Abstract
Rh B glycoprotein (Rhbg) is a member of the Rh glycoprotein family of ammonia transporters. In the current study, we examine Rhbg's role in basal and acidosis-stimulated acid-base homeostasis. Metabolic acidosis induced by HCl administration increased Rhbg expression in both the cortex and outer medulla. To test the functional significance of increased Rhbg expression, we used a Cre-loxP approach to generate mice with intercalated cell-specific Rhbg knockout (IC-Rhbg-KO). On normal diet, intercalated cell-specific Rhbg deletion did not alter urine ammonia excretion, pH, or titratable acid excretion significantly, but it did decrease glutamine synthetase expression in the outer medulla significantly. After metabolic acidosis was induced, urinary ammonia excretion was significantly less in IC-Rhbg-KO than in control (C) mice on days 2–4 of acid loading, but not on day 5. Urine pH and titratable acid excretion and dietary acid intake did not differ significantly between acid-loaded IC-Rhcg-KO and C mice. In IC-Rhbg-KO mice, acid loading increased connecting segment (CNT) cell and outer medullary collecting duct principal cell Rhbg expression. In both C and IC-Rhbg-KO mice, acid loading decreased glutamine synthetase in both the cortex and outer medulla; the decrease on day 3 was similar in IC-Rhbg-KO and C mice, but on day 5 it was significantly greater in IC-Rhbg-KO than in C mice. We conclude 1) intercalated cell Rhbg contributes to acidosis-stimulated renal ammonia excretion, 2) Rhbg in CNT and principal cells may contribute to renal ammonia excretion, and 3) decreased glutamine synthetase expression may enable normal rates of ammonia excretion under both basal conditions and on day 5 of acid loading in IC-Rhbg-KO mice.
Keywords: acid-base homeostasis, net acid excretion
acid-base homeostasis is maintained by the kidneys through the process of net acid excretion (NAE) which involves ammonia, titratable acid, and bicarbonate excretion.1 Under basal conditions, renal ammonia metabolism is the predominant component of NAE, accounting for 60–70% of the total, whereas titratable acid excretion accounts for only 30–40% and urinary bicarbonate is essentially 0 (8, 17). In response to metabolic acidosis, kidneys increase NAE, which helps to restore acid-base homeostasis. Nearly the entire increase in NAE is due to increased ammonia excretion (6, 8, 17, 27).
Members of the Rhesus factor family of ammonia transporters, Rh B and Rh C glycoprotein (Rhbg and Rhcg, respectively), are orthologs of Mep/Amt ammonia transporters found in primitive organisms and are expressed in mammalian tissues where ammonia is transported. Rhbg is expressed in the kidney, liver, lung, gastrointestinal tract, and skin (10, 12, 21, 37), and Rhcg is expressed in the kidney, liver, lung, gastrointestinal tract, testes, and brain (10, 12, 20, 37). Recent studies, using both global and collecting duct-specific Rhcg deletion, showed that Rh glycoprotein-mediated ammonia secretion is critical to both basal and acidosis-stimulated renal ammonia excretion (1, 15).
Whether Rhbg contributes to renal ammonia excretion is less clear. Rhbg is a homolog of Rhcg and is an integral membrane protein. Rhbg transports ammonia and the ammonia analog, methylammonia, and has no other known substrates (22, 23, 25). It is expressed in the kidney in the same cells as Rhcg, with the difference that Rhbg expression is exclusively basolateral (26, 31). Renal collecting duct cells studied in vitro express basolateral Rhbg and the primary mechanism of basolateral ammonia movement has functional characteristics of Rh glycoprotein-mediated transport (11). Acid loading increases Rhbg expression in mice with collecting duct-specific Rhcg deletion, suggesting that adaptive increases in Rhbg-mediated ammonia transport can contribute to the renal response to metabolic acidosis (15). Evidence against a role of Rhbg in renal ammonia transport, however, is a report that mice with global Rhbg deletion had normal and acidosis-stimulated renal ammonia excretion (3). Because of this conflicting evidence, we reinvestigated Rhbg's role in renal ammonia excretion under basal conditions and in response to metabolic acidosis.
First, we determined the effect of HCl-induced metabolic acidosis on renal Rhbg expression. We then generated mice with intercalated cell-specific Rhbg deletion (IC-Rhbg-KO) using mice with loxP sites flanking exons 5 and 9 of the Rhbg gene and mice expressing Cre-recombinase under control of the B1 subunit of H+-ATPase promoter (B1-Cre). Using these mice, we examined the effect of intercalated cell-specific Rhbg deletion on basal and acidosis-stimulated urinary ammonia excretion. We also examined adaptive responses to intercalated cell-specific Rhbg deletion. Our results show that metabolic acidosis increases Rhbg expression, that intercalated cell-specific Rhbg expression is necessary for the normal response to metabolic acidosis, that metabolic acidosis increases principal cell Rhbg expression in both control (C) and IC-Rhcg-KO mice, and that glutamine synthetase expression is decreased in IC-Rhbg-KO mice. We conclude that 1) Rhbg contributes to renal ammonia excretion during metabolic acidosis, 2) adaptive changes in glutamine synthetase expression can partially compensate for the absence of Rhbg, and 3) principal cell-mediated ammonia secretion involving Rhbg contributes to renal ammonia excretion.
METHODS
Floxed Rhbg mouse generation.
