Abstract
Descending vasa recta (DVR) are 12- to 15-μm microvessels that supply the renal medulla with blood flow. We examined the ability of intrinsic nitric oxide (NO) and reactive oxygen species (ROS) generation to regulate their vasoactivity. Nitric oxide synthase (NOS) inhibition with Nω-nitro-l-arginine methyl ester (l-NAME; 100 μmol/l), or asymmetric NG,NG-dimethyl-l-arginine (ADMA; 100 μmol/l), constricted isolated microperfused DVR by 48.82 ± 4.34 and 27.91 ± 2.91%, respectively. Restoring NO with sodium nitroprusside (SNP; 1 mmol/l) or application of 8-Br-cGMP (100 μmol/l) reversed DVR vasoconstriction by l-NAME. The superoxide dismutase mimetic Tempol (1 mmol/l) and the NAD(P)H inhibitor apocynin (100, 1,000 μmol/l) also blunted ADMA- or l-NAME-induced vasoconstriction, implicating a role for concomitant generation of ROS. A role for ROS generation was also supported by an l-NAME-associated rise in oxidation of dihydroethidium that was prevented by Tempol or apocynin. To test whether H2O2 might play a role, we examined its direct effects. From 1 to 100 μmol/l, H2O2 contracted DVR whereas at 1 mmol/l it was vasodilatory. The H2O2 scavenger polyethylene glycol-catalase reversed H2O2 (10 μmol/l)-induced vasoconstriction; however, it did not affect l-NAME-induced contraction. Finally, the previously known rise in DVR permeability to 22Na and [3H]raffinose that occurs with luminal perfusion was not prevented by NOS blockade. We conclude that intrinsic production of NO and ROS can modulate DVR vasoactivity and that l-NAME-induced vasoconstriction occurs, in part, by modulating superoxide concentration and not through H2O2 generation. Intrinsic NO production does not affect DVR permeability to hydrophilic solutes.
Keywords: kidney, medulla, microcirculation, hydrogen peroxide, reactive oxygen species
the importance of interactions between nitric oxide (NO) and reactive oxygen species (ROS) in the renal medulla has been underscored by several studies. Reduction of superoxide (O2−) by intramedullary infusion of the O2− scavenger tiron increased both medullary blood flow (MBF) and sodium excretion (56). Similarly, chronic administration of the O2− dismutase mimetic Tempol, either orally or intravenously, abrogated hypertension in experimental models (25, 28, 36). Conversely, an increase in O2− by intramedullary infusion of the SOD inhibitor diethyldithiocarbamate (DETC) reduced MBF and sodium excretion (56), and chronic DETC administration induced hypertension (19). Thus a link among oxidative stress, medullary perfusion, and hypertension is increasingly well established. Evidence favors saliuretic and vasodilatory roles for NO that are partially limited by antagonistic effects of O2−, a counterbalance that is achieved in part through their reaction to form peroxynitrite (11, 16, 17).
Given the tubular-vascular relationships in the renal medulla, it seems likely that the descending vasa recta (DVR) are exposed to the influences of ROS and NO from multiple sources. It has been reported that NO and O2− generated by the medullary thick ascending limb (mTAL) can diffuse to DVR within vascular bundles to affect vascular tone (8, 10, 23, 24). The latter points to an important role for extrinsic production of NO and ROS that occurs outside of vasa recta endothelia and pericytes. In this study, we tested the hypothesis that intrinsic generation of NO and ROS generation can also exert substantial control over DVR vasoactivity. We found that nonspecific blockade of NO synthase (NOS) intensely constricts isolated, perfused DVR and enhances the rate of conversion of dihydroethidium (DHE) to ethidium without affecting permeability to hydrophilic solutes. These results favor the interpretation that intrinsic NO and ROS generation participates in the modulation of DVR vascular tone.
METHODS
Isolation and microperfusion of DVR.
Kidneys were harvested from Sprague-Dawley rats (100–150 g; Harlan Sprague Dawley, Indianapolis, IN) that were anesthetized by an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Renal tissue was sliced, placed in cold buffer, and maintained on ice. DVR were dissected from outer medullary vascular bundles and transferred to the stage of an inverted microscope where microperfusion, videomicroscopy, or fluorescence microscopy was performed as previously described (5, 52, 54). Isolated DVR were mounted on concentric pipettes, cannulated at one end, and held at the other end to permit free flow into the collection pipette. The vessel was positioned with micromanipulators (Instruments Technology and Machinery, San Antonio, TX) near a thermocouple in the narrow entrance region of a custom-built chamber. Temperature was maintained at 37°C with a feedback controller (CN9000A, Omega Engineering). The buffer used for dissection, perfusion, and the bath was (in mM) 140 NaCl, 10 Na acetate, 5 KCl, 1.2 MgCl2, 2 NaHPO4/NaH2PO4, 1 CaCl2, 5 alanine, 0.1 l-arginine, 5 glucose, 5 HEPES, and 0.5 g/dl albumin, pH 7.4 at 37°C (5, 52, 54). All investigations involving animal use were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland.
Videomicroscopy and measurement of DVR diameter.
Vessel images were captured every 20 s with a digital camera (Spot camera, Diagnostic Instruments). DVR were observed with a ×40 objective to yield the final magnification of 1:1,300. Constriction was quantified as percent reduction of internal diameter at the point of maximal constriction using National Institutes of Health Image J software for offline image analysis. Percent constriction was calculated as [1 − (D/D0)] × 100, where D and D0 are the experimental and baseline diameters, respectively (5, 52, 53).
Measurement of ROS generation by oxidation of DHE.
DVR ROS generation was measured by quantifying conversion of DHE to fluorescent ethidium (ETH). DHE (10 μmol/l) was loaded into isolated DVR via the bath as previously described. ETH was excited at 535 nm, and emissions were monitored at 635 nm using a photon-counting photomultiplier (Photon Technology International) (5, 53, 54).
Immunochemistry.
DVR were transferred onto microscope slides and processed for double staining of neuronal (nNOS), inducible (iNOS), or endothelial NOS (eNOS) and α-smooth muscle actin (SMA) using specific antibodies (5). DVR were fixed with 3% paraformaldehyde in 0.1 mol/l cacodylate buffer, pH 7.4 and incubated in PBS containing 5% BSA, 0.1% Triton X-100 (PBS/Triton solution) before incubation with a primary antibody. DVR were then incubated overnight at −4°C with a polyclonal rabbit anti-nNOS, eNOS, or iNOS, and a monoclonal mouse anti-SMA antibody. After a wash with PBS containing 0.1% Triton X-100, DVR were incubated in Alexa Fluor 568 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) for 1 h at room temperature. DVR were mounted under coverslips with Vectorshield, and fluorescent optical sections were captured with a Zeiss LSM510 Meta confocal microscope.
