Abstract
Nonsteroidal anti-inflammatory drug usage has long revealed renoprotective prostaglandin actions on the renal microvasculature during increased pressor hormone influence, but whether increased cyclooxygenase (COX)-2 expression supports prostaglandin vasodilatory influence by interfering with the actions of ANG II remains unresolved. Therefore, we tested the hypothesis that COX-2 inhibition causes hemodynamic and excretory effects that are increased in proportion to ANG II activity. In anesthetized Sprague-Dawley rats having augmented cortical COX-2 expression but different ANG II activity, we conducted renal clearance experiments during acute inhibition of COX-2 with nimesulide (NMSLD) and inhibition of COX-1 with SC-560. In one series of experiments, acute captopril [acute angiotensin-converting enzyme (ACE) inhibitor (aACEi)] was administered alone (n = 13) or in combination with chronic captopril [chronic ACEi (cACEi)] pretreatment (n = 19). In another series of experiments, rats were fed a normal-sodium [0.4% (NS), n = 12] or a low-sodium [0.03% (LS), n = 18] diet. NMSLD did not alter mean arterial blood pressure in any group but, in the LS and cACEi groups, decreased renal plasma flow (from 3.99 ± 0.33 to 2.85 ± 0.26 and from 4.30 ± 0.19 to 3.22 ± 0.21 ml·min−1·g−1), cortical blood flow (−12 ± 8% and −13 ± 4%), and glomerular filtration rate (from 0.88 ± 0.04 to 0.65 ± 0.05 and from 0.95 ± 0.07 to 0.70 ± 0.05 ml·min−1·g−1). In contrast, medullary blood flow (MBF) was significantly decreased by COX-2 inhibition in NS (−24 ± 5%), LS (−27 ± 8%), aACEi (−16 ± 3.8%), and cACEi (−24 ± 4.2%) groups. Absolute and fractional sodium excretion rates were unchanged by NMSLD, except in the LS group (0.75 ± 0.05 μeq/min and 0.43 ± 0.15% and 0.51 ± 0.06 μeq/min and 0.26 ± 0.10%). SC-560 did not augment the effects of NMSLD. These results demonstrate an augmented COX-2-mediated vasodilation that is not contingent on ANG II, in contrast to COX-2-mediated augmented sodium excretion, where ANG II activity is requisite. Furthermore, the COX-2 effects on MBF are not contingent on ANG II or changes in cortical microvascular responses. These results reflect COX-2 continual regulation of MBF and adaptive opposition to ANG II prohypertensinogenic effects on renal plasma flow, cortical blood flow, glomerular filtration rate, and absolute and fractional sodium excretion.
Keywords: angiotensin-converting enzyme inhibition, sodium restriction, medullary blood flow
the dynamic prostaglandin biosynthetic capacity in the kidney relies on the catalysis of the rate-limiting step by the cyclooxygenase (COX) isoforms COX-1 and COX-2 (25). Adaptive renal responses to reduced sodium intake involve augmented COX activity, along with activation of the renin-angiotensin system (RAS) (21, 36, 38, 41). Interactions of the COX isoforms with the RAS system have been revealed by several expression studies; however, critical uncertainties remain. For example, angiotensin (AT1) receptor stimulation augments COX-2 expression in vascular smooth muscle cells (24), whereas AT1 receptor blockade increases cortical COX-2 expression in the kidney (40). Furthermore, the augmented renocortical COX-2 expression by AT1 receptor blockade or angiotensin-converting enzyme (ACE) inhibition mirrors that produced by sodium restriction (14), despite their opposite effects on kidney ANG II concentrations (8).
Clinical observations and in vivo studies provide functional support for an antagonism between prostaglandin renoprotective and RAS hypertensinogenic actions. Nonselective COX inhibition decreases total renal blood flow (RBF) (23) and medullary blood flow (MBF) (7) and increases vascular resistance in kidneys preconstricted with ANG II (7, 23). When studies involving nonselective inhibition of renal COX are reconsidered in view of more recent isoform-distinct experiments, COX-2 appears to mediate the dynamic hemodynamic and excretory effects previously attributed to COX-1 or both isoforms. For example, nonselective COX inhibition-mediated reductions of glomerular filtration rate (GFR), RBF, sodium excretion, and urine flow in sodium-restricted animals are similar to those precipitated by acute or chronic COX-2 inhibition in similar models (30, 31). The effects of dietary sodium restriction to augment macula densa COX-2 expression help explain the increased COX-2 influence on renal hemodynamics (13, 42). Furthermore, the potential of COX-2 to mediate the prostaglandin antagonism of hypertension is evident from the effects of COX-2 inhibitors to increase blood pressure in ACE inhibitor-treated patients (18).
Functional opposition of prostaglandin and ANG II is more commonly observed when nonsteroidal anti-inflammatory drugs and COX-2 inhibitors are applied during high activity of the RAS compared with control conditions. As a result, such effects are often considered to be the consequence of greater unopposed ANG II, rather than withdrawal of prostaglandin vasodilatory influence. However, it is equally plausible that prostaglandins act by modulating ANG II effects. Support for this kind of interference has been shown, where ANG II-mediated elevations of intracellular calcium are attenuated in vascular smooth muscle cells by prostaglandin analogs (28) and are augmented by interrupted prostaglandin synthesis in preglomerular vessels of both kidneys in 2K1C rats (15). Were this applicable, the dearth of ANG II activity would render COX-2-dependent prostaglandin availability functionally irrelevant.
Studies during augmented COX-2 expression could provide insight into pleiotropic prostaglandin-mediated vasodilation or vasoconstriction; whether such responses to prostaglandin are contingent on intrarenal ANG II activity is unresolved (28). Therefore, we employed pharmacological COX-2 inhibition in two rat models, similar in directional change of COX-2 expression but dissimilar in ANG II activity, to test the hypothesis that hemodynamic and excretory manifestations of COX-2 inhibitor effects are increased in proportion to ANG II concentrations. In particular, we compared COX-2 inhibitor-mediated hemodynamic and excretory changes in mean arterial blood pressure (MABP), RPF, cortical blood flow (CBF), MBF, GFR, urine flow, and absolute and fractional sodium excretion (UNa and FENa) in rats subjected to ACE inhibition and sodium restriction.
Because the interventions respectively attenuate and augment intrarenal ANG II activity (8, 9), the ANG II-dependent component can be demonstrated by comparisons of hemodynamic with excretory indexes. Qi et al. (29) demonstrated that the COX isoforms differ in their modulation of ANG II effects on renal function in normal mice. Therefore, we also investigate the effects of COX-1 on COX-2 modulation of ANG II effects.
