are shown. C: CEM fractions were solublized in immunoprecipitation (Ippt) buffer and immunoprecipitated with anti-annexin A2 antibody. Resulting immunobeads were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine (a) or anti-annexin A2 (b) antibody. Experiments were performed in triplicate, with highly reproducible findings, and representative data are shown. D: EC were treated with no siRNA (control), scramble siRNA, annexin A2 siRNA, or protein S100-A10 siRNA for 48 h. EC lysates were obtained and run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-annexin A2 (a), anti-protein S100-A10 (b), or anti-actin (c) antibody. Experiments were performed in triplicate, with highly reproducible findings, and representative data are shown. E: percent inhibition of maximal HMW-HA-induced TER response in human EC with scramble, annexin A11, annexin A2, protein S100-A10, or annexin A2 + protein S100-A10 siRNA treatment. Silencing both annexin II and protein S100-A10 is required for maximal inhibition of HMW-HA-induced TER in EC.
Use of quantitative proteomic analysis to identify novel human EC proteins recruited to CEM by HMW-HA.
Our data suggest a crucial role for CEM in HMW-HA-mediated, CD44-dependent EC barrier enhancement. To identify potential signaling molecules recruited to CEM with HMW-HA challenge, we performed quantitative proteomic analysis (iTRAQ), as we previously described (20). Aliquots (50 μg) of CEM proteins, isolated and precipitated from confluent human EC (untreated control vs. HMW-HA challenged, 100 nM, 5 min), were reduced and alkylated, and the resulting peptides were labeled with iTRAQ reagents (114 and 115). The two samples were mixed and analyzed by LC-MS/MS. The MS/MS fragmentation of the iTRAQ-labeled peptides results in signature peaks (114.1 and 115.1) for quantitation, and the fragmentation along the peptide backbone results in b- and y-type fragments, which may be used to identify the peptide sequence. In the second independent experiment, the samples were labeled with iTRAQ reagents 116 and 117. Several proteins showed specific recruitment into CEM upon HMW-HA treatment, and these results were consistent between the two independent experiments. The 10 highest HMW-HA-recruited CEM proteins from the 2 independent experiments are displayed in Table 1.
Table 1.
CEM proteins upregulated with HMW-HA stimulation
| HMW-HA/Control |
||||
|---|---|---|---|---|
| %Coverage | Accession No. | Name | Expt 1 | Expt 2 |
| 42.0 | ANXA2_HUMAN(P07355) | Annexin A2 (annexin II, lipocortin II, calpactin I heavy chain, p36 | 1.40 | 1.57 |
| 39.6 | S10AA_HUMAN(P60903) | Protein S100-A10 (S100 Ca2+-binding protein A10, calpactin-1 light chain | 1.43 | 1.67 |
| 28.0 | FLNA_HUMAN(P21333) | Filamin-A (α-filamin, filamin-1, endothelial actin-binding protein | 1.37 | 1.49 |
| 31.4 | FLNB_HUMAN(O75369) | Filamin-B (FLN-B, β-filamin, actin-binding-like protein, filamin 3 | 1.48 | 1.36 |
| 46.1 | AHNK_HUMAN(Q09666) | AHNAK (desmoyokin, fragments) | 1.33 | 1.17 |
| 65.1 | ACTG_HUMAN(P63261) | Actin, cytoplasmic 2 (γ-actin) | 1.11 | 1.19 |
| 43.5 | PLEC1_HUMAN(Q15149) | Plectin-1 (PLTN, PCN) | 1.25 | 1.07 |
| 24.1 | TSP1_HUMAN(P07996) | Thrombospondin-1 precursor | 1.45 | 1.18 |
| 33.5 | AMPN_HUMAN(P15144) | Aminopeptidase N (alanyl aminopeptidase, gp150, CD13 antigen) | 1.34 | 1.16 |
| 20.2 | FETUA_HUMAN(P02765) | α-2-HS-glycoprotein precursor (fetuin-A) | 1.50 | 1.79 |
| 40.1 | CAV1_HUMAN(Q03135) | Caveolin-1 | 1.07 | 1.09 |
Endothelial cells (EC) were grown to confluency, left untreated or treated with high-molecular-weight hyaluronan (HMW-HA, 100 nM) for 15 min, and washed with PBS, and caveolin-enriched microdomains (CEM) were isolated using low-density detergent-resistant fractionation. Plasma membrane proteins were enriched by wheat germ agglutinin affinity and precipitated and reconstituted in 1% SDS and 100 mM triethylammonium bicarbonate (pH 8.5). Proteins were digested, labeled with isobaric tagging for relative and absolute quantitation (iTRAQ) reagents, and combined. Resulting peptides were fractionated using strong cation exchange chromatography and analyzed on LC-MS/MS. The 10 proteins with the highest HMW-HA-to-control ratio are listed. Caveolin-1 is positive control.