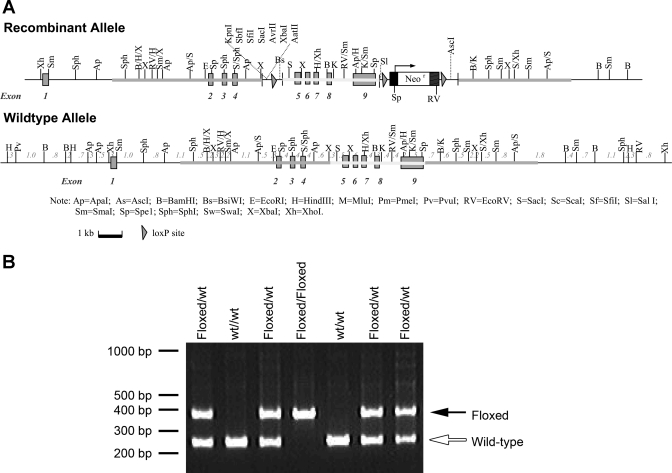
Transgenic mice expressing loxP sites in introns flanking exons 5 and 9 of the Rhbg gene were generated by Caliper Life Sciences (formerly Xenogen Biosciences) under contract from the University of Florida. Briefly, mouse chromosome 3 sequence was retrieved from Ensembl database build 30. Mouse C57Bl/6 BAC clone RP23–4L11 was used to generate the homologous arms and the conditional KO region for the gene-targeting vector and the southern probes for screening-targeted events. PCR or RED cloning/gap-repair methods were used to clone the appropriate sequences. The 5′ homologous arm (5.6 kb) and conditional knockout region (3.8 kb) were generated by RED cloning/gap repair. The 3′ homologous arm (3.9 kb) was generated by PCR reaction using proofreading LA Taq DNA polymerase. These fragments were cloned into the pCRXL vector and confirmed by restriction digest and end sequencing. The final vector also contains loxP sequences flanking the conditional knockout region, loxP sequences flanking the Neo expression cassette (for positive selection of the ES cells), and a DTA expression cassette (for negative expression of the ES cells). The final vector was confirmed by both restriction digestion and end sequencing analysis. NOTl was used to linearize the final vector before electroporation. Figure 1A shows key features of the final vector used. 5′ And 3′ external probes were generated by PCR reaction and tested on genomic southern analysis for ES screening. They were cloned in pCR2.1 backbone and confirmed by sequencing. Subsequently, ES cells were transfected with the gene-targeting construct and homologous recombinant clones were identified. The ES cells were derived from a C57BL/6 Tac inbred mouse strain. After successful homologous recombination, we electroporated a supercoiled Neo expression plasmid into the correctly targeted ES clones. Under no G418 selection, ES clones were picked. G418 sensitivity was tested on duplicates of these clones. PCR was then performed to confirm the Neo deletion on the G418-sensitive clones. Positive ES clones were injected into blastocysts of FVB/NTac recipient mice to create chimera. High-percentage chimeras were bred with C57BL/6 Tac to generate heterozygous floxed Rhbg mice, which were then used to generate homozygous floxed Rhbg mice.
Fig. 1.
Generation of floxed Rhbg mice. A: vector used to generate loxP-flanked (floxed) Rhbg gene. B: PCR amplification of tail clip DNA showing differentiation of wild-type (wt) and floxed Rhbg alleles.
Animals.
Transgenic mice expressing Cre-recombinase under control of a 6.5-kb portion of the H+-ATPase B1 subunit promoter (B1-Cre) have been described previously (24). Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel. IC-Rhbg-KO mice were generated using standard breeding approaches using floxed Rhbg mice and mice expressing Cre-recombinase under control of the B1 subunit of H+-ATPase promoter (B1-Cre). All mice used in this project were either floxed Rhbg, B1-Cre-positive or floxed Rhbg, B1-Cre-negative. All animal studies were approved by the Institutional Animal Care and Use Committees of the Gainesville VA Medical Center and of the University of Florida College of Medicine.
Genotyping transgenic mice.
Mice were genotyped using tail-clip samples obtained at ∼14 days of age as described previously (13). The B1-Cre transgene was identified using primers, B1-Cre-F (5′-CCCTCTTCCCTTCTCCCTCCA-3′) and Cre-Tag-R (5′-GCGAACATCTTCAGGTTCTGCGG-3′), which anneal to the B1 promoter and Cre recombinase, respectively (24). The floxed Rhbg gene is amplified using the primers UF1delcKF (5′-GGTTCTGGGACTGTGGATGAGGAAG-3′) and UF1del3R (5′-CCACCTGCCGAAGGGATGAAGTC-3′). Amplification of an allele containing a 3′ loxP site (“floxed”) generates an ∼400-bp product; amplification of an allele without a 3′ loxP site (“wild-type”) generates an ∼240-bp product (Fig. 1B).
Antibodies.
Affinity-purified antibodies to Rhcg and Rhbg were generated in our laboratory and have been previously characterized (13, 15, 23, 31). In particular, we showed specificity of both the Rhbg and the Rhcg antibodies in studies using heterologous expression in Xenopus laevis oocytes (23) and in studies using genetic deletion of Rhcg (13). N. Curthoys, Ph.D. (Colorado State University) provided antibodies to phosphate-dependent glutaminase (PDG) and F. Karet, Ph.D. (Cambridge Institute for Medical Research, Cambridge, UK) provided antibodies to the a4 subunit of H+-ATPase. Antibodies to glutamine synthetase were obtained from Millipore (Billerica, MA), and antibodies to phosphenolpyruvate carboxykinase (PEPCK) were obtained from Cayman Chemical (Ann Arbor, MI).
Acid loading.
Acid diet was prepared by adding 0.4 M HCl to powdered rodent chow as detailed previously (15). Control diet was identical except that deionized water was substituted for HCl. Adult animals, greater than 8 wk age, were placed into metabolic cages (Tecniplast diuresis metabolic cage, Fisher Scientific) and allowed to acclimate for 1 to 2 days while receiving control diet. They then received either HCl diet or control diet. Daily food intake was measured. At all times, animals were provided ad libitum access to water. Daily urine excretion was collected under mineral oil, urine pH was measured, and urine volume was calculated. Urine samples were stored at −80°C until analyzed.
Electrolyte measurements.
Urine ammonia was measured using a commercially available kit (A7553, Pointe Scientific, Canton, MI) modified for use in 96-well plates. Serum bicarbonate was measured as total CO2 using a commercially available kit (C750–120, Pointe Scientific) modified for use with microliter quantities of serum. Urine pH was measured using a micro-pH electrode (Thermo Scientific, ROSS semi-micro pH, ORION 8115BN). Urinary titratable acid was measured using techniques described previously (15). Plasma and urine creatinine were measured using capillary electrophoresis and Na+ and K+ concentrations were measured using a flame photometer (Instrumentation Laboratory, Lexington, MA) as described previously (15).
Tissue preparation for immunolocalization.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 4-mm-thick slices and immersed 24 to 48 h at 4°C in the same fixative. For light microscopy, samples of kidney from each animal were embedded in polyester wax [polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol], and 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization was accomplished using standard immunoperoxidase procedures detailed previously (13, 15). The sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in peroxidase blocking reagent (DakoCytomation, Carpinteria, CA) for 45 min. The sections were blocked for 15 min with serum-free protein block (DakoCytomation) and then incubated at 4°C overnight with primary antibody. The sections were washed in PBS and incubated for 30 min with polymer-linked peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical), again washed with PBS, and then exposed to diaminobenzidine for 5 min. The sections were washed in distilled water, then dehydrated in a graded series of ethanols and xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon). Color adjustment was performed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA).
Double immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (13). Briefly, tissue sections were labeled with the first primary antibody following the procedure described above, using vector SG (Vector Laboratories) as the chromogen to produce a blue label. After the vector SG reaction, sections were washed in PBS and then blocked using the peroxidase-blocking reagent and serum-free protein block as described in the single label procedure. The above procedure was repeated with the substitution of a second primary antibody and the substitution of DAB for vector SG. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted with permount, and observed by light microscopy.
Protein preparation.
