Abstract
We showed that luminal flow stimulates nitric oxide (NO) production in thick ascending limbs. Ion delivery, stretch, pressure, and shear stress all increase when flow is enhanced. We hypothesized that shear stress stimulates NO in thick ascending limbs, whereas stretch, pressure, and ion delivery do not. We measured NO in isolated, perfused rat thick ascending limbs using the NO-sensitive dye DAF FM-DA. NO production rose from 21 ± 7 to 58 ± 12 AU/min (P < 0.02; n = 7) when we increased luminal flow from 0 to 20 nl/min, but dropped to 16 ± 8 AU/min (P < 0.02; n = 7) 10 min after flow was stopped. Flow did not increase NO in tubules from mice lacking NO synthase 3 (NOS 3). Flow stimulated NO production by the same extent in tubules perfused with ion-free solution and physiological saline (20 ± 7 vs. 24 ± 6 AU/min; n = 7). Increasing stretch while reducing shear stress and pressure lowered NO generation from 42 ± 9 to 17 ± 6 AU/min (P < 0.03; n = 6). In the absence of shear stress, increasing pressure and stretch had no effect on NO production (2 ± 8 vs. 8 ± 8 AU/min; n = 6). Similar results were obtained in the presence of tempol (100 μmol/l), a O2− scavenger. Primary cultures of thick ascending limb cells subjected to shear stresses of 0.02 and 0.55 dyne/cm2 produced NO at rates of 55 ± 10 and 315 ± 93 AU/s, respectively (P < 0.002; n = 7). Pretreatment with the NOS inhibitor l-NAME (5 mmol/l) blocked the shear stress-induced increase in NO production. We concluded that shear stress rather than pressure, stretch, or ion delivery mediates flow-induced stimulation of NO by NOS 3 in thick ascending limbs.
Keywords: luminal flow, NaCl delivery, stretch, pressure, nitric oxide synthase
nitric oxide (NO) can induce natriuresis and diuresis by increasing renal blood flow (22, 23, 31) and glomerular filtration rate (9, 47) and/or by inhibiting salt and water reabsorption along the nephron (8, 35, 37, 49, 51). NO is produced by several tissues in the kidney, including the vascular endothelium (18, 19), the macula densa (26, 50), and the epithelium of the nephron (36, 45). Although the thick ascending limb of the loop of Henle expresses all three NO synthases, NO produced by NO synthase type 3 (NOS 3; also known as endothelial NOS) appears to be most important physiologically under normal circumstances (40, 44). However, this does not rule out the possibility that other NOSs produce NO under some situations. The effects of NO on the thick ascending limb likely contribute to its natriuretic and diuretic properties because NO produced by NOS 3 acts as an autacoid to inhibit net NaCl (44) and NaHCO3 absorption (34) in this segment.
NO production in the thick ascending limb is stimulated by several factors. These include endothelin-1 acting via endothelin B receptors (43), angiotensin II acting via angiotensin II receptor type 2 (14), and adrenergic agonists acting via α2 receptors (42). NO production by NOS 3 in the thick ascending limb is also enhanced by increased luminal flow (38, 39). It is not known whether flow increases NO production by NOS 1 and/or 2.
Luminal flow through the thick ascending limb varies over a wide range both acutely and chronically. Acutely, changes in tubuloglomerular feedback (15), proximal nephron volume reabsorption (24), and peristalsis of the renal pelvis (7) have the greatest impact on luminal flow. Tubuloglomerular feedback may alter flow by as much as 2.5 nl/min (15). Peristalsis of the renal pelvis can completely block luminal flow, and in some situations cause it to reverse direction (7, 48). Chronically, flow is enhanced by a high-salt diet (30) and it also rises in the early stages of diabetes (46) as well as hypertension (1).
Variations in flow result in changes in shear stress, cellular stretch, transmural pressure, and NaCl delivery, all of which can enhance NO production. Shear stress has been shown to increase NO production by vascular smooth muscle (41), endothelial (13, 25, 33), and inner medullary collecting duct cells (4). Stretch increases NO in the epithelium of the primary bronchial airways (29), while increased NaCl delivery stimulates NO production by macula densa cells via enhanced Na/H exchange, which in turn elevates intracellular pH (26). However, it is unclear whether stimulation of NO production by NOS 3 in the thick ascending limb is due to elevated shear stress, stretch, pressure, and/or ion delivery. We hypothesized that enhanced shear stress rather than elevated pressure, cellular stretch, or NaCl delivery mediates flow-stimulated NO production by NOS 3 in the thick ascending limb.
MATERIALS AND METHODS
Animals
We used male Sprague-Dawley rats weighing 100 to 150 g (Charles River Breeding Laboratories, Wilmington, MA) maintained on a diet containing 0.22% sodium and 1.1% potassium for at least 5 days before the experiments. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Hospital.