Role of annexin A2, protein S100-A10, and filamin A/B in HMW-HA-mediated actin cytoskeletal reorganization and EC barrier function in vitro.
Of the 10 proteins that exhibited the greatest recruitment to CEM with HMW-HA treatment as identified by quantitative proteomic analysis, we focused on 4 proteins chosen for known CEM localization and CD44 association (annexin A2 and protein S100-A10) (34) and regulation of CD44-mediated actin cytoskeletal reorganization (filamin A/B) (4). To validate our MS results, immunoblot analysis of isolated CEM was performed. Under control conditions, basal levels of the cytoskeletal regulatory proteins annexin A2, protein S100-A10, filamin A, and filamin B exist in isolated lipid rafts (Fig. 3B). HMW-HA challenge (100 nM, 5 min) results in robust recruitment of these proteins to isolated CEM. The CEM marker caveolin-1 did not exhibit altered CEM recruitment following HMW-HA stimulation, consistent with our previously reported MS data results (20, 51).
Annexin A2 is a Ca2+-dependent phospholipid- and actin-binding protein that is regulated by tyrosine phosphorylation and complex formation with protein S110-A10 (12, 41, 45). Immunoprecipitation of solublized CEM proteins from control and HMW-HA-treated human EC reveals that HMW-HA induces tyrosine phosphorylation of annexin A2 in CEM (Fig. 3C). Furthermore, silencing (siRNA) of annexin A2 or protein S100-A10 expression (Fig. 3D) partially inhibits HMW-HA-mediated EC barrier enhancement (Fig. 3E).The same effects are not observed with silencing of annexin A11 expression, indicating the specific requirement for annexin A2. Silencing of annexin A2 and protein S100-A10 produces additive effects on inhibition of HMW-HA-mediated EC barrier enhancement, indicating the absence of redundant function of these molecules.
Filamins are a family of cortical actin cross-linking proteins (12, 41). Our data indicate that HMW-HA induces recruitment of filamin A and filamin B to CEM, which is blocked by silencing expression of annexin A2 and protein S100-A10 (Fig. 4A), consistent with a novel regulatory role of these molecules. Silencing (siRNA) of filamin A or filamin B expression (Fig. 4B) inhibits HMW-HA-mediated EC barrier enhancement, indicating the importance of these molecules in barrier regulation (Fig. 4C). There was a mild synergistic effect with silencing of filamin A and filamin B, indicating that these molecules may have overlapping functions.
Fig. 4.
Annexin A2 and protein S100-A10 regulation of HMW-HA-induced filamin A/B recruitment to CEM and human EC barrier enhancement. A: immunoblot analysis of HMW-HA-treated (100 nM, 5 min) or untreated human EC lysates from scramble siRNA, annexin A2 siRNA, protein S100-A10 siRNA, or annexin A2 + protein S100-A10 siRNA transfection using anti-filamin A (a), anti-filamin B (b), or anti-caveolin-1 (c) antibody. Experiments were performed in triplicate, each with similar results, and representative data are shown. Silencing annexin A2 and protein S100-A10 blocks HMW-HA-induced filamin A and filamin B recruitment to CEM. B: EC were treated with no siRNA (control), scramble siRNA, filamin A siRNA, or filamin B siRNA for 48 h. EC lysates were obtained and run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-filamin A (a), anti-filamin B (b), or anti-actin (c) antibody. Experiments were performed in triplicate, with highly reproducible findings, and representative data are shown. C: percent inhibition of maximal HMW-HA-induced TER response. EC were plated on gold microelectrodes and treated with scramble siRNA (control), annexin A11 siRNA, filamin A siRNA, filamin B siRNA, or filamin A + filamin B siRNA for 48 h. After EC were serum-starved for 1 h, 100 nM HMW-HA was added. Values represent pooled TER data ± SE at 30 min after addition of agonist from 3 independent experiments.