MATERIALS AND METHODS
Experimental Studies
The experiments were approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (325–350 g; Charles River Laboratories, Wilmington, MA) were subjected to protocols that varied dietary salt or length of ACE inhibition. Prior to clearance studies, normal-sodium [0.4% (NS), n = 12] and low-sodium [0.03% (LS), n = 18] groups were fed semisynthetic rat chow (Harlan Teklad, Madison, WI) for 5 days. Rats in the acute ACE inhibition (aACEi) group (n = 13) were fed the NS diet and drinking water. Rats in the combined ACE inhibition (cACEi) group (n = 19) were given captopril (100 mg/l) in the drinking water for 7 days. Therefore, by combining acute and chronic ACE inhibition, we sought to amplify the ANG II concentration difference to further appreciate the effects of constitutive and augmented COX-2 activity during attenuated ANG II biosynthesis.
Renal clearance studies were performed in rats anesthetized with thiobutabarbital sodium (Inactin, 100 mg/kg body wt ip) and placed on a heated platform to maintain body temperature at 37°C. After tracheotomy via a polyethylene (PE-250) catheter to maintain a patent airway, a PE-50 catheter was introduced into the left jugular vein for continuous infusions (0.02 ml/min) of 6% BSA in isotonic (0.9%) saline to compensate for volume losses during the surgery. Arterial blood was sampled and MABP was monitored via a PE-50 catheter inserted into the right femoral artery. A PE-10 catheter was introduced via the left femoral artery and advanced to the renal artery for administration of drugs during the experiment.
The left kidney was exposed by flank incision and secured in a Lucite holder. The left ureter was cannulated, and fiber tips of needle flow probes (500 μm diameter), connected to a laser-Doppler flowmeter (Periflex 4001, Perimed, Stockholm, Sweden), were inserted 1 and 4 mm below the surface of the kidney to measure the changes in CBF and MBF, respectively. After the surgery, an initial 1-ml bolus of a solution containing 1% BSA, 7.5% sinistrin (Inutest, Laevosan, Linz, Austria), and 1.5% p-aminohippuric acid (Merck, Sharp & Dohme, West Point, PA) in isotonic saline (0.9%) was administered and infused (1.2 ml/h) for the duration of the experiment via the left jugular vein catheter. A 1-h stabilization and equilibration period was then allowed prior to the start of the urine collection periods.
Sodium series.
In the NS and LS groups, urine was collected for two 30-min control periods. Next, injection of a bolus of the selective COX-2 inhibitor nimesulide (3 mg/kg body wt) or a 50 mM sodium carbonate vehicle (1 ml/kg body wt) was followed by a 15-min delay. Nimesulide has been previously shown to be selective for COX-2 (4, 34) and to elicit alterations in renal function (26, 30, 31). The third and fourth 30-min urine samples were collected during COX-2 inhibition. Subsets of the NS (n = 4) and LS (n = 8) groups were then subjected to selective COX-1 inhibition with SC-560 (5 mg/kg body wt; Cayman Chemicals, Ann Arbor, MI) or DMSO vehicle. Thereafter, two additional urine samples were collected during COX-1 inhibition. Plasma was acquired from centrifuged blood samples collected after the first, third, and, when applicable, fifth 30-min urine collection periods.
ACE inhibition series.
After the stabilization period in the aACEi (n = 13) and cACEi (n = 19) groups, urine was collected during two 20-min control periods. Then captopril (5 mg/kg body wt) was administered. After 10 min, samples were obtained over two additional 20-min clearance periods. Then nimesulide (3 mg/kg body wt) or a 50 mM sodium carbonate vehicle (1 ml/kg body wt) was administered. After 15 min, samples were obtained over two 20-min COX-2 inhibition periods. Urine was collected during each 20-min period, and plasma samples were obtained from centrifuged blood samples after the first, third, and fifth 20-min periods. Subsets of the acute (n = 4) and combined (n = 8) inhibition groups were also subjected to selective COX-1 inhibition with SC-560 (5 mg/kg body wt) or DMSO vehicle. Thereafter, two additional urine samples were collected during COX-1 inhibition. Plasma was acquired from centrifuged blood samples collected after the first, third, fifth, and, when applicable, seventh 20-min urine collection periods. In all rats, red blood cells were reconstituted in isotonic saline and returned to the rat through the jugular vein catheter.
After the experiment, rats were euthanized with KCl, and the excised kidneys were sliced to verify flow probe depth. Kidneys were weighed and frozen in liquid nitrogen. Blood and urine samples were assayed for inulin and p-aminohippuric acid by colorimetric assay to derive RPF and GFR. Urine flow was determined gravimetrically. Total prostaglandin and PGE2 concentrations in the urine were measured by commercial enzyme immunoassay kits (Cayman Chemicals) and multiplied by respective urine flows to calculate prostaglandin excretion rates. Plasma and urinary sodium and potassium concentrations were measured by flame photometry. Plasma renin activity (PRA) was measured by radioimmunoassay.
Immunohistochemistry
COX-2 expression was localized in mounted kidney slices with specific antibodies against murine COX-2 (Cayman Chemicals) and processed as previously described (37). Briefly, renal cortical slices were fixed in Bouin's fixative, and the first antibody immunostaining was developed with diaminobenzidine-hydrogen peroxide to give a brown color. Appropriate immunostaining controls were done. The sections were observed and photographed on a Nikon Eclipse 50i microscope with a Nikon DS-U2/L2 USB camera.
Statistical Analysis
Values are means ± SE. Bonferroni-corrected paired Student's t-tests were used to analyze changes in MABP, GFR, RPF, urine flow, and UNa and FENa within groups before and after nimesulide or SC-560, within ACE-inhibited groups before and after captopril, and within groups before and after combined selective COX-1 and COX-2 inhibition. Unpaired Welch's t-tests were used to analyze parameters within and between series, including percent changes averaged from each experiment within and between series comparing pharmacological agents with vehicle. Statistical significance was identified at P < 0.05.
RESULTS
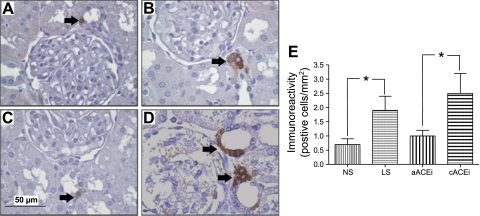
COX-2 immunoreactivity in cortical kidney slices in the NS and aACEi groups was 0.7 ± 0.2 and 1 ± 0.2 cells/mm2, respectively (Fig. 1). Sodium restriction and combined ACE inhibition significantly increased the number of positive cells to 1.9 ± 0.5 and 2.5 ± 0.7 cells/mm2, respectively (P < 0.01).
Fig. 1.
Cyclooxygenase (COX)-2 protein immunoreactivity in kidney cortical slices (3 μm). The immunoperoxidase technique was used for specific staining in rats fed normal-salt (NS) and low-salt (LS) diets for 5 days (A and B, respectively), as well as in rats without captopril pretreatment (aACEi, C) and rats pretreated with captopril (100 mg/kg body wt) for 7 days (cACEi, D). E: quantification of COX-2 protein immunoreactivity. *P < 0.05.
