Abstract
Little is known about the residues that control the binding and affinity of thiazide-type diuretics for their protein target, the renal Na+-Cl− cotransporter (NCC). Previous studies from our group have shown that affinity for thiazides is higher in rat (rNCC) than in flounder (flNCC) and that the transmembrane region (TM) 8–12 contains the residues that produce this difference. Here, an alignment analysis of TM 8–12 revealed that there are only six nonconservative variations between flNCC and mammalian NCC. Two are located in TM9, three in TM11, and one in TM12. We used site-directed mutagenesis to generate rNCC containing flNCC residues, and thiazide affinity was assessed using Xenopus laevis oocytes. Wild-type or mutant NCC activity was measured using 22Na+ uptake in the presence of increasing concentrations of metolazone. Mutations in TM11 conferred rNCC an flNCC-like affinity, which was caused mostly by the substitution of a single residue, S575C. Supporting this observation, the substitution C576S conferred to flNCC an rNCC-like affinity. Interestingly, the S575C mutation also rendered rNCC more active. Substitution of S575 in rNCC for other residues, such as alanine, aspartate, and lysine, did not alter metolazone affinity, suggesting that reduced affinity in flNCC is due specifically to the presence of a cysteine. We conclude that the difference in metolazone affinity between rat and flounder NCC is caused mainly by a single residue and that this position in the protein is important for determining its functional properties.
Keywords: diuretics, distal convoluted tubule, salt transport
the renal na+-cl− cotransporter (NCC) is the major transport protein that is expressed in the apical membrane of the distal convoluted tubule, which is located beyond the macula densa. The macula densa is where intratubular fluid chloride concentration is sensed to adjust the glomerular filtration rate by the tubuloglomerular feedback mechanism. Thus, salt handling by NCC escapes this regulatory mechanism that affects the urinary salt excretion and thereby the mean circulatory filling pressure (19). Inactivating mutations of NCC in Gitelman's disease or increased activity of NCC due to dysregulation of the cotransporter by the mutant with no lysine kinases (WNK1 or WNK4) in Gordon's syndrome lead to arterial blood pressure decreases or increases, respectively, demonstrating the importance of NCC activity in blood pressure regulation (8). This role of NCC has been hypothesized for many years because this cotransporter is the target of the thiazide-type diuretics that were introduced into clinical medicine in 1957 (17). Fifty years later, thiazide diuretics are still recommended by the Joint National Committee for the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure as the first line pharmacological therapy for patients with arterial hypertension (3). Consequently, thiazides are one of the most frequently prescribed drugs in the world. Little is known, however, about the residues or domains that control the kinetic properties or specificity for thiazide binding to NCC.
NCC is a protein of 1,002 to 1,028 amino acid residues composed of a central hydrophobic domain that contains 12 putative transmembrane-spanning segments [transmembrane regions (TMs) 1–12]. These segments are interconnected by six extracellular and five intracellular hydrophilic loops. The longer interconnecting segment between TMs 7 and 8 is glycosylated (12) and thus faces the extracellular side. The central hydrophobic domain is flanked by a short amino-terminal domain and a long carboxyl-terminal hydrophilic domain, presumably located within the cell (6) (Fig. 1). Tovar-Palacio et al. (21) determined that the critical residues defining the specificity for thiazide inhibition reside within the central hydrophobic domain by studying a chimeric protein. The chimeric protein contained the transmembrane segments of NCC flanked by the hydrophilic loops of the renal Na+-K+-2Cl− cotransporter (NKCC), which is sensitive to loop diuretics and not to thiazides and behaves in a similar fashion as NCC.
Fig. 1.
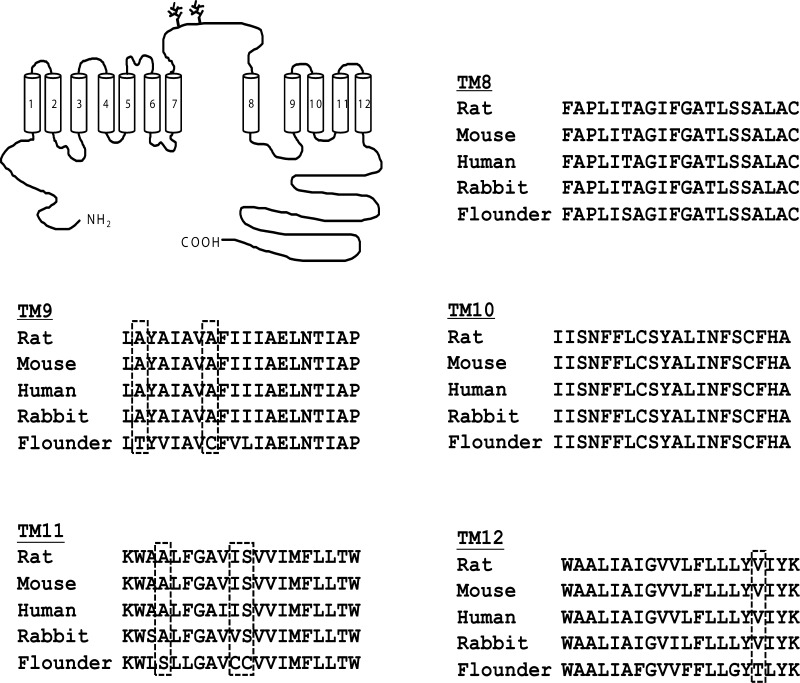
General topology and alignment analysis of transmembrane regions (TM) 8–12 in mammalian and flounder Na+-Cl− cotransporters (NCC). The proposed topology for NCC includes a central hydrophobic domain made up of 12 putative TM segments divided into 2 fragments by a large extracellular glycosylated loop between segments 7 and 8. The central hydrophobic domain is flanked by intracellular short and long amino and carboxyl-terminal domains. Alignment analysis of TM segments 8–12 is shown. Amino acid residues in boxes are those less conserved between flounder and all mammalian sequences and were thus the residues chosen for study.
The functional properties of rNCCs and flNCCs, such as ion transport kinetics and sensitivity to thiazide-type diuretics, are significantly different; the rNCC exhibits higher affinity for ions and diuretics (15, 22). These differences were exploited recently in a study in which multiple chimeric proteins between rNCCs and flNCCs were produced and analyzed at the functional level (16). In that study, it was observed that the difference in thiazide affinity between cotransporters from different species was conferred by the region containing the TM8 to TM12 segments because a chimeric protein in which this segment from flounder was inserted into the rNCC exhibited a flounder-like affinity for metolazone and vice versa. Thus, it was proposed that the affinity-defining residues for thiazides are located within the TM8 to TM12 segments of the cotransporter.