Our previous published data indicate that the actin cytoskeleton plays an essential role in EC barrier regulation (14, 16, 50, 51). HMW-HA induces a dramatic redistribution of the actin cytoskeleton to form a cortical actin “ring,” which is important for EC barrier function (Fig. 5A). This HMW-HA-induced cortical actin formation is blocked when CEM are abolished (MβCD treatment), indicating the importance of this microdomain in actin cytoskeletal dynamics. Furthermore, silencing (siRNA) of CD44, caveolin-1, annexin A2, protein S100-A10, filamin A, or filamin B inhibits HMW-HA-mediated cortical actin formation in human EC (Fig. 5B, Table 2).
Fig. 5.
Analysis of HMW-HA-mediated human EC actin cytoskeletal rearrangement. A: immunofluorescent images of HMW-HA-induced EC cortical actin rearrangement. EC were serum-starved for 1 h and either left untreated (control) or treated with 100 nM HMW-HA for 15 min or pretreated with 5 mM MβCD for 1 h and then with 100 nM HMW-HA for 15 min. Cells were then fixed and stained with tetramethylrhodamine isothiocyanate (TRITC)-phalloidin (to visualize F-actin) and analyzed using fluorescent microscopy. Observations are representative of the entire cell monolayer and were reproduced in multiple independent experiments (n ≥ 3 for each condition). B: immunofluorescent images of HMW-HA-induced EC cortical actin rearrangement as described in A. Human EC were treated with scramble siRNA (control), CD44 siRNA, caveolin-1 siRNA, annexin A2 siRNA, or protein S100-A10 siRNA for 48 h. After EC were serum-starved for 1 h, 100 nM HMW-HA was added for 15 min.
Table 2.
Quantitation of HMW-HA-induced cortical actin formation in human EC
| Cortical Actin Phalloidin Staining/Total Phalloidin Staining | SAGV at 5 min |
|---|---|
| 1. Control (no siRNA) | 7 ± 0.4 |
| 2. Scramble siRNA | 8 ± 0.4 |
| 3. HMW-HA | 75 ± 3.6 |
| 4. HMW-HA + scramble siRNA | 73 ± 3.5 |
| 5. HMW-HA + CD44 siRNA | 10 ± 0.7 |
| 6. HMW-HA + caveolin-1 siRNA | 16 ± 0.8 |
| 7. HMW-HA + annexin A2 siRNA | 40 ± 2.9 |
| 8. HMW-HA + protein S100-A10 siRNA | 56 ± 3.2 |
| 9. HMW-HA + annexin A2 siRNA + protein S100-A10 siRNA | 14 ± 0.9 |
| 10. HMW-HA + filamin A siRNA | 26 ± 1.4 |
| 11. HMW-HA + filamin B siRNA | 32 ± 1.2 |
| 12. HMW-HA + filamin A/B siRNA | 8 ± 0.8 |
Values are means ± SD of ≥10 cells per sample; experiments were performed in triplicate. Polymerized actin rearrangement was assessed with Texas Red-conjugated phalloidin (Molecular Probes, Eugene, OR) and analyzed using a Nikon Eclipse TE 300 microscope, as we previously described (16). Computer-recorded .tiff images were analyzed with ImageQuant software (Amersham Biosciences, Piscataway, NJ). A standardized average gray value (SAGV) was generated for total phalloidin staining vs. cortical phalloidin staining for each cell (50). Percent cortical actin staining was calculated as follows: (cortical actin SAGV × area) ÷ (total actin SAGV × area) × 100. siRNA, small interfering RNA
Role of HMW-HA and CD44 in vascular integrity in vivo.