Animals were anesthetized with inhalant isoflurane and the kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4), rapidly removed, and stored frozen at −80°C until used. In some experiments, the right renal vasculature was clamped after in vivo cardiac perfusion with PBS, the right kidney was removed, and then the left kidney was perfused with PLP fixative for immunohistochemistry. Tissues were homogenized using microtube pestles (USA Scientific, Ocala, FL) and proteins were extracted using T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL) according to the manufacturer's recommended procedures. For membrane protein preparation for Rhcg, tissues were homogenized in buffer A (in mM: 50 sucrose, 10 Tris buffer, and 1 EDTA, pH 7.4) and then diluted in buffer B (in mM: 250 sucrose, 10 Tris buffer, and 1 EDTA, pH 7.4). The sample was then centrifuged at 1,000 g for 5 min at 4°C. The pellet was resuspended in buffer B and again centrifuged at 21,000 g for 30 min at 4°C. The 21,000-g pellet was finally resuspended in buffer B. An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −80°C until used.
Immunoblotting procedure.
Five to twenty micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated for 2 h with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween-20, pH 7.5) with 5 g/dl nonfat dry milk. Loading and transfer equivalence was assessed with Ponceau S staining. After being washed, membranes were exposed to secondary antibody (goat anti-rabbit IgG; Promega, Madison, WI or goat anti-mouse IgG; Upstate, Temecula, CA conjugated to horseradish peroxidase) at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce, Rockford, IL) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 3.5.4 software (Kodak Scientific Imaging, New Haven, CT). Band density was normalized such that mean density in the same region (cortex or outer medulla) in C kidneys was 100.0.
RESULTS
Rhbg expression in response to metabolic acidosis.
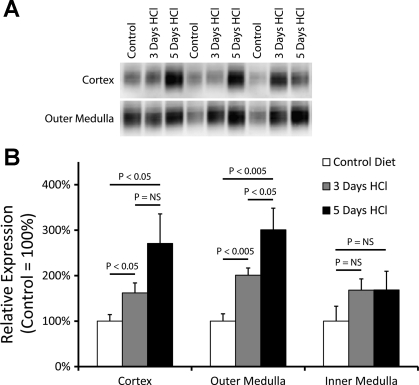
We first examined whether metabolic acidosis alters renal Rhbg expression. Mice with intact Rhbg expression were placed either on control diet for 3 days or HCl diet for either 3 or 5 days, as previously described (15). Immunoblot analysis showed that metabolic acidosis induced a time-dependent, progressive increase in Rhbg expression in the cortex (control diet, 100 ± 14 vs. 3 days HCl, 162 ± 22 vs. 5 days HCl diet, 271 ± 65; P < 0.05 for control vs. 3 days HCl and control vs. 5 days HCl, n = 7, 5, and 5/group, respectively) and outer medulla (control diet, 100 ± 16 vs. 3 days HCl, 201 ± 16 vs. 5 days HCl diet, 301 ± 48; P < 0.005 for control vs. 3 days HCl and control vs. 5 days HCl, n = 7, 5, and 5/group, respectively; Fig. 2).
Fig. 2.
Effect of metabolic acidosis on renal Rhbg expression. A: changes in Rhbg protein expression by immunoblot analysis. B: quantified data. HCl-induced metabolic acidosis results in time-dependent increases in Rhbg expression in the cortex and outer medulla. Rhbg expression in the inner medulla was not significantly increased. NS, not significant.
Immunohistochemistry confirmed these findings. Increased Rhbg expression was most evident in the connecting segment (CNT) and cortical collecting duct (CCD) in the cortex and in both intercalated and principal cells in the outer medullary collecting duct (OMCD; Fig. 3).
Fig. 3.
Effect of metabolic acidosis on renal Rhbg immunolabel. Immunolocalization of Rhbg confirmed increases in Rhbg expression after 5 days of HCl acid loading. In all panels, signal for Rhbg immunoreactivity is brown; H+-ATPase immunoreactivity is blue. A and B: 5 days HCl acid loading (B) increased Rhbg immunoreactivity in the connecting segment (CNT) and initial collecting tubule compared with control (A). C and D: no differences in cortical collecting duct (CCD) Rhbg immunoreactivity were apparent in control vs. 5-day HCl diet mice. Intercalated cells (arrows) are identified by intense basolateral Rhbg immunolabel and prominent apical H+-ATPase, and principal cells (arrowheads) by moderate basolateral Rhbg immunolabel and minimal or no apical H+-ATPase. E and F: in the outer medullary collecting duct in the outer stripe (OMCDo), Rhbg immunoreactivity in both intercalated cells (arrows) and principal cells (arrowheads) was increased by 5-day HCl loading. G and H: increased intercalated and principal cell Rhbg immunolabel and intercalated cell hypertrophy in the OMCD in the inner stripe (OMCDi) in response to HCl metabolic acidosis.
Generation of mice with IC-Rhbg-KO.
To determine the functional significance of increased Rhbg expression in the renal response to metabolic acidosis, we generated transgenic mice with intercalated cell-specific Rhbg deletion. Transgenic mice expressing loxP sites flanking exons 5 and 9 of the Rhbg gene (floxed Rhbg) were mated with mice expressing B1-Cre. Offspring expressing B1-Cre were backcrossed with homozygous floxed Rhbg mice until mice homozygous for floxed Rhbg and expressing B1-Cre were generated. They were then mated with homozygous floxed Rhbg, B1-Cre negative mice. This breeding scheme produced both floxed Rhbg, B1-Cre-positive and floxed Rhbg, B1-Cre-negative offspring in the same litters.
We confirmed that floxed Rhbg, B1-Cre-positive mice had intercalated cell-specific Rhbg deletion using immunohistochemistry. In floxed Rhbg, B1-Cre-positive mice, a subset of collecting duct cells lacked detectable Rhbg expression (Fig. 4). Double-immunolabeling with antibodies to the a4 subunit of H+-ATPase demonstrated that the cells lacking Rhbg expression expressed intense H+-ATPase immunolabel, thus identifying these cells as intercalated cells. Although rare intercalated cells had persistent Rhbg expression, these cells were <5% of total intercalated cells. Principal cell Rhbg expression was intact. Floxed Rhbg, B1-Cre-negative mice exhibited intact basolateral Rhbg expression in the distal convoluted tubule (DCT), CNT, initial collecting tubule, and collecting duct. As observed in wild-type mice, Rhbg immunolabel in floxed Rhbg, B1-Cre-negative mice was more intense in intercalated cells than in principal cells. Intercalated cell distribution, assessed from intense H+-ATPase immunolabel, appeared normal in both IC-Rhbg-KO and C mice (data not shown). These findings confirm generation of mice with intercalated cell-specific Rhbg deletion. In the remainder of this report, we use the term IC-Rhbg-KO to refer to floxed Rhbg, B1-Cre-positive mice and C to refer to floxed Rhbg, B1-Cre-negative mice.
