Abstract
In autosomal dominant polycystic kidney disease (ADPKD), aberrant proliferation of the renal epithelial cells is responsible for the formation of numerable fluid-filled cysts, massively enlarged kidneys, and progressive loss of renal function. cAMP agonists, including arginine vasopressin, accelerate cyst epithelial cell proliferation through protein kinase A activation of the B-Raf/MEK/extracellular signal-regulated kinase (ERK) signaling pathway. The mitogenic effect of cAMP is equally potent and additive to growth factor stimulation. Here, we determined whether Sorafenib (BAY 43–9006), a small molecule Raf inhibitor, inhibits proliferation of cells derived from the cysts of human ADPKD kidneys. We found that nanomolar concentrations of Sorafenib reduced the basal activity of ERK, inhibited cAMP-dependent activation of B-Raf and MEK/ERK signaling, and caused a concentration-dependent inhibition of cell proliferation induced by cAMP, epidermal growth factor, or the combination of the two agonists. Sorafenib completely blocked in vitro cyst growth of human ADPKD cystic cells cultured within a three-dimensional collagen gel. These data demonstrate that cAMP-dependent proliferation of human ADPKD cyst epithelial cells is blocked by Sorafenib and suggest that small molecule B-Raf inhibitors may be a therapeutic option to reduce the mitogenic effects of cAMP on cyst expansion.
Keywords: polycystic kidney disease, cyclic AMP, epidermal growth factor, growth factors, MAP kinase
autosomal dominant polycystic kidney disease (ADPKD) is one of the most common hereditary renal disorders and accounts for ∼10% of patients with end-stage renal disease requiring renal replacement therapy. Although cysts are benign neoplasms, their unrelenting growth leads to extensive nephron loss, interstitial fibrosis, and progressive loss of renal function. ADPKD is caused by mutations in PKD1 (85% of cases) and PKD2 (15%), which encode polycystin-1 (PC-1) and PC-2, respectively (3, 11, 39). It remains unclear how mutations in the PKD genes transform tubule epithelial cells into poorly differentiated cells that give rise to fluid-filled cysts. However, it is generally accepted that aberrant proliferation of cyst-lining epithelial cells is important for cyst enlargement. Cystic cells of ADPKD kidneys have increased activity of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway (18), a key signaling cascade involved in the regulation of multiple processes required for cell proliferation and cell survival.
Raf kinases are a family of serine/threonine kinases that are intermediates in transmitting extracellular signals, including those from growth factors, to MEK, a MAPK kinase that phosphorylates and activates ERK (9). Activation of the Raf/MEK/ERK signaling pathway is essential in normal development and coordinates cell cycle reentry in response to growth factor stimulation during tissue repair. An initial step in the Raf/MEK/ERK pathway is activation of Ras, a small GTPase protein that recruits Raf to the plasma membrane and is required for Raf activity. Activated Raf phosphorylates and activates MEK which, in turn, stimulates ERK. Translocation of activated ERK to the nucleus leads to phosphorylation of transcription factors and the upregulation of the transcriptional activity of a number of genes involved in cell proliferation.
B-Raf and Raf-1 (also called C-Raf) are members of the Raf kinase family and are expressed in many cell types, including renal epithelial cells. B-Raf and Raf-1 share homology in amino acid sequence; however, the two kinases are differentially regulated. For example, Raf-1 requires phosphorylation at S338 for kinase activation. By contrast, S446, the corresponding site in B-Raf, is constitutively phosphorylated. In addition, Raf-1 contains two tyrosine residues that must be phosphorylated for full kinase activity. These residues are replaced in B-Raf with aspartic acid residues (D448, D449), mimicking phosphorylated tyrosines (14). As a result, B-Raf requires fewer phosphorylation events for maximal activation. B-Raf has a higher affinity for MEK than does Raf-1 and produces the strongest induction of MEK activity; and when present, B-Raf seems to be the preferred MAPK kinase kinase.
cAMP signaling can either stimulate or inhibit the Raf/MEK/ERK pathway and cell proliferation, depending on cell type and cellular conditions (26). In astrocytes, smooth muscle cells, fibroblasts, and mesangial cells, cAMP inhibits ERK activity and cell proliferation by PKA phosphorylation of an inactivation site on Raf-1. On the other hand, cAMP signaling activates ERK and proliferation of other cell types, including thyroid cells, hepatocytes, and PC-12 neuronal cells. In ADPKD, cAMP has a central role in cyst growth by stimulating both fluid secretion and cell proliferation. We found that activation of cAMP signaling stimulates B-Raf, leading to increased ERK activity and proliferation of ADPKD cells, but it inhibits the proliferation of normal renal cells (43, 44). The phenotypic difference in the mitogenic response to cAMP between normal and ADPKD cells appears to be related to steady-state Ca2+ levels (42, 45).