Our data indicate that CD44 and caveolin-1 are colocalized in the lung vasculature (Fig. 6A), a finding similar to our in vitro results. Our data indicate that intravenous administration of HMW-HA confers vascular protection in a LPS-induced murine model of ALI, with the protective HMW-HA effects greatly diminished in the CD44 and caveolin-1 knockout mouse (Fig. 6B). To further implicate endothelial CD44 as a regulator of HMW-HA-mediated vascular protection, we target CD44 siRNA selectively to the lung vasculature. We utilized ACE antibodies, as previously described, for delivery of catalase to the lung endothelium, with consequent attenuation of ischemia-reperfusion injury (33). Intravenous delivery of ACE antibody-conjugated liposomes containing CD44 siSTABLE siRNA (Dharmacon; 10 mg/kg, 5 days) selectively silenced CD44 expression in lung vasculature, but not in kidney vasculature or immune cells (Fig. 6, C and D). Inhibition of CD44 expression in the pulmonary vasculature increases basal vascular leakiness, as determined by total BAL protein concentrations in a CD44 siRNA dose-dependent manner (Fig. 6E).
Fig. 6.
Pulmonary vascular CD44 regulation of HMW-HA-induced protection from LPS-induced vascular hypermeability. A: immunohistochemical fluorescently stained images of control (untreated) mouse lung [bright-field (DIC) imaging (a) or treatment with anti-Factor VIII (von Willebrand factor) antibody (b), anti-caveolin-1 antibody (c), or FITC-conjugated anti-CD44 antibody (d) and secondary fluorescent antibody (Alexa Fluor 610 for vWF and 350 for caveolin-1; e)]. Magnification ×100. Arrows in e (an overlay of b, c, and d) indicate immunostaining of endothelial cells. Insets: negative controls for immunohistochemical analysis, which were obtained by the method described above, but without primary antibody. B: bronchoalveolar lavage (BAL) protein concentration from wild-type (C57BL/6J), CD44 knockout, or caveolin-1 knockout mice that were anesthetized and given saline (control) or LPS (2.5 mg/kg) intratracheally. After 4 h, mice were injected intravenously (internal jugular vein) with saline (control) or HMW-HA (1.5 mg/kg). Treated mice were allowed to recover for 24 h, BAL fluids were obtained, and protein concentrations were determined. *Significant (P < 0.05) difference between LPS and HMW-HA + LPS (n = 6 per condition). C: immunohistochemical analysis of murine lungs and kidneys either left untreated or injected intravenously (internal jugular vein) with angiotensin I-converting enzyme (ACE) antibody-conjugated liposomes (DOTAP/DOPE) containing siControl siRNA (10 mg/kg) or siSTABLE CD44 siRNA (10 mg/kg) for 5 days. Vascular CD44 expression is inhibited in lung, but not kidney. D: immunocytochemical analysis of cytospin material from BAL of mice injected intravenously (internal jugular vein) with ACE antibody-conjugated liposomes (DOTAP/DOPE) containing siControl siRNA (10 mg/kg) or siSTABLE CD44 siRNA (10 mg/kg) for 5 days. There is no difference in CD44 immunoreactivity. E: total BAL protein of B6129N2 mice injected intravenously (internal jugular vein) with ACE antibody-conjugated liposomes (DOTAP/DOPE) containing siControl siRNA (5 or 10 mg/kg) or siSTABLE CD44 siRNA (5 or 10 mg/kg) for 5 days (n = 6 per condition). *Statistically significant difference (P < 0.05) between siControl and siCD44. F: BAL protein concentration from C57BL/6J mice injected intravenously (internal jugular vein) with ACE antibody-conjugated liposomes (DOTAP/DOPE) containing scramble siRNA (10 mg/kg) or siSTABLE CD44 siRNA (10 mg/kg) for 5 days. Mice were then anesthetized and given saline (control) or LPS (2.5 mg/kg) intratracheally. After 4 h, mice were injected intravenously (internal jugular vein) with saline (control) or HMW-HA (1.5 mg/kg). Treated mice were allowed to recover for 24 h, BAL fluids were obtained, and protein concentrations were determined. *Significant (P < 0.05) difference between LPS and HMW-HA + LPS (n = 6 per condition).