The major goal of the present study was to determine the specific amino acid residues within the TM8 to TM12 segments of rat NCC (rNCC) that are responsible for the different affinity for thiazide between the mammalian and flounder orthologues. To do this, we constructed several mutant NCCs and changed key residues within specific TM segments. The functional properties of the resulting proteins were determined by functional expression in Xenopus laevis oocytes. Our results show that a single amino acid residue, the serine at position 575 of the rat NCC, corresponding to a cysteine at position 576 in flounder NCC (flNCC), explains the difference in thiazide affinity between the mammalian and flounder cotransporters.
METHODS
Xenopus laevis oocyte preparation.
Oocytes were harvested surgically from adult female Xenopus laevis frogs (Nasco) under 0.17% tricaine anesthesia and incubated in ND96 (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl, and 5 HEPES-Tris, pH 7.4) in the presence of collagenase B (2 mg/ml) for 1 h. After four washes in ND96, the oocytes were manually defolliculated and incubated at 18°C in ND96 supplemented with 2.5 mM sodium pyruvate and 5 mg/100 ml gentamicin overnight. The next day, stage V-VI oocytes (5) were injected with 50 nl of water or 20 ng of cRNA/oocyte. Then, the oocytes were incubated for 2 or 3 days in ND96 with sodium pyruvate and gentamicin, which were changed every 24 h. Two hours before the uptake experiments were performed, oocytes were incubated in Cl−-free ND96 (96 mM Na+ isethionate, 2 mM K+ gluconate, 1.8 mM Ca2+ gluconate, 1.0 mM Mg2+ gluconate, 5 mM HEPES, 2.5 mM sodium pyruvate, and 5 mg/100 ml gentamicin, pH 7.4) (7).
Site-directed mutagenesis.
The NCC cDNAs used in this study were the rat and flounder orthologs that we previously isolated from rat kidney (rNCC) (6) and flounder urinary bladder (flNCC) (7). These cDNAs were previously engineered to contain unique silent restriction sites for SacII and HpaI at the beginning of the TM8 segment and at the end of the TM12 segment, respectively (16). Single, double, or triple mutants for TMs 9, 11, and 12 were constructed for these cDNAs using the Quickchange site-directed mutagenesis system (Stratagene), following the manufacturer's recommendations. All mutations were confirmed by automatic DNA sequencing from TM8 to TM12 to avoid unwanted mutations elsewhere, and the SacII to HpaI fragment from each mutant was subcloned into wild-type NCC by gel purification and ligation of the appropriate cDNA band. All primers used for mutagenesis were custom made (Sigma).
In vitro cRNA translation.
cRNA for microinjection was synthesized from wild-type, mutant, or chimeric cDNAs previously digested at their 3′ end using NotI from New England Biolabs, using the T7 RNA polymerase mMESSAGE mMACHINE (Ambion) transcription system. cRNA integrity was confirmed using agarose gels, and the concentration was assessed by absorbance reading at 260 nm (DU 640; Beckman, Fullerton, CA). cRNA was stored frozen in aliquots at −80°C until use.
Transport assays.
The activity of the NCC was determined by assessing 22Na+ tracer uptake (New England Nuclear) in groups of 10–15 oocytes, following our protocol (15). Briefly, a 30-min incubation in a Cl−-free ND96 medium containing 1 mM ouabain, 0.1 mM amiloride, and 0.1 mM bumetanide was followed by a 60-min uptake period in a K+-free NaCl medium (40 mM NaCl, 56 mM Na-gluconate, 4.0 mM CaCl2, 1.0 mM MgCl2, and 5.0 mM HEPES-Tris, pH 7.4) containing ouabain, amiloride, bumetanide, and 2 μCi of 22Na+/ml. Because Xenopus laevis oocytes do not express a thiazide-sensitive NCC (7, 15), only one group of water-injected oocytes was included in every experiment to determine the basal, nonspecific trace 22Na+ uptake. The affinity for thiazide diuretics was assessed by exposing groups of cRNA-injected oocytes to increasing concentrations of drug (from 10−9 to 10−4 M). The desired concentration of the diuretic was present in both the incubation and uptake periods. All metolazone dependency curves were assessed at least twice for each clone. Ion transport kinetics were determined by performing experiments with varying concentrations of Na+ and Cl−. Isethionate was used as a Cl− substitute and N-methyl-d-glucamine as a Na+ substitute to preserve osmolarity and ionic strength.
All uptake experiments were performed at 32°C. At the end of the uptake period, oocytes were washed five times in ice-cold uptake solution without isotope to remove tracer in the extracellular fluid. After oocytes were dissolved in 10% sodium dodecyl sulfate, the tracer activity was determined for each oocyte by β-scintillation counting.
Western blotting.
Western blot analysis was used to assess the amount of protein expression in wild-type or mutant NCC-injected oocytes. Expression of NCC was determined as FLAG-NCC because our cDNA contains a FLAG epitope after the first methionine (16). Proteins extracted from 50 oocytes injected with wild-type or mutant NCCs were quantified by Bradford's technique, and 50 μg of each protein per lane was run using sample buffer containing 6% SDS, 15% glycerol, 0.3% bromophenol blue, 150 mM Tris, pH 7.6, and β-mercaptoethanol (when indicated), resolved by Laemmli SDS-polyacrylamide (7.5%) gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. Immunoblotting was performed using anti-FLAG monoclonal antibody (Sigma). Membranes were exposed to anti-FLAG antibody overnight at 4°C, washed and incubated for 60 min at room temperature with alkaline phosphatase-conjugated secondary (anti-mouse) antibody (Bio-Rad) diluted 1:2,000 in blocking buffer, and washed again. Bands were detected by using Immun-Star Chemiluminescent Protein Detection Systems (Bio-Rad).
Data analysis.
All results presented are based on a minimum of three different experiments with ≥12 oocytes/group in each experiment. Results are presented as means of uptake values within groups ± SE unless stated otherwise. Prism 5.0 was used to fit the kinetic data. Chloride and sodium km values were determined by fitting the uptake data with the Michaelis-Menten equation. For thiazide, nonlinear fittings were done using the Hill equation, from which the IC50 values shown in the text were obtained. In all cases, a Hill coefficient close to unity was observed.
RESULTS
Single amino acid residue differences between mammalian and flNCC.