To address the specific NOS isoform involved, we used 6-wk-old male NOS 3 knockout mice (NOS 3 −/−) on a C57BL/6J background and their wild-type controls (The Jackson Laboratory, Bar Harbor, ME) maintained on a diet containing 0.22% sodium and 1.1% potassium for at least 5 days.
Chemicals and Solutions
4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF FM-DA) and calcein AM were purchased from Invitrogen (Eugene, OR) and 4,5-diaminofluorescein diacetate (DAF 2 diacetate) from Calbiochem (San Diego, CA). Collagenase type 1, 4-hydroxy-TEMPO (tempol), l-arginine, and NG-nitro-l-arginine methyl ester (l-NAME) were obtained from Sigma (St. Louis, MO). The physiological saline used to perfuse and bathe tubules contained (in mmol/l) 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 6 l-alanine, 0.1 l-arginine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C. The ion-free solution used to perfuse the tubules contained (in mmol/l) 270 mannitol and 10 HEPES, pH 7.4 (titrated with KOH) at 37°C; thus, the only inorganic ion present was K (≈5 mM). Both solutions were adjusted to 290 ± 3 mosmol/kgH2O.
Measurement of NO in Isolated Tubules
Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). The abdominal cavity was opened and the left kidney was superfused with ice-cold saline. The kidney was removed, placed in physiological saline (4°C), and coronal slices were cut. Thick ascending limbs were isolated at 10°C and mounted on perfusion pipettes as described previously (10, 37). They were loaded with dye by bathing them in 4 μmol/l DAF FM-DA for 15 min and then washing them in dye-free solution for 20 min. DAF FM was excited at 488 nm. Emitted fluorescence was measured using a 515-nm long-pass dichroic mirror and a 535/50-nm barrier filter in regions of interest (ROI). Fluorescence was imaged digitally using an inverted microscope (TE Nikon 2000; Nikon, Japan) with a ×100 immersion oil objective and a Coolsnap HQ digital camera (Photometrics, Tucson, AZ). Data were recorded using Metafluor version 7 imaging software (Universal Imaging, Downington, PA). Fluorescence was measured at the beginning of each period once every 30 s for 5 min. The change in emitted fluorescence over time was taken as NO production.
ROIs were set larger than the tubule to accommodate changes in shape.
Protocols Involving Isolated Tubules
Protocol 1: effect of increasing and decreasing luminal flow.
Thick ascending limbs were mounted on perfusion pipettes with the distal end open and NO synthesis was measured over two periods. In period 1, NO was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. Ten minutes after flow was stopped, NO was measured again in the absence of luminal flow for 5 min and then immediately after flow was increased to 20 nl/min with physiological saline for 5 min (period 2). Fluorescence was measured at the beginning of each period after the initial shape change occurred once every 30 s.
In control experiments, tubules remained nonperfused during both periods.
Protocol 2: are changes in DAF-FM fluorescence due to NO?
Thick ascending limbs were mounted on perfusion pipettes with the distal end open and NO synthesis was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. l-NAME (5 mmol/l) was present throughout the whole experiment. Fluorescence was measured after the initial change occurred once every 30 s.
Protocol 3: do changes in cell volume or shape bias our ability to measure fluorescence?
Thick ascending limbs were mounted on perfusion pipettes with the distal end open. Tubules were loaded with calcein AM by bathing them in 1 μmol/l dye for 5 min and then washing them in dye-free solution for 10 min to measure cell volume. Calcein was excited at 488 nm. Emitted fluorescence was measured using a 515-nm long-pass dichroic mirror and a 535/50-nm barrier filter in ROI. Calcein fluorescence was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. Fluorescence was measured at the beginning of each period after the initial shape change occurred once every 30 s for 5 min.
Protocol 4: is NOS3 responsible for flow-induced NO?
Thick ascending limbs from NOS3 −/− mice on a C57BL/6J background and their wild-type controls were mounted on perfusion pipettes with the distal end open. NO synthesis was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. Fluorescence was measured at the beginning of each period after the initial shape change occurred once every 30 s.
Protocol 5: effect of ion removal.
Thick ascending limbs were mounted on perfusion pipettes with the distal end open and NO synthesis was measured over two periods. In period 1, NO was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. Ten minutes after flow was stopped (period 2), NO was measured again in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min using an ion-free solution instead of physiological saline for 5 min. Fluorescence was measured at the beginning of each period after the initial shape change occurred once every 30 s for 5 min.
In control experiments, tubules remained nonperfused during both periods.
Protocol 6: effect of increasing stretch while reducing pressure and shear stress.