In the NS group, initial total prostaglandin and PGE2 excretion rates were 42.3 ± 8.1 and 10.1 ± 2.0 pg/min, respectively, and significantly less than the initial rates in the LS group (83.4 ± 6.8 and 21.7 ± 5.2 pg/min, respectively, P < 0.05). The initial total prostaglandin and PGE2 excretion rates in the aACEi group (45.6 ± 9.2 and 11.3 ± 2.7 pg/min, respectively, P < 0.05) were similar to those of the NS group and were significantly increased by acute captopril (67.5 ± 11.3 and 18.6 ± 4.1 pg/min, respectively, P < 0.05). The initial total prostaglandin and PGE2 excretion rates were significantly greater in the cACEi group (93.8 ± 13.5 and 27.4 ± 3.7 pg/min, respectively) than the aACEi group (P < 0.05), were similar to the LS group, and were unchanged by acute captopril (100.1 ± 15.3 and 29.2 ± 2.7 pg/min, respectively). COX-2 inhibition significantly decreased these rates in the NS (12.6 ± 2.3 and 2.9 ± 1.5 pg/min, respectively), LS (24.4 ± 1.9 and 6.1 ± 1.5 pg/min, respectively), aACEi (19.7 ± 3.2 and 5.3 ± 1.2 pg/min, respectively), and cACEi (27.1 ± 3.8 and 7.8 ± 1.1 pg/min, respectively) groups.
COX-1 inhibition significantly decreased total prostaglandin excretion in the NS (7.4 ± 1.9 pg/min, P < 0.05 vs. COX-2 inhibition) and cACEi (15.8 ± 3.2 pg/min, P < 0.05 vs. COX-2 inhibition) groups and remained significantly lower than initial rates in all groups (Table 1). Compared with values during COX-2 inhibition, PGE2 excretion was significantly decreased by COX-1 inhibition only in the cACEi group (5.2 ± 1.5 pg/min, P < 0.05; Table 1).
Table 1.
Effects of COX-2 and COX-1 inhibition on MABP and renal hemodynamic and excretory function
| Sodium Series |
ACE Inhibition Series |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Before COX-2 inhibition | After COX-2 inhibition | After COX-1 inhibition | Group | Before COX-2 inhibition | After COX-2 inhibition | After COX-1 inhibition | |
| MABP, mmHg | NS | 116 ± 7 | 114 ± 7 | 115 ± 6 | aACEi | 113 ± 11 | 108 ± 10 | 101 ± 7 |
| LS | 119 ± 6 | 120 ± 6 | 132 ± 10 | cACEi | 107 ± 9 | 108 ± 9 | 106 ± 12 | |
| RPF, ml·min−1·g kidney wt−1 | NS | 4.31 ± 0.37 | 3.38 ± 0.26 | 3.68 ± 0.31 | aACEi | 4.54 ± 0.21 | 3.89 ± 0.18 | 4.14 ± 0.18 |
| LS | 4.20 ± 0.53 | 2.50 ± 0.28* | 3.54 ± 0.50 | cACEi | 4.09 ± 0.19 | 2.72 ± 0.42* | 3.23 ± 0.27 | |
| ΔCBF, % | NS | 2.86 ± 3.34 | 5.02 ± 4.21 | aACEi | 0.98 ± 2.32 | 3.66 ± 5.09 | ||
| LS | −12.09 ± 7.09§ | 4.09 ± 6.82 | cACEi | −13.14 ± 5.73§ | 5.67 ± 4.73 | |||
| ΔMBF, % | NS | −19.98 ± 5.32§ | −0.34 ± 6.57 | aACEi | −18.54 ± 4.98§ | 2.15 ± 3.53 | ||
| LS | −23.96 ± 4.83§ | 0.57 ± 6.10 | cACEi | −26.14 ± 5.48§ | −4.13 ± 2.95 | |||
| GFR, ml·min−1·g kidney wt−1 | NS | 0.96 ± 0.06 | 0.81 ± 0.12 | 0.83 ± 0.10 | aACEi | 1.11 ± 0.07 | 0.91 ± 0.10 | 1.06 ± 0.07 |
| LS | 0.93 ± 0.03 | 0.75 ± 0.09* | 0.84 ± 0.07 | cACEi | 1.02 ± 0.08 | 0.78 ± 0.04* | 0.81 ± 0.08* | |
| Urine flow, μl/min | NS | 21.40 ± 1.33 | 19.39 ± 1.25 | 18.80 ± 1.06 | aACEi | 20.25 ± 3.07 | 18.75 ± 3.88 | 17.67 ± 2.04 |
| LS | 17.68 ± 3.61 | 10.84 ± 3.53* | 8.84 ± 1.93* | cACEi | 16.33 ± 3.76 | 11.78 ± 3.41* | 11.4 ± 3.00* | |
| UNa, μeq/min | NS | 1.58 ± 0.21 | 1.58 ± 0.25 | 1.37 ± 0.21 | aACEi | 1.54 ± 0.17 | 1.45 ± 0.20 | 1.40 ± 0.24 |
| LS | 0.81 ± 0.12† | 0.62 ± 0.28* | 0.59 ± 0.15* | cACEi | 0.69 ± 0.19† | 0.57 ± 0.09 | 0.51 ± 0.22 | |
| FENa, % | NS | 0.57 ± 0.08 | 0.40 ± 0.11 | 0.50 ± 0.38 | aACEi | 0.62 ± 0.15 | 0.63 ± 0.12 | 0.58 ± 0.07 |
| LS | 0.48 ± 0.10 | 0.21 ± 0.07* | 0.23 ± 0.11* | cACEi | 0.40 ± 0.14 | 0.31 ± 0.14 | 0.31 ± 0.17 | |
| Prostaglandin excretion, pg/min | NS | 42.3 ± 8.1 | 12.6 ± 2.3* | 7.4 ± 1.9‡ | aACEi | 67.5 ± 11.3 | 19.7 ± 3.2* | 11.3 ± 5.1* |
| LS | 83.4 ± 6.8† | 24.4 ± 1.9* | 18.2 ± 2.9* | cACEi | 100.1 ± 15.3† | 27.1 ± 3.8* | 15.8 ± 3.2‡ | |
| PGE2 excretion, pg/min | NS | 10.1 ± 2.0 | 2.9 ± 1.5* | 1.8 ± 1.1* | aACEi | 18.6 ± 2.7 | 5.3 ± 1.2* | 3.6 ± 0.9* |
| LS | 21.7 ± 5.2† | 6.1 ± 1.5* | 4.2 ± 1.8* | cACEi | 29.2 ± 4.1† | 7.8 ± 1.1* | 5.2 ± 1.5‡ | |
Values are means ± SE. Nimesulide (3 mg/kg body wt) was used for cyclooxygenase (COX)-2 inhibition, and SC-560 (5 mg/kg body wt) was used for COX-1 inhibition. Mean arterial blood pressure (MABP) and renal hemodynamic and excretory function were studied in a subgroup of rats fed the normal-salt (NS) or low-salt (LS) diet for 5 days as well as in a subgroup of rats without (aACEi) or with (cACEi) captopril pretreatment (100 mg/kg body wt) for 7 days. RPF, renal plasma flow; CBF, cortical blood flow; MBF, medullary blood flow; GFR, glomerular filtration rate; UNa and FENa, absolute and fractional sodium excretion.