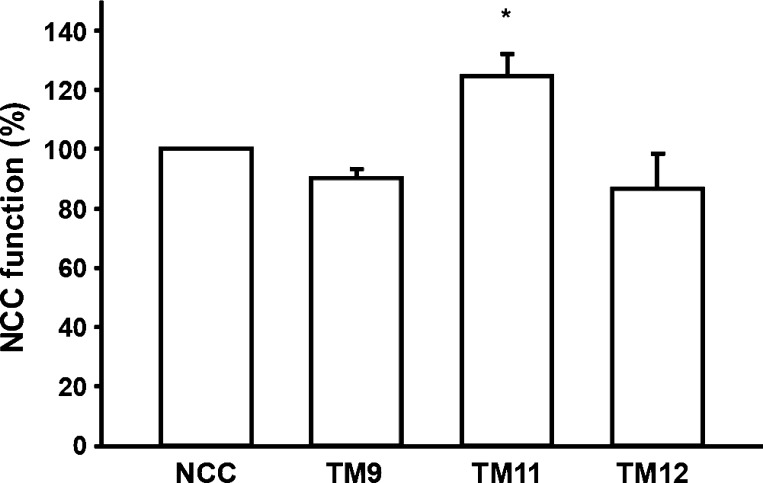
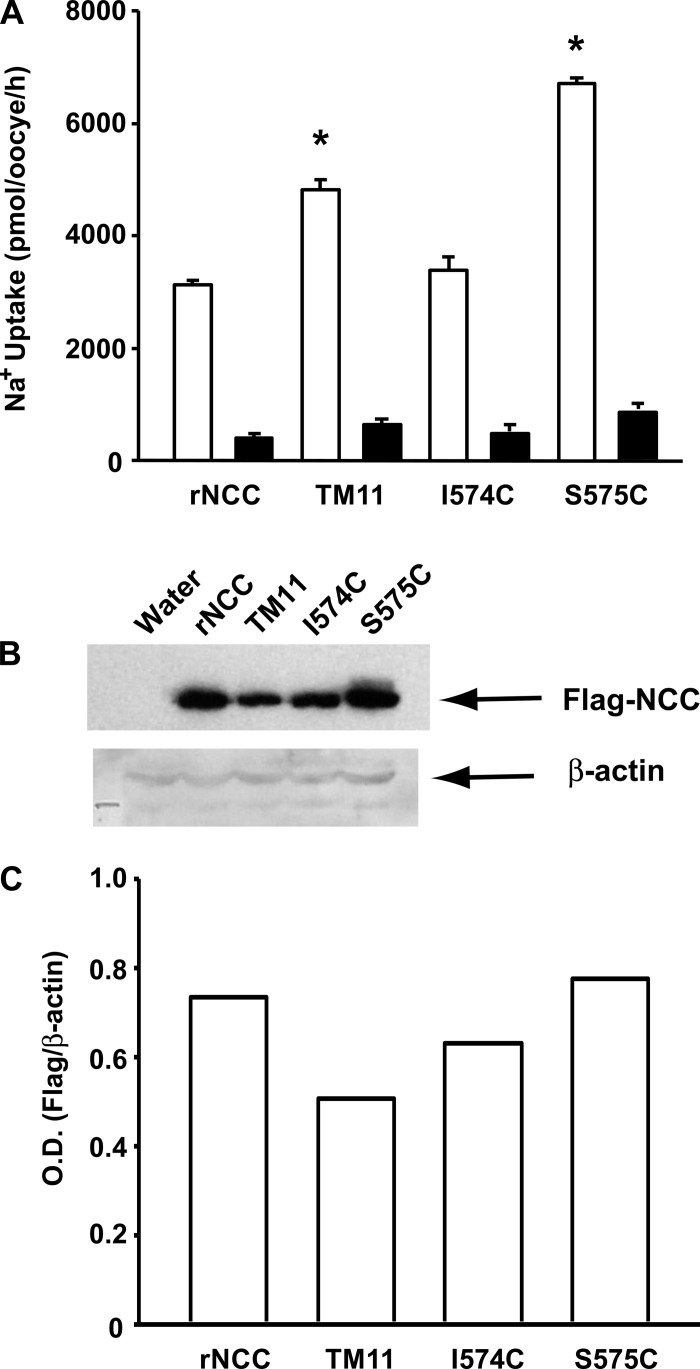
We have shown previously that the difference in thiazide affinity between rat and flNCC is encoded within the TM segments 8 to 12 (16). In addition, we have also observed that thiazide affinity is similar between rat and mouse NCCs (18). Thus, we reasoned that the difference in thiazide affinity between mammalian and flNCCs could be due to residue differences between them. We performed an alignment analysis of NCC TMs 8 to 12, using all of the available NCC sequences to find the residues that are identical in mammalian orthologs but different in the flounder, with a change between mammalian and flounder that is not conservative. As shown in Fig. 1, we found only six amino acid residues with these characteristics. The two located in TM segment 9 are the alanine residues 510 and 516, which correspond to threonine and cysteine, respectively, in flounder. The three located in TM segment 11 are alanine, isoleucine, and serine at positions 568, 574, and 575, which correspond to serine, cysteine, and cysteine, respectively, in the flNCC. Finally, a valine at position 601 in the rNCC TM12 is substituted by a threonine in the flNCC. No such differences were found in TM8 or TM10, which are almost identical between the mammalian and flNCCs. Thus, by site-directed mutagenesis, we introduced the single-point mutations to change one TM segment at a time from rNCC to flNCC. Three mutant clones were generated: mutant TM9 (A510T and A516C), mutant TM11 (A568S, I574C, and S575C), and mutant TM12 (V601T). As shown in Fig. 2, all three mutant cotransporters were functional, since they exhibited a thiazide-sensitive 22Na+ uptake that was similar to that observed for rNCC. Of note, functional expression observed in the TM11 mutant was significantly higher than in oocytes injected with any of the other clones.
Fig. 2.
Mutant constructs for TM9, TM11, and TM12 are functional. Percentage of rat NCC activity in wild-type and mutant NCC. The thiazide-sensitive 22Na+ uptake in wild-type NCC was set as 100%, and the values observed in mutant clones were normalized accordingly. At least 5 different 10-oocyte experiments were done. Normalization of data was done for each experiment before the groups were compared. The data shown represent the mean ± SE of 5 different experiments. *P < 0.05 vs. wild-type NCC.
The difference in thiazide affinity between rNCC and flNCC resides in TM11.