Several studies demonstrated the importance of cAMP activation of the B-Raf/MEK/ERK pathway in the proliferation of the cystic cells in PKD (21, 22, 42, 45). Inhibition of cAMP production by blocking the vasopressin V2 receptor (V2R) with a receptor antagonist or reduction of plasma vasopressin levels through increased water intake lowered renal cAMP levels and inhibited ERK activity and cystic area (7, 16, 30, 34, 37, 38). Tolvaptan, a selective V2R antagonist, is currently in clinical trials in ADPKD patients and studies to examine the beneficial outcome of increased water intake in ADPKD patients are being considered (31). Octreotide is a long-acting somatostatin capable of reducing cAMP production via Gi activation and is also in clinical trials for ADPKD (23). There are complicating side effects of treatments designed to lower renal cAMP, including persistent polyuria which makes it unclear whether these methods can be tolerated by the patient long-term (31). By contrast, we think that B-Raf is an ideal target to slow cyst growth since B-Raf appears to be uniquely activated in ADPKD cyst cells and centrally located for ERK activation and cell proliferation.
Sorafenib (BAY 43–9006) is a multi-kinase inhibitor that was developed as a Raf inhibitor, but also has activity against receptor tyrosine kinases, including VEGFR and PDGFR (41). Sorafenib (also called Nexavar) has been approved for treatment of advanced renal cell and hepatocellular carcinomas. In this study, we determined whether Sorafenib could block cAMP activation of the B-Raf/MEK/ERK pathway and proliferation of human ADPKD cyst epithelial cells. The results demonstrate that relatively low concentrations of Sorafenib completely blocked ERK activation and cell proliferation induced by cAMP alone, or in combination with epidermal growth factor (EGF). These data support the hypothesis that B-Raf has a central role in cAMP-dependent activation of ERK and cell proliferation in ADPKD. We conclude that small molecule Raf inhibitors may have therapeutic value in slowing the proliferation of cyst epithelial cells.
METHODS
Primary cultures of ADPKD cells.
Kidneys were obtained from hospitals participating in the Polycystic Kidney Research Retrieval Program with the assistance of the PKD Foundation (Kansas City, MO) and from the Tissue and Serum Repository in the Kansas Cancer Center at the University of Kansas Medical Center. The protocol for the use of surgically discarded kidney tissue complies with federal regulations and was approved by the Institutional Review Board at the University of Kansas Medical Center. Primary cultures were prepared by the PKD Biomaterials Research Core of the Kansas Interdisciplinary Center for PKD Research as previously described (35, 42). Cells were seeded and grown in T75 flasks containing DMEM/F12 (1:1) media supplemented with 5% FBS, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml sodium selenite (ITS). At 70 to 80% confluency, cells were lifted from the plastic and either frozen in culture medium containing 10% DMSO for storage in liquid N2 or seeded directly for experiments.
Sorafenib.
Sorafenib {BAY 43–9006; N-(3-trifluoromethyl-4-chlorophenyl)-N′-[4-(2-methylcarbamoyl pyridin-4-yl) oxyphenyl] urea} was a generous gift from Bayer (West Haven, CT) (41). Sorafenib was dissolved in DMSO and stored as a ×1,000 stock solution in a −20°C freezer.
Cell proliferation measurements.
ADPKD cells (4 × 103 cells/well) were seeded into 96-well culture plates (6 wells/experimental condition) and incubated in DMEM/F12 supplemented with 1% FBS and ITS for 24 h. The serum concentration was reduced to 0.002% and ITS was removed for an additional 24 h before the experiment. For each experiment, cells were incubated in 0.002% FBS media containing 100 μM 8-Br-cAMP, 25 ng/ml EGF, and/or Sorafenib (10, 50, or 100 nM) for 48–72 h, and then cell proliferation was determined by Promega Cell Titer 96 MTT assay (44).
Immunoblot analysis.
Cells (0.5 × 106) were seeded onto plastic petri dishes (100-mm diameter) containing DMEM/F12 medium with 1% FBS. The serum was reduced to 0.002% when the cells were ∼75% confluent and the cells were allowed to grow for an additional 24 h. Cells were treated with Sorafenib for 30 min, and then 8-Br-cAMP and/or EGF were added for an additional 15 min before cell lysates were prepared (43). Immunoblots were probed with antibodies for phospho-ERK (E-4), ERK1 (C-16), and ERK2 (C14) from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody for phospho-MEK was purchased from Biosource (Camarillo, CA). Secondary anti-rabbit, -mouse, -rat, and -goat IgG conjugated to horseradish peroxidase were purchased from Santa Cruz Biotechnology.
Kinase assay.