Thick ascending limbs were mounted on perfusion pipettes with the distal end open and NO synthesis was measured over two periods. In period 1, NO was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. Fluorescence was measured after the initial shape change occurred once every 30 s. After flow was stopped, we added 0.05% collagenase to the bath for 5 min and washed the tubules for 10 min to partially digest the basement membrane and thereby increase compliance before measuring NO in the next period. In period 2, NO was measured in the absence of luminal flow for 5 min and then after flow was increased to 20 nl/min with physiological saline for 5 min. After we treated tubules using collagenase 0.05%, when flow was applied luminal diameter increased by 24 ± 2% and because flow rate was maintained at 20 nl/min, we enhanced stretch while reducing both pressure and shear stress.
Protocol 7: does collagenase treatment alter cell volume or shape in the absence of changes in flow?
Tubules were loaded with the dye by bathing them in 1 μmol/l dye for 5 min and then washing them in dye-free solution for 10 min. Calcein AM was excited and emitted fluorescence was measured as described in protocol 3. Thick ascending limbs remained nonperfused and calcein fluorescence was measured before collagenase treatment for 5 min. Then, we added 0.05% collagenase to the bath for 5 min and washed the tubules for 10 min. Calcein fluorescence was measured after tubules were treated with collagenase 0.05% for 5 min. Fluorescence was measured once every 30 s.
Protocol 8: does collagenase treatment alter DAF fluorescence in the absence of changes in flow?
Thick ascending limbs remained nonperfused and DAF fluorescence was measured before tubules were treated with collagenase 0.05% for 5 min. Then, we added 0.05% collagenase to the bath for 5 min and washed the tubules for 10 min. DAF fluorescence was measured after tubules were treated with collagenase 0.05% for 5 min. Fluorescence was measured once every 30 s.
Protocol 9: effect of stretch and pressure alone.
Thick ascending limbs were mounted on perfusion pipettes with the distal end pinched closed and NO production was measured in the absence of applied luminal pressure for 5 min. Then, luminal pressure was increased by infusing physiological saline so that the outer diameter was the same as the average diameter observed in protocol 1 and NO was measured for 5 min. In these experiments, pressure and stretch were increased in the absence of shear stress. Fluorescence was measured after the initial shape change occurred once every 30 s.
Protocol 10: effect of stretch and pressure alone in the presence of the O2− scavenger tempol.
Thick ascending limbs were mounted on perfusion pipettes with the distal end pinched closed and NO production was measured in the absence of applied luminal pressure in the presence of 100 μmol/l tempol. Then, luminal pressure was increased by infusing physiological saline so that the outer diameter was the same as the average diameter observed in protocol 1 and NO was measured for 5 min. Tubules were treated with tempol 100 μmol/l throughout the whole experiment to eliminate the potential effects of O2− on NO. Fluorescence was measured after the initial shape change occurred once every 30 s.
Protocol 11: does tempol treatment alter DAF fluorescence in the absence of changes in flow?
Thick ascending limbs remained nonperfused and DAF fluorescence was measured before tubules were treated with 100 μmol/l tempol for 5 min. Then, we added 100 μmol/l tempol to the bath and DAF fluorescence was measured 5 to 10 min after tubules were treated with 100 μmol/l tempol. Fluorescence was measured once every 30 s.
Effect of Shear Stress on NO in Primary Cultures of Rat Medullary Thick Ascending Limb Cells
Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). The abdominal cavity was opened and the kidneys were flushed with 40 ml ice-cold 0.1% collagenase in Hanks' balanced salt solution (HBSS) via retrograde perfusion of the aorta. Coronal slices were cut from the kidneys, and the inner stripe of the outer medulla was minced into 1-mm3 fragments and digested in 0.1 mg/ml collagenase at 37°C for 30 min. During each 5-min period, the tissue was gently agitated and gassed with 100% oxygen. After continuous agitation for 30 min in cold HBSS, the tissue was filtered through a 250-μm nylon mesh and the filtered material was rinsed twice with culture medium. Cells were resuspended in renal epithelial growth medium supplemented with 5% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and seeded onto glass coverslips. Two days later, we rinsed the cells with prewarmed PBS (37°C) and loaded them with prewarmed 5 μmol/l DAF-2 in physiological saline for 30 min. Coverslips were mounted in a temperature-controlled chamber and washed in physiological saline for 20 min at 37°C. l-arginine (50 μmol/l) was present throughout the experiment. ROIs were defined for 3–5 cells. To measure NO production, DAF-2 was excited at 490 nm and fluorescence was collected at >515 nm. During the control period, the flow rate was 0.5 ml/min, which generated a shear stress of 0.02 dyne/cm2. Measurements were taken once every 30 s for a total of 10 measurements. Then, shear stress was increased to 0.55 dyne/cm2 and NO production was measured once every 30 s for a total of 20 measurements.