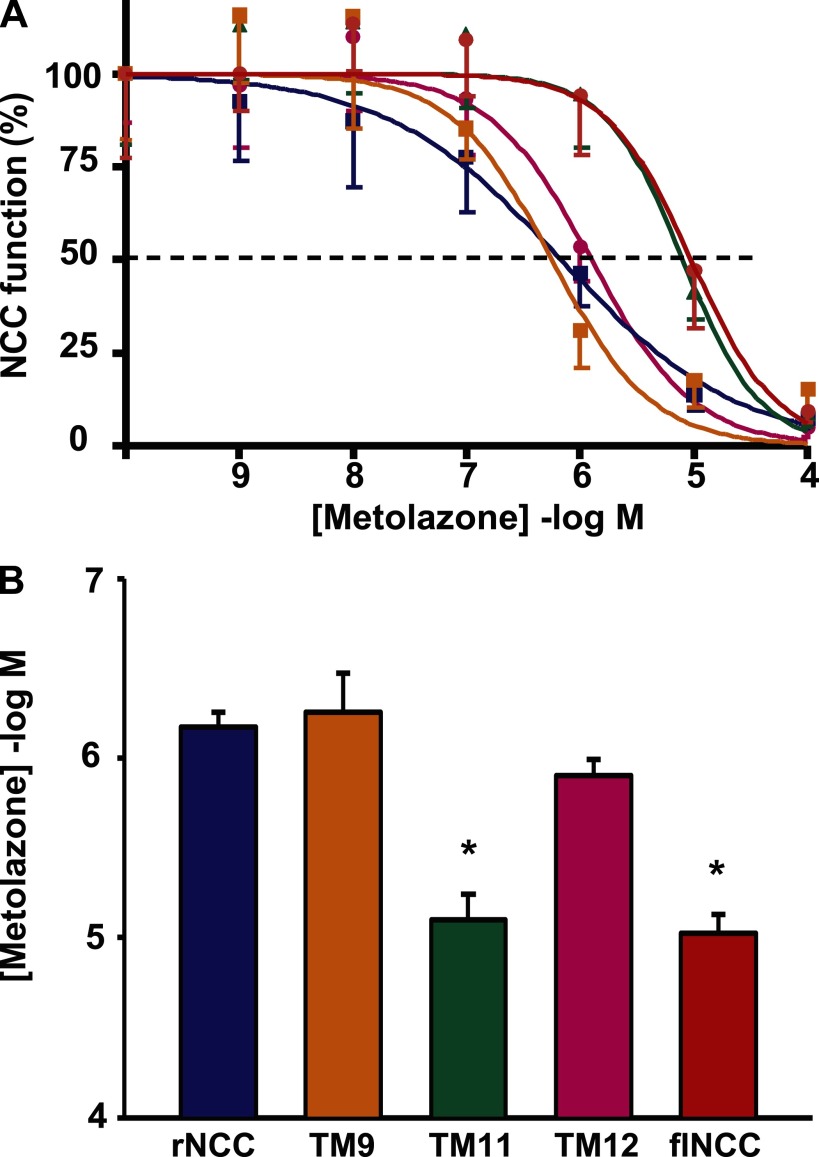
rNCCs and flNCCs exhibit a difference in thiazide affinity of approximately one order of magnitude (15, 16, 22). Thus, in the present study, we assessed the thiazide dose-response curves in oocytes injected with wild-type rNCC or flNCC cRNA as well as oocytes injected with mutant NCC cRNAs at TM9, TM11, or TM12. The thiazide affinity for all clones was assessed in the same experiment to assure that the oocytes, solutions, and metolazone concentration were identical among groups. Figure 3 shows the compilation of five different experiments. As shown previously (16), rNCC (in blue) and flNCC (in red) display a significant difference in affinity for metolazone, which is of about one order of magnitude. Interestingly, the dose-response curves for the rNCC-TM9 mutant (orange) and for the rNCC-TM12 mutant (pink) were similar to that of rNCC. In contrast, the affinity for metolazone in oocytes injected with the rNCC-TM11 mutant cRNA (green) was identical to that observed in oocytes injected with flNCC cRNA. These observations strongly suggested that the difference in metolazone affinity between rNCCs and flNCCs is encoded in TM11 and that one to three amino acid residues could be responsible.
Fig. 3.
The effect of TM mutations on rat NCC (rNCC) affinity for metolazone. Metolazone dose response analysis was assessed in 5 different experiments, in all of which the 5 different clones were tested simultaneously, using the same uptake solutions and metolazone dilutions. A: a compilation of the dose-response curves from the 5 experiments is shown. Wild-type rNCC (blue), wild-type flounder NCC (flNCC; red), TM9 mutant (orange), TM12 mutant (pink), and TM11 mutant (green). Data were fit to the Hill equation, and in all cases Hill coefficients close to unity were obtained. All group data are shown as means ± SE of %activity. The coefficient of determination (r2) was between 0.95 and 0.99 for the fits shown. B: metolazone IC50 for rNCC and mutants calculated from the nonlinear regression fit of uptake data to the Hill equation. Data are shown as mean IC50 ± SE of 5 different experiments. *P < 0.05 vs. rNCC.
rNCCs and flNCCs also exhibit a significant difference in affinity for Na+ and Cl− ions. Our recent observations suggested that the difference in chloride affinity is encoded within TM segments 1–7, whereas no specific domain seems to be responsible for the difference in sodium affinity (15, 16, 22). We thus analyzed the effect of TM9, TM11, and TM12 mutations for affinity to cotransported ions. The results are shown in Table 1. As expected, the sodium and chloride transport kinetics of all three mutants are similar to each other and to that observed for wild-type rNCC, indicating that these residues play no role in defining transport ion kinetics.
Table 1.
Ion transport affinities in rNCC, flNCC, and mutant clones
| rNCC | TM9 | TM11 | TM12 | flNCC | |
|---|---|---|---|---|---|
| Na+ km, mM | 6.95 ± 1.8 | 9.2 ± 1.5 | 7.7 ± 1.3 | 8.6 ± 1.3 | 31 ± 4.3 |
| Cl− km, mM | 5.25 ± 2.0 | 4.1 ± 1.3 | 4.8 ± 1.0 | 3.3 ± 1.2 | 14 ± 1.3 |
Values are means ± SE. rNCC, rat Na+-Cl− cotransporter; flNCC, flounder Na+-Cl− cotransporter; TM, transmembrane region.
A serine to cysteine change in TM11 is responsible for the difference in thiazide affinity between rNCCs and flNCCs.