P < 0.05 vs. before COX inhibition.
P < 0.05 vs. NS or aACEi.
P < 0.05 vs. after COX-2 inhibition.
P < 0.05 vs. vehicle.
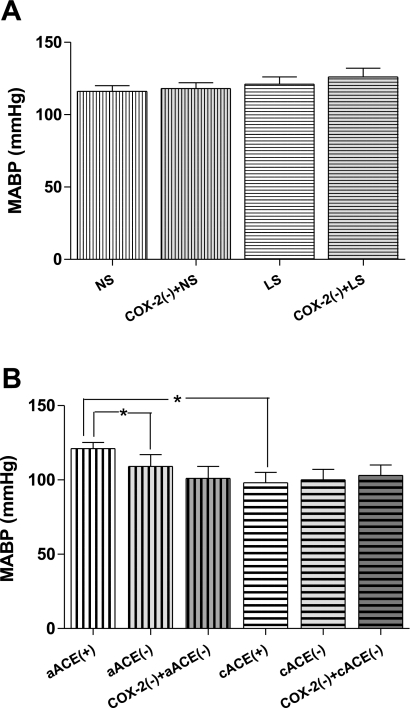
MABP in NS rats averaged 116 ± 4 mmHg during the control period (Fig. 2A) and 118 ± 4 mmHg after nimesulide. The initial arterial pressure in the LS group was 126 ± 6 mmHg and was not altered after nimesulide bolus (131 ± 7 mmHg). PRA was significantly increased in the LS compared with the NS subgroup (17.4 ± 4.1 vs. 3.2 ± 1.2 ng ANG I·ml−1·h−1, P < 0.05). In the LS group, PRA was significantly decreased by COX-2 inhibition (13.71 ± 3.1 ng ANG I·ml−1·h−1, P < 0.05) but, conversely, was significantly increased by COX-1 inhibition (26.5 ± 6.7 ng ANG I·ml−1·h−1, P < 0.05). Nevertheless, there were no significant differences in MABP between LS and NS groups during control periods or subsequent to COX-2 inhibition.
Fig. 2.
A: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on mean arterial blood pressure (MABP) in rats maintained on NS or LS for 5 days. B: effects of COX-2 inhibition on MABP in rats before (+) and after (−) acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). *P < 0.05.
During the initial period, MABP was lower in the cACEi than in the aACEi group (94 ± 4 vs. 107 ± 6 mmHg, P < 0.05; Fig. 2B). Administration of captopril to the pretreated rats did not further affect MABP (100 ± 7 mmHg), nor did the subsequent nimesulide treatment (102 ± 7 mmHg). In the aACEi group, MABP was significantly decreased (96 ± 8 mmHg, P < 0.05 vs. control value) by captopril to values similar to those in the cACEi rats. MABP was not significantly changed by COX-2 inhibition in the aACEi group (101 ± 8 mmHg). In the subgroups treated with SC-560, MABP after COX-1 inhibition remained similar to MABP after COX-2 alone in each experimental group (Table 1). PRA was significantly increased in the cACEi subgroup compared with the aACEi subgroup (23.4 ± 3.6 vs. 3.9 ± 0.6 ng ANG I·ml−1·h−1, P < 0.05). In the cACEi subgroup, PRA was significantly increased by acute captopril (35.2 ± 5.9 ng ANG I·ml−1·h−1, P < 0.05), COX-2 inhibition (53.04 ± 3.1 ng ANG I·ml−1·h−1, P < 0.05), and COX-1 inhibition (60.47 ± 10.02 ng ANG I·ml−1·h−1, P < 0.05).
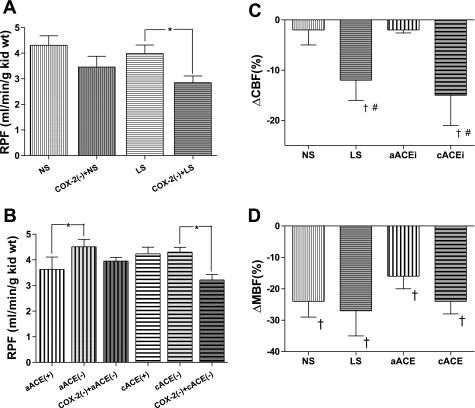
In the NS group, RPF during the control period was 4.23 ± 0.37 ml·min−1·g kidney wt−1 and was not significantly reduced by nimesulide (3.46 ± 0.42 ml·min−1·g kidney wt−1, −10 ± 3.5%), as shown in Fig. 3A. RPF in the LS group was significantly reduced by nimesulide from 3.99 ± 0.33 to 2.85 ± 0.26 ml·min−1·g kidney wt−1 (−17.6 ± 4.5%, P < 0.05). CBF was not significantly changed by nimesulide (Fig. 3C) in the NS group (−2.1 ± 3.0%) but was significantly decreased in the LS group (−12.1 ± 8%, P < 0.05 vs. vehicle). In contrast, MBF (Figs. 3D) was decreased significantly by COX-2 inhibition in the NS (−22.0 ± 4.5%, P < 0.05 vs. vehicle) and LS (−25.6 ± 4.7%, P < 0.05 vs. vehicle) groups, and to a similar extent.
Fig. 3.
A: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on renal plasma flow (RPF) in rats maintained on NS or LS for 5 days. B: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on RPF in rats after acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). *P < 0.05. C and D: changes in cortical blood flow (CBF) and medullary blood flow (MBF) after nimesulide (3 mg/kg body wt) in rats maintained on NS or LS for 5 days and after acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). #P < 0.05 vs. vehicle. †P < 0.05 vs. NS or aACE.
In the NS and LS subgroups, RPF after COX-1 inhibition was similar to that after COX-2 inhibition, and CBF and MBF percent changes after COX-1 inhibition were not significantly different from those after vehicle infusion (Table 1). In the LS subgroup, the difference between RPF during the control period (4.20 ± 0.53 ml·min−1·g kidney wt−1) and COX-2 inhibition (2.50 ± 0.28 ml·min−1·g kidney wt−1) was not observed with combined COX-1 and COX-2 inhibition (3.54 ± 0.50 ml·min−1·g kidney wt−1).
RPF during the control period in the aACEi group was 4.23 ± 0.37 ml·min−1·g kidney wt−1 (Fig. 3B). After acute captopril bolus, RPF was significantly elevated to 4.51 ± 0.29 ml·min−1·g kidney wt−1 (P < 0.05), but consequent COX-2 inhibition failed to elicit a significant change in RPF (3.95 ± 0.14 ml·min−1·g kidney wt−1). CBF, similarly, was significantly increased by captopril (9 ± 0.8%, P < 0.05), with nonsignificant change (−2 ± 0.6%) after nimesulide (Fig. 3C). On the other hand, MBF in the aACEi group did not change with captopril (−6 ± 4.4%) but was reduced −16.3 ± 4.2% by subsequent nimesulide (Figs. 3D).