Permeability measurements.
The effects of blockade of NO formation on solute permeability were tested using methods previously described. In brief, tracer concentrations of radioisotopes (22Na, [3H]raffinose) were loaded into the microperfusion pipette to measure lumen-to-bath flux (33). The perfusion rate was varied by pressurizing the perfusion pipette lumen, while timed collections were performed with volumetric constriction pipettes. Isotopes in the perfusate and collectate were measured by dual isotope counting with an LS6500 β counter (Beckman) as previously described (31). Diffusional permeabilities (Pi) were calculated from the collection rate, geometry of the vessel outside the pipettes, and collectate-to-perfusate ratio of tracer activity (Ri*) according to Pi = [Qc /(πDL)] In (Ri*) where D is the diameter, L is the length of vessel outside the perfusion and collection pipettes, and QC is the collection rate obtained under conditions of zero transmural volume flux (symmetrical bath and perfusate).
Reagents.
Nω-nitro-l-arginine methyl ester (l-NAME), asymmetric NG,NG-dimethyl-l-arginine (ADMA), apocynin, 8-Br-cGMP, sodium nitroprusside (SNP), 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (Tempol), hydrogen peroxide (H2O2), polyethylene glycol (PEG)-catalase, and l-N6-(1-iminoethyl)lysine (l-NIL) were dissolved in water and aliquoted frozen at −20°C. Apocynin and NG-propyl-l-arginine (l-NPA) were dissolved in DMSO and frozen at −20°C in aliquots. Reagents were thawed once, and the excess was discarded at the end of the day. DHE (Molecular Probes) was stored frozen in DMSO, thawed, and diluted for daily use.
Statistics.
Data in the text and figures are reported as means ± SE. The significance of differences was evaluated with SigmaStat 3.11 (Systat Software, Point Richmond, CA). Comparisons between groups were performed with Student's t-test (paired or unpaired, as appropriate) or the rank sum test (nonparametric). Comparisons between multiple groups employed repeated measures ANOVA, or repeated measures ANOVA on ranks (nonparametric). Post hoc comparisons were performed using Tukey's or Holm-Sidak tests. P < 0.05 was used to reject the null hypothesis.
RESULTS
Effect of NOS inhibition on DVR vasoactivity.
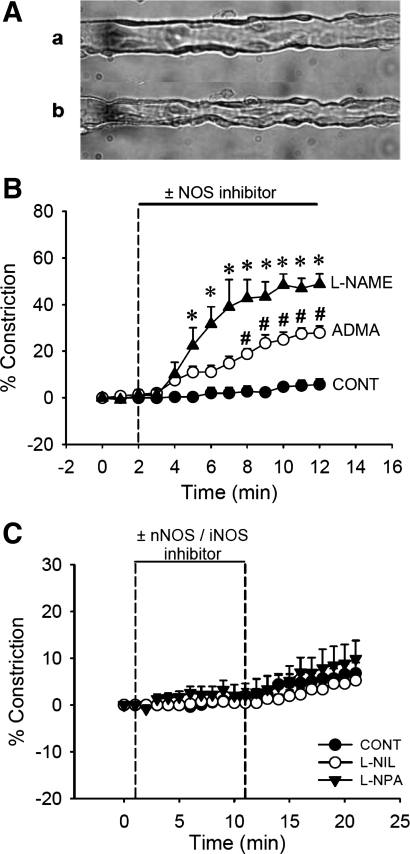
Figure 1 illustrates the effect of global NOS inhibition on DVR vasoactivity. Abluminal application of the nonselective NOS inhibitor l-NAME (100 μmol/l, n = 5) for 10 min induced sustained vasoconstriction; internal diameter was reduced by 48.82 ± 4.34% (Fig. 1, A and B). The endogenous NOS inhibitor ADMA (100 μmol/l, n = 9) also constricted DVR, but to a lesser degree than l-NAME. ADMA reduced luminal diameter by 27.91 ± 2.91% (Fig. 1B). To test which NOS isoforms contribute to intrinsic NO generation, we examined the effects of applying the selective NOS inhibitors l-NPA (1 μmol/l, nNOS blockade, n = 10) and l-NIL (10 μmol/l, iNOS blockade, n = 6) on DVR vasoactivity. Neither affected DVR vascular tone (Fig. 1C). DVR constriction by l-NAME was reversed by restoring NO with the donor SNP (Fig. 2A, 1 mmol/l, n = 6), or cGMP with the nonhydrolyzable analog 8-Br-cGMP (Fig. 2B, 100 μmol/l, n = 5), verifying that vasodilatory signaling pathways downstream of NO remain intact during NOS inhibition. Microfluorescent examination of immunostained DVR verified that both eNOS and nNOS are expressed in the vessel wall (Fig. 3, B and E). In contrast, iNOS could not be detected (data not shown).
Fig. 1.
Effect of nitric oxide synthase (NOS) inhibition on descending vasa recta (DVR) vasoactivity. A: images of perfused DVR before (a) and after (b) application of Nω-nitro-l-arginine methyl ester (l-NAME). B: microperfused DVR were exposed to nonselective NOS inhibitor l-NAME (100 μmol/l, n = 5), endogenous NOS inhibitor asymmetric NG,NG-dimethyl-l-arginine (ADMA; 100 μmol/l, n = 9), or sham exchange (n = 6). After 2-min baseline recording, l-NAME or ADMA or vehicle was introduced to the bath for 10 min. l-NAME and ADMA induced DVR vasoconstriction. *P < 0.05, l-NAME vs. vehicle. #P < 0.05, ADMA vs. vehicle. C: microperfused DVR were exposed to selective neuronal NOS (nNOS) inhibitor NG-propyl-l-arginine (l-NPA; 1 μmol, n = 10) or selective inducible (iNOS) inhibitor l-N6-(1-iminoethyl)lysine (l-NIL; 10 μmol, n = 6) or vehicle (n = 8). After 1-min baseline recording, l-NIL or l-NPA or vehicle was introduced to the bath for 10 min and removed. Neither l-NIL nor l-NPA affected DVR vasoactivity.
