Abstract
The kidney is an important target for the actions of the renin-angiotensin system (RAS) and this tissue contains a complete local RAS that expresses the bioactive peptides angiotensin II (ANG II) and Ang-(1–7). We find both angiotensin type 1 (AT1R) and type 2 (AT2R) receptors expressed on renal nuclei that stimulate reactive oxygen species and nitric oxide (NO), respectively. Since Ang-(1–7) also exhibits actions within the kidney and the Ang-(1–7)/Mas receptor protein contains a nuclear localization sequence, we determined the expression of Ang-(1–7) receptors in nuclei isolated from the kidneys of young adult sheep. Binding studies with 125I-[Sar1Thr8]-ANG II revealed sites sensitive to the Ang-(1–7) antagonist [d-Ala7]-Ang-(1–7) (DALA, A779), as well as to AT2 and AT1 antagonists. Incubation of Ang-(1–7) [10−15 to 10−9 M] with isolated cortical nuclei elicited a dose-dependent increase in the fluorescence of the NO indicator [4-amino-5-methylamino-2′,7′]-difluorofluorescein diacetate. The NO response to Ang-(1–7) was abolished by the NO inhibitor N-nitro-l-arginine methyl ester and DALA, but not the AT1 antagonist losartan or the AT2 blocker PD123319. Immunofluorescent studies utilizing the Ang-(1–7)/Mas receptor antibody revealed immunolabeling of the proximal tubules but not staining within the glomerulus in cortical sections of the sheep kidney. In the nuclear fraction of isolated proximal tubules, immunoblots revealed the precursor angiotensinogen and renin, as well as functional activity for ACE, ACE2, and neprilysin. We conclude that renal nuclei express Ang-(1–7)/Mas receptors that are functionally linked to NO formation. The marked sensitivity of the intracellular NO response to Ang-(1–7) implicates a functional role of the Ang-(1–7) axis within the nucleus. Moreover, evidence for the precursor and enzymatic components of the RAS within the nuclear compartment of the proximal tubules provides a potential pathway for the intracellular generation of Ang-(1–7).
Keywords: kidney, Mas protein, angiotensinogen, renin, ACE, ACE2, neprilysin
ongoing characterization of the renin-angiotensin system (RAS) continues to reveal novel aspects regarding the molecular and functional pathways of this hormonal system (7, 13). The RAS was traditionally viewed as an endocrine system whereby circulating renin and angiotensinogen initiate an enzymatic cascade to ultimately form ANG II that recognizes a G protein-coupled receptor (GPCR) to influence blood pressure. Abundant evidence also defines the RAS as a tissue system whose components are expressed in essentially every organ and whose actions are implicated in numerous physiological events that influence renal, neuronal, cardiac, pancreatic, vascular, adrenal, pituitary, cognitive, aging, and reproductive functions (29). The majority of these actions are thought to be mediated through the AT1 receptor subtype and AT1 receptor antagonists (ARBs) are now one of the major classes of blood pressure-lowering therapies. However, ANG II may also interact with the AT2 receptors which generally oppose or attenuate the actions of the ANG II-AT1 axis (4). The role of the AT2 receptor may be particularly relevant during ARB therapy as the circulating levels of ANG II markedly increase due to the disinhibiton of ANG II feedback on renin release. There is compelling evidence as well for other angiotensin products such as Ang-(1–7) and its receptor subtype that may buffer or evoke antagonistic actions to the angiotensin-converting enzyme (ACE)-ANG II-AT1 receptor pathway (7, 13, 14, 17, 21, 37). Indeed, evidence for the Ang-(1–7)-forming enzyme ACE2 and the Ang-(1–7)/Mas receptor argues for a distinct Ang-(1–7) pathway within the RAS (7, 13, 14, 37).
Emerging evidence in various tissues including the brain, heart, and kidney also suggests an intracellular or intracrine system in which receptors localized within the cell on distinct organelle can mediate the actions of ANG II (6, 16–18, 20, 23–26, 31, 35, 43). Intracellular administration of ANG II to cardiomyocytes, vascular smooth muscle, or proximal tubule epithelial cells results in the immediate increase in intracellular calcium (12, 15, 24, 45). We and others find a surprisingly high density of intracellular angiotensin receptor sites localized to the nuclear fraction in both the cortical and medullary areas of the rat kidney (25, 26, 33, 45). The intracellular AT1 receptor is coupled to the formation of reactive oxygen species (ROS) through protein kinase C- and phosphoinositide 3-kinase (PI3)-dependent pathways; flow cytometry revealed that essentially all nuclei (99%) in the renal cortex of rat express the AT1 receptor subtype (32). We also find nuclear AT1 sites in the cortex of sheep kidney; however, the AT2 receptor is predominant in young adults and these sites are functionally coupled to the formation of nitric oxide (NO) (18). Indeed, both endothelial nitric oxide synthase (eNOS or NOSIII) and soluble guanylate cyclase are evident on isolated nuclei from the kidney and liver (16, 18). To determine whether an intracellular Ang-(1–7) system exists within the kidney, the current study examined whether isolated nuclei from the renal cortex of sheep express functional Ang-(1–7) receptors. In addition, we determined the intracellular expression of RAS components that may contribute to the generation of the peptide.
MATERIALS AND METHODS
Animals.
Tissues were obtained from eight adult (4 males, 4 females; 1.5 years of age) mixed-breed sheep housed in the Wake Forest University School of Medicine animal facility. Animals were fed a normal diet with access to water ad libitum and maintained on a 12:12-h light-dark cycle. Kidneys were obtained from animals anesthetized with ketamine and halothane, dissected in saline on ice into renal cortex and medulla, and then immediately frozen on dry ice and stored at −80°C or processed at 4°C for density gradient separation. All procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
Preparation of nuclei.