Fig. 4.
Rhbg expression in intercalated cell-specific Rhbg knockout (IC-Rhbg-KO) mouse kidney. A and B: low-magnification images showing reduced Rhbg immunoreactivity in kidneys of IC-Rhbg-KO mice compared with control (C). C and D: double-immunolabeling in the CCD with antibodies to Rhbg (brown) and H+-ATPase (blue) used to characterize cell-specific Rhbg expression in C and IC-Rhbg-KO mice. C: C mice exhibited intact Rhbg expression in both intercalated cells (arrows) and principal cells (arrowheads), with greater immunolabel in intercalated cells. D: IC-Rhbg-KO mice had normal principal cell Rhbg immunoreactivity (arrowheads), but intercalated cells lacked Rhbg immunolabel (arrows). E and F: Rhbg expression in the OMCD of C and IC-Rhbg-KO mice. Again, basolateral Rhbg immunolabel is absent in intercalated cells (arrows) and intact in principal cells (arrowheads) in IC-Rhbg-KO mice.
IC-Rhbg-KO mice on control diet.
To determine Rhbg's role in basal acid-base homeostasis, we measured serum electrolytes, urinary ammonia, and titratable acid excretion in age-matched IC-Rhbg-KO and C mice. Table 1 summarizes these results. IC-Rhbg-KO did not alter urinary ammonia excretion, titratable acid excretion, or urine pH significantly compared with C mice. Plasma HCO3− did not differ significantly between the two groups. Thus, while on control diet, mice with intercalated cell-specific Rhbg deletion exhibit normal acid-base homeostasis.
Table 1.
Physiologic parameters under basal conditions
| Parameter | IC-Rhbg-KO | C | P Value |
|---|---|---|---|
| Urine ammonia, μmol/day | 105.0 ± 18.6 (4) | 82.8 ± 10.3 (4) | NS |
| Urine pH | 6.13 ± 0.05 (4) | 6.08 ± 0.05 (4) | NS |
| Urine volume, ml/day | 1.73 ± 0.38 (4) | 1.58 ± 0.29 (4) | NS |
| Urine titratable acids, μmol/day | 84.5 ± 10.9 (4) | 84.6 ± 9.1 (4) | NS |
| Serum HCO3−, mmol/l | 19.1 ± 0.9 (8) | 19.1 ± 0.7 (8) | NS |
| Serum creatinine, mg/dl | 0.11 ± 0.010 (4) | 0.08 ± 0.002 (4) | NS |
| Creatinine clearance, μl/min | 244.9 ± 28.3 (4) | 325.1 ± 59.2 (4) | NS |
| Body wt, g | 34.1 ± 2.1 (4) | 32.5 ± 0.9 (4) | NS |
Values are means ± SE. Numbers in parentheses are numbers of animals in each group. IC-Rhbg-KO, intercalated cell-specific Rhbg knockout; C, control; NS, not significant.
Urinary ammonia excretion in response to metabolic acidosis.
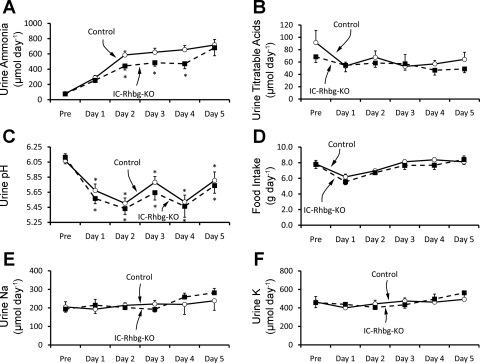
Increased urinary ammonia excretion is the predominant renal mechanism increasing NAE in response to metabolic acidosis. Intercalated cells have been long recognized as important in the regulation of acid-base balance. Therefore, to examine the role of intercalated cell Rhbg expression in renal ammonia metabolism, we quantified urinary ammonia excretion in acid-loaded IC-Rhbg-KO and C mice (Fig. 5A). Before acid loading, urinary ammonia excretion did not differ significantly between IC-Rhbg-KO and C mice (76.1 ± 8.4 vs. 79.3 ± 7.7 μmol/day, n = 18/group, P = NS). However, with acid loading, urinary ammonia excretion was significantly less in IC-Rhbg-KO mice than in C mice on days 2, 3, and 4 (day 2: 441.9 ± 52.1 vs. 586.3 ± 50.8 μmol/day, n = 18/group, P < 0.05; day 3: 484.4 ± 45.1 vs. 621.7 ± 52.6 μmol/day, n = 18/group, P < 0.05; day 4: 471.2 ± 65.3 vs. 655.7 ± 55.2 μmol/day, n = 10/group, P < 0.05) of acid loading. Quantitatively, intercalated cell-specific Rhbg deletion inhibited urinary ammonia excretion by ∼25% on days 2, 3, and 4 of metabolic acidosis.
Fig. 5.
Effect of IC-Rhbg-KO on renal ammonia excretion in response to metabolic acidosis. A: urinary ammonia excretion in IC-Rhbg-KO and C mice. Mice were placed on control diet for 1 day and then changed to HCl diet. On days 2, 3, and 4 of acid loading, IC-Rhbg-KO mice excreted significantly less urinary ammonia than did C mice. B: urine titratable acid excretion in IC-Rhbg-KO and C mice. There was no significant difference in urine titratable acid excretion either before acid loading or at any time point during the HCl acid-loading protocol. C: urine pH during the same time period. Urine pH decreased significantly with induction of metabolic acidosis in both IC-Rhbg-KO and C mice, but it did not differ significantly between IC-Rhbg-KO and C mice either on control diet or any day after acid loading (*P < 0.05 vs. pH before acid loading). D: food intake in IC-Rhbg-KO and C mice. HCl acid loading was accomplished by adding 0.4 M HCl to powdered standard rodent chow. Food intake, and therefore the acid load, did not differ significantly between C and IC-Rhbg-KO mice either before or during acid loading. E: urinary Na+ excretion in C and IC-Rhbg-KO mice. There was no significant difference in urinary Na+ excretion between C and IC-Rhbg-KO mice either under basal conditions or in response to acid loading. F: urinary K+ excretion in C and IC-Rhbg-KO mice. There was no significant difference in urinary K+ excretion between C and IC-Rhbg-KO mice either under basal conditions or in response to acid loading. N = 18 for both C and IC-Rhbg-KO before acid loading and on days 1-3 of acid loading and N = 10 on days 4 and 5 of acid loading in all panels. *P < 0.05 vs. C mice.
Effect of IC-Rhbg-KO on acidosis-stimulated titratable acid excretion.