In the cACEi group, RPF in the control period was 3.81 ± 0.20 ml·min−1·g kidney wt−1, which was not significantly changed by acute captopril administered to these already chronically ACE-inhibited rats (Fig. 3B; 4.30 ± 0.19 ml·min−1·g kidney wt−1). Furthermore, RPF after acute captopril in aACEi or cACEi rats (4.50 ± 0.45 ml·min−1·g kidney wt−1) was not statistically different. In the cACEi group, COX-2 inhibition significantly reduced (P < 0.05) RPF to 3.22 ± 0.21 ml·min−1·g kidney wt−1 (−20 ± 9%). CBF was also unchanged by captopril (5.3 ± 1.2%) but was decreased significantly (−13.3 ± 3.8%, P < 0.05) by nimesulide (Fig. 3C). MBF in this group showed a similar pattern of change, with no significant difference introduced by ACE inhibition (−9.7 ± 6%) but a significant −24.4 ± 3.9% reduction (P < 0.05) in response to COX-2 inhibition (Fig. 3D). The nimesulide-mediated MBF reduction was not significantly different between aACEi and cACEi rats. Administration of vehicle or subsequent COX-1 inhibitor alone did not affect RPF or MBF in any experimental group.
RPF after COX-1 inhibition was similar to RPF after COX-2 inhibition in all ACE-inhibited subgroups. Similarly, in aACEi and cACEi groups, SC-560 and vehicle infusion resulted in similar percent changes in CBF and MBF. However, in the cACEi subgroup, as also observed in the LS group, the difference between RPF values after acute captopril (4.09 ± 0.19 ml·min−1·g kidney wt−1) and after COX-2 inhibition (2.72 ± 0.42 ml·min−1·g kidney wt−1) was not observed when RPF after acute captopril was compared with RPF during combined COX-1 and COX-2 inhibition (3.23 ± 0.27 ml·min−1·g kidney wt−1).
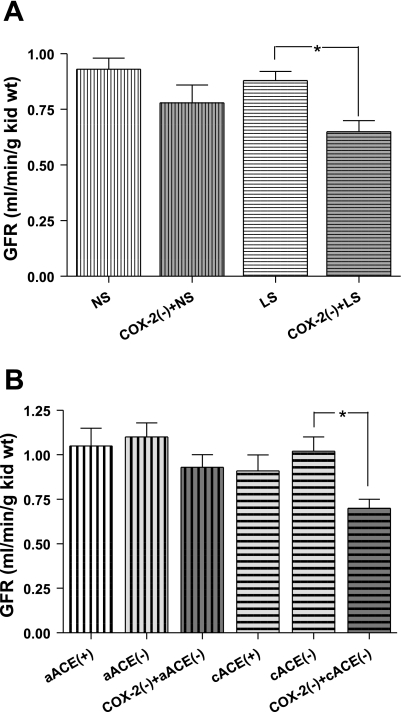
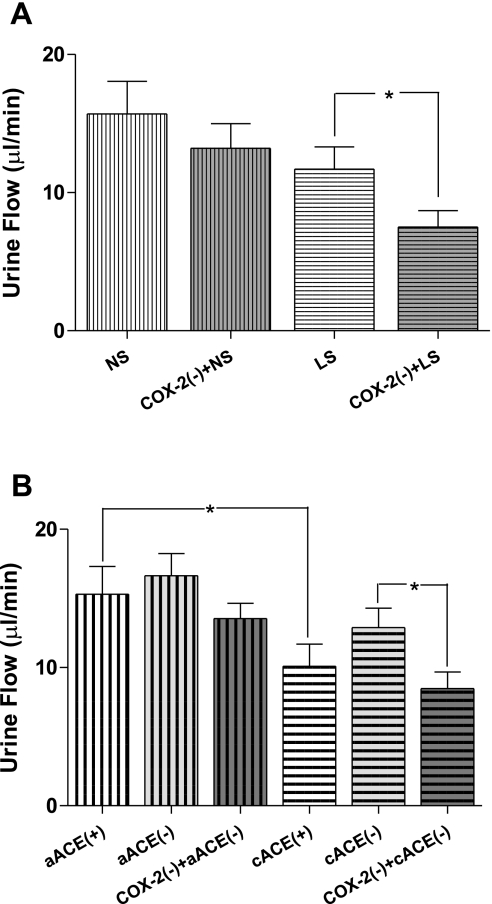
GFR prior to COX-2 inhibition was not significantly influenced by dietary sodium. Control GFR in the NS group was 0.96 ± 0.06 ml·min−1·g kidney wt−1 and was not significantly altered by nimesulide (0.81 ± 0.12 ml·min−1·g kidney wt−1, −15 ± 2.9%; Fig. 4A). However, in the LS group, nimesulide caused a significant fall in GFR (−38 ± 2.2%, P < 0.05) from 0.93 ± 0.03 ml·min−1·g kidney wt−1 during the control period to 0.75 ± 0.09 ml·min−1·g kidney wt−1. Similarly, no differences in urine flow were found during the control periods in NS and LS rats (Fig. 5A; 15.70 ± 2.36 and 11.7 ± 1.6 μl/min), and only in the LS group did nimesulide cause a significant decrease (7.5 ± 1.2 μl/min, P < 0.05).
Fig. 4.
A: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on glomerular filtration rate (GFR) in rats maintained on NS or LS for 5 days. B: effects of COX-2 inhibition on GFR in rats after acute captopril (5 mg/kg) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). *P < 0.05.
Fig. 5.
A: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on urine flow in rats maintained on NS or LS for 5 days. B: effects of COX-2 inhibition on urine flow in rats after acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). *P < 0.05.
In the salt series subgroups subjected to COX-1 inhibition, GFR and urine values were not changed by COX-2 inhibition in the NS subgroup but were altered in the LS subgroup (from 0.93 ± 0.03 ml·min−1·g kidney wt−1 and 17.68 ± 3.61 μl/min to 0.75 ± 0.09 ml·min−1·g kidney wt−1 and 10.84 ± 3.53 μl/min, P < 0.05; Table 1). A difference in GFR was not demonstrated by combined selective COX isoform inhibition (0.84 ± 0.07 ml·min−1·g kidney wt−1) compared with values during the control or the COX-2 inhibition period in any subgroup. However, in the LS subgroup, urine flow subsequent to the addition of SC-560 was maintained at a significantly lower level than during the control period (8.84 ± 1.93 μl/min), although it was not different from urine excretion measured during COX-2 inhibition.