LPS administered via an intratracheal route induces murine inflammation and increased vascular leakiness (37). Figure 6F indicates that intravenous injection of the CD44 ligand HMW-HA (1.5 mg/kg) 4 h after LPS delivery attenuated pulmonary hyperpermeability in C57BL/6J wild-type mice. In contrast, this potent protective effect of HMW-HA on LPS-induced inflammatory lung injury was markedly attenuated in mice that had previously received ACE antibody-conjugated liposomes containing CD44 siRNA to selectively inhibit pulmonary vascular CD44 expression. These data indicate that the protective effects of HMW-HA on LPS-induced pulmonary hyperpermeability are dependent on lung vascular CD44 expression.
DISCUSSION
This study presents several novel observations, including the findings that caveolin-1 and pulmonary vascular expression of CD44 regulate HMW-HA-mediated protection from ALI in vivo. Silencing (siRNA) of CD44 or caveolin-1 expression in EC in vitro reveals the importance of these molecules in HMW-HA cellular binding and barrier enhancement. Furthermore, CD44 signaling to actin cytoskeletal regulatory proteins in CEM regulates HMW-HA-mediated EC barrier enhancement. Specifically, HMW-HA binding to CD44s induces annexin A2 tyrosine phosphorylation and annexin A2/protein S100-A10 translocation to CEM. Annexin A2 and protein S100-A10 are crucial for subsequent HMW-HA-induced recruitment of filamin A and filamin B to CEM, actin cytoskeletal reorganization (cortical actin formation), and EC barrier enhancement (Fig. 7).
Fig. 7.
Proposed model of HMW-HA-induced vascular integrity. HMW-HA binding to CD44s in caveolin-enriched microdomains (CEM) in human EC (1) induces annexin A2 tyrosine phosphorylation and annexin A2/protein S100-A10 translocation to CEM (2). Annexin A2 and protein S100-A10 are crucial for subsequent HMW-HA-induced recruitment of filamin A and filamin B to CEM (3), actin cytoskeletal reorganization (cortical actin formation) (4), and EC barrier enhancement (5).
Utilizing immunocytochemistry, we determined that HMW-HA induces CD44 receptor clustering and CEM redistribution to areas of cell-cell contact, similar to HMW-HA-mediated F-actin redistribution (51). In caveolin-1 knockout fibroblasts, there is aberrant actin cytoskeletal structure, indicating that CEM can regulate actin cytoskeletal dynamics (18). Redistribution of CEM could regulate actin reorganization at areas of cell-cell contact, thus enhancing barrier function.
Using quantitative proteomic analysis, we determined that annexin A2, protein S100-A10, filamin A, and filamin B are specifically recruited to CEM upon HMW-HA stimulation and that annexin A2 phosphorylation was increased with HMW-HA challenge, findings validated by immunoblot analysis. We speculate that, upon HMW-HA stimulation, phospholipid and actin cytoskeletal regulatory proteins are recruited to CEM and stabilize a rigid F-actin cytoskeleton, which increases EC barrier protection. Our results are consistent with previous findings that annexin A2 associates with CD44 in lipid rafts and regulates HA-mediated actin cytoskeletal changes (34). Annexin A2 requires tyrosine phosphorylation and is associated with protein S100-A10 for plasma membrane recruitment (12, 41), results consistent with our findings. Our siRNA experiments confirmed the regulatory role of annexin A2 and protein S100-A10 in HMW-HA-induced EC barrier enhancement and suggested that annexin A2 and protein S100-A10 are nonredundant in HMW-HA-induced EC barrier protection.
Interestingly, annexin A2 has been reported to be a profibrinolytic coreceptor for plasminogen and tissue plasminogen activator (40, 45). Increased levels of annexin II in humans promote excessive bleeding, while annexin A2 knockout mice have excessive fibrin in blood vessels and reduced clearance of injury-induced thrombi (40). HMW-HA could therefore conceivably influence the regulation of the blood coagulation cascade through annexin A2 plasma membrane recruitment. This is in agreement with our published data indicating that HMW-HA inhibits the enzymatic activity of the blood coagulation regulatory serine protease HA-binding protein 2 (also called factor VII-activating protease) (26).