Titratable acid excretion did not increase with acid loading and did not differ significantly between IC-Rhbg-KO and C mice either on control diet (84.5 ± 10.9 vs. 84.6 ± 9.1 μmol/day, n = 4/group, P = NS) or on any day during acid loading (54.7 ± 5.1 vs. 53.5 ± 9.3 μmol/day, n = 7/group, P = NS for day 1, 58.4 ± 7.1 vs. 67.7 ± 10.3 μmol/day, n = 7/group, P = NS, for day 2, and 57.2 ± 14.9 vs. 52.9 ± 6.2 μmol/day, n = 7/group, P = NS, for day 3). Figure 5B summarizes these data. Thus, intercalated cell Rhbg deletion does not alter titratable acid excretion, either under basal conditions or in response to metabolic acidosis.
Effect of IC-Rhbg-KO on urine pH in response to acid loading.
Because collecting duct ammonia secretion involves parallel H+ and NH3 secretion, we examined the possibility that disruption of the Rhbg gene in intercalated cells impaired ammonia excretion by altering urinary acidification instead of by altering Rhbg-mediated ammonia transport. These results are shown in Fig. 5C. Urine pH did not differ significantly between IC-Rhbg-KO and C mice either under basal conditions or in response to HCl acid loading. Thus, impaired urine ammonia excretion in IC-Rhbg-KO mice is not due to impaired urinary acidification.
Effect of IC-Rhbg-KO on extent of acid loading.
We then examined the possibility that differences in extent of acid loading between IC-Rhbg-KO and C mice accounted for the differences in urinary ammonia excretion. Because HCl was administered with the food, we could quantify acid loading from the net food ingested by IC-Rhbg-KO and C mice. These results are shown in Fig. 5D. Food intake, and thus HCl intake, did not differ significantly between IC-Rhbg-KO and C mice at any time during acid loading.
Effect of IC-Rhbg-KO on serum bicarbonate.
We also examined the effect of acid loading on systemic acid-base parameters in IC-Rhbg-KO mice. Serum bicarbonate measured as total CO2 did not differ significantly between IC-Rhbg-KO and C mice in response to HCl acid loading (IC-Rhbg-KO, 17.3 ± 0.2 vs. C, 16.7 ± 0.4 mmol/l, n = 5/group, P = NS).
Effect of IC-Rhbg-KO on serum potassium concentration.
Serum potassium levels did not differ significantly between IC-Rhbg-KO and C mice under basal conditions (IC-Rhbg-KO, 4.0 ± 0.1 vs. C, 3.9 ± 0.3 mmol/l, n = 4/group, P = NS) or in response to acid loading (IC-Rhbg-KO, 4.0 ± 0.2 vs. C, 3.5 ± 0.2 mmol/l, n = 6/group, P = NS).
Effect of IC-Rhbg-KO on urinary electrolyte excretion.
Urinary Na+ and K+ excretion did not differ significantly between C and IC-Rhbg-KO mice while on control diet. After induction of metabolic acidosis with acid loading, there remained no significant difference in urinary Na+ or K+ excretion between C and IC-Rhbg-KO mice on any day of acid loading (Fig. 5, E and F). Thus, intercalated cell-specific Rhbg deletion does not appear to alter renal Na+ or K+ excretion under either basal conditions or in response to metabolic acidosis.
Principal cell Rhbg expression in response to metabolic acidosis.
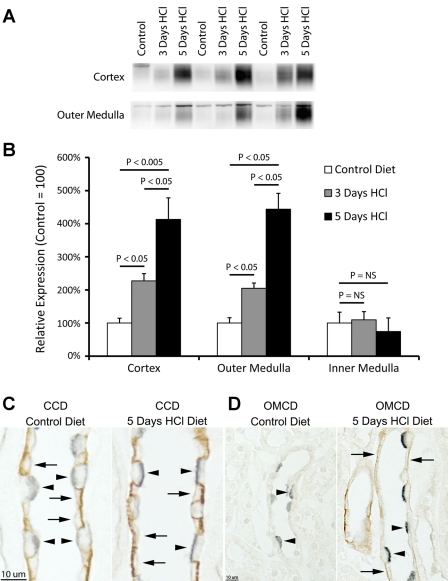
Our immunohistochemistry studies in acid-loaded mice with intact Rhbg expression suggested that acid loading increases intercalated cell, principal cell, and CNT cell Rhbg expression. To examine this further, we determined whether metabolic acidosis increased Rhbg expression in nonintercalated cells by examining Rhbg expression in IC-Rhbg-KO mice fed either control or HCl diet (Fig. 6). Immunoblot analysis showed that HCl-induced metabolic acidosis increased Rhbg expression significantly in both the cortex and outer medulla. Immunohistochemistry confirmed these findings. Double-immunolabeling with antibodies to Rhbg and H+-ATPase confirmed that acid loading did not induce Rhbg expression in intercalated cells in IC-Rhbg-KO mice. Thus, metabolic acidosis induced adaptive increases in Rhbg expression in CNT cells and CCD and OMCD principal cells, which may enable increased renal ammonia secretion.
Fig. 6.
Effect of acid loading on Rhbg expression in IC-Rhbg-KO mice. A: immunoblot analyses of Rhbg expression in IC-Rhbg-KO mice fed control diet (powdered rodent chow + H2O) or acid-loading diet (powdered rodent chow + HCl, 0.4 M). B: quantified Rhbg expression. IC-Rhbg-KO mice show a time-dependent, progressive increase in Rhbg expression in both the cortex and the outer medulla, while expression in the inner medulla does not change significantly. C: immunolocalization of Rhbg in the CCD of IC-Rhbg-KO mice on control diet and after 5-day HCl acid loading. Double immunolabel for H+-ATPase (blue) and Rhbg (brown) was used to differentiate intercalated and principal cells. Intercalated cells (arrowheads) lack Rhbg expression; acid loading increases Rhbg immunolabel in principal cells (arrows). D: same pattern of expression in the OMCD. Acid loading increases principal cell (arrows) Rhbg immunolabel.
Adaptive responses to intercalated cell-specific Rhbg deletion.
Deletion of a critical protein in a biological process frequently results in adaptive changes in other components of that process to maintain homeostasis. Thus, we examined the effect of IC-Rhbg-KO on expression of several proteins involved in renal ammonia metabolism.
Glutamine synthetase is a cytosolic protein expressed in the proximal straight tubule that catalyzes the reaction of NH4+ with glutamate to form glutamine. Decreased glutamine synthetase expression contributes to increased renal ammonia excretion, at least in the mouse in response to metabolic acidosis (4). We observed that IC-Rhbg-KO mice expressed significantly less glutamine synthetase in the outer medulla than did C mice under basal conditions (Fig. 7). Altered glutamine synthetase expression, by increasing net proximal tubule NH4+ production, may enable normal total ammonia excretion in IC-Rhbg-KO mice while on normal diet.
Fig. 7.