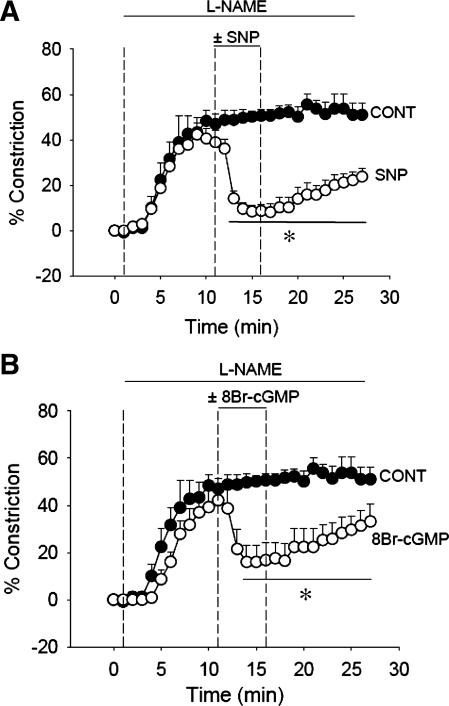
Fig. 2.
Effect of restoration of NO and cGMP on l-NAME-induced vasoconstriction. A: donation of NO by sodium nitroprusside (SNP) reversed vasoconstriction. After 1-min baseline recording, DVR were preconstricted with l-NAME for 10 min after which SNP (1 mmol/l, n = 6) or vehicle (n = 5) was added for 5 min and removed. *P < 0.05, SNP vs. control. B: using the same protocol as in A, abluminal 8-Br- cGMP (100 μmol/l, n = 5) dilated l-NAME (100 μmol/l)-preconstricted vessels. *P < 0.05, 8-Br-cGMP vs. vehicle (n = 5).
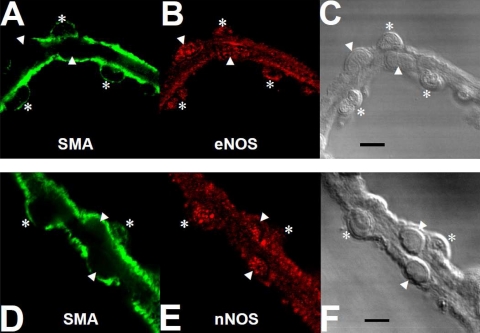
Fig. 3.
Immunofluorescent detection of NOS isoforms in DVR. Immunofluorescent detection of α-smooth muscle actin (SMA; green, A and D), endothelial NOS (eNOS; red, B), nNOS (red, E), and white light differential interference contrast images of the corresponding vessels (C and F). Asterisks and arrowheads indicate pericytes and endothelial cell bodies, respectively. Both pericytes and endothelial cells expressed eNOS and nNOS. The results are representative of 4 experiments. Bars = 10 μm.
Effect of O2− dismutation or NADPH oxidase inhibition on vasoconstriction induced by ADMA.
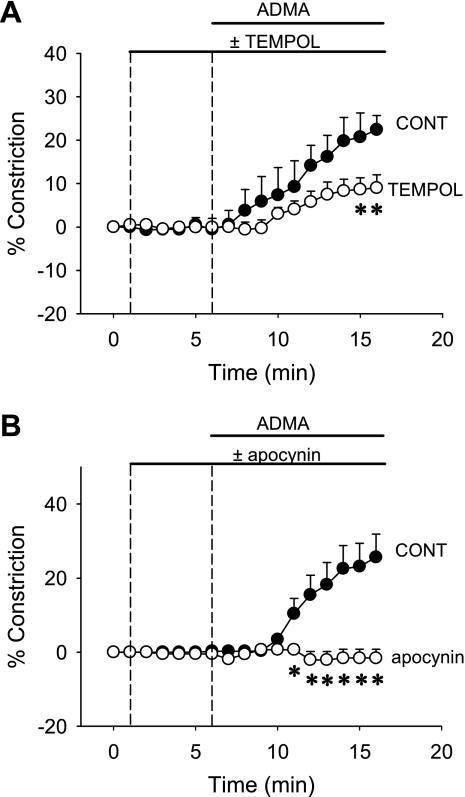
We tested the hypothesis that O2− generation plays a role in ADMA- and l-NAME-induced DVR vasoconstriction. We applied the SOD mimetic Tempol to determine whether it can limit vasoconstriction. Microperfused DVR were pretreated with Tempol (1 mmol/l, n = 7) or vehicle (n = 5) for 5 min after which ADMA (100 μmol/l) was also included in the bath (Fig. 4A). In a second series, we tested the ability of NAD(P)H oxidase inhibition with apocynin to blunt ADMA vasoconstriction. Apocynin (1,000 μmol/l, n = 7) or vehicle (n = 7) was employed using the same experimental design (Fig. 4B). Both Tempol and apocynin blunted ADMA-induced constriction. Apocynin was the more effective of the two agents. Neither of those inhibitors affected the basal tone of microperfused DVR.
Fig. 4.
Effect of O2− dismutation or inhibition of O2− generation on ADMA-induced DVR vasoconstriction. A: after 1-min baseline recording, the O2− dismutase mimetic Tempol (1 mmol/l, n = 7) or vehicle (n = 5) was added to the bath for 5 min. Subsequently, ADMA (100 μmol/l) was also applied. *P < 0.05, Tempol vs. vehicle. B: same protocol as in A, except that the vessels were treated with the NAD(P)H oxidase inhibitor apocynin (1 mmol/l, n = 7) or vehicle (n = 7). *P < 0.05, apocynin vs. vehicle.
Effect of O2− dismutation or NADPH oxidase inhibition on vasoconstriction induced by l-NAME.