Statistical Analysis
Results are expressed as means ± SE. Student's paired t-test was used to evaluate the data, taking P < 0.05 as significant. Statistical analysis was performed by the Department of Biostatistics and Epidemiology of Henry Ford Hospital.
RESULTS
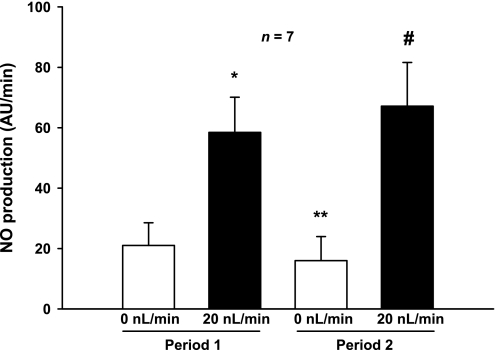
To determine whether shear stress, cellular stretch, and/or pressure mediate flow-stimulated NO production, we first measured NO generation in the absence and presence of luminal flow. In the absence of flow (and therefore in the absence of ion delivery, stretch, pressure, and shear stress), thick ascending limbs produced NO at a rate of 21 ± 7 AU/min. When flow was increased to 20 nl/min with physiological saline, thereby increasing shear stress, stretch, pressure, and ion delivery, NO production increased significantly to 58 ± 12 AU/min (P < 0.02; n = 7). NO decreased from 58 ± 12 to 16 ± 8 AU/min (P < 0.02; n = 7) 10 min after flow was stopped. When flow was once again elevated, the resulting increase in NO was not significantly different from the first period (67 ± 14 AU/min; Fig. 1).
Fig. 1.
Effect of increasing and decreasing luminal flow on nitric oxide (NO) production by isolated thick ascending limbs (TALs; *P < 0.02 compared with 0 nl/min, period 1; **P < 0.02 compared with 20 nl/min, period 1; #P < 0.04 compared with 0 nl/min, period 2; n = 7). There was no difference between flow-stimulated NO production during periods 1 and 2.
To show that changes in DAF fluorescence were due to NO and not changes in cell shape/volume, we performed two types of experiments. First, we measured DAF-FM fluorescence in the presence of 5 mmol/l l-NAME to inhibit all NOSs in the presence or absence of luminal flow. In the presence of l-NAME, we found that flow did not change emitted fluorescence (change: 5 ± 7 AU/min).
We also performed experiments in which we measured emitted fluorescence over time using the cell volume-sensitive dye calcein AM. When we applied luminal flow, calcein fluorescence did not change significantly (from −0.2 ± 0.04 to 0.01 ± 0.16 AU/min). Together, these data indicate that 1) increases in DAF-FM fluorescence are due to NO and 2) changes in cell shape and volume do not interfere with our ability to measure NO with DAF-FM.
We next addressed the role of the specific NOS isoform by measuring NO production in NOS 3 −/− and wild-type mice in the presence and absence of luminal flow. Flow-stimulated NO production in NOS 3 −/− mice was 4 ± 7 AU/min and flow-stimulated NO production in C57Bl/6J was 78 ± 26 AU/min (P < 0.04).
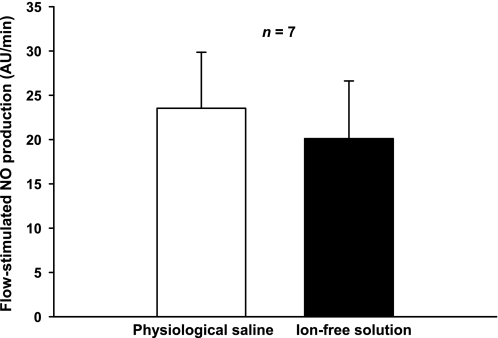
Since increased NaCl delivery enhances NO production in the macula densa (26), we examined whether increasing luminal flow with an ion-free solution could increase NO in thick ascending limbs. When flow was increased to 20 nl/min with physiological saline, NO production increased by 24 ± 6 AU/min. When we increased flow to 20 nl/min using the ion-free solution, NO synthesis increased by the same amount (20 ± 7 AU/min; n = 7; Fig. 2).
Fig. 2.
Effect of ion removal on flow-stimulated NO production by isolated, perfused TALs (n = 7). Tubules were perfused with either physiologic saline or an ion-free solution as described in the text. Luminal flow was 20 nl/min in the 2 periods.