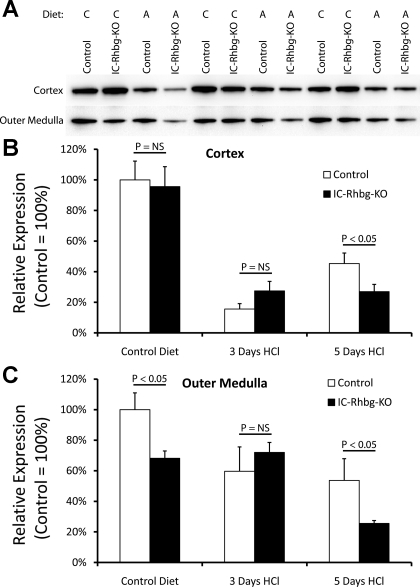
Effect of IC-Rhbg-KO and acid loading on glutamine synthetase expression. A: glutamine synthetase expression by immunoblot analyses in the cortex and outer medulla of IC-Rhbg-KO and C mice. Diet is either control (C) or acid (A). B: quantified data. In the cortex, IC-Rhbg-KO did not alter glutamine synthetase expression on control diet. Metabolic acidosis decreased glutamine synthetase expression in the cortex in both C and IC-Rhbg-KO mice. After 3 days of acid loading, there was no difference in glutamine synthetase expression between C and IC-Rhbg-KO mice. However, after 5 days of acid loading, glutamine synthetase expression was decreased significantly more in IC-Rhbg-KO mice than in C mice. C: changes in glutamine synthetase expression in the outer medulla in response to IC-Rhbg-KO and to acid loading. Under basal conditions, glutamine synthetase expression was significantly less in IC-Rhbg-KO than in C mice. Acid loading decreased glutamine synthetase expression. After 3 days of acid loading, glutamine synthetase expression did not differ significantly in the outer medulla between C and IC-Rhbg-KO mice. However, after 5 days of acid loading, glutamine synthetase expression was decreased significantly more in IC-Rhbg-KO than in C mice.
Metabolic acidosis decreased glutamine synthetase expression further in both the cortex and outer medulla in both C and IC-Rhbg-KO mice. At 3 days of acid loading, glutamine synthetase levels did not differ significantly between C and IC-Rhbg-KO mice. However, after 5 days, glutamine synthetase expression was significantly less in both the cortex and the outer medulla of IC-Rhbg-KO mice compared with C mice (Fig. 7). Decreased glutamine synthetase expression appears to be an adaptive response to Rhbg deletion both in mice fed control diet and following acid loading. The differences in glutamine synthetase adaptation between C and IC-Rhbg-KO mice at 5 days may mediate, at least in part, the lack of difference in urinary ammonia excretion at this time point.
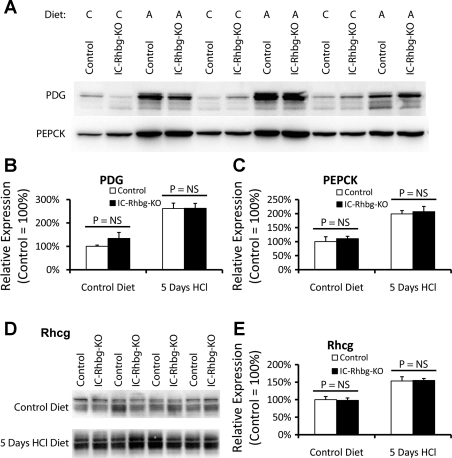
This adaptive change was specific to glutamine synthetase. In mice fed control diet, IC-Rhbg-KO did not alter significantly the expression of either PEPCK or PDG (Fig. 8, A–C). Although metabolic acidosis increased both PEPCK and PDG expression, the expression did not differ significantly between acid-loaded IC-Rhbg-KO and C mice (Fig. 8, A–C). However, we cannot exclude the possibility that posttranslational regulation of either PEPCK or PDG enables an adaptive response to intercalated cell-specific Rhbg deletion.
Fig. 8.
Effect of IC-Rhbg-KO on phosphate-dependent glutaminase (PDG), phosphenolpyruvate carboxykinase (PEPCK), and Rhcg expression. A: immunoblot analysis of PDG and PEPCK expression in the cortex of C and IC-Rhbg-KO mice. Diet: C or A. B: quantification of PDG protein expression. Cortical PDG expression is not significantly different between IC-Rhbg-KO and C mice while on control diet. Acid loading increases PDG expression but PDG expression does not differ significantly between C and IC-Rhbg-KO mice. C: quantification of PEPCK expression. Cortical PEPCK expression is not significantly different between IC-Rhbg-KO and C mice fed control diet. Acid loading increases PEPCK expression, but PEPCK expression does not differ significantly between C and IC-Rhbg-KO acid-loaded mice. D: immunoblot analysis of Rhcg expression in control and IC-Rhbg-KO mice on control diet and after acid loading. E: quantification of Rhcg expression. Rhcg protein expression does not differ between C and IC-Rhbg-KO mice on control diet. Metabolic acidosis increases Rhcg expression, but there is no difference in Rhcg expression between acid-loaded C and IC-Rhbg-KO mice.
Similarly, although metabolic acidosis increased Rhcg expression, Rhcg expression did not differ significantly between IC-Rhbg-KO and C mice, either while on normal diet or after acid loading (Fig. 8, D and E). Thus, while increased PEPCK, PDG, and Rhcg expression contribute to the renal response to metabolic acidosis, changes in the abundance of these proteins do not appear to compensate for the lack of Rhbg expression.
DISCUSSION
This study provides a detailed examination of Rhbg's role in renal ammonia metabolism. Our findings showing that metabolic acidosis increases Rhbg expression and that intercalated cell-specific Rhbg deletion impairs the ability to increase urinary ammonia excretion suggest an important role for Rhbg in acidosis-stimulated ammonia excretion. The differences in glutamine synthetase expression induced by IC-Rhbg-KO may enable normal rates of ammonia excretion in IC-Rhcg-KO mice under both basal conditions and after 5 days of metabolic acidosis. Metabolic acidosis increases Rhbg expression in CNT cells and in CCD and OMCD principal cells, suggesting that principal cells also contribute to renal, Rhbg-mediated ammonia excretion. Thus, Rhbg appears critical for normal renal ammonia secretion by both intercalated cells and principal cells.
The current study indicates that Rhbg plays an important role in the renal response to metabolic acidosis. Rhbg is expressed in the basolateral plasma membrane of several cell types from the DCT through the inner medullary collecting duct (IMCD) (26, 31). Heterologous expression studies show that Rhbg transports ammonia and its analog, methylammonia, and that it has no other known substrates (22, 23, 25, 39). Basolateral ammonia transport in cultured mouse collecting duct cells is predominantly a transporter-mediated, not diffusive, process with functional characteristics identical to those of Rhbg (11). Finally, metabolic acidosis increases basolateral Rhbg expression, and Rhbg expression, at least in intercalated cells, is necessary for the normal increase in renal ammonia excretion in response to metabolic acidosis (current study). Thus, Rhbg appears to mediate an important role in the renal response to metabolic acidosis by facilitating basolateral NH3 uptake which facilitates transcellular ammonia secretion.