Soluble cellular extracts (500 μg) were immunoprecipitated for 2 h by gentle rotation at 4°C with either an anti-B-Raf or anti-Raf-1 antibody that was covalently coupled to protein A/G PLUS agarose beads (43). Immunoprecipitates were washed and resuspended in 20 μl of solution containing 0.5 mmol β-glycerophosphate (pH 7.3), 1.5 mmol/l EGTA, 1 mmol/l dithiothreitol, and 0.03% Brij 35. The kinase activities of B-Raf and Raf-1 were determined by the phosphorylation of exogenous MEK, a natural substrate for the kinases. The kinase assay was performed in a reaction mixture containing 20 μl of kinase reaction solution [16 μl of 50 mmol/l MgCl2, 2 μl of 1 mmol ATP, and 2 μg human MEK-1 fusion protein (full-length, Santa Cruz Biotechnology)], mixed with 20 μl of the resuspended beads, and incubated for 30 min. The reaction was stopped by the addition of SDS sample buffer. The reaction product was immunoblotted using an antibody to phosphorylated MEK and visualized by the ECL system. Band images were captured and quantified by a Fluor-S MAX Multi Imager System (Bio-Rad, Hercules, CA).
Measurements of cultured ADPKD cysts.
ADPKD cells (4 × 103 cells/well) were dispersed within ice-cold type I collagen (Vitrogen; Collagen, Palo Alto, CA) in wells of a 96-well culture plate (35, 36). Warming the plate to 37°C caused polymerization of the collagen, trapping the cells within the gel. Cells were incubated in a defined medium (DMEM/F12 with ITS, 5 × 10−8 M hydrocortisone, 5 × 10−5 M triiodothyronine) containing 5 μM forskolin and 5 ng/ml EGF with or without Sorafenib for a total of 8 days. In a second series of experiments, EGF and forskolin were added for 3 days to initiate cyst growth. At this time, the outer diameters of the cysts were <100 μm. The agonists were removed and the gels were rinsed twice with defined media. To initiate the experiment, control media or media containing 5 μM forskolin, 50 nM Sorafenib, or the combination of the two agents were added. After 5 days, the outer diameters of cross-sectional images of spherical cysts with distinct lumens were measured using a digital camera attached to an inverted microscope and analyzed with video analysis software. Surface area was calculated from the outer diameters and total surface area of the cysts was determined from the sum of individual cysts within each well. Cysts with diameters <100 μm were excluded from measurement.
Statistics.
Data are expressed as means ± SE. Statistical significance was determined by one-way ANOVA and Student-Newman-Keuls posttest for multiple comparisons or unpaired t-test for comparison between control and treated groups.
RESULTS
Effect of Sorafenib on cAMP-dependent activation of ERK and proliferation of human ADPKD cells.
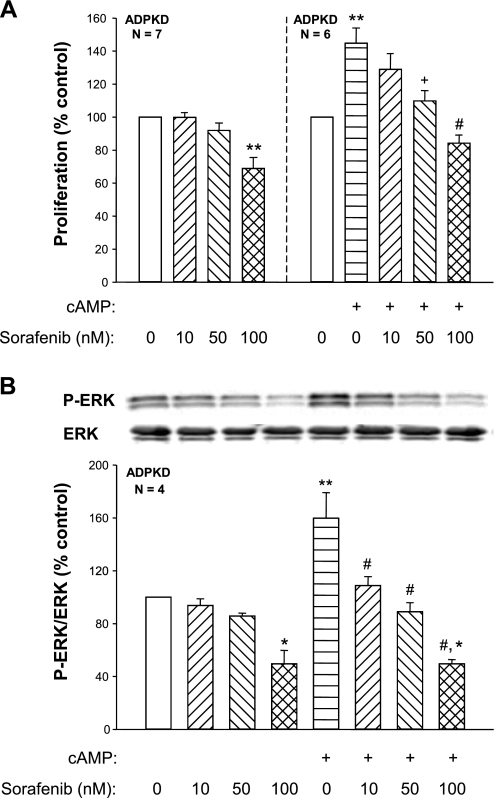
To determine whether pharmacological inhibition of Raf kinases inhibits cAMP-dependent activation of ADPKD cell proliferation, we measured phosphorylated ERK (P-ERK) levels and proliferation rates of human ADPKD cells incubated in the absence or presence of various concentrations of Sorafenib (10–100 nM). The addition of 100 nM Sorafenib decreased P-ERK/ERK by 51 ± 10% (P < 0.01, n = 4; Fig. 1B) and inhibited ADPKD cell proliferation by 31 ± 7% (P < 0.001, n = 7; Fig. 1A). These results are in accordance with a previous study, in which H-89, a protein kinase A (PKA) inhibitor, and PD-98059, a MEK inhibitor, reduced ERK activity and cell proliferation below control levels (43), suggesting that basal ADPKD cell proliferation is dependent, at least in part, on endogenous cAMP activation of the B-Raf/MEK/ERK pathway.