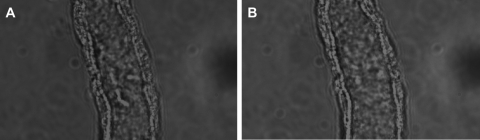
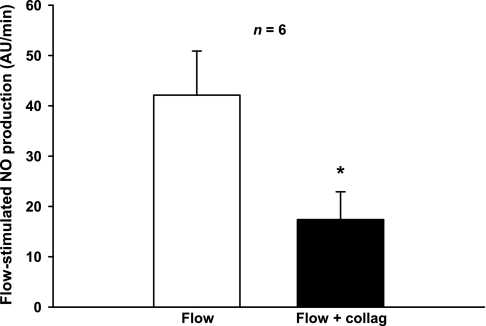
Increasing stretch (20, 29) and shear stress (3, 4) both have been reported to increase NO in other cells. Thus, we tested the effect of decreasing shear stress and pressure while increasing stretch by treating the tubules with collagenase (0.05% for 5 min). Brief exposure to collagenase partially digests the basement membrane and increases compliance. Thus, when flow is held constant, shear stress and pressure decrease while stretch increases. In the presence of luminal flow, NO production was 42 ± 9 AU/min and luminal diameter was 19.4 ± 1.7 μm. After we treated the tubules with collagenase, diameter increased to 24 ± 2 μm (Fig. 3). Although the morphology of the tubule other than its diameter did not change, NO generation fell to 17 ± 6 AU/min (P < 0.03; n = 6; Fig. 4).
Fig. 3.
Transmitted light images of an isolated and perfused TAL (×100 immersion oil objective). A: isolated and perfused TAL before collagenase treatment. B: isolated and perfused TAL after collagenase treatment.
Fig. 4.
Effect of increasing stretch while reducing pressure and shear stress on flow-stimulated NO production by isolated, perfused TALs. Luminal flow was maintained at 20 nl/min. Treatment of TALs with collagenase (collag) increased stretch (seen as a 24 ± 2% increase in diameter), lowering both shear stress and pressure (*P < 0.03; n = 6).
To test whether collagenase treatment has any effect on cell volume/shape in the absence of changes in luminal flow, we performed experiments using the cell volume-sensitive dye calcein. Calcein fluorescence was measured before and after tubules were treated with collagenase 0.05% for 5 min. In thick ascending limbs treated with collagenase, calcein fluorescence did not change significantly (136 ± 4 to 134 ± 5 AU).
We also tested whether collagenase treatment affected DAF absolute fluorescence intensity in the absence of changes in luminal flow. Thick ascending limbs remained nonperfused and DAF fluorescence was measured before and after tubules were treated with collagenase 0.05% for 5 min. The absolute fluorescence intensity of DAF did not change before and after collagenase treatment (134.2 ± 13.1 vs. 134.7 ± 13.7 AU).
We next examined whether stretch and pressure could stimulate NO production in the absence of shear stress. For this, we pinched the distal end of each tubule closed and increased luminal pressure so that the average diameter was the same as when tubules were perfused at 20 nl/min. Thus, we ensured that they were exposed to the same stretch and pressure as when they were perfused. In the absence of pressure, pinched thick ascending limbs produced NO at a rate of 2 ± 8 AU/min. After we applied pressure to stretch the cells in the absence of shear stress, NO production was 8 ± 8 AU/min, not significantly different from the first period (n = 6; Fig. 5).
Fig. 5.
Effect of increasing pressure and stretch but not shear stress on NO production by isolated TALs (n = 6). Distal ends of tubules were pinched closed and pressurized. Thus, stretch and pressure increased but not shear stress. P, pressure; Str, stretch.
Because we previously showed that cellular stretch increases O2− production in the thick ascending limb (11) and that O2− can scavenge NO (17), we tested the effect of stretch and pressure in the presence of the superoxide dismutase mimetic tempol. When we repeated the experiments depicted in Fig. 5 in the presence of tempol (100 μmol/l) to scavenge O2−, similar results were obtained.
In control experiments, thick ascending limbs remained nonperfused and DAF absolute fluorescence intensity was measured before and after tubules were treated with 100 μmol/l tempol. The absolute fluorescence intensity of DAF did not change before and after tempol treatment in the absence of flow (135 ± 6 vs. 133 ± 5 AU).
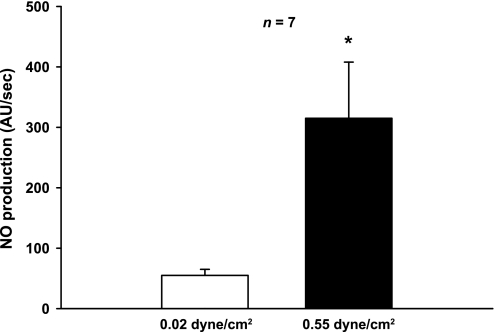
Our previous results indicate that an increase in shear stress is required for flow-induced NO production. However, they do not indicate whether it alone is sufficient. To examine whether shear stress alone is sufficient to induce NO production, we measured the effect of increasing shear stress from 0.02 to 0.55 dyne/cm2 on NO production without varying pressure or stretch. For this, we used primary cultures of thick ascending limb cells grown on glass coverslips. These cells had typical cobblestone shape of epithelial cells (Fig. 6). When shear stress was 0.02 dyne/cm2, thick ascending limb cells produced NO at a rate of 55 ± 10 AU/s. When shear stress was increased to 0.55 dyne/cm2, NO production increased to 315 ± 93 AU/s (P < 0.002; n = 7; Fig. 7). Pretreatment with the NOS inhibitor l-NAME (5 mM) blocked the shear stress-induced increase in NO production (44 ± 24 vs. 48 ± 12 AU/s for 0.02 and 0.55 dyne/cm2, respectively; n = 6).