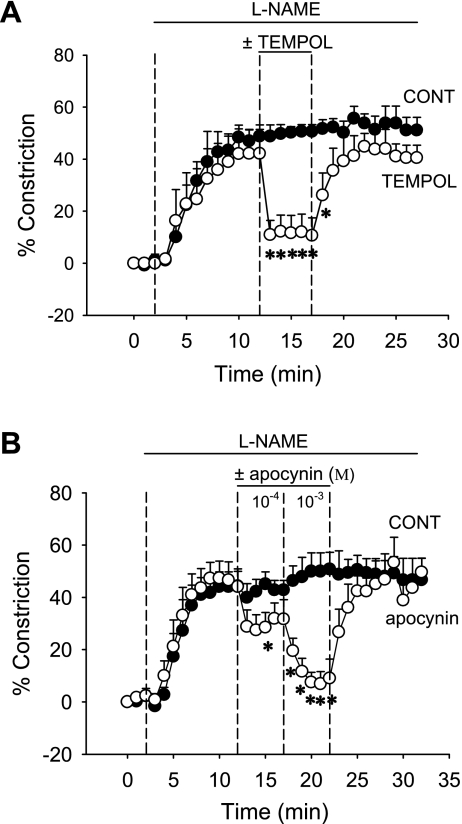
To test the effects of Tempol and apocynin on l-NAME-induced constriction, DVR were exposed to l-NAME (100 μmol/l) for 10 min after which Tempol (1 mmol/l, n = 5) was added to the bath for 5 min and removed. Tempol reversibly dilated the vessels (Fig. 5A). In a second series, we tested whether inhibition of O2− generation by NAD(P)H oxidase could similarly reverse l-NAME-induced vasoconstriction. After application of l-NAME (100 μmol/l), apocynin was sequentially added to the bath at 100 and 1,000 μmol/l (Fig. 5B, n = 5). At each concentration, apocynin vasodilated l-NAME-preconstricted vessels. Thus both O2− dismutation and inhibition of O2− generation reversed l-NAME-induced vasoconstriction.
Fig. 5.
Effect of O2− dismutation or inhibition of O2− generation on l-NAME-induced DVR vasoconstriction. A: after 2-min baseline recording, DVR were preconstricted with l-NAME (100 μmol/l) for 10 min after which the O2− dismutase mimetic Tempol (1 mmol/l, n = 5) or vehicle (n = 6) was added for 5 min and then removed. *P < 0.05, Tempol vs. vehicle (n = 6). B: same protocol as in A except that the vessels were treated with the NAD(P)H oxidase inhibitor apocynin (1 mmol/l, n = 5) or vehicle (n = 5). *P < 0.05, apocynin vs. vehicle.
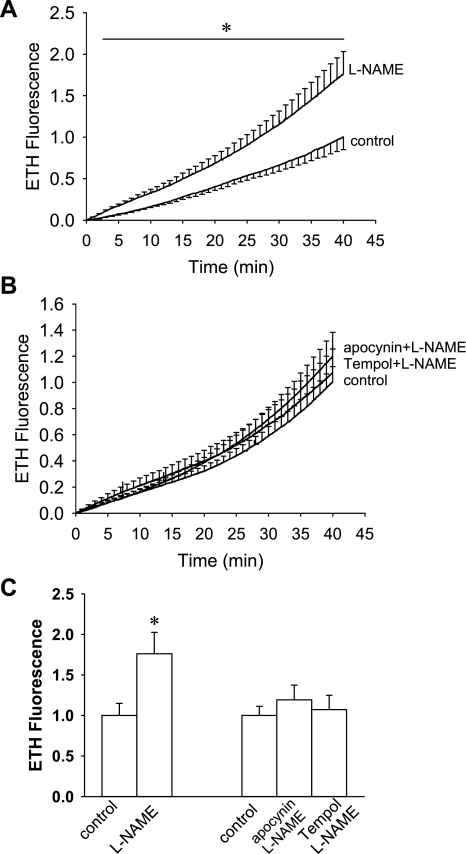
To test whether constriction of DVR by l-NAME enhances ROS availability, using methods previously described, we measured oxidation of DHE to ETH (53, 54). Isolated DVR were microperfused and ETH fluorescence was measured for 40 min in the presence (n = 8) or absence (n = 7) of l-NAME (100 μmol/l). l-NAME increased DHE fluorescence (Fig. 6A). When either apocynin (1 mmol/l, n = 7) or Tempol (1 mmol/l, n = 6) was added along with l-NAME (100 μmol/l), the rate of DHE oxidation was similar to controls (n = 7) that were not exposed to l-NAME (Fig. 6B). The mean fluorescence during the last 2 min of recording is summarized in Fig. 6C.
Fig. 6.
Effect of l-NAME on oxidation of dihydroethidium (DHE) to ethidium (ETH). A: background-subtracted fluorescence is shown as a function of time in isolated DHE-loaded DVR exposed in the presence and absence of l-NAME (100 μmol/l). Compared with vehicle (n = 7), l-NAME (n = 8) significantly (*) enhanced the rate of ETH generation. B: in a separate series, the effects of l-NAME on DHE oxidation was measured in vehicle (n = 7), l-NAME (100 μmol/l)+Tempol (1 mmol/l, n = 6), or l-NAME (100 μmol/l)+apocynin (1 mmol/l, n = 7). C: statistical summary of data from A and B. The mean of the final 2 min of fluorescence is compared for the designated groups. *P < 0.05, l-NAME vs. vehicle.
Effect of H2O2 on DVR vasoactivity.
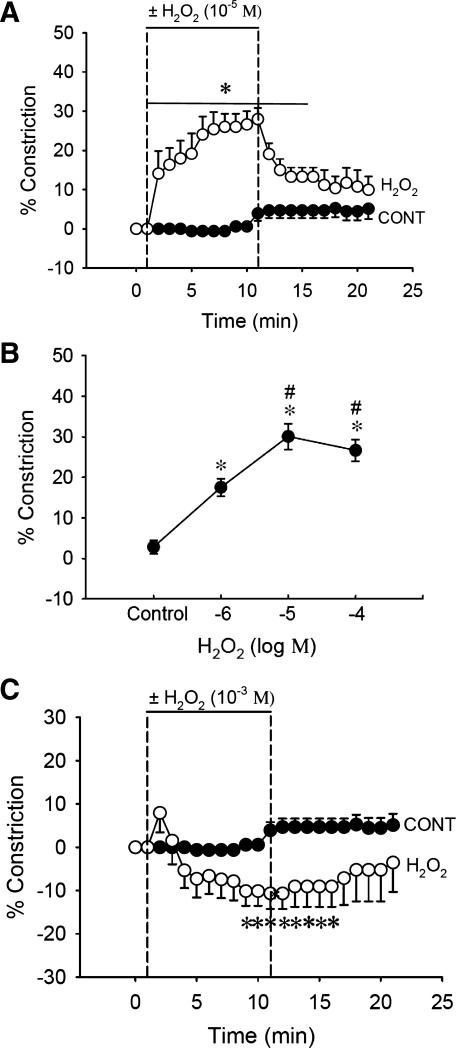
Once generated, O2− can be dismutated by SOD to form H2O2. Given that H2O2 has been reported to act as either a constrictor or a dilator of blood vessels (2, 6, 13, 49), we first examined the effect of exposing microperfused DVR to abluminal H2O2. After a 1-min baseline was obtained, H2O2 was introduced at 10 μmol/l for 10 min and then removed. At that concentration, H2O2 induced sustained vasoconstriction (Fig. 7A, n = 6). In a second protocol, we examined its concentration dependence; H2O2 was added to the bath at 5-min intervals in increasing concentrations (1, 10, 100 μmol/l). H2O2 reversibly constricted DVR in a concentration-dependent manner (Fig. 7B, n = 6). In contrast, at 1 mmol/l, H2O2 acted as a vasodilator (Fig. 7C, n = 6).