The cortical actin cross-linking protein filamin is intimately involved in EC barrier regulation (3) and is a downstream target of HA/CD44 signaling (4). We have extended these findings to show that HMW-HA challenge of human EC induces recruitment of filamin A and filamin B to CEM, with this recruitment dependent on annexin A2/protein S100-A10. Our siRNA experiments confirmed the regulatory role of filamin A and filamin B in HMW-HA-induced EC barrier enhancement and suggested that filamin A and filamin B have significant overlapping roles in HMW-HA-induced EC barrier protection. This is consistent with the phenotype of filamin A knockout mice, which have numerous cardiovascular abnormalities, and filamin B knockout mice, which exhibit microvascular defects (15, 60).
CEM, a subset of lipid rafts containing caveolin-1, have been implicated in numerous EC functions (27, 42, 43, 47). Deletion of caveolin-1 expression in mice inhibits CEM (caveolae) formation in EC and promotes lung fibrosis and microvascular hyperpermeability (13, 30, 42, 59). We observed that HMW-HA protection from LPS-induced ALI is blocked in the caveolin-1 knockout mouse. This suggests an important regulatory role for caveolin-1 and CEM in HMW-HA-mediated enhancement of vascular integrity in vivo.
Our results indicate that CD44 knockout mice fail to respond to HMW-HA-mediated protection from LPS-induced ALI. In CD44 knockout mice, inflammatory cells are recruited and HA fragments accumulate at sites of lung injury (56). In addition to being an important regulator of immune cell function, our previously published data indicate that CD44 is involved in regulating epithelial barrier function (5). To specifically target the lung vasculature, we utilized targeted delivery of CD44 siRNA using ACE antibody-conjugated liposomes (1). ACE is a zinc metallopeptidase highly expressed on the surface of lung endothelium (23). ACE antibodies have previously been used to deliver catalase to the lung endothelium, with consequent attenuation of ischemia-reperfusion injury (33). Our results indicate that pulmonary vascular CD44 is the major regulator of HMW-HA-mediated protection from LPS-induced ALI. In addition to endothelial barrier function, HMW-HA and CD44 could also regulate neutrophil-endothelium interactions during LPS-induced neutrophil extravasation and pulmonary vascular leak similar to that reported for lymphocyte-endothelium interactions with inflammatory injury (10, 28, 46).
Whether our results indicating that HMW-HA promotes pulmonary vascular integrity extend beyond LPS-induced lung injury remains to be determined. Intraperitoneal injection of IL-2 causes systemic vascular leak, which is attenuated in CD44 knockout mice and with intraperitoneal injection of human umbilical cord HA, anti-CD44 (9F3) mouse antibody, or the HA-specific binding peptide Pep-1 (19, 31, 39). We examined the effects of IL-2 on HMW-HA-mediated, CD44-dependent human EC barrier enhancement in vitro (see Supplemental Material, Supplemental Fig. S1). Our results indicate that HPMVEC express low levels of the IL-2 receptor (22). We can induce IL-2 receptor expression by treatment of the EC with IL-2 or LPS. This sensitizes the EC to subsequent IL-2-mediated EC barrier disruption, which is not affected by silencing (siRNA) of CD44 expression. Exogenous HMW-HA addition protects from IL-2-induced EC barrier disruption in our in vitro model. Whether intravenous (vs. intraperitoneal) administration of HMW-HA protects from IL-2-induced vascular leak syndrome remains to be determined. Previous work with IL-2 used HA that was not purified to remove LMW-HA (31). We previously reported that LMW-HA induces human EC barrier disruption in vitro (51). Furthermore, intratracheal administration of a single dose of LPS induces a rapid (within hours) ALI with vascular hyperpermeability, while the IL-2 model involves intraperitoneal injection three times a day for 3 days followed by a single dose on day 4 (19, 31, 39, 53). Therefore, the targeting of injury (LPS/lung vs. IL-2/systemic model), the IL-2 receptor expression level of the pulmonary endothelium, the molecular weight of HA, and the route of HA delivery (intravenous vs. intraperitoneal) can potentially explain the observed differences.