Fig. 6.
Transmitted light image of primary culture of rat medullary TAL cells (×40 immersion oil objective).
Fig. 7.
Effect of increasing shear stress on NO production by primary cultures of TAL cells (*P < 0.002; n = 7).
DISCUSSION
The main purpose of this study was to elucidate the specific component of flow that enhances NO production in the thick ascending limb. NO was measured using an NO-specific fluorescence indicator DAF FM-DA. We chose this reagent because it has higher sensitivity than other reagents to measure NO in real time. Inside the cell the dye is cleaved by intracellular esterases leading to DAF FM. After reacting with NO, its fluorescence increases. The later reaction is irreversible. Thus, absolute fluorescence intensity increases as long as NO is available to react with the dye. Increases in the rate of change of fluorescent intensity reflect an increase in the amount of NO in the cell. The amount of NO in the cell is a function of both degradation and production. Because there is no evidence that the rate of degradation changes with flow, we assumed the increase in the rate of change of emitted fluorescence reflects an increase in production.
We hypothesized that it is shear stress rather than cellular stretch, pressure, or ion delivery. To test our hypothesis, we first measured NO production in the absence and presence of luminal flow. We found that NO production tripled when luminal flow rate was increased from 0 to 20 nl/min, the upper limit of physiological flow in the thick ascending limb. Pretreatment with the NOS inhibitor l-NAME (5 mmol/l) prevented the flow-induced increase in NO production indicating that changes in DAF fluorescence were due to NO. Both the l-NAME and calcein experiments indicated that changes in DAF-FM fluorescence could not be due to changes in cell shape/volume.
To test which NOS isoform was responsible for flow-stimulated NO, we used NOS 3 −/− mice. We found that thick ascending limbs from these mice did not respond to flow with an increase in NO while wild-type mice did. We concluded that flow-stimulated NO production was mediated by NOS 3.
When luminal flow increases, ion delivery is enhanced. This could stimulate NO production in the thick ascending limb via several mechanisms. First, raising NaCl in the luminal perfusate could stimulate the Na/H exchanger, increasing intracellular pH and thus NO production as it does in the macula densa (26). Increased luminal NaCl could also elevate intracellular Na, reducing Na/Ca exchange. Reduced Na/Ca exchange would raise intracellular calcium which has been shown to activate phosphatidylinositol 3-kinase (PI3K) (5), which in turn stimulates NO production by thick ascending limbs (39). Thus, we tested whether ion delivery contributes to flow-induced NO production using a nearly ion-free solution. When luminal flow was increased using this solution, NO production increased as much as it did when physiological saline was used. These data suggest that flow-induced NO production is independent of ion delivery and hence NaCl and Na bicarbonate reabsorption.
Our finding that flow-induced NO production in the thick ascending limb is independent of NaCl delivery contrasts with previous reports by us (26) and others (27) that NO production by the macula densa is enhanced by raising luminal NaCl even though the thick ascending limb and macula densa express the same luminal transporters. The most likely explanation is that NO production by thick ascending limbs is due to NOS 3 (40, 44), which is not sensitive to changes in pH (32). In contrast, macula densa-derived NO is due to NOS 1, which is activated at an alkaline pH (12, 26).
It is also possible that a NaCl-induced increase in NO is masked by a NaCl-induced elevation in O2−, which scavenges the NO. We previously reported that enhanced NaCl delivery stimulates O2− production by thick ascending limbs (16). However, this possibility seems unlikely. Here, we found that NO production was the same in the presence and absence of all ions except K. Thus, NaCl-induced O2− production would have to exactly match NaCl-induced NO production to account for our data.
The “ion-free” solution used in this study actually contains two ions at significant concentrations: HEPES and K. It is unlikely that increasing delivery of a large organic ion such as HEPES could affect NO production. It is not transported to a significant extent and thus would not be expected to alter any physiological parameters that might modify NO production. On the other hand, K transport could decrease membrane potential and thereby increase intracellular calcium. Such an increase in calcium could activate NOS 3; however, changes in intracellular calcium do not appear to play an important role in activating NOS 3 in the thick ascending limb (42).