Fig. 7.
Effect of H2O2 on DVR vasoactivity. A: H2O2 (10 μmol/l) constricted DVR. After 1-min baseline recording, H2O2 (10 μmol/l, n = 6) or vehicle (n = 5) was added to the bath for 10 min and removed. *P < 0.01, H2O2 vs. vehicle. B: dose-response curve of H2O2 on DVR vasoactivity. After 3-min baseline recording, H2O2 (1, 10, 100 μmol/l, n = 6) was added to the bath in log-molar increments, at 5-min intervals, and then removed. The mean of the final 2 min of data is compared for vehicle and H2O2-treated vessels. H2O2 constricted DVR in a dose-dependent and reversible manner. *P < 0.01, H2O2 vs. control. #P < 0.01, H2O2 (10 or 100 μmol/l) vs. H2O2 (1 μmol/l). C: H2O2 (1 mmol/l) dilated DVR. After 1-min baseline recording, H2O2 (1 mmol/l, n = 6) or vehicle (n = 5) was added to the bath for 10 min and removed. *P < 0.05, H2O2 vs. vehicle.
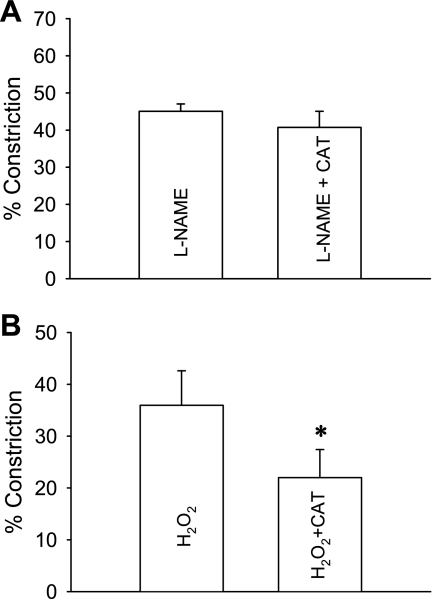
To test whether the vasoconstriction associated with NOS inhibition involves conversion of O2− to H2O2, we examined the effect of converting H2O2 to O2 and water with catalase (Fig. 8). DVR were preconstricted with l-NAME (100 μmol/l) for 10 min after which cell-permeant PEG-catalase (100 U/ml) was added to bath for 10 min and then removed. PEG-catalase did not affect l-NAME-induced vasoconstriction (Fig. 8A, n = 7). As a positive control for the reagent, we verified that PEG-catalase can reverse H2O2 (10 μmol/l) induced vasoconstriction (Fig. 8B, n = 6). These data favor the interpretation that DVR constriction by l-NAME does not rely upon H2O2 generation.
Fig. 8.
Effect of polyethylene glycol (PEG)-catalase on l-NAME-induced vasoconstriction. A: DVR were preconstricted with l-NAME (100 μmol/l) for 10 min after which PEG-catalase (CAT; 100 U/ml, n = 7) or vehicle (n = 5) was added to the bath for 5 min and removed. The mean of the final minute of data is compared for PEG-catalase and vehicle. PEG-catalase had no significant effect on l-NAME-induced constriction. B: as a positive control for PEG-catalase, DVR were preconstricted with H2O2 for 10 min after which PEG-catalase (100 U/ml, n = 6) or vehicle (n = 5) was added to the bath for 5 min and removed. The mean of the final minute of data is compared for PEG-catalase and vehicle. PEG-catalase reversed H2O2-induced constriction. *P < 0.05, PEG-catalase vs. vehicle.
Effect of l-NAME on DVR solute permeability.
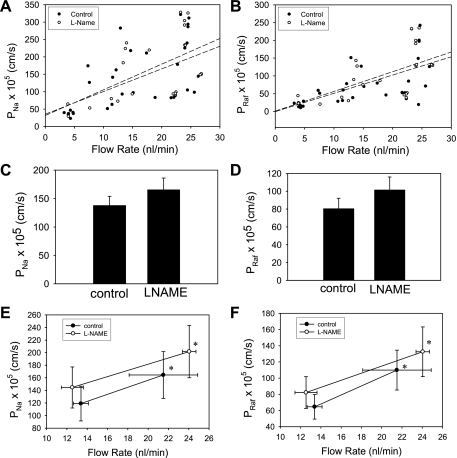
We have previously shown that both diffusive permeability of the DVR wall to hydrophilic solutes (31, 44) and NO generation (52) rise when the rate of luminal perfusion is increased. To determine whether the perfusion-stimulated NO generation plays a causal role in the permeability change, we tested whether l-NAME could prevent it. Figure 9 shows scatter plots of DVR permeability to 22Na (Fig. 9A) and [3H]raffinose (Fig. 9B) as a function of microperfusion rate during exposure to vehicle (filled symbols, 33 measurements, 13 vessels) or l-NAME (open symbols, 22 measurements, 9 vessels). The dashed lines in the figure panels show linear regressions to the vehicle and l-NAME data sets and are nearly overlapping. These results favor the interpretation that the rise in permeability associated with luminal perfusion is unrelated to NO generation. Figure 9, C and D, provides the overall averages of measurements in Fig. 9, A and B, in which means ± SE perfusion rates were 15.8 ± 1.4 (n = 33) vs. 18.2 ± 1.5 (n = 22) for the vehicle and l-NAME groups, respectively. Measurements in which individual DVR were perfused, in random order, at low and high rates in either vehicle or l-NAME (n = 6 vessels/group) are summarized in Fig. 9, E and F. In all cases, independent of the presence of l-NAME, the isotopic permeability was higher when the perfusion rate was increased (P < 0.05).