In summary, utilizing in vitro and in vivo models of pulmonary vascular permeability, we have demonstrated that HMW-HA ligates CD44 in CEM in human EC, redistributes these CEM to areas of cell-cell contact, and recruits actin cytoskeletal regulatory proteins to CEM, which regulates HMW-HA-mediated EC barrier enhancement. These results indicate that HMW-HA may serve as a potentially useful therapeutic treatment for diseases characterized by high permeability states.
GRANTS
This research was supported by American Heart Association National Scientist Development Grant 0730277N (to P. A. Singleton) and National Heart, Lung, and Blood Institute Grant RO1-HL-095723 (to P. A. Singleton).
DISCLOSURES
P. A. Singleton and J. G. N. Garcia are provisional patent holders involving applications of hyaluronan with the University of Chicago and have not received any financial gain.
Supplementary Material
REFERENCES
- 1. Balyasnikova IV, Sun ZL, Metzger R, Taylor PR, Vicini E, Muciaccia B, Visintine DJ, Berestetskaya YV, McDonald TD, Danilov SM. Monoclonal antibodies to native mouse angiotensin-converting enzyme (CD143): ACE expression quantification, lung endothelial cell targeting and gene delivery. Tissue Antigens 67: 10–29, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 154: 1819–1828, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Borbiev T, Verin AD, Shi S, Liu F, Garcia JG. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 280: L983–L990, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase Cε-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem 281: 14026–14040, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bourguignon LY, Ramez M, Gilad E, Singleton PA, Man MQ, Crumrine DA, Elias PM, Feingold KR. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol 126: 1356–1365, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem 279: 26991–27007, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, Cleutjens J, van Suylen R, Joos GF, Brusselle GG, Wouters EF. Enhanced deposition of low weight hyaluronan in lungs of cigarette smoke-exposed mice. Am J Respir Cell Mol Biol 42: 753–761, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Cantor JO, Turino GM. Can exogenously administered hyaluronan improve respiratory function in patients with pulmonary emphysema? Chest 125: 288–292, 2004. [DOI] [PubMed] [Google Scholar]
- 9. de Belder AN, Wik KO. Preparation and properties of fluorescein-labelled hyaluronate. Carbohydr Res 44: 251–257, 1975. [DOI] [PubMed] [Google Scholar]
- 10. DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 278: 672–675, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Dentener MA, Vernooy JH, Hendriks S, Wouters EF. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax 60: 114–119, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem 279: 43411–43418, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA 103: 19836–19841, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-κB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol 177: 4853–4860, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Grande-Garcia A, del Pozo MA. Caveolin-1 in cell polarization and directional migration. Eur J Cell Biol 87: 641–647, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Guan H, Nagarkatti PS, Nagarkatti M. Blockade of hyaluronan inhibits IL-2-induced vascular leak syndrome and maintains effectiveness of IL-2 treatment for metastatic melanoma. J Immunol 179: 3715–3723, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Guo Y, Singleton PA, Rowshan A, Gucek M, Cole RN, Graham DR, Van Eyk JE, Garcia JG. Quantitative proteomics analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol Cell Proteomics 6: 689–696, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J 34: 616–628, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci USA 107: 11906–11911, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des 13: 1231–1245, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Le Lay S, Kurzchalia TV. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochim Biophys Acta 1746: 322–333, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA. High-molecular-weight hyaluronan—a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12: R102, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mambetsariev N, Mirzapoiazova T, Mambetsariev B, Sammani S, Lennon FE, Garcia JG, Singleton PA. Hyaluronic acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler Thromb Vasc Biol 30: 483–490, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285: L1179–L1183, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest 101: 97–108, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mrabat H, Beagle J, Hang Z, Garg HG, Hales CA, Quinn DA. Inhibition of HA synthase 3 mRNA expression, with a phosphodiesterase 3 inhibitor, blocks lung injury in a septic ventilated rat model. Lung 187: 233–239, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mustafa A, McKallip RJ, Fisher M, Duncan R, Nagarkatti PS, Nagarkatti M. Regulation of interleukin-2-induced vascular leak syndrome by targeting CD44 using hyaluronic acid and anti-CD44 antibodies. J Immunother 25: 476–488, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Nettelbladt O, Hallgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am Rev Respir Dis 140: 1028–1032, 1989. [DOI] [PubMed] [Google Scholar]