Because enhanced ion delivery did not appear to be required for flow-induced NO production, we next examined the role of shear stress, stretch, and pressure, all of which have been shown to stimulate NO production in various tissues (3, 4, 20, 29). We first tested the effect of reducing shear stress and pressure while increasing stretch. To do this, we treated perfused tubules with collagenase which partially digests the basement membrane. In our experiments, collagenase treatment increased diameter by 24 ± 2% at a constant flow rate; thus, both shear stress and transmural pressure decreased while stretch was enhanced. We found that NO production decreased. Control experiments showed that collagenase treatment does not affect DAF absolute fluorescence intensity or cell volume/shape in the absence of changes in luminal flow. Together, these results suggest that either shear stress or pressure mediates flow-stimulated NO production in the thick ascending limb. Stretch does not appear to be involved because stretch increased and NO generation decreased.
Collagenase could alter a variety of signaling cascades via a change in cell shape in perfused tubules. However, there is no evidence that indicates that collagenase alone alters cell signaling in thick ascending limbs. Thick ascending limb suspensions have been used to study the effects of a variety of hormones and factors on cell metabolism and transport. In all of these studies, the suspensions respond to the hormone/factor qualitatively in a manner similar to dissected tubules. Since suspensions are made using collagenase, these data indicate that collagenase alone does not dramatically alter cellular signaling pathways, but such an effect cannot be completely ruled out. While collagenase could affect a signaling cascade in perfused tubules in such a way that it reduced NO production, this has not been demonstrated and the simplest explanation of the data is that collagenase reduces shear stress by allowing increased stretch.
To separate shear stress from pressure and stretch, we pinched the distal ends of tubules closed and applied luminal pressure, thereby enhancing pressure and stretch but not shear stress. In this experiment, we were more concerned with the effects of pressure because stretch had been all but eliminated as the mediator of flow-stimulated NO in the previous experiment. To apply the same amount of pressure to tubules as when they are exposed to a luminal flow of 20 nl/min, we pressurized tubules until we obtained the same average diameter as tubules exposed to flow. When tubules were exposed to pressure and stretch in the absence of shear stress, we observed no increase in NO production. These data indicate that pressure does not mediate flow-induced NO production. These data also confirm that stretch is not necessary for stimulation of NO.
Previously, we showed that stretching thick ascending limbs can stimulate production of O2− (11), which can scavenge NO. Thus, it is possible that we did not observe an increase in NO when tubules were pressurized because the NO was scavenged by the stretch-stimulated O2−. To address this concern, we repeated these experiments in the presence of tempol, a O2− scavenger, to eliminate its potential effects on NO. We did not observe any increases in NO production when tubules were pressurized in the presence of tempol. Control experiments showed that tempol treatment does not affect DAF absolute fluorescence intensity in the absence of changes in luminal flow. These data indicate that the absence of increased NO production was not due to NO scavenging by O2−.
Although the previous data indicate that shear stress is necessary for enhanced NO production, they do not prove that increased shear stress alone is sufficient. To confirm our hypothesis, we therefore applied two different magnitudes of shear stress to primary cultures of thick ascending limb cells grown on glass coverslips in a laminar flow chamber. These cells had a cobble stone appearance characteristic of many epithelial cells in culture. We found that a shear stress of 0.55 dyne/cm2 increased NO production sixfold compared with 0.02 dyne/cm2. The values for shear stress in the thick limb range from 0 to ∼5 dyne/cm2. These values correspond to flows of 0 to ∼20 nl/min, the reported flows found in vivo. In cell culture experiments, we chose shear stresses of 0.02 dyne/cm2 to reflect a flow approaching zero. We used 0.55 dyne/cm2 to reflect a high flow value, but were limited by the absolute flow rate that could be used in our laminar flow chamber. Due to the cross-sectional area of the chamber, a luminal flow of ∼15 ml/min only causes a shear stress of 0.55 dyne/cm2. These data establish that increased shear stress is sufficient to stimulate NO production by thick ascending limbs.
Our finding that shear stress mediates flow-stimulated NO production by NOS 3 in the thick ascending limb is similar to other reports of NOS 3 activation in endothelial cells of various vessels. In the pulmonary artery, shear stress increases NOS 3 phosphorylation and NO generation by lowering PKCδ activity (21). In the bovine aorta, it stimulates phosphorylation of NOS 3 via a mechanism mediated by PKA (2). Although we did not study the signaling mechanism involved in shear stress-induced NO production, we previously showed that luminal flow increases NO production and induces NOS 3 translocation and activation in thick ascending limbs via PI3K (39).
In contrast, our finding that stretch does not activate NOS 3 differs from studies of pulmonary endothelial cells in which stretch was found to activate PI3K and consequently NO production (20). In the thick ascending limb, PI3K activation also stimulates NOS 3 (39). Thus, the discrepancy must lie in whether PI3K is activated by stretch. Stretch could also activate NOS 3 in thick ascending limbs by increasing intracellular calcium, which is known to activate NOS 3 (6). Stretch increases intracellular calcium in cortical collecting ducts (28) and could also do so in thick ascending limbs by activating TRPV4 channels. However, changes in intracellular calcium do not appear to play an important role in activating NOS 3 in the thick ascending limb (42).