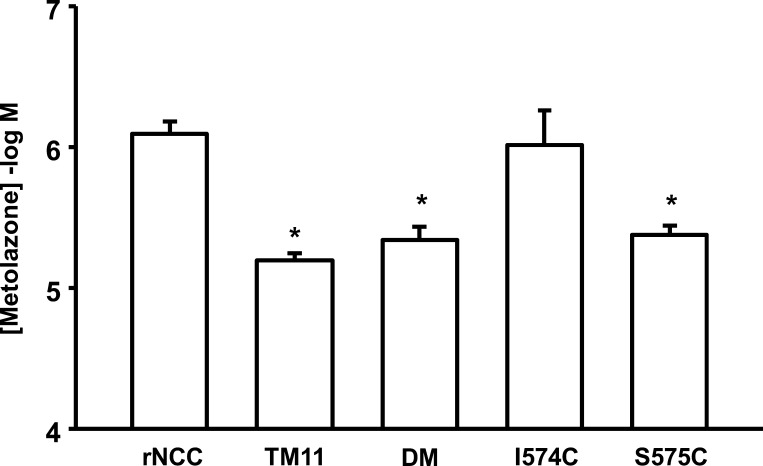
Because thiazide affinity between wild-type rNCC and rNCC-TM9 or rNCC-TM12 mutants was similar, no additional analysis was done for these clones. In contrast, rNCC harboring three mutations in TM11, which correspond to the flNCC sequence, exhibited an affinity for metolazone that was identical to that in flNCC. Therefore, we further explored the role of each of these amino acid residues. Two of these mutations in TM11 switched the original amino acid for a cysteine residue (I574C and S575C). Cysteines are special residues because they can establish strong covalent interactions that can affect the structural and functional properties of proteins; therefore, we decided to study these two residues first. We used site-directed mutagenesis to create a double mutant rNCC that harbors both the I574C and S575C mutations. In addition, we made two rNCC single mutants containing either the I574C or S575C mutation alone. The effect of these mutations on metolazone affinity was determined. Figure 4 depicts the mean of the IC50 values obtained from five different dose-response curves that were assessed simultaneously in oocytes injected with rNCC-TM11, the double mutant I574C S575C, or the single mutants I574C or S575C. Similar to the dose-response curves shown in Fig. 3, rNCC-TM11 shifted the metolazone affinity to the right by about one order of magnitude (P < 0.05 vs. rNCC). A similar shift was observed in oocytes injected with double mutant or the single mutant S575C. In contrast, the I574C mutation had no effect on thiazide affinity because the IC50 in oocytes injected with this mutant cRNA was identical to that shown for wild-type rNCC.
Fig. 4.
The effect of individual mutations in the rNCC TM11 segment on the affinity for metolazone. Methods are similar to those explained in Fig. 3, and 5 different experiments were performed. The bars represent mean IC50 ± SE for each tested clone, as stated. For each experiment, all Hill slopes were ∼1 and coefficients of determination (r2) were between 0.92 and 0.99. *P < 0.05 vs. rNCC.
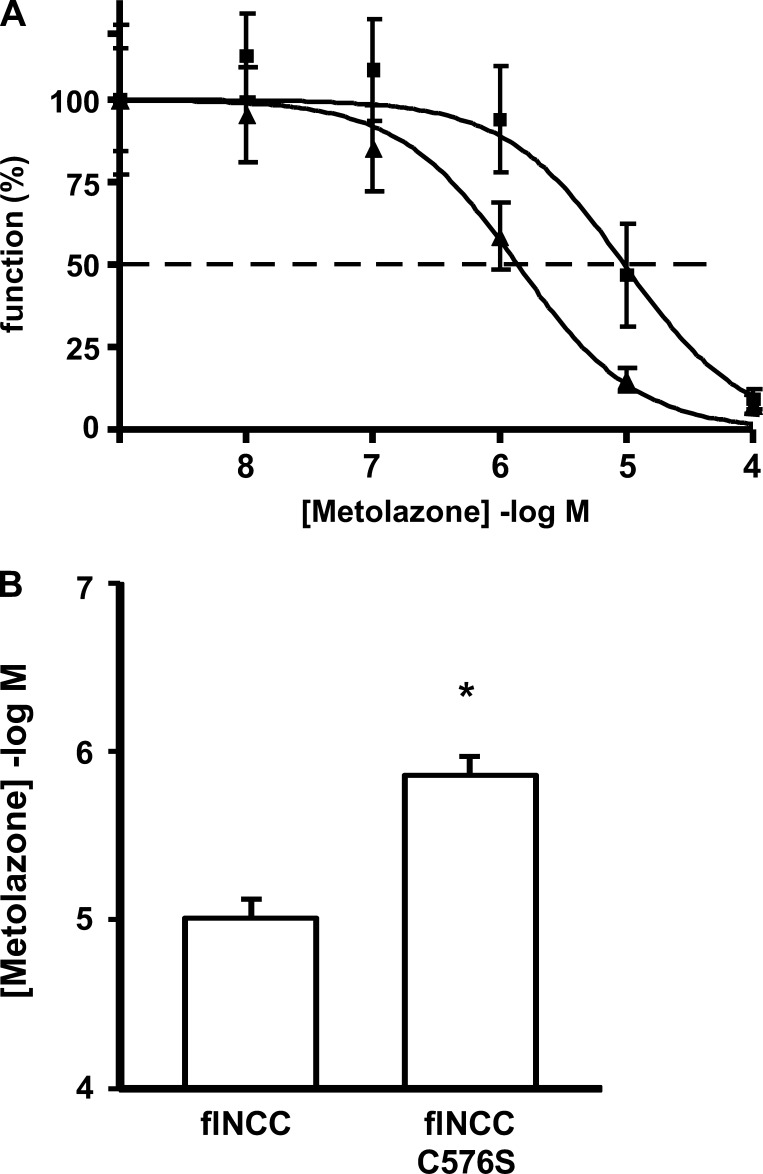
If the serine vs. cysteine substitution at position 575 in rNCC or 576 in flNCC is responsible for the difference in affinity for metolazone between species, one would expect that this mutation should be enough to switch the affinity for metolazone in flounder to an IC50 similar to that observed in wild-type rNCC. We thus introduced by site-directed mutagenesis the C576S mutation into flNCC cDNA. Metolazone dose-response curves were performed in three different experiments. A representative experiment is shown in Fig. 5A, and the mean IC50 values from three different experiments are shown in Fig. 5B. As expected, the affinity for metolazone was increased significantly by the C576S substitution in flNCC.
Fig. 5.
The effect of TM mutations in flNCC on the affinity for metolazone. Metolazone dose-response analysis was assessed as explained in Fig. 3. Similar observations were done in 3 different experiments. A: a representative experiment is shown. Dose-response curves are shown for wild-type flNCC (■) and C576S-flNCC (▲). The coefficient of determination (r2) for wild-type flNCC and C576S-flNCC was 0.96 and 0.98, respectively. B: metolazone IC50 for flNCC and C576S mutant was calculated from the nonlinear regression fit of uptake data to the Hill equation. Data are shown as mean IC50 ± SE of 3 different experiments. *P < 0.05 vs. flNCC.
We have observed previously that, whereas rNCCs and flNCCs exhibit a difference in affinity for thiazide-type diuretics, the profile of inhibition among different thiazides is similar (15, 22). The observed inhibition is greatest for polythiazide, followed by metolazone, bendroflumethiazide, trichloromethaizide, hydrochlorothiazide, and chlorthalidone. This inhibitory profile is similar to thiazide potencies in clinical medicine as well as their potency to block the [3H]metolazone binding to renal cortical membranes (1). We assessed the thiazide inhibitory profile of mutant S575C. For all thiazides tested, the IC50 in S575C was shifted to the right compared with rNCC (data not shown). In addition, the inhibitory profile observed was similar to that previously seen for rNCC (15) or to that observed in simultaneous experiments. Thus, substitution of serine 575 to cysteine in rNCC similarly affected the affinity for all thiazide-type diuretics tested.