GFR during the control period in cACEi rats (0.85 ± 0.09 ml·min−1·g kidney wt−1) and those receiving no captopril pretreatment (1.05 ± 0.10 ml·min−1·g kidney wt−1) were not significantly different (Fig. 4B). In the aACEi group, GFR was not significantly altered after captopril (1.10 ± 0.08 ml·min−1·g kidney wt−1) or subsequent COX-2 inhibition (0.93 ± 0.07 ml·min−1·g kidney wt−1). In cACEi rats, GFR in the control period was not significantly altered by administration of captopril (0.95 ± 0.07 ml·min−1·g kidney wt−1). However, after nimesulide treatment, GFR was significantly reduced (P < 0.05) to 0.70 ± 0.05 ml·min−1·g kidney wt−1. Urine flow was significantly (P < 0.05) lower in cACEi than in nonpretreated rats (Fig. 5B). Captopril did not significantly change urine flow in cACEi or aACEi rats (from 10.1 ± 1.6 to 12.9 ± 1.4 and from 15.3 ± 2.0 to 18.6 ± 1.6 ml/min, respectively). Successive COX-2 inhibition significantly decreased urine flow in the cACEi group (8.5 ± 1.2 ml/min), but not in the aACEi group (16.6 ± 1.1 ml/min).
In the aACEi subgroup, GFR and urine flow during combined selective COX-1 and COX-2 inhibition were not significantly different from GFR and urine flow before COX-1 or COX-2 inhibition. In the cACEi subgroup, GFR and urine flow were significantly decreased by COX-2 inhibition (from 1.02 ± 0.08 to 0.78 ± 0.04 ml·min−1·g kidney wt−1 and from 16.33 ± 3.76 to 11.78 ± 3.61 μl/min, P < 0.05; Table 1) and, during combined selective COX isoform inhibition, were not significantly different (0.81 ± 0.08 ml·min−1·g kidney wt−1 and 11.43 ± 3.00 μl/min) from values during the control period.
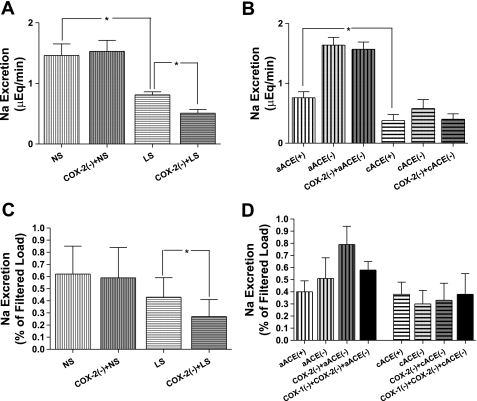
UNa (Fig. 6A) and FENa (Fig. 6C) in the NS group were not significantly altered by COX-2 inhibition (1.46 ± 0.19 μeq/min and 0.62 ± 0.23% vs. 1.53 ± 0.18 μeq/min and 0.59 ± 0.25%). Dietary salt restriction reduced control UNa, and COX-2 inhibition further decreased UNa and FENa in the LS group from 0.75 ± 0.05 μeq/min and 0.43 ± 0.15% to 0.51 ± 0.06 μeq/min and 0.26 ± 0.10% (P < 0.05 for both comparisons). Vehicle infusions did not alter urine flow or UNa or FENa in the LS or NS group.
Fig. 6.
A: effects of COX-2 inhibition [COX-2(−); nimesulide, 3 mg/kg body wt] on absolute sodium excretion in rats maintained on NS or LS for 5 days. B: effects of COX-2 inhibition on absolute sodium excretion in rats after acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). *P < 0.05. C: effects of COX-2 inhibition on fractional sodium excretion in rats maintained on NS or LS for 5 days. D: effects of nimesulide on fractional sodium excretion in rats after acute captopril alone or in combination with chronic captopril for 7 days. *P < 0.05.
After COX-1 inhibition, sodium excretion in the subgroups of the salt series was similar to that after COX-2 inhibition. In the LS subgroup, the difference between UNa and FENa during the control period (0.81 ± 0.12 μeq/min and 0.48 ± 0.10%) and COX-2 inhibition (0.62 ± 0.28 μeq/min and 0.21 ± 0.07%) was not observed during combined COX-1 and COX-2 inhibition (0.59 ± 0.15 μeq/min and 0.23 ± 0.11%).
Control UNa in cACEi rats (Fig. 6B) was significantly less (P < 0.05) than that in non-captopril-pretreated rats (0.38 ± 0.15 vs. 1.36 ± 0.12 μeq/min). FENa during the control period in the aACEi and cACEi groups were 0.40 ± 0.09% and 0.25 ± 0.09%, respectively, and not significantly different (Fig. 6D). In nonpretreated rats, acute ACE inhibition significantly elevated UNa (P < 0.05, 1.64 ± 0.13 μeq/min) but did not significantly alter FENa (0.51 ± 0.17%), and UNa and FENa were not affected by consequent COX-2 inhibition (1.57 ± 0.12 μeq/min and 0.79 ± 0.15%). In rats subjected to chronic ACE inhibition, UNa in the control period was not statistically changed by administration of captopril (0.58 ± 0.15 μeq/min, 0.30 ± 0.13%). These values were also not altered by nimesulide (0.40 ± 0.09 μeq/min, 0.33 ± 0.14%) or vehicle in any rat subjected to ACE inhibition. COX-1 inhibition in aACEi and cACEi animals did not result in sodium excretion values that were different from those during COX-2 inhibition or before COX inhibition (Table 1).
DISCUSSION
These experiments were performed to examine the interaction between ANG II and the effects of nimesulide on renal function during increased cortical COX-2 expression. Our findings show that ACE inhibition and sodium restriction, maneuvers associated with augmented COX-2 expression, result in greater nimesulide-mediated reductions in RPF, GFR, and urine flow than those observed in normal rats. These effects appear to be independent of the prevailing intrarenal RAS activity, unlike the COX-2 inhibition-mediated reduction in sodium excretion that occurred only during augmented RAS activity. In contrast, COX-2 inhibition consistently reduced MBF and did not rely on increased ANG II activity, changes in cortical COX-2 expression, or renal microvascular responses.
In reference to the hemodynamic consequence of upregulated COX-2 expression, the results suggest that the COX-2 inhibition-induced alterations in renal hemodynamics of sodium-restricted and ACE-inhibited rats reflect acute interruption of augmented prostaglandin biosynthesis. We observed that COX-2 inhibition does not elicit significant alterations of RPF and GFR under NS conditions. This finding is consistent with previous in vivo studies under NS conditions that have not demonstrated a discernible consequence of acutely attenuated prostaglandin biosynthesis on renal hemodynamics (11, 26, 30). Furthermore, afferent arteriolar diameters of juxtamedullary nephrons in NS rats were not changed by COX-2 inhibition with NS-398 (16).