In previous studies of the role of renal Rh glycoproteins in the response to metabolic acidosis, we examined Rhbg expression in mice with intercalated cell-specific Rhcg deletion (IC-Rhcg-KO) (16) and in normal rats (28). In acid-loaded IC-Rhcg-KO mice compared with baseline, renal Rhbg expression increased in the outer medulla, in agreement with the findings in C and IC-Rhbg-KO mice in the current study. However, Rhbg expression in the cortex was unchanged in IC-Rhcg-KO mice (16) after acid loading, unlike the current results in C and IC-Rhbg-KO mice which had increased renal Rhbg protein after acid loading. The acid-loading model and methods for renal protein isolation and immunoblot analyses and immunohistochemistry were identical in the two studies, thus suggesting that intact Rhcg expression may be necessary for normal regulation of Rhbg protein expression in the mouse renal cortex. In the rat, a similar model of metabolic acidosis did not alter renal Rhbg protein abundance (28). It is possible that other mechanisms of Rhbg regulation, such as phosphorylation-dephosphorylation or subcellular trafficking (32), may enable a role for Rhbg in response to metabolic acidosis in the rat.
Rhbg may also be important for basal rates of ammonia excretion, in addition to the response to metabolic acidosis. This interpretation is based on the observation that under basal conditions, intercalated cell-specific Rhbg deletion decreases glutamine synthetase expression. Glutamine synthetase catalyzes the reaction of NH4+ with glutamate to form glutamine, thereby resulting in net ammonia degradation, and decreased glutamine synthetase expression is known to increase net proximal tubule ammonia production (4). Accordingly, decreased glutamine synthetase expression in the outer medulla is likely an adaptive response to intercalated cell-specific Rhbg deletion which enables normal ammonia excretion under basal conditions.
In contrast to the current study, a previous study examining global Rhbg deletion did not identify an effect of Rhbg deletion on renal ammonia metabolism (3). Several explanations may account for the differences in findings between these studies. First, there are several important methodological differences between the acid-loading protocols used in the studies. In the current study, 0.4 M HCl was added to the chow, whereas in (3) NH4Cl, 280 mM was added to the drinking water. Whether the use of HCl vs. NH4Cl to induce metabolic acidosis alters the renal response is not known at present. Another difference is that very different increases in renal ammonia excretion were observed in the two studies; drinking water NH4Cl increased ammonia excretion to ∼200 μmol/day in Ref. 3, whereas dietary HCl increased ammonia excretion to ∼700 μmol/day. On the first day of acid loading in the current study, when urinary ammonia excretion was only ∼250 μmol/day, IC-Rhbg-KO did not alter ammonia excretion. Thus, one possible explanation for the different conclusions in the current study and in Ref. 3 is that Rhbg expression is not absolutely necessary for modest increases in urinary ammonia excretion, but that maximal increases require intact Rhbg expression.
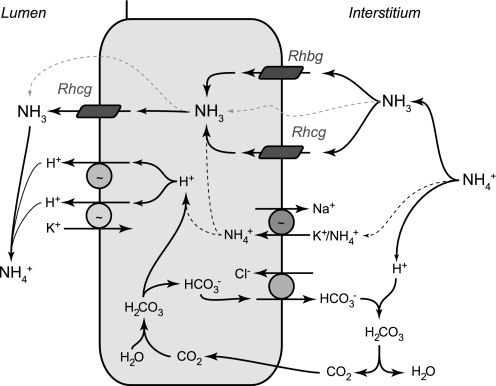
Rhbg is unlikely to be the only transport mechanism contributing to basolateral ammonia uptake. In addition to its well-recognized apical plasma membrane expression, Rhcg is also present in the basolateral plasma membrane in collecting duct cells in rat, human, and mouse kidney (2, 9, 13, 28, 29). Furthermore, both metabolic acidosis and reduced renal mass increase basolateral Rhcg expression (14, 29). In the IMCD, Na+-K+-ATPase contributes to basolateral ammonia uptake and transepithelial ammonia secretion (32–34). Since the IMCD is the portion of the collecting duct where there is the least Rhbg expression, it is possible that Rhbg and Na+-K+-ATPase may mediate complementary roles in basolateral ammonia uptake. Finally, diffusive basolateral NH3 transport is present (11), and increased interstitial ammonia, as occurs in metabolic acidosis, results in parallel increases in diffusive NH3 uptake. Figure 9 summarizes our current model of collecting duct ammonia secretion.
Fig. 9.
Model of collecting duct ammonia secretion. Interstitial NH4+ is in equilibrium with NH3 and H+. NH3 is transported across the basolateral membrane through both Rhbg and Rhcg. In the inner MCD (IMCD), basolateral Na+-K+-ATPase contributes to NH4+ transport; NH4+ then dissociates to NH3 and H+ (black dotted line). Intracellular NH3 is secreted across the apical membrane by apical Rhcg. H+-ATPase and H+-K+-ATPase secrete H+; H+ combines with luminal NH3 to form NH4+, which is “trapped” in the lumen. In addition, there are also components of diffusive NH3 movement across both the basolateral and apical plasma membranes (gray dotted lines). Intracellular H+ is generated by CA II-accelerated CO2 hydration that forms carbonic acid, which dissociates to H+ and HCO3−. Basolateral Cl−/HCO3− exchange transports HCO3− across the basolateral membrane; HCO3− combines with H+ released from NH4+ to form carbonic acid, which dissociates to CO2 and water. This CO2 can recycle into the cell, supplying the CO2 used for cytosolic H+ production. The net result is NH4+ transport from the peritubular space into the luminal fluid.
Whether Rhbg is involved in human kidney ammonia metabolism is unclear. Multiple studies demonstrated that Rhbg mRNA is present in the human kidney (2, 21) and suggested that Rhbg mRNA expression is greater in the kidney than in any other organ (21). However, a recent study failed to detect Rhbg protein expression in the human kidney (2). The explanation for this discrepancy between the high level of mRNA expression and lack of detectable protein expression cannot be determined at present.