Fig. 9.
Effect of l-NAME on DVR permeability (P) to 22Na and [3H]raffinose. A and B: scattergram of dual isotope permeability measurements performed during exposure of DVR to vehicle (filled symbols, n = 33) or l-NAME (100 μmol/l, open symbols, n = 22). Dashed lines that show regressions to the vehicle and l-NAME data sets nearly overlap. C and D: bar graphs show summary comparisons of means ± SE of the 22Na and [3H]raffinose permeability measurements from A and B. E and F: paired measurements in which perfusions were performed at low and high rates, in random order, in the same vessel (n = 6 each, vehicle or l-NAME treatment). In the presence or absence of l-NAME, increasing luminal perfusion led to similar rises in permeability to either isotope (*P < 0.05).
DISCUSSION
Blood flow to the renal medulla is subject to tonic regulation by NO (9), and evidence supports an important role for NO generation to preserve perfusion when the medulla is exposed to vasoconstrictors (40, 41, 55). Inhibition of NO generation by intravenous or intramedullary infusion of l-NAME induces hypertension, reduces MFB by 30–40% while sparing changes in cortical blood flow (20, 21, 26). A role for inhibition of NO production in the medulla to induce hypertension has also been supported by the observation that infusion of l-arginine prevents its development (22). Recent studies have shown that inhibition of NOS causes sustained vasoconstriction of renal afferent and efferent arterioles (12). Taken together, these fundamental observations implicate NO as a tonic modulator of renal vascular tone.
Outer medullary DVR are 12- to 15-μm branches of juxtamedullary efferent arterioles that carry blood from the cortex to the medulla of the kidney (34). They are lined by a continuous endothelium and enveloped by contractile pericytes. Based on their relationships within outer medullary vascular bundles, DVR are key intrarenal vascular segments responsible for regulation of regional blood flow in the outer and inner medulla. They are vasoactive and respond to several classes of vasoconstrictors and dilators (5, 30, 38, 39, 48). Many mechanisms for modulation of DVR vasoactivity in vivo seem plausible. Localized interactions between endothelia and pericytes within the vessel wall, involving NO, prostaglandins, endothelins, and endothelium-dependent hyperpolarizing factors are likely to exist. DVR branch from efferent arterioles of juxtamedullary glomeruli, and efferent glomerular blood flow may carry high concentrations of angiotensin II (37) and other vasoactive factors (27, 46). Proximity of vascular bundles to nephrons that reside within the interbundle region suggests the opportunity for feedback control that mitigates hypoxic effects related to reduction of perfusion by vasoconstrictors (8, 10). The DVR endothelium is a syncytium that can conduct electrical signals and diffusive transport of small molecules from distant cells via gap junctions (51). Finally, inner medullary collecting ducts and renal medullary interstitial cells release many vasoactive paracrine agents and autocoids that might conceivably circulate to the outer medulla by countercurrent trapping (32). In summary, it seems likely that DVR tone is regulated by many influences. We focused this study on interactions between NO and ROS intrinsic to the DVR wall.
Other investigators have demonstrated potential for paracrine modulation of DVR tone through interactions between mTAL and DVR pericytes. Fluorescent imaging of outer medullary tissue strips verified transport of NO from adjacent mTAL to vascular bundles. In that preparation, ANG II increased DVR pericyte NO in those vessels that lie adjacent to the mTAL, suggesting an important role for mTAL to influence the level of NO in DVR pericyte cytoplasm (8, 10). In contrast to that extrinsic source of NO, we have shown that isolated DVR generate NO in response to bradykinin, acetylcholine, l-arginine, and luminal pressurization (35, 52). The highest rates of NO generation measured in structures isolated from the medulla occur in inner medullary collecting ducts and vasa recta (47). Based on these observations, we performed the current series of studies to more fully examine the effects of intrinsic NO generation in outer medullary DVR isolated from surrounding structures. The overall results favor the interpretation that intrinsic generation of NO and ROS can substantially influence DVR vascular tone.
The dramatic effects of global NOS inhibition by ADMA and l-NAME provide evidence for intrinsic NO generation in isolated DVR. Specifically, exposure to abluminal l-NAME strongly constricts DVR, reducing luminal diameter by 48.82 ± 4.34% (Fig. 1, A and B). That level of vasoconstriction rivals that achieved by maximal ANG II stimulation (53). Similar constriction occurred when DVR were exposed to the endogenous NOS inhibitor ADMA; luminal diameter fell by 27.91 ± 2.91% (Fig. 1B). Resupply of NO by application of SNP or a cGMP mimetic reversed l-NAME-induced vasoconstriction (Fig. 2, A and B), demonstrating that l-NAME does not interfere with the vasodilatory signaling mechanisms that are activated downstream of NO and cGMP.
ADMA was less effective than l-NAME in contracting DVR. The reason for that is uncertain; however, a number of possibilities exist. Most simply, ADMA might be less efficacious than l-NAME as a NOS inhibitor in these vessels. Other plausible explanations surround its access to the intracellular environment when delivered as an abluminal agent to an in vitro preparation, or its metabolism by dimethylaminohydrolase (DDAH) in the DVR endothelium. ADMA equilibrates with the cytoplasm via cationic amino acid transporters (CATs) (43, 45). Delivery to the cytoplasm might be limited if DVR isoforms of CATs are expressed to limit NOS inhibition and favor preservation of MBF by NO generation. Alternately, for the same purpose, the DDAH2 isoform might be present at a high level to metabolize ADMA (45). DVR vasoactivity is subject to many vasodilatory influences, including prostaglandins (38, 39), cAMP (38), cell-to-cell coupling (51), and activation of inward rectifier K+ channels, implicating K+ as an endothelium-dependent hyperpolarizing factor (3, 4). We cannot rule out the possibility that ADMA activates such pathways by unknown mechanisms to limit its contractile effects. These many hypotheses remain to be tested.