Fig. 1.
Effects of Sorafenib on cAMP-dependent cell proliferation and extracellular signal-regulated kinase (ERK) activity in human autosomal dominant polycystic kidney disease (ADPKD) cells. Primary cultures of ADPKD cells were incubated with 100 μM 8-Br-cAMP (cAMP) and concentrations of Sorafenib ranging from 10 to 100 nM. Rates of cell proliferation were determined by a Promega Cell Titer 96 MTT assay (A) and levels of phosphorylated ERK (P-ERK) were determined by immunoblot analysis (B). Addition of 100 nM Sorafenib caused a significant reduction in basal P-ERK levels and cell proliferation. Sorafenib caused a dose-dependent inhibition of cAMP-stimulated cell proliferation that was accompanied by a reduction in the level of P-ERK. *P < 0.01 and **P < 0.001 compared with control (open bars); +P < 0.01 and #P < 0.001 compared with cAMP treatment only. Lower concentrations of Sorafenib (0.01–1 nM) had no effect on baseline or cAMP-dependent cell proliferation (data not shown).
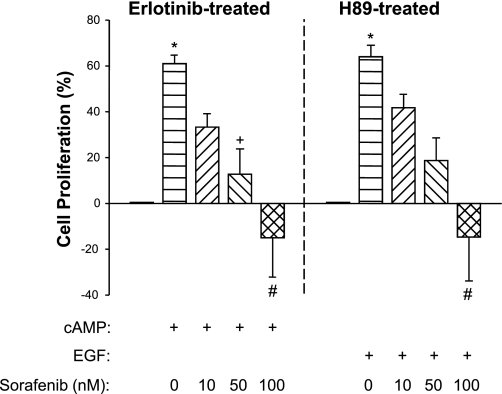
Addition of 100 μM 8-Br-cAMP increased P-ERK/ERK by 60 ± 19% (P < 0.001, n = 4) and stimulated ADPKD cell proliferation by 45 ± 9% (P < 0.001, n = 6) compared with control-treated ADPKD cells (Fig. 1, A and B). Treatment with 50 nM Sorafenib completely blocked cAMP stimulation of ERK and cell proliferation. Interestingly, 100 nM Sorafenib reduced P-ERK and proliferation to levels that were below the control group, suggesting that this concentration completely inhibited cAMP-dependent ERK activation. To examine the effect of Sorafenib on cAMP-induced proliferation in the absence of a potential contribution of receptor tyrosine kinase stimulation, we treated ADPKD cells with Erlotinib (Tarceva; gift from Genetech, South San Francisco, CA), a small molecule inhibitor of EGF receptor (HER1/EGFR). In the presence of Erlotinib, cAMP-stimulated ADPKD cell proliferation and Sorafenib completely blocked the mitogenic effect of cAMP (Fig. 2).
Fig. 2.
Effect of Sorafenib on B-Raf and Raf-1-mediated proliferation of ADPKD cells. ADPKD cells were treated with 10 nM Erlotinib (Tarceva; Genentech, South San Francisco, CA), a receptor tyrosine kinase inhibitor, to reduce the activity of Raf-1 by basal stimulation of the epidermal growth factor receptor (EGFR). In ADPKD cells, 100 μM cAMP increased B-Raf activity, but it did not stimulate Raf-1 (43). Thus, in the presence of Erlotinib, cAMP activation of MEK/ERK and cell proliferation are presumably mediated only through B-Raf signaling. Sorafenib inhibited cAMP-dependent cell proliferation in a concentration-dependent relationship, consistent with an inhibitory effect on B-Raf. To isolate the effect of Sorafenib on growth factor-mediated cell proliferation, H-89, a protein kinase A inhibitor, was used to inhibit endogenous cAMP activation of B-Raf. In the presence of H-89, cell proliferation induced by EGF (25 ng/ml) is probably mediated through both Raf-1 and B-Raf activation of the MEK/ERK pathway. Sorafenib inhibited EGF-dependent cell proliferation in a concentration-dependent fashion that was similar to its effect on cAMP-dependent cell proliferation; n = 3 different ADPKD cell preparations per experiment. *P < 0.05 compared with untreated control; +P < 0.05 and #P < 0.01 compared with cAMP or EGF treatment alone.
Effect of Sorafenib on B-Raf and Raf-1 kinase activities.