Similar to our present findings in rats, sodium-restricted dogs exhibited decreases in GFR and RPF with acute or chronic nimesulide treatment (30, 31). In addition, we present the novel finding that nimesulide decreases GFR and RPF in chronically ACE-inhibited rats. In neither the chronically sodium-depleted nor ACE-inhibited rats did we observe concurrent decreases in arterial pressure, indicating that these effects were due to an increase in renal vascular resistance following nimesulide administration. The upregulation of cortical COX-2, which occurs in chronic ACE inhibition and sodium restriction, provides an attractive explanation for these results. Therefore, these data suggest that an increased prostaglandin-mediated vasodilatory influence is the hemodynamic consequence of cortical COX-2 upregulation by dietary sodium and chronic ACE inhibition.
In contrast to our findings, Harding et al. (11) reported that COX-2 inhibition does not change RPF in NS or LS animals. Harding et al. administered a 100-μl bolus of 10−5 M NS-398 solution intravenously to 300- to 400-g rats, an ∼1 μg/kg dose. In the present study, we administered a larger dose (3 mg/kg) of nimesulide intra-arterially, and dose- or drug-specific effects may account for the differences in our results, given a comparable IC50 for NS-398 and nimesulide (1.77 and 1.27 μM, respectively) (1).
Another question addressed by our data was whether augmented intrarenal ANG II activity is necessary for manifestation of the functional consequence of inhibiting the upregulated COX-2. The influence of COX-2 on GFR and RPF was greater in the combined ACE-inhibited group than in the acute ACE-inhibited group, although both groups showed elevated urinary prostaglandin excretion compared with control levels. However, the total prostaglandin and PGE2 excretion was significantly greater in the cACEi than the aACEi group and likely accounts for the greater functional influence. Such similarity between the LS and cACEi groups supports an increased vasodilatory influence from the upregulated COX-2 expression. Further comparison of results from the LS group with results from the cACEi group endorses a direct COX-2-derived prostaglandin hemodynamic influence that is not contingent on the prevailing RAS activity.
Various studies have focused on the roles of the renal principal vasoconstrictive [thromboxane A2 (TxA2)] and vasodilatory (PGE2 and PGI2) prostaglandins during sodium restriction. In contrast to the diminished vasoconstricting influence suggested by decreased TxA2 levels (39) and TxA2 synthase activity (39) during sodium restriction, the role of PGE2 via augmented microsomal PGE synthase-1 (20) and prostaglandin (EP4) receptor (19) expression is enhanced. Furthermore, macula densa cells have been shown to release PGE2 in response to low salt (27). This is consistent with the increased overall COX-2 vasodilatory influence demonstrated by these experiments when the enzyme expression is upregulated during LS diet and ACE inhibition.
The interaction of the COX isoforms was explored in several studies in which COX-1-mediated vasoconstriction that was demonstrated in the systemic vasculature was not observed in the renal microcirculation. During elevated intrarenal ANG II, the addition of meclofenamate, a nonselective COX inhibitor, augmented nimesulide-mediated reductions in blood pressure but did not alter nimesulide-induced reductions in renal vascular resistances, GFR, or RPF (26). Also, in studies using selective COX-1 (genetic deletion and pharmacological) inhibition, COX-1-augmented ANG II increases in systemic resistances were not apparent in the vascular resistances that influence CBF or MBF (29). The present experiments demonstrate RPF values that are decreased by COX-2 inhibition and are not different from those measured during combined selective inhibition of the COX isoforms. This suggests that the effects of COX-1 on RPF are the opposite the vasodilatory influence of COX-2 under augmented cortical COX-2 expression in the LS and cACEi groups.
In the present study, we also investigated the functional role of COX-2 to interact in regulating sodium excretion during varying ANG II activity. Nimesulide caused significant FENa and UNa only in the LS group. However, the antinatriuresis caused by nimesulide in LS animals is absent during combined ACE inhibition and suggests the involvement of unopposed ANG II. Therefore, in contrast to COX-2 influence on hemodynamics, these results provide evidence for an ANG II-dependent COX-2 influence on sodium excretion.
Maintenance of medullary prostaglandin levels during sodium restriction could oppose the MBF effects of augmented medullary ANG II activity, similar to our observation in the cortex. Indeed, kidney ANG II and PGE2 have an opposite effect on vasa recta flow (5). Decreases in MBF and augmentation of ANG II-mediated MBF reductions by inhibition of COX-2, but not COX-1, suggest that COX-2 is the source of that PGE2 (29). Consistent with these findings, these experiments demonstrate that nimesulide reduced MBF in all groups. While the decreased MBF could alternatively be explained as a consequence of decreased total RBF, the decrease in NS rats was not accompanied by a significant reduction of RPF or CBF, suggesting that the reduction of MBF is not primarily mediated by reduced total RBF.
However, neither sodium restriction nor ACE inhibition, maneuvers associated with increased (17) and decreased intrarenal ANG II activity (9), affected the magnitude of nimesulide-induced MBF reduction among the groups. Therefore, we conclude that COX-2 activity helps maintain MBF, regardless of changes in intrarenal ANG II activity. Studies have demonstrated MBF reductions by captopril (6, 10, 22, 32) that are augmented by nonselective COX inhibition (5). The difference in our results likely reflects that selective inhibition in these experiments maintains medullary vasodilatory PGE2 and PGI2 derived from COX-1 during the loss of regulated COX-2 supplement.
In addition, our results suggest that the mechanism by which COX-2 inhibition alters sodium excretion in the LS group is not principally dependent on associated changes in MBF, given that similar reductions in the NS group were not accompanied by significant decreases in sodium excretion. Another possible mechanism responsible for the reduced sodium excretion could involve COX-2-dependent sodium transport activity. Indeed, PGE2 has been shown to reduce sodium transport in isolated thick ascending limbs (35) and collecting duct (33). Nevertheless, the absence of COX-2 effects during combined ACE inhibition could reflect diminished ANG II-dependent sodium reabsorption available for prostaglandin modulation that is otherwise present in the thick ascending limb or collecting duct or, indirectly, through aldosterone-regulated epithelial sodium channel activity (2, 12).
In the collecting duct, the effect of COX-2-derived PGE2 on sodium transport may be further dependent on whether it acts in the tubular or interstitial environment. The implication of dual prostaglandin sources on renal excretory function is highlighted by studies in cortical collecting ducts, where PGE2 applied to the basolateral membrane decreases EP1 or EP3 receptor-mediated amiloride-sensitive sodium transport, whereas EP4 receptor activation increases transport when applied apically (33). Furthermore, dietary sodium is positively correlated with apical expression of prostaglandin transporters (3). Therefore, during sodium restriction, polarized cellular EP receptor localization and decreased transcellular prostaglandin transport could lead to PGE2 derived from upregulated cortical COX-2 to favor activation of EP4 proreabsorption in opposition to EP1 and/or EP3 antireabsorptive functions.