To test which NOS isoform contributes to basal NO generation, we examined the effects of selective NOS isoform inhibitors. Neither the selective nNOS (l-NPA, 1 μmol/l) nor iNOS (l-NIL, 10 μmol/l) inhibitors constricted DVR (Fig. 1C). Immunochemical staining showed that both eNOS and nNOS are expressed in vasa recta (Fig. 3, B and E), but the experiments in Figs. 1 and 2 favor the interpretation that predominant basal NO generation that inhibits the pericyte contractile machinery is derived from eNOS. The current finding that intrinsic NO generation has a strong influence on basal DVR tone contrasts with the small increase in fluorescence observed when explanted DVR have been loaded with the NO-sensitive probe diaminofluoroscein-2 (DAF2) (35, 52). We interpret this as an indication that DAF2 cannot detect NO generation within DVR pericytes and endothelia. One possible explanation is that DAF2 undergoes organelle sequestration away from sites of NO synthesis. In the DVR wall, intrinsic NO may have highly localized effects and a short half-life, particularly due to its interactions with locally generated superoxide and hemoglobin (35, 52–54).
Our studies have shown that DVR generate O2− in response to several stimuli and that Tempol and NAD(P)H oxidase inhibition can affect vasoconstriction by ANG II or PKC activation (52–54). To determine whether O2− is involved in the DVR contractile response to NOS inhibition, we tested whether O2− dismutation by Tempol affects ADMA- or l-NAME-induced contraction. Neither Tempol nor apocynin alone affected basal tone of microperfused vessels (Fig. 4). That finding implies that basal O2− production is probably insufficient to offset large intrinsic NO production. In contrast, Tempol blunted contraction by ADMA (Fig. 4A) and reversed l-NAME preconstriction (Fig. 5A). Reduction of O2− generation by apocynin had similar effects (Figs. 4B and 5B). We interpret those results as consistent with a substantial and important role for basal NO production in these vessels. To test whether l-NAME enhances bioavailability of ROS in the DVR wall, we measured oxidation of DHE to ETH. l-NAME increased the oxidation rate (Fig. 6, A and C), suggesting that either enhanced O2− generation or a decline in O2− consumption occurs during exposure to l-NAME. Elimination of the enhancement of DHE oxidation by Tempol or apocynin (Fig. 6, B and C) reinforces the role of ROS during l-NAME treatment. Consumption of O2− by reaction with NO to form peroxynitrite is a likely explanation for the data.
Once generated, O2− dismutates to H2O2, catalyzed by SOD. Subsequently, H2O2 is converted to water and oxygen by catalase. H2O2 is a cell-permeable, relatively stable, nonradical species produced by both endothelial and smooth muscle cells, and much data point to H2O2 as an important regulator of vasomotor tone (1). However, the effect of H2O2 on vasoactivity is variable. Depending upon concentration, species, vessel type, and details of the experimental protocol, H2O2 may induce either vasodilation or vasoconstriction. For example, catalase blocked Tempol-induced vasodilation of mesenteric arterioles, implicating a H2O2-mediated effect (6). Recent studies have shown that H2O2 regulates MBF (7, 18). An increase in O2− induced by intramedullary infusion of the SOD inhibitor DETC reduced MBF and sodium excretion (56), and chronic DETC administration was shown to induce hypertension (18). Intramedullary administration of catalase along with Tempol prevented DETC-induced hypertension, implicating H2O2 as an important mediator in the process. Direct infusion of H2O2 into the renal medulla was shown to reduce MBF, and coinfusion of H2O2 with catalase abolished the effect (7), favoring the interpretation that medullary H2O2 acts as a vasoconstrictor. To determine whether H2O2 generation from O2− is involved in l-NAME-induced vasoconstriction of isolated DVR, we tested the direct effect of H2O2 and the effect of H2O2 reduction with PEG-catalase. H2O2 caused concentration-dependent vasoconstriction at low concentrations from 1 to 100 μmol/l (Fig. 7, A and B). However, at highest concentration (1 mmol/l), H2O2 was a mild vasodilator (Fig. 7C). Similar results have been reported in other preparations (14).
Renal medullary interstitial H2O2 concentrations have been reported between 50 and 300 nM, and can be two- to threefold higher in hypertension (42). Presumably, local intracellular concentrations might be much greater. Our results are consistent with the interpretation that H2O2 constriction of DVR could be part of the mechanism by which it reduces MBF (Fig. 7, A and B). To confirm the efficacy of PEG-catalase, we showed that H2O2-induced vasoconstriction was reversed by that reagent (Fig. 8). Notably, however, PEG-catalase did not affect l-NAME-induced vasoconstriction, favoring the interpretation that it is mediated by an increase in the concentration of O2− rather than H2O2.
Studies performed in explanted perfused vessels cannot address the relative influences of extrinsic (8, 10) vs. intrinsic (Fig. 1) NO and ROS generation on DVR vasoactivity that exists in vivo. Nonetheless, the current observations show the latter can exert significant tonic effects, particularly when the endothelium is stimulated by the shear and stretch associated with luminal perfusion (52). Red blood cells and hemoglobin provide a sink for metabolism of NO. Thus diffusion of NO from mTAL toward the DVR lumen seems likely to govern the negative slope of a falling concentration gradient directed from the mTAL toward the vascular bundles in the outer medulla. Based on that consideration, localized elevation of NO within the DVR wall, due to its endothelial generation, could hypothetically affect the gradient, thereby tending to raise NO within the mTAL. By that mechanism, vascular-to-tubular communication, i.e., “reverse cross talk,” seems possible. A connection between luminal flow in vasa recta and NO levels in the mTAL (and surrounding collecting ducts) might mechanistically link medullary perfusion with natriuresis.
In the renal medulla, DVR serve dual roles as vasoactive and transporting microvessels that regulate distribution of intramedullary blood flow and accommodate the countercurrent exchange for preservation of corticomedullary osmotic gradients. In other microvessel preparations, NO has been shown to affect microvessel permeability, generally but not always leading to enhancement of solute permeability or hydraulic conductivity (15, 50). This is of interest in the current context because luminal perfusion of DVR leads to marked increases in both NO generation (52) and permeability to hydrophilic solutes (31, 44), implying that an augmentation of permeability might serve to offset the reduction of exchanger efficiency that would otherwise occur when luminal flow rises. A causal link between perfusion-induced NO generation and the flow-induced rise in permeability has not been previously investigated. For that reason, we tested whether blockade of NO generation by l-NAME would prevent it. To accomplish this, we measured lumen-to-bath tracer flux of 22Na and [3H]raffinose at varied perfusion rates in the absence and presence of l-NAME (Fig. 9). Unlike urea, which partly traverses the DVR wall by carrier-mediated transport (29, 33), these tracers permeate via a pathway that is shared with the bulk flow of water, likely the paracellular cleft; we have previously shown that 22Na and [3H]raffinose permeability correlates with hydraulic conductivity of individual vessels (31, 44). A flow-mediated rise in permeability was readily confirmed in controls but was unaffected by inhibition of NO generation with l-NAME (Fig. 9, A and C). We conclude that flow/shear-enhanced permeability of the DVR is not mediated by the parallel rise in NO generation.