Increasing evidence suggests glutamine synthetase may contribute to renal acid-base homeostasis. The late proximal tubule reabsorbs ammonia in normal animals (7) and can metabolize ammonia to glutamine in an enzymatic reaction catalyzed by glutamine synthetase in which NH4+ reacts with glutamate to form glutamine. Metabolic acidosis reverses late proximal tubule net ammonia transport from reabsorption to secretion (7) and decreases mouse renal glutamine synthetase expression (4, 16, and current study). In the rat kidney, metabolic acidosis decreases glutamine synthetase activity (5, 18), although it does not alter glutamine synthetase protein expression (35). Finally, the findings in the current study suggest that regulated glutamine synthetase expression may contribute to the adaptation to diminished Rhbg-mediated renal ammonia transport, both under basal conditions and in response to metabolic acidosis. However, determining the quantitative role of glutamine synthetase in renal ammonia metabolism requires defining the specific rates of ammonia metabolism involving glutamine synthetase and involving each of the other components of renal ammonia metabolism in in vivo states, and thus it involves much more than simple determination of steady-state changes in glutamine synthetase protein expression.
Another implication of the current study is that principal cell-mediated ammonia secretion contributes to acid-base homeostasis. Principal cells exhibit apical H+-ATPase and H+-K+-ATPase activities and basolateral Cl−/HCO3− exchange activity, and these processes are regulated in response to physiologic stimuli that alter collecting duct acid-base transport (36, 38). The Rh glycoprotein, Rhcg, is expressed in both intercalated cells and principal cells (13, 14, 19, 28, 29, 31), and principal cell Rhcg expression increases in response to both metabolic acidosis and reduced renal mass (14, 29). We reported recently that principal cell-specific Rhcg expression is sufficient to maintain normal rates of basal ammonia excretion (16), whereas Rhcg deletion from both intercalated and principal cells (15) or global deletion impairs basal ammonia excretion (1). In acid-loaded mice with collecting duct-specific Rhcg deletion, OMCD principal cell Rhbg expression increases more than in acid-loaded C mice (15), suggesting that increased principal cell Rhbg-mediated ammonia secretion contributes to the increased rate of ammonia excretion. Finally, the current study, by showing that Rhbg expression is necessary for the normal renal response to metabolic acidosis and that principal cell Rhbg expression increases in mice lacking intercalated cell Rhbg, further supports the conclusion that principal cells contribute to transcellular ammonia secretion, and thereby to acid-base homeostasis.
The CNT is an anatomically distinct portion of the distal tubule that connects the DCT to the initial collecting tubule. Increased Rhbg expression in the CNT in response to metabolic acidosis suggests that the CNT may contribute to increased ammonia excretion. Direct studies of CNT ammonia transport are difficult because of the inability to perform in vitro microperfusion with this segment, but in vivo micropuncture studies show that luminal ammonia increases between the early and end distal tubule, a region that includes the CNT (30). Taken with the results of the current study, metabolic acidosis appears to increase CNT ammonia secretion through mechanisms that involve increased Rhbg expression.
In summary, the current study provides substantial new information regarding the molecular mechanisms of ammonia transport in the mammalian kidney. Metabolic acidosis increases Rhbg expression and intercalated cell-specific Rhbg expression is necessary for normal ammonia excretion in response to metabolic acidosis, suggesting that Rhbg contributes to renal ammonia transport. Glutamine synthetase expression decreases in response to intercalated cell-specific Rhbg deletion, which may help maintain basal rates of renal ammonia excretion. Finally, CNT cell- and principal cell-specific Rhbg expression increases in response to metabolic acidosis, suggesting that these cells, in addition to intercalated cells, contribute to transporter-mediated ammonia secretion. These observations have significant implications for our understanding the molecular mechanisms of both ammonia transport and acid-base homeostasis.
GRANTS
These studies were supported by funds from the NIH (R01-DK-45788), Research Service of the North Florida/South Georgia Veterans Health System, and the Gatorade Research Foundation.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, the NIH, or the Department of Veterans Affairs.
ACKNOWLEDGMENTS
The authors thank Dr. D. E. Kohan for numerous helpful discussions regarding generation of floxed Rhbg mice, G. Cowsert for secretarial support, Dr. S. W. Matthews of the University of Florida College of Medicine Electron Microscopy Core for expert assistance with tissue processing, the personnel of the University of Florida Cancer and Genetics Transgenic Animal Core Facility for expert care and breeding of the mice, and the University of Texas Southwestern O'Brien Kidney Research Core Center [National Institutes of Health (NIH) P30-DK-079328] for performing the serum and urine creatinine measurements.
Footnotes
Ammonia consists of two molecular species, NH3 and NH4+, which are in equilibrium with each other according to the reaction NH3 + H+ ↔ NH4+. We use the term “ammonia” to refer to the sum of these two molecular forms and refer to each molecular form as either “NH3” or “NH4+,” respectively.
REFERENCES
- 1.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Brown ACN, Hallouane D, Mawby WJ, Karet FE, Saleem MA, Howie AJ, Toye AM. RhCG is the major putative ammonia transporter expressed in human kidney and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol 296: F1279–F1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Damian AC, Pitts RF. Rates of glutaminase I and glutamine synthetase reactions in rat kidney in vivo. Am J Physiol 218: 1249–1255, 1970 [DOI] [PubMed] [Google Scholar]
- 6.Elkinton JR, Huth EJ, Webster GD, Jr, McCance RA. The renal excretion of hydrogen ion in renal tubular acidosis. Am J Med 36: 554–575, 1960 [DOI] [PubMed] [Google Scholar]
- 7.Good DW, DuBose TD. Ammonia transport by early and late proximal convoluted tubule of the rat. J Clin Invest 79: 684–691, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297: L153–L163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemann J, Jr, Lennon EJ, Goodman AD, Litzow JR, Relman AS. The net balance of acid in subjects given large loads of acid or alkali. J Clin Invest 44: 507–517, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemieux G, Baverel G, Vinay P, Wadoux P. Glutamine synthetase and glutamyltransferase in the kidney of man, dog, and rat. Am J Physiol 231: 1068–1073, 1976 [DOI] [PubMed] [Google Scholar]
- 19.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Chen Y, Mo R, Hui CC, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Peng J, Mo R, Hui CC, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Sartorius OW, Roemmelt JC, Pitts RF. The renal regulation of acid-base balance in man. IV. The nature of the renal compensations in ammonium chloride acidosis. J Clin Invest 28: 423–439, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Simon E, Martin D, Buerkert J. Contribution of individual superficial nephron segments to ammonium handling in chronic metabolic acidosis in the rat. Evidence for ammonia disequilibrium in the renal cortex. J Clin Invest 76: 855–864, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Wall SM. NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Wall SM. Ouabain reduces net acid secretion and increases pHi by inhibiting NH4+ uptake on rat tIMCD Na+-K+-ATPase. Am J Physiol Renal Physiol 273: F857–F868, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Wall SM, Koger LM. NH4+ transport mediated by Na+-K+-ATPase in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F660–F670, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Watford M. Regulation of expression of the genes for glutaminase and glutamine synthetase in the acidotic rat. Contrib Nephrol 92: 211–217, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol Renal Physiol 276: F606–F613, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of the inner stripe of the rabbit outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F406–F415, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Kim CVAN, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391: 33–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]