In summary, these experiments demonstrate that upregulation of COX-2 expression by ACE inhibition is associated with prostaglandin-mediated vasodilation and preservation of the renal hemodynamic indexes GFR, RPF, and MBF. By employing dietary variation, we confirm similar consequences in rats. In addition, these experiments support the notion that COX-2-derived prostaglandin effects on hemodynamics are not contingent on ANG II activity. On the other hand, COX-2-mediated enhancements of FENa and UNa are contingent on ANG II activity, but MBF changes are not. Thus it appears that COX-2 activity in ACE-inhibited and sodium-restricted rats directly promotes vasodilation in opposition to intrarenal ANG II vasoconstrictive influence on cortical and medullary microvasculature to maintain renal function while modulating ANG II effects on sodium reabsorption. Further in vivo prostaglandin receptor-specific studies would indicate the importance of these mechanisms among disease states managed by regulation of body fluid volume, including ACE inhibition and dietary sodium restriction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-18426 and HL-26371 and by the Institutional Award Program of the National Center for Research Resources Centers of Biomedical Research Excellence Grant P20 RR-017659. T. Green was a predoctoral fellow supported by the Louisiana Board of Regents Superior Educational Quality Support Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. M. Prieto-Carrasquero for support with immunohistochemical techniques, Dale Seth for technical contributions, and D. Olavarietta for administrative assistance.
REFERENCES
- 1.Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta 1209: 130–139, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chi Y, Pucci ML, Schuster VL. Dietary salt induces transcription of the prostaglandin transporter gene in renal collecting ducts. Am J Physiol Renal Physiol 295: F765–F771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen L, Kelly L, Connor SO, Fitzgerald DJ. Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther 287: 578–582, 1998 [PubMed] [Google Scholar]
- 5.Cupples WA, Sakai T, Marsh DJ. Angiotensin II and prostaglandins in control of vasa recta blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 254: F417–F424, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Dukacz SA, Feng MG, Yang LF, Lee RM, Kline RL. Abnormal renal medullary response to angiotensin II in SHR is corrected by long-term enalapril treatment. Am J Physiol Regul Integr Comp Physiol 280: R1076–R1084, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Dunham EW, Zimmerman BG. Release of prostaglandin-like material from dog kidney during nerve stimulation. Am J Physiol 219: 1279–1285, 1970 [DOI] [PubMed] [Google Scholar]
- 8.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol Renal Fluid Electrolyte Physiol 262: F902–F909, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53: 351–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansell P, Sjoquist M, Ulfendahl HR. Effect of a converting-enzyme inhibitor on vasa recta blood flow in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 254: F492–F499, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Harris PJ, Navar LG. Tubular transport responses to angiotensin. Am J Physiol Renal Fluid Electrolyte Physiol 248: F621–F630, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Harris RC, McKanna JA, Akai Y, Jacobson HR, DuBois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RC, Zhang MZ, Cheng HF. Cyclooxygenase-2 and the renal renin-angiotensin system. Acta Physiol Scand 181: 543–547, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Helle F, Vagnes OB, Iversen BM. Angiotensin II-induced calcium signaling in the afferent arteriole from rats with two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 291: F140–F147, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Ichihara A, Imig JD, Inscho EW, Navar LG. Cyclooxygenase-2 participates in tubular flow-dependent afferent arteriolar tone: interaction with neuronal NOS. Am J Physiol Renal Physiol 275: F605–F612, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol 283: F995–F1002, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Izhar M, Alausa T, Folker A, Hung E, Bakris GL. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and Hispanics. Hypertension 43: 573–577, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jensen BL, Mann B, Skott O, Kurtz A. Differential regulation of renal prostaglandin receptor mRNAs by dietary salt intake in the rat. Kidney Int 56: 528–537, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kammerl MC, Nusing RM, Seyberth HW, Riegger GA, Kurtz A, Kramer BK. Inhibition of cyclooxygenase-2 attenuates urinary prostanoid excretion without affecting renal renin expression. Pflügers Arch 442: 842–847, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mattson DL, Roman RJ. Role of kinins and angiotensin II in the renal hemodynamic response to captopril. Am J Physiol Renal Fluid Electrolyte Physiol 260: F670–F679, 1991 [DOI] [PubMed] [Google Scholar]
- 23.McGiff JC, Crowshaw K, Terragno NA, Lonigro AJ. Renal prostaglandins: possible regulators of the renal actions of pressor hormones. Nature 227: 1255–1257, 1970 [DOI] [PubMed] [Google Scholar]
- 24.Morinelli TA, Kendall RT, Luttrell LM, Walker LP, Ullian ME. Angiotensin II-induced cyclooxygenase 2 expression in rat aorta vascular smooth muscle cells does not require heterotrimeric G protein activation. J Pharmacol Exp Ther 330: 118–124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Opay AL, Mouton CR, Mullins JJ, Mitchell KD. Cyclooxygenase-2 inhibition normalizes arterial blood pressure in CYP1A1-REN2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 291: F612–F618, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdy KE, Arendshorst WJ. Iloprost inhibits inositol-1,4,5-trisphosphate-mediated calcium mobilization stimulated by angiotensin II in cultured preglomerular vascular smooth muscle cells. J Am Soc Nephrol 12: 19–28, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest 110: 61–69, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez F, Llinas MT, Gonzalez JD, Rivera J, Salazar FJ. Renal changes induced by a cyclooxygenase-2 inhibitor during normal and low sodium intake. Hypertension 36: 276–281, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Roig F, Llinas MT, Lopez R, Salazar FJ. Role of cyclooxygenase-2 in the prolonged regulation of renal function. Hypertension 40: 721–728, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Roman RJ, Kaldunski ML, Scicli AG, Carretero OA. Influence of kinins and angiotensin II on the regulation of papillary blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 255: F690–F698, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Sakairi Y, Jacobson HR, Noland TD, Breyer MD. Luminal prostaglandin E receptors regulate salt and water transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F257–F265, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Shah AA, Murray FE, Fitzgerald DJ. The in vivo assessment of nimesulide cyclooxygenase-2 selectivity. Rheumatology (Oxford) 38Suppl 1: 19–23, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Stokes JB. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest 64: 495–502, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander AJ. Direct effects of prostaglandin on renal function and renin release in anesthetized dog. Am J Physiol 214: 218–221, 1968 [DOI] [PubMed] [Google Scholar]
- 37.Vio CP, Balestrini C, Recabarren M, Cespedes C. Postnatal development of cyclooxygenase-2 in the rat kidney. Immunopharmacology 44: 205–210, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Weber P, Holzgreve H, Stephan R, Herbst R. Plasma renin activity and renal sodium and water excretion following infusion of arachidonic acid in rats. Eur J Pharmacol 34: 299–304, 1975 [DOI] [PubMed] [Google Scholar]
- 39.Wilcox CS, Welch WJ. Thromboxane synthase and TP receptor mRNA in rat kidney and brain: effects of salt intake and ANG II. Am J Physiol Renal Physiol 284: F525–F531, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Wolf K, Castrop H, Hartner A, Goppelt-Strube M, Hilgers KF, Kurtz A. Inhibition of the renin-angiotensin system upregulates cyclooxygenase-2 expression in the macula densa. Hypertension 34: 503–507, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819–F825, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998 [DOI] [PubMed] [Google Scholar]