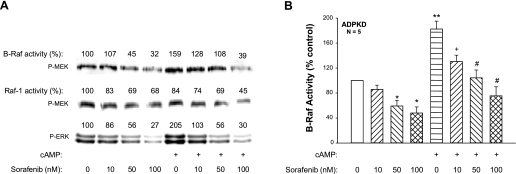
Sorafenib does not distinguish between Raf isoforms and is capable of inhibiting both Raf-1 and B-Raf. In biochemical assays, Sorafenib potently inhibited Raf-1 (IC50 6 nmol/l), B-Raf (IC50 22 nmol/l), and constitutively active B-RafV600E (IC50 38 nmol/l) (29); however, single-digit micromolar concentrations of Sorafenib were required to inhibit B-Raf and Raf-1 in cancer cells (24, 40). Sorafenib is known to bind serum proteins, therefore requiring a higher concentration of drug for Raf inhibition in cells grown in serum-containing media. To examine the effect of Sorafenib on B-Raf and Raf-1 in ADPKD cells, the serum concentration was reduced to 0.002% (heat-inactivated FBS) and 8-Br-cAMP ± Sorafenib (10–100 nM) were added. B-Raf or Raf-1 was immunoprecipitated from cell lysates and then a kinase assay was performed using exogenous MEK as a substrate for phosphorylation (Fig. 3). Treatment with cAMP alone for 15 min increased B-Raf activity above that of control-treated cells. By contrast, cAMP inhibited Raf-1 activity, a result consistent with PKA phosphorylation of Serine 338, an inhibitory site on Raf-1 (43). These results confirm that cAMP-dependent proliferation of ADPKD cells is mediated by the activation of B-Raf, not Raf-1. Treatment of ADPKD cells with Sorafenib ranging in concentration from 10 to 100 nM reduced basal activities of B-Raf, Raf-1, and ERK. Incubation with Sorafenib for 30 min before the addition of 8-Br-cAMP caused a dose-dependent inhibition of B-Raf activity and further reduction in Raf-1 activity. There was a corresponding reduction in the level of P-ERK. As shown in Fig. 3B, Sorafenib significantly reduced basal B-Raf kinase activity and completely blocked cAMP-dependent activation of B-Raf in ADPKD cells.
Fig. 3.
Effect of Sorafenib on cAMP-dependent B-Raf and Raf-1 kinase activity in ADPKD cells. ADPKD cells in control media or media containing 100 μM cAMP were treated with various concentrations of Sorafenib, ranging from 10 to 100 nM. Cells were incubated with Sorafenib for 30 min and then cAMP was added for an additional 15 min in the presence of Sorafenib. B-Raf and Raf-1 were immunoprecipitated from cell lysates for in vitro kinase assays using MEK as a substrate. Raf kinase activity was determined by immunoblot analysis using an anti-phospho-MEK antibody. Band intensities were quantified and reported as density units relative to control (set to 100). A: representative immunoblot for B-Raf and Raf-1 activities and levels of P-ERK in Sorafenib-treated ADPKD cells. B: effect of Sorafenib on cAMP-dependent B-Raf activity in cyst-derived cells from 5 ADPKD kidneys. *P < 0.05 and **P < 0.001 compared with the untreated control; +P < 0.01 and #P < 0.001 compared with treatment with cAMP alone.
Effect of Sorafenib on growth factor activation of ERK and proliferation of human ADPKD cells.
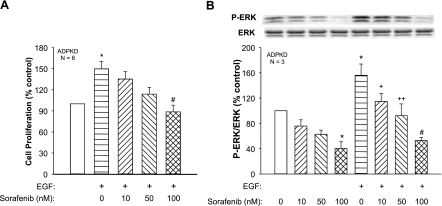
EGF and insulin-like growth factor have been shown to stimulate ADPKD cell proliferation through activation of the Ras/Raf/MEK/ERK signaling pathway (8, 22, 43). To determine whether Sorafenib also inhibits the mitogenic effect of growth factors on ADPKD cells, we incubated ADPKD cells with 25 ng/ml EGF alone or in combination with cAMP. EGF increased cell proliferation 50 ± 11% (P < 0.05, n = 6; Fig. 4A) and P-ERK levels 56 ± 18% (P < 0.05, n = 3) above control (Fig. 4B). Addition of Sorafenib inhibited the effect of EGF on ERK activation and cell proliferation with a similar dose dependency as its effect on cAMP-dependent cell proliferation (compare Fig. 4 with Fig. 1). To eliminate the potential contribution of cAMP, ADPKD cells were treated with H-89, a PKA inhibitor (Fig. 2). In the absence of PKA signaling, EGF increased cell proliferation to ∼60%, and Sorafenib caused a dose-dependent inhibition of cell proliferation that paralleled its effect in the absence of H-89.
Fig. 4.
Effect of Sorafenib on cell proliferation and ERK activity in ADPKD cells treated with EGF. EGF, a well-known agonist for renal epithelial cell proliferation, stimulated ADPKD cell proliferation (A) and increased ERK activity (B), consistent with previous studies (43, 44). Sorafenib decreased basal P-ERK and cell proliferation and caused a concentration-dependent decrease in EGF-stimulated cell proliferation and ERK activation. *P < 0.05 compared with the untreated control; +P < 0.05, ++P < 0.01, and #P < 0.001 compared with EGF alone.