In conclusion, l-NAME-induced vasoconstriction of DVR appears to involve a concomitant reduction and increase in NO and O2− concentrations, respectively. H2O2 is a DVR vasoconstrictor but is not responsible for l-NAME-induced vasoconstriction. Our findings show that intrinsic NO and O2− generation within the DVR wall can modulate its basal tone and vasoactivity but does not appear to mediate flow-induced rises in solute permeability. The possibility that generation of NO within the DVR wall might affect ambient NO concentrations in the vicinity of vascular bundles to modify epithelial salt reabsorption cannot be dismissed. Finally, the balance between NO and O2− is consistent with the possibility that its dysregulation may play a role in hypertension (5).
GRANTS
This work was supported by American Heart Association National Scientist Development Grant 09SDG2130035 (to C. Cao) and National Institutes of Health Grants P01 HL78870, R37 DK42495, R01 DK67621 (to T. L. Pallone), and RO1 DK53775 (to A. Edwards).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 231: 237–251, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic monophosphate. Am J Physiol Lung Cell Mol Physiol 261: L393–L398, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Goo JH, Lee-Kwon W, Pallone TL. Vasa recta pericytes express a strong inward rectifier K+ conductance. Am J Physiol Regul Integr Comp Physiol 290: R1601–R1607, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cao C, Lee-Kwon W, Payne K, Edwards A, Pallone TL. Descending vasa recta endothelia express inward rectifier potassium channels. Am J Physiol Renal Physiol 293: F1248–F1255, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Payne K, Lee-Kwon W, Zhang Z, Lim SW, Hamlyn J, Blaustein MP, Kwon HM, Pallone TL. Chronic ouabain treatment induces vasa recta endothelial dysfunction in the rat. Am J Physiol Renal Physiol 296: F98–F106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 293: H2085–H2092, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley AW, Jr, Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol 284: R1355–R1369, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Evans RG, Fitzgerald SM. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14: 9–15, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Feng MG, Navar LG. Nitric oxide synthase inhibition activates L- and T-type Ca2+ channels in afferent and efferent arterioles. Am J Physiol Renal Physiol 290: F873–F879, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto S, Asano T, Sakai M, Sakurai K, Takagi D, Yoshimoto N, Itoh T. Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur J Pharmacol 412: 291–300, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138: 1085–1092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol Heart Circ Physiol 262: H611–H615, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol Heart Circ Physiol 266: H1918–H1926, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Mattson DL, Roman RJ, Cowley AW., Jr Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension 19: 766–769, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Miyata N, Cowley AW., Jr Renal intramedullary infusion of l-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension 33: 446–450, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Mori T, O'Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nabha L, Garbern JC, Buller CL, Charpie JR. Vascular oxidative stress precedes high blood pressure in spontaneously hypertensive rats. Clin Exp Hypertens 27: 71–82, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of l-NAME hypertension in rats. Am J Physiol Regul Integr Comp Physiol 268: R317–R323, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Navar LG. Integrating multiple paracrine regulators of renal microvascular dynamics. Am J Physiol Renal Physiol 274: F433–F444, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Onuma S, Nakanishi K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism 53: 1305–1308, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Pallone TL. Characterization of the urea transporter in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 267: R260–R267, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol 282: F1064–F1074, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Pallone TL, Nielsen S, Silldorff EP, Yang S. Diffusive transport of solute in the rat medullary microcirculation. Am J Physiol Renal Fluid Electrolyte Physiol 269: F55–F63, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: 212–222, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Rhinehart KL, Pallone TL. Nitric oxide generation by isolated descending vasa recta. Am J Physiol Heart Circ Physiol 281: H316–H324, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86: 1352–1357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silldorff EP, Pallone TL. Adenosine signaling in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 280: R854–R861, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Silldorff EP, Yang S, Pallone TL. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer medullary descending vasa recta of the rat. J Clin Invest 95: 2734–2740, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szentivanyi M, Jr, Park F, Maeda CY, Cowley AW., Jr Nitric oxide in the renal medulla protects from vasopressin-induced hypertension. Hypertension 35: 740–745, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Szentivanyi M, Jr, Zou AP, Maeda CY, Mattson DL, Cowley AW., Jr Increase in renal medullary nitric oxide synthase activity protects from norepinephrine-induced hypertension. Hypertension 35: 418–423, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res 60: 448–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner MR, Pallone TL. Hydraulic and diffusional permeabilities of isolated outer medullary descending vasa recta from the rat. Am J Physiol Heart Circ Physiol 272: H392–H400, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res 101: 627–635, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Garvin JL, Falck JR, Ren Y, Sankey SS, Carretero OA. Glomerular cytochrome P-450 and cyclooxygenase metabolites regulate efferent arteriole resistance. Hypertension 46: 1175–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Silldorff EP, Pallone TL. Effect of norepinephrine and acetylcholine on outer medullary descending vasa recta. Am J Physiol Heart Circ Physiol 269: H710–H716, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur J Pharmacol 344: 169–181, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol Heart Circ Physiol 263: H641–H646, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Cao C, Mangano M, Zhang Z, Silldorff EP, Lee-Kwon W, Payne K, Pallone TL. Descending vasa recta endothelium is an electrical syncytium. Am J Physiol Regul Integr Comp Physiol 291: R1688–R1699, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol Renal Physiol 287: F535–F542, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Rhinehart K, Kwon W, Weinman E, Pallone TL. ANG II signaling in vasa recta pericytes by PKC and reactive oxygen species. Am J Physiol Heart Circ Physiol 287: H773–H781, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Rhinehart K, Solis G, Pittner J, Lee-Kwon W, Welch WJ, Wilcox CS, Pallone TL. Chronic ANG II infusion increases NO generation by rat descending vasa recta. Am J Physiol Heart Circ Physiol 288: H29–H36, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Zou AP, Cowley AW., Jr Nitric oxide in renal cortex and medulla. An in vivo microdialysis study. Hypertension 29: 194–198, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001 [DOI] [PubMed] [Google Scholar]