The serine-to-cysteine substitution affects the NCC basal activity.
In the experiments described above, we noticed that functional expression of the rNCC-TM11 mutant was always higher than wild-type NCC by ≥20%. Thus, we assessed the 22Na+ uptake in oocytes injected with the same amount of cRNA for wild-type NCC, rNCC-TM11 mutant, or single mutants I574C and S575C rNCC, as determined by densitometry of the cRNA bands in agarose gels. A representative experiment is shown in Fig. 6A. The 22Na+ uptake in oocytes injected with the rNCC-TM11 mutant or S575C mutant rNCC cRNA was significantly higher than that observed in wild-type rNCC. In contrast, the activity of the I574C cotransporter was similar to wild-type rNCC. Proteins from the same oocytes used in the experiment shown in Fig. 6A were extracted and used for Western blot analysis. The results of this experiment and the corresponding densitometric analysis are shown in Fig. 6, B and C. No increase in protein expression of TM11 or S575C that would explain the higher 22Na+ uptake in these mutants was observed. Together, these observations suggest that the difference in basal activity between the rNCC-TM11 or S575C mutants and wild-type NCC cannot be explained by changes in protein expression. Therefore, S575C increases the tonic activity of the cotransporter by improving either its surface expression or its translocation rate. Testing these possibilities is beyond the scope of this study.
Fig. 6.
TM11 mutant rNCC and the single mutant S575C-rNCC exhibited higher activity than wild-type NCC or I574C-rNCC. Oocytes were injected with the same amount of cRNA of each clone, as explained in the text. A: 3 days later the 22Na+ uptake was assessed in the absence (open bars) or presence (filled bars) of metolazone. Data presented are means ± SE of the uptake observed for each oocyte. Similar observations were done in at least 5 experiments. *P < 0.05 vs. control wild-type rNCC. B: proteins were extracted for a Western blot analysis using anti-FLAG or anti-actin antibody. A representative Western blot is shown in B. C: densitometric analysis of bands observed in the Western blot. OD, optical density.
The decrease of thiazide affinity in rNCC is specific for a cysteine substitution.
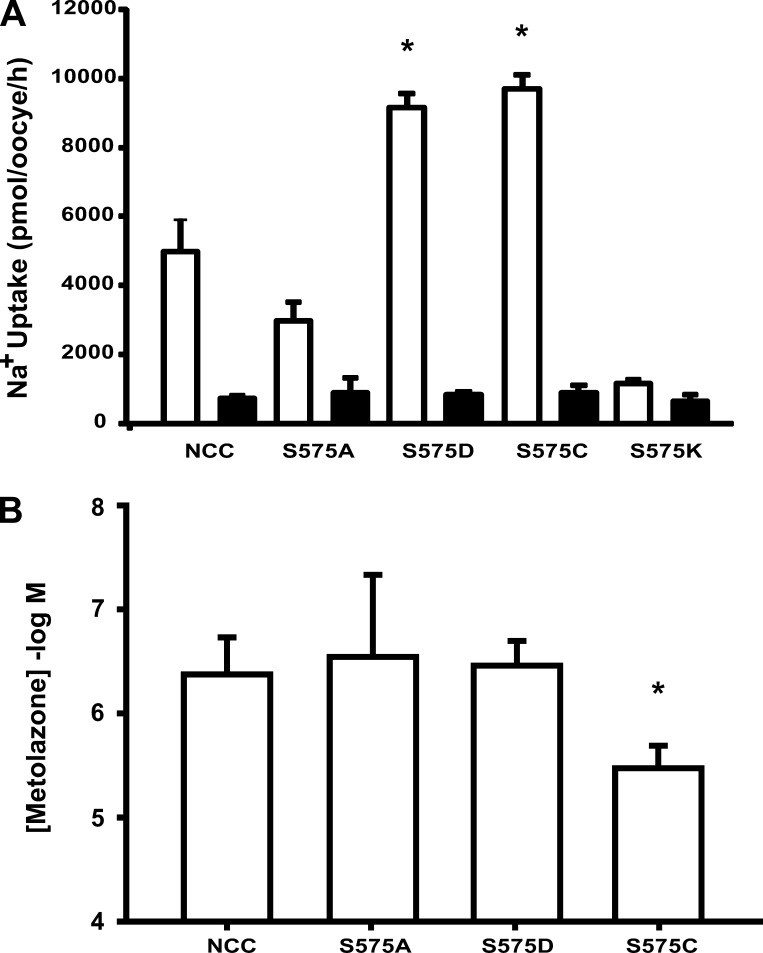
We have shown that substituting serine for cysteine in rNCC or cysteine for serine in flNCC switched the cotransporter affinity from rat to flounder like and vice versa. We wanted to know whether the decreased thiazide affinity in rNCC-S575C is specifically due to the introduction of the cysteine and, thus, probably related to its capacity to establish disulfide bonds. Another possibility is that this phenomenon could be due to a change in the local environment of this residue produced by the different physical and chemical properties of serine and cysteine. Sulfur and oxygen atoms have different electronegativities, so the polarity of these chemical groups is not identical, with hydroxyls having a higher electronegativity. This produces a weak H-bonding propensity in the sulfhydryl groups of cysteines. In addition, the proton in the sulfhydryl groups of cysteines is much more acidic (pKa 8.18) than the hydroxylic proton of serine. All of these different properties could explain the difference in affinity if, for example, a hydrogen bond established with the thiazide or another residue in the protein is stronger in the presence of a serine. With this in mind, we constructed three more mutants in which serine 575 of rNCC was substituted for a positive, a negative, or a neutral amino acid residue, namely lysine, aspartate, or alanine, respectively. We reasoned that positive and negative charges could establish an electrostatic interaction that would resemble a hydrogen bond formed by the cysteine/serine and that the alanine would prevent the formation of any interaction. However, none of the introduced residues would favor the formation of disulfide bonds. As shown in Fig. 7A, substitution of serine for lysine resulted in a nonfunctional cotransporter. In contrast, substitution of serine 575 with alanine or aspartate produced rNCC mutants that exhibited enough 22Na+ uptake to determine a dose-response curve for metolazone. As shown in Fig. 7B, affinity for metolazone was similar in oocytes injected with wild-type rNCC, rNCC-S575A, and rNCC-S575D but different from that observed in rNCC-S575C. These observations suggest that it is specifically a change for cysteine in position 575 that makes rNCC less sensitive to metolazone.
Fig. 7.