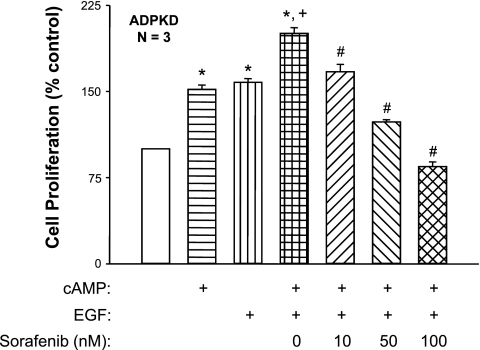
Next, we examined the effect of Sorafenib on ADPKD cell proliferation induced by the combination of cAMP and EGF. We found that cAMP and EGF caused a significantly greater increase in cell proliferation compared with the individual effects of two compounds alone (Fig. 5), as reported previously (10, 44). Sorafenib, at a concentration as low as 10 nM, inhibited cell proliferation induced by the combination of cAMP and EGF, supporting the hypothesis that both agonists function through the Raf kinases to induce the proliferation of ADPKD cyst epithelial cells.
Fig. 5.
Effect of Sorafenib on the proliferation of ADPKD cells stimulated with cAMP and EGF. ADPKD cells were treated with 100 μM cAMP, 25 ng/ml EGF, or a combination of the 2 agonists in the absence or presence of Sorafenib (10, 50, or 100 nM). Sorafenib potently inhibited ADPKD cell proliferation induced by the combination of cAMP and EGF. *P < 0.001 compared with the untreated control; +P < 0.001 compared with treatment with either cAMP or EGF alone; #P < 0.001 compared with treatment with cAMP + EGF alone.
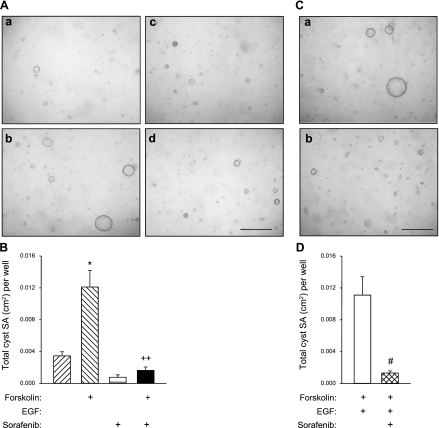
Effect of Sorafenib on human ADPKD cyst growth in vitro.
Microcysts were generated by culturing ADPKD cells within a polymerized collagen gel as described previously by this laboratory (35, 42). After the cysts had formed, agonists were removed, and forskolin was added in the absence or presence of 50 nM Sorafenib. Forskolin, a direct activator of adenylyl cyclases that maximally elevates intracellular cAMP and stimulates ADPKD cell proliferation and fluid secretion (27), caused an increase in the size of in vitro cysts (Fig. 6A). Total cyst surface area (SA) increased from 0.003 ± 0.001 cm2 to 0.012 ± 0.002 cm2, P < 0.001, n = 6 wells (Fig. 6B). The addition of Sorafenib reduced total SA of the cysts 75% compared with forskolin alone, consistent with the effect of Raf inhibition on cAMP-mediated cell proliferation. The effect of Sorafenib was also tested on ADPKD microcysts treated with a combination of forskolin and EGF. Addition of 50 nM Sorafenib at the beginning of the treatment period decreased total SA of the microcysts by 88.3% (Fig. 6, C and D).
Fig. 6.
Effect of Sorafenib on in vitro cyst growth of ADPKD cells cultured within collagen gel. A: ADPKD cells were dispersed within collagen gels and media containing 5 μM forskolin and 5 ng/ml EGF were added for 3 days to induce cyst formation. Agonists were removed and control media (a), forskolin (b), Sorafenib (c), or the combination of the 2 compounds (d) were added for an additional 5 days. Gels were fixed in 1% formalin and total surface area (SA) of cysts with diameters greater than 100 μm was measured. B: forskolin alone stimulated cyst growth above control and the addition of 50 nM Sorafenib completely blocked cAMP-induced cyst growth. C: in a second experiment, ADPKD cells were incubated with forskolin and EGF without (a) and with (b) 50 nM Sorafenib for 8 days. Afterwards, the gels were fixed and total cyst SA was measured. D: Sorafenib inhibited cyst formation induced by the combination of forskolin and EGF. *P < 0.001 compared with the untreated control; ++P < 0.001 compared with treatment with forskolin alone; #P < 0.01 compared with forskolin + EGF.
DISCUSSION
Role of B-Raf in cAMP-dependent cell proliferation in PKD.