- 33. Nowak K, Weih S, Metzger R, Albrecht RF, 2nd, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol 293: L162–L169, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol 146: 843–854, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ormiston ML, Slaughter GR, Deng Y, Stewart DJ, Courtman DW. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 298: L148–L157, 2010. [DOI] [PubMed] [Google Scholar]
- 36. Peng ST, Su CH, Kuo CC, Shaw CF, Wang HS. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int J Oncol 31: 1119–1126, 2007. [PubMed] [Google Scholar]
- 37. Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Pogrel MA, Low MA, Stern R. Hyaluronan (hyaluronic acid) and its regulation in human saliva by hyaluronidase and its inhibitors. J Oral Sci 45: 85–91, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Rafi-Janajreh AQ, Chen D, Schmits R, Mak TW, Grayson RL, Sponenberg DP, Nagarkatti M, Nagarkatti PS. Evidence for the involvement of CD44 in endothelial cell injury and induction of vascular leak syndrome by IL-2. J Immunol 163: 1619–1627, 1999. [PubMed] [Google Scholar]
- 40. Rescher U, Gerke V. Annexins—unique membrane binding proteins with diverse functions. J Cell Sci 117: 2631–2639, 2004. [DOI] [PubMed] [Google Scholar]
- 41. Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflügers Arch 455: 575–582, 2008. [DOI] [PubMed] [Google Scholar]
- 42. Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1−/− knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, l-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277: 40091–40098, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res 70: 42–49, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Scott JE, Heatley F. Biological properties of hyaluronan in aqueous solution are controlled and sequestered by reversible tertiary structures, defined by NMR spectroscopy. Biomacromolecules 3: 547–553, 2002. [DOI] [PubMed] [Google Scholar]
- 45. Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des 13: 3568–3575, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Siegelman MH, DeGrendele HC, Estess P. Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol 66: 315–321, 1999. [DOI] [PubMed] [Google Scholar]
- 47. Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res 295: 102–118, 2004. [DOI] [PubMed] [Google Scholar]
- 48. Singleton PA, Bourguignon LY. CD44v10 interaction with Rho-kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil Cytoskeleton 53: 293–316, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and α-actinin. FASEB J 19: 1646–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 281: 34381–34393, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, Natarajan V. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and ROS production in caveolin-enriched microdomains of the endothelium. J Biol Chem 284: 34964–34975, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem 282: 30643–30657, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Sun RJ, Muller S, Zhuang FY, Stoltz JF, Wang X. Caveolin-1 redistribution in human endothelial cells induced by laminar flow and cytokine. Biorheology 40: 31–39, 2003. [PubMed] [Google Scholar]
- 55. Teder P, Heldin P. Mechanism of impaired local hyaluronan turnover in bleomycin-induced lung injury in rat. Am J Respir Cell Mol Biol 17: 376–385, 1997. [DOI] [PubMed] [Google Scholar]
- 56. Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002. [DOI] [PubMed] [Google Scholar]
- 57. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539, 2004. [DOI] [PubMed] [Google Scholar]
- 58. Wang Y, Ao X, Vuong H, Konanur M, Miller FR, Goodison S, Lubman DM. Membrane glycoproteins associated with breast tumor cell progression identified by a lectin affinity approach. J Proteome Res 7: 4313–4325, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wunderlich C, Schober K, Schmeisser A, Heerwagen C, Tausche AK, Steinbronn N, Brandt A, Kasper M, Schwencke C, Braun-Dullaeus RC, Strasser RH. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol 44: 938–947, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Zhou X, Tian F, Sandzen J, Cao R, Flaberg E, Szekely L, Cao Y, Ohlsson C, Bergo MO, Boren J, Akyurek LM. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proc Natl Acad Sci USA 104: 3919–3924, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.