In summary, we found that shear stress rather than stretch, pressure, or ion delivery mediates flow-induced NO production in the thick ascending limb. Understanding how luminal flow regulates NO production will give us insight into pathophysiological states such as diabetes, renal damage, and hypertension in which NO production is reduced.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-070985, HL-090550, and HL-028982 to J. L. Garvin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Baer PG, Bianchi G, Liliana D. Renal micropuncture study of normotensive and Milan hypertensive rats before and after development of hypertension. Kidney Int 13: 452–466, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536: 193–197, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 75: 247–260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci 18: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Eitle E, Hiranyachattada S, Wang H, Harris PJ. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am J Physiol Cell Physiol 274: C1075–C1080, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Gabbai FB, Blantz RC. Role of nitric oxide in renal hemodynamics. Semin Nephrol 19: 242–250, 1999 [PubMed] [Google Scholar]
- 10.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorren AC, Schrammel A, Schmidt K, Mayer B. Effects of pH on the structure and function of neuronal nitric oxide synthase. Biochem J 331: 801–807, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecker M, Mulsch A, Bassenge E, Busse R. Vasoconstriction and increased flow: two principal mechanisms of shear stress-dependent endothelial autacoid release. Am J Physiol Heart Circ Physiol 265: H828–H833, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT2 receptors and Akt1-dependent nitric oxide synthase 3 (NOS3) activation. J Biol Chem 285: 14932–14940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1448–F1459, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun 18: 195–199, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Carretero OA, Abe K. Role of nitric oxide in the control of glomerular microcirculation. Clin Exp Pharmacol Physiol 24: 578–581, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 168: 1391–1398, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Sud N, Fonseca FV, Hou Y, Black SM. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C delta. Am J Physiol Lung Cell Mol Physiol 298: L105–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahera V, Salom MG, Fiksen-Olsen MJ, Raij L, Romero JC. Effects of NG-monomethyl-l-arginine and l-arginine on acetylcholine renal response. Hypertension 15: 659–663, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-l-arginine methyl ester on renal function and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1033–F1037, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Leyssac PP, Karlsen FM, Holstein-Rathlou NH, Skott O. On determinants of glomerular filtration rate after inhibition of proximal tubular reabsorption. Am J Physiol Regul Integr Comp Physiol 266: R1544–R1550, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells. Biol Reprod 69: 1053–1059, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13: 2688–2696, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mohammed KA, Nasreen N, Tepper RS, Antony VB. Cyclic stretch induces PlGF expression in bronchial airway epithelial cells via nitric oxide release. Am J Physiol Lung Cell Mol Physiol 292: L559–L566, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mozaffari MS, Jirakulsomchok S, Shao ZH, Wyss JM. High-NaCl diets increase natriuretic and diuretic responses in salt-resistant but not salt-sensitive SHR. Am J Physiol Renal Fluid Electrolyte Physiol 260: F890–F897, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Naess PA, Kirkeboen KA, Christensen G, Kiil F. Inhibition of renal nitric oxide synthesis with NG-monomethyl-l-arginine and NG-nitro-l-arginine. Am J Physiol Renal Fluid Electrolyte Physiol 262: F939–F942, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Nagy S, Harris MB, Ju H, Bhatia J, Venema RC. pH and nitric oxide synthase activity and expression in bovine aortic endothelial cells. Acta Paediatr 95: 814–817, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res 76: 536–543, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO3− transport by rat thick ascending limb. Kidney Int 58: 2069–2074, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. II. Role of PI3-kinase and Hsp90. Am J Physiol Renal Physiol 287: F281–F288, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of l-arginine on NaCl absorption. Hypertension 42: 674–679, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Papadaki M, Tilton RG, Eskin SG, McIntire LV. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am J Physiol Heart Circ Physiol 274: H616–H626, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Plato CF, Garvin JL. α2-Adrenergic-mediated tubular NO production inhibits thick ascending limb chloride absorption. Am J Physiol Renal Physiol 281: F679–F686, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ET(B) receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Plato CF, Shesely EG, Garvin JL. eNOS mediates l-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension 35: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F946–F952, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Raij L, Baylis C. Glomerular actions of nitric oxide. Kidney Int 48: 20–32, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu XC, Harris PJ, Johns EJ. Nitric oxide and renal nerve-mediated proximal tubular reabsorption in normotensive and hypertensive rats. Am J Physiol Renal Physiol 277: F560–F566, 1999 [DOI] [PubMed] [Google Scholar]