The rationale for targeting B-Raf in ADPKD is based on studies that demonstrate that cAMP signaling stimulates B-Raf, leading to ERK activation and proliferation of human ADPKD cyst epithelial cells. In addition, cystic kidneys of PKD animals, including Cy/+ rats, jck mice, and pcy/pcy mice, have elevated levels of intracellular cAMP and increased activity of the Raf/MEK/ERK pathway (17, 18, 20–22, 25, 42–45). Arginine vasopressin (AVP), an anti-diuretic hormone that binds to V2 receptors on cystic cells, is an important factor in cAMP production and disease progression in PKD (7, 16, 34, 37, 38). V2 receptors are G protein-coupled receptors that stimulate adenylyl cyclases, enzymes that synthesize cAMP. It has been proposed that low intracellular Ca2+ in cystic cells causes an increase in the activity of Ca2+-inhibited adenylyl cyclases and a decrease in the activity of Ca2+-dependent phosphodiesterases, enzymes that degrade cAMP (33). Changes in the activities of these enzymes may raise basal cAMP to levels that are closer to the threshold for PKA activation. Consequently, a higher resting cAMP level could make ADPKD cells more sensitive to AVP stimulation and/or amplify the cAMP signal. Thus, normal plasma AVP may maintain intracellular cAMP at elevated levels in the cystic cells that stimulate the B-Raf/MEK/ERK pathway and cell proliferation (1, 16).
In normal renal cells, B-Raf is repressed by Akt phosphorylation of an inhibitory site (S365), in a PI3 kinase- and Ca2+-dependent manner. Thus, cAMP is unable to stimulate B-Raf and ERK in normal cells, but instead it inhibits Raf-1, leading to decreased P-ERK and cell proliferation. Pharmacological inhibition of PI3 kinase or Akt in normal renal cells relieves B-Raf inhibition, allowing cAMP-dependent activation of B-Raf, ERK, and cell proliferation (45).
In ADPKD, mutations in either PKD1 or PKD2 result in insufficient expression of their protein products, polycystin-1 and polycystin-2, and predispose tubule epithelial cells to an abnormal mitogenic response to cAMP. The underlying hypothesis is that a defect in polycystin function reduces steady-state levels of intracellular Ca2+ that relieves Akt inhibition of B-Raf, thus allowing cAMP activation of the B-Raf/MEK/ERK pathway. The role of Ca2+ in regulating the mitogenic response to cAMP can be demonstrated experimentally. Restriction of intracellular Ca2+ in wild-type cells with Ca2+ channel blockers reduces Akt activity, allowing cAMP activation of B-Raf, ERK, and cell proliferation, mimicking the PKD phenotype (45). Treatment of Cy/+ rats, a model of dominantly inherited PKD, with a Ca2+ channel blocker caused a further increase in renal B-Raf and P-ERK levels, number of proliferating cells within the kidney, and total kidney volume (17). Thus, reduced Ca2+ and elevated cAMP in cystic epithelial cells appear to act synergistically to activate B-Raf, leading to inappropriate activation of MEK/ERK pathway which may be uniquely responsible for proliferation of these cells (42). As such, we think that B-Raf is at the nexus between aberrant intracellular Ca2+ and cAMP signaling that is responsible for activation of the ERK pathway and ADPKD cell proliferation.
Targeting B-Raf with Sorafenib.
In contrast to the other Raf kinases, B-Raf is frequently activated by mutations and somatic mutations in B-Raf are found in as many as 8% of all human cancers (4). A single point mutation in B-Raf (BRAFV600E) leads to a constitutively active kinase that is associated with cancer (4); however, in the absence of other mutations, BRAFV600E leads to benign neoplasms, e.g., nevi (15). In ADPKD, benign neoplastic growth of renal cysts appears to be caused by persistent B-Raf activation, not due to activating mutations in B-Raf, but rather due to decreased intracellular Ca2+ levels and continual activation by cAMP (2).
Many of the signaling molecules involved in cAMP-dependent proliferation of ADPKD cells are also components of the mitogenic pathways activated by growth factor stimulation. Raf kinases are central for ERK activation and cell proliferation by cAMP and growth factors; accordingly, small molecule Raf inhibitors may target both of these mitogenic signals. Recent studies showed that activation of MEK/ERK signaling by growth factors negatively regulates Rheb, a GTPase-activating protein, leading to increased activity of mTOR, a serine/threonine kinase involved in the regulation of protein translation, responses to cellular energy, and cell cycle progression (5, 13). This pathway has been implicated in PKD and rapamycin, an mTOR inhibitor, is being considered for treatment of PKD (32). It is reasonable to speculate that a decrease in ERK activity due to Raf inhibition may also reduce the activity of the mTOR pathway.