Functional expression and metolazone affinity of mutant rNCCs S575C, S575A, S575D, and S575K. Oocytes were injected with cRNA from each mutant clone. A: 3 days later, the 22Na+ uptake was assessed in the absence (open bars) or presence (closed bars) of 100 μM metolazone. Data are means ± SE. B: metolazone IC50 values for rNCC and mutants were calculated from the nonlinear regression fit of uptake data to the Hill equation. Data are shown as mean IC50 ± SE of 3 different experiments. *P < 0.05 vs. rNCC.
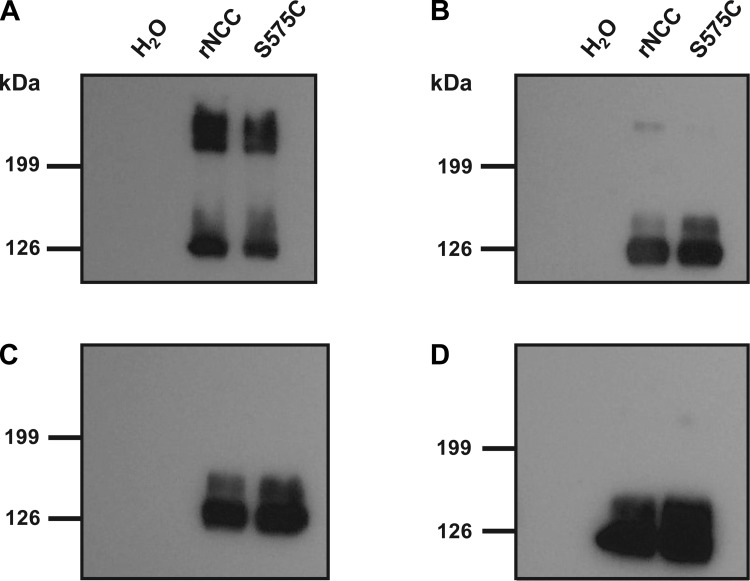
It has been suggested that NCC functions as a homodimer, but it is not clear whether the interaction between monomers involves the formation of disulfide bonds (4). If this type of bond is not present between NCC homodimers, then a cysteine difference between rNCC and flNCC could be allowing the flounder cotransporter to form a disulfide bond between monomers that is not present in the mammalian cotransporter. To determine whether disulfide bonds are present in NCC dimers and to find out whether this is affected by S575C substitution in rNCC, we performed a Western blot analysis of proteins extracted from Xenopus laevis oocytes microinjected with water, wild-type rNCC, or S575C-rNCC cRNA. Protein extracts were prepared for standard SDS-PAGE with Laemmli buffer with or without reducing agent (5% β-mercaptoethanol). As shown in Fig. 8, in the absence of reducing agent, bands of expected size for NCC dimers and monomers are observed in both wild-type NCC and S575C-NCC. When a reducing agent was used, single bands of the expected size for the NCC monomer were present, whereas the bands corresponding to the dimer size were absent. This finding suggests that disulfide bonds are involved in NCC dimerization. However, no difference was observed between wild-type rNCC and the S575C NCC mutant. As shown in Fig. 8, to rule out the possibility that mutant rNCC becomes slightly resistant to the reducing agent because of the presence of an extra disulfide bond, we repeated the experiment using β-mercaptoethanol at different concentrations. No difference between wild-type and mutant NCC was observed at any point.
Fig. 8.
Electrophoretic behavior of rNCC and S575C-rNCC in the absence or presence of a reducing agent. Protein extracts from oocytes injected with water, rNCC cRNA, or rNCC-S575C cRNA were diluted for standard SDS-PAGE in Laemmli buffer without reducing agent (A) or containing either 0.5 (B), 2.5 (C), or 5% β-mercaptoethanol (D). In the absence of reducing agent (A), bands of expected size for monomeric and dimeric forms of the cotransporter were observed, and in both cases a diffused signal of slightly higher molecular weight was detected, which probably corresponds to glycosylated forms of the protein. This was seen for the wild-type and mutant form of rNCC. In the presence of reducing agent, the bands corresponding to the dimeric form of the cotransporter disappeared even at the lowest concentration of reducing agent used. At this concentration, we were able to observe a slight signal corresponding to the dimers, but no difference in intensity was observed between the wild type and the mutant.
DISCUSSION
In the present study, we show that substituting serine 575 in rNCC for the cysteine present in flNCC at the same position is sufficient to render rNCC more resistant to thiazides, exhibiting a flounder-like affinity. Supporting these observations, substituting the cysteine 576 in the flNCC for the corresponding serine in the rNCC increased the affinity of flNCC to a mammalian-like IC50. Therefore, a single residue difference in TM11 segment between rNCC and flNCC explains the difference in affinity for thiazides between species. In addition, we observed that substituting the serine 575 in rNCC with residues other than cysteine, such as alanine or aspartate, had no effect upon affinity for thiazides, suggesting that it is not the presence of the serine in this position that increases affinity, but instead it is the absence of a cysteine residue that is present in flNCC. Although it is unknown whether loop diuretics or thiazides bind to a similar region in NKCCs and NCC, our observations are supported by previous work on chimeric proteins between shark and human NKCC1 that suggests roles for transmembrane segments 11 and 12 (13).
Two different possible mechanisms can potentially explain the decrease in affinity for metolazone observed in the NCC-S575C mutant. The first mechanism is that this residue may be part of the thiazide-binding site on the cotransporter, and the second mechanism is that substitution of S575C may modify the tridimensional configuration of the protein, indirectly affecting the interaction of thiazides with their binding site or the protein's response upon drug binding. Our results favor the second possibility because, if serine 575 were part of the thiazide-binding site, it would be expected that substituting the serine with different amino acid residues would result in altered affinity for thiazide. However, our data show (Fig. 7) that changes in affinity occur only when serine is substituted with cysteine, suggesting that it is a unique property of cysteines that decreases the thiazide affinity in mutant rNCC-S575C.