Sorafenib is an orally available, small molecule Raf inhibitor that decreases ERK activity and inhibits proliferation of human cancer cells caused by activating mutations in the Ras/Raf pathway, including BRAFV600E (12, 24, 40, 41). In biochemical studies, Sorafenib inhibits B-Raf and Raf-1 with an IC50 in the low nanomolar range; however, higher concentrations are required to inhibit tumor growth in vivo (12). In this study, we found that 10 to 100 nmol/l Sorafenib inhibited the basal activity of B-Raf, decreased P-ERK levels, and slowed ADPKD cell proliferation, consistent with the idea that endogenous production of cAMP contributes to the basal activities of B-Raf and ERK and cell proliferation.
Effect of Sorafenib on ADPKD cell proliferation stimulated by cAMP and growth factors.
In the current study, we found that 8-Br-cAMP, a permeable form of cAMP, inhibited Raf-1, but increased B-Raf and ERK activities and stimulated ADPKD cell proliferation, consistent with previous reports (43, 44). Sorafenib completely blocked cAMP-dependent stimulation of B-Raf, ERK, and cell proliferation, confirming that the mitogenic effect of cAMP is mediated by activation of B-Raf and not by other cAMP-signaling pathways. Activation of receptor tyrosine kinases by EGF, transforming growth factor-α, and insulin-like growth factor also stimulates the Raf/MEK/ERK pathway and proliferation of cyst epithelial cells (6, 19, 28, 43). We found that Sorafenib inhibited ERK activation and cell proliferation stimulated by EGF alone or in combination with cAMP, and blocked in vitro cyst growth induced by cAMP and EGF. Taken together, these data suggest that inhibition of B-Raf and Raf-1, with these relatively low concentrations of Sorafenib, blocked the mitogenic effects of cAMP and EGF in these cells.
In summary, B-Raf appears to be uniquely activated in human ADPKD cyst epithelial cells by the combination of elevated intracellular cAMP and reduced intracellular Ca2+ levels (42, 43, 45). Here, we show that nanomolar concentrations of Sorafenib inhibit B-Raf, MEK/ERK signaling, cell proliferation, and in vitro cyst growth of human ADPKD cells stimulated by cAMP and/or EGF. These results demonstrate that ADPKD cells are exquisitely sensitive to Sorafenib and suggest that B-Raf inhibition with small molecule inhibitors may be a potential therapeutic approach to reduce the mitogenic effects of cAMP agonists on cyst growth.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-081579 (to D. P. Wallace) and P50-DK-057301 (to D. P. Wallace and J. P. Calvet).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. D. P. Healy for helpful comments on the manuscript.
REFERENCES
- 1.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66: 964–973, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Calvet JP. MEK inhibition holds promise for polycystic kidney disease. J Am Soc Nephrol 17: 1498–1500, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature 417: 949–954, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, Germino GG, Pandolfi PP, Boletta A. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol 29: 2359–2371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattone VH, 2nd, Kuenstler KA, Lindemann GW, Lu X, Cowley BD, Jr, Rankin CA, Calvet JP. Renal expression of a transforming growth factor-alpha transgene accelerates the progression of inherited, slowly progressive polycystic kidney disease in the mouse. J Lab Clin Med 127: 214–222, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Grantham JJ. Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl 63: S93–S97, 1997 [PubMed] [Google Scholar]
- 9.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem 275: 27354–27359, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11: 1179–1187, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R. B-RAF is a therapeutic target in melanoma. Oncogene 23: 6292–6298, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J 18: 2137–2148, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene 27: 877–895, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int 73: 269–277, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Nagao S, Yamaguchi T, Kusaka M, Maser RL, Takahashi H, Cowley BD, Grantham JJ. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int 63: 427–437, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Neufeld TK, Grantham JJ. Epidermal growth factor promotes cyst formation by human renal epithelial cells in vitro. Trans Assoc Am Physicians 103: 48–52, 1990 [PubMed] [Google Scholar]
- 20.Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17: 1604–1614, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem 284: 7214–7222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, Epstein FH. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 68: 206–216, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Salvatore G, De Falco V, Salerno P, Nappi TC, Pepe S, Troncone G, Carlomagno F, Melillo RM, Wilhelm SM, Santoro M. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res 12: 1623–1629, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12: 258–266, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev 78: 1165–1191, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Sweeney WE, Jr, Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res 326: 671–685, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol 5: 350–356, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Torres VE. Therapies to slow polycystic kidney disease. Nephron Exp Nephrol 98: e1–e7, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol 4: 1140–1150, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Torres VE, Boletta A, Chapman A, Gattone V, Pei Y, Qian Q, Wallace DP, Weimbs T, Wuthrich RP. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol 5: 1312–1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH., 2nd Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Wallace DP, Quante MA, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through integrin alpha (v) receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Gattone V, 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol 19: 327–343, 1999 [PubMed] [Google Scholar]
- 40.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5: 835–844, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004 [DOI] [PubMed] [Google Scholar]