Cysteines are very important residues in the tridimensional structure of proteins because they form disulfide bonds. Two types of interactions can be established by the sulfhydryl group in their side chain, hydrogen bonds and disulfide bonds (10). In NCC, the establishment of a hydrogen bond by this cysteine probably would not generate a significant difference in terms of structure, compared with the structure of the wild-type cotransporter, because the serine originally present in rNCC also possesses the ability to form hydrogen bonds. Thus, a plausible possibility is that the substitution of serine 575 for cysteine may introduce a new covalent bond, changing the tertiary structure of NCC. This substitution could reduce the access of thiazides to their binding site located elsewhere, introduce a structural change that allosterically affects the binding site for the inhibitor, or affect the protein's response upon drug binding. According to the hydropathy predictions (6), serine 575 lies approximately in the middle of TM11 segment. A model for the putative configuration of the α-helix formed by TM11 predicts that serine 575 is located in a hydrophilic face of the helix (Supplemental Fig. S1; Supplemental Material for this article can be found on the AJP-Renal Physiology web site), implying that this face could be involved in contacts with other protein domains and not facing the membrane, thus making it possible that changing the serine for cysteine creates the possibility of a disulfide bond formation with another residue in a membrane domain of the same polypeptide or of another NCC monomer. There is evidence that NCC forms homodimers that may be the functional form of the cotransporter; however, the TM segments involved in establishing the interface between monomers are unknown. Although no crystal structure of any member of the SLC12 family is available, the crystal structures of transporters of the APC (amino acids, polyamine, organocation) transporter superfamily, in which the SLC12 family is included, have been reported (9, 20). Two of these transporters, as detailed in the legend of Supplemental Fig. S1, present a generally similar folding pattern, with segments 1–10 forming the core structure of the cotransporter that comprises the ion translocation pathway and TM11 and -12 lying outside as accessory segments. This general folding pattern is not only conserved in APC transporters but is also present in a more distantly related bacterial leucine cotransporter, LeuT (2, 11, 23). Thus, expecting similarities, at least at a general level of structure, between NCC and these transporters is sound. This finding is supported by a recent thorough analysis of the structural conservation among sodium transporters (14). Continuing this line of reasoning, the residue that was found to alter NCC affinity for thiazides would be located in an accessory TM segment. However, it is not possible to speculate on its exact position within this segment. AdiC, one of the crystallized APC transporters, has a dimeric structure in which TM segments 11 and 12 lie outside the core structure and form the interface between the dimers. LeuT also forms dimers, and there is evidence that, in the eukaryotic homologs of this cotransporter, TM11 participates in dimerization. Therefore, the cysteine in position 576 of flNCC could be forming an interaction either with another residue within the same polypeptide chain or located in the second monomer of the NCC homodimer.
Considering the possibility that substituting a serine for cysteine at position 575 in rNCC could be mediating an interaction between NCC monomers, we performed a western blot analysis of wild-type and mutant rNCC-S575C in the absence or presence of a reducing agent. The existence of NCC homodimers has been demonstrated previously (4), but that disulfide bonds are involved in the interaction between NCC monomers has never been explored. Thus, we reasoned that the new cysteine introduced could perhaps be creating a new covalent bond between subunits that was originally nonexistent. If this were the case, and if there are no disulfide bonds between wild-type rat NCC monomers, we would expect to see bands corresponding to the homodimers in the absence of a reducing agent only in the mutant protein. However, the result shown in Fig. 8 suggests that wild-type rNCC forms homodimers in which disulfide bonds are involved, and no difference is observed between the wild-type and mutant NCCs. Also, no difference in resistance to the effects of the reducing agent was observed between the wild-type and the mutant clones when different concentrations of β-mercaptoethanol were used. This finding suggests that no additional intermolecular bonds are formed when serine 575 is switched for a cysteine. However, this observation does not rule out the possibility that this cysteine may be forming a new disulfide bond, perhaps altering the original pattern of disulfide bonds formed between NCC subunits.
In summary, we propose that the difference in thiazide affinity observed between mammalian and flNCCs is due mainly to a single amino acid residue difference between cotransporters. This residue is located within the transmembrane segment 11. Further investigation will be required to define the mechanism by which substitution of a cysteine in flNCC for a serine in rNCC increases the affinity for thiazides.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-64635 to G. Gamba and Mexican Council of Science and Technology Grants 59992 to G. Gamba and 104451 to E. Moreno.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all members of the Molecular Physiology Unit for suggestions and stimulating discussion.
REFERENCES
- 1. Beaumont K, Vaughn DA, Fanestil DD. Thiazide diuretic drug receptors in rat kidney: identification with [3H]metolazone. Proc Natl Acad Sci USA 85: 2311–2314, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch 447: 519–531, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003. [DOI] [PubMed] [Google Scholar]
- 4. De Jong JC, Willems PH, Mooren FJ, van den Heuvel LP, Knoers NV, Bindels RJ. The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J Biol Chem 278: 24302–24307, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Dumont JN. Oogenesis in Xenopus laevis (Daudin). Stages of oocyte development in laboratory maintained animals. J Morphol 136: 153–180, 1970. [DOI] [PubMed] [Google Scholar]
- 6. Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 7. Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA 90: 2749–2753, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gamba G. Molecular physiology and pathophysiology of the electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. Structure and mechanism of an amino acid antiporter. Science 324: 1565–1568, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Gray TM, Matthews BW. Intrahelical hydrogen bonding of serine, threonine and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J Mol Biol 175: 75–81, 1984. [DOI] [PubMed] [Google Scholar]
- 11. Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch 447: 465–468, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Hoover RS, Poch E, Monroy A, Vázquez N, Nishio T, Gamba G, Hebert SC. N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+):Cl(−) cotransporter. J Am Soc Nephrol 14: 271–282, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Isenring P, Jacoby SC, Chang J, Forbush B. Mutagenic mapping of the Na-K-Cl cotransporter for domains involved in ion transport and bumetanide binding. J Gen Physiol 112: 549–558, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature 459: 347–355, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na+-Cl− cotransporter: a new model for ions and diuretics interaction. Am J Physiol Renal Physiol 279: F161–F169, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Moreno E, Cristóbal PS, Rivera M, Vázquez N, Bobadilla NA, Gamba G. Affinity-defining domains in the Na-Cl cotransporter: a different location for Cl− and thiazide binding. J Biol Chem 281: 17266–17275, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Novello FC, Sprague JM. Benzothiadiazine dioxides as novel diuretics. J Am Chem Soc 79: 2028–2029, 1957. [Google Scholar]
- 18. Sabath E, Meade P, Berkman J, de los Heros P, Moreno E, Bobadilla NA, Vázquez N, Ellison DH, Gamba G. Pathophysiology of functional mutations of the thiazide-sensitive Na-Cl cotransporter in Gitelman disease. Am J Physiol Renal Physiol 287: F195–F203, 2004. [DOI] [PubMed] [Google Scholar]
- 19. San-Cristobal P, de los Heros P, Ponce-Coria J, Moreno E, Gamba G. WNK kinases, renal ion transport and hypertension. Am J Nephrol 28: 860–870, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science 325: 1010–1014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tovar-Palacio C, Bobadilla NA, Cortés P, Plata C, de los Heros P, Vázquez N, Gamba G. Ion and diuretic specificity of chimeric proteins between apical Na+-K+-2Cl− and Na+-Cl− cotransporters. Am J Physiol Renal Physiol 287: F570–F577, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Vázquez N, Monroy A, Dorantes E, Muñoz-Clares RA, Gamba G. Functional differences between flounder and rat thiazide-sensitive Na-Cl cotransporter. Am J Physiol Renal Physiol 282: F599–F607, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437: 215–223, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.