Nuclei were prepared from cortical tissue as previously described (18). Cortex was homogenized in buffer containing 25 mM KCl, 5 mM MgCl2, 20 mM Tricine-KOH, and 25 mM sucrose (pH 7.8) utilizing a Polytron Ultra-Turrax T25 Basic (setting 4), followed by a Barnant Mixer (series 10; setting 3). The homogenate was passed through a 100-μm mesh-filter and centrifuged twice at 1,000 g (4°C) for 10 min to obtain the nuclear fraction. This pellet was resuspended in 20% OptiPrep solution (Accurate Chemical and Scientific, Westbury, NY) according to the manufacturer's recommendations and layered on a discontinuous density gradient column. The columns, consisting of descending layers of 10, 20, 25, 30, and 35% OptiPrep solution to form the gradient, were centrifuged at 10,000 g for 20 min (4°C). The enriched fraction of isolated nuclei was recovered at the 30–35% layer interface. Intact nuclei were visualized by hematoxylin and eosin staining by light microscopy as described (18).
Angiotensin receptor radioligand binding.
Characterization of angiotensin receptor binding was performed as previously described (18). Briefly, isolated nuclei were suspended in HEPES buffer supplemented with 0.2% BSA (pH 7.4), peptidase inhibitors, and coincubated with the radioligand 125I-[Sar1, Thr8] ANG II (125I-Sarthran) in the presence of Losartan (the AT1-receptor antagonist), PD123319 (the AT2-receptor antagonist), [d-Ala7]-Ang-(1–7) (the Ang-(1–7) receptor antagonist), or nonlabeled Sarthran to define specific binding. The final concentration of the receptor antagonists in the binding studies was 10 μM.
Western blotting and immunodetection.
Purified nuclear fractions were suspended in PBS and added to Laemmli buffer containing mercaptoethanol. Proteins were separated on 10% SDS polyacrylamide gels for 1 h at 120 V in Tris-glycine buffer and electrophoretically transferred onto polyvinylidene difluoride membranes. Immunodetection was performed on blots blocked for 1 h with 5% dry milk (Bio-Rad) and Tris-buffered saline containing 0.05% Tween and then probed with antibodies against lamin A/C (1:500; Abcam ab78450, lot no. 732616,), the Mas protein (1:200, Alomone AAR-013, lot no. AN-02), rat renin (1:3,000; Inagami antibody no. 826), and both total and ANG I-intact forms of rat angiotensinogen (1:2,000). The angiotensinogen antibodies were raised against residues 25–34 (DRVYIHPFHLC*, ANG I sequence) and residues 42–57 (CAQLENPSVETLPEPT) of the rat protein (9). An additional cysteine residue was added for covalent coupling of the peptides to keyhole limpet hemocyanin. Both rat and sheep share the identical ANG I sequence while the sheep 42–57 sequence [CDQLEKPSVETAPDPT] shares similar identity to the rat with bold letters indicating the different residues. Plasma extracts from intact and nephrectomized sheep as well as from the cytosolic fraction of rat kidney cortex were prepared as controls. Reactive proteins were detected with Pierce Super Signal West Pico Chemiluminescent substrates and exposed to Amersham Hyperfilm enhanced chemiluminescence (Piscataway, NJ).
Immunoctyochemistry of the Mas protein and Ang-(1–7).
Kidney paraffin-embedded 5-μm sections of paraformaldehyde-fixed tissues were rehydrated from ethanol to PBS and blocked with 5% bovine serum albumin and 0.2% Tween in PBS for 30 min at room temperature. Sections were incubated overnight at 4°C with the Alomone Mas antibody diluted 1:100 in the blocking solution. The antibody was preabsorbed with the antigenic peptide (Alomone, 10 μM) for 30 min before incubation with the tissue sections. Following three washes with PBS, sections were incubated with goat anti-rabbit IgG conjugated to Alexa fluor 488 (1:200 dilution, Invitrogen A1100) for 30 min at room temperature. The sections were washed in PBS and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen P36935). To confirm the localization of the Mas protein along the nephron, we employed additional antibodies against aquaporin-1 for proximal tubules (1:100; Millipore AB3272, lot no. JC1606846), aquaporin-2 for the collecting duct (1:100; Millipore AB3274, lot no. JC1604252), as well as Tamm-Horsfall (1:50; Santa Cruz sc-20631, lot no. F1908), and the Na-K-2Cl transporter for the thick ascending limb of Henle [anti-sodium potassium chloride cotransporter 1 (1:50; Millipore AB3560P, lot no. JC1583414)]. All images were taken in one sitting on a Leica fluorescent microscope (DM4000B, Leica Microsystems, Wetzlar, Germany) using the ×40 objective. Illumination settings were held constant for image capture session (Retiga 1300R camera, QImaging, Surrey, BC, Canada; SimplePCI v6.0, Compix, Cranberry Twp., PA), and image channel input levels were windowed [45–145] uniformly in Adobe Photoshop (CS2 v9.0, Adobe Systems, San Jose, CA).
Measurement of NO production.
Isolated cortical nuclei, prepared by OptiPrep density gradient separation, were preincubated with the fluorescence dye [4-amino-5-methylamino-2′,7′]-difluorofluorescein diacetate (DAF; 5 μg/ml; Molecular Probes, Invitrogen) in buffer containing (in mM) 140 NaCl, 14 glucose, 4.7 KCl, 2.5 CaCl2, 1.8 MgSO4, 1.8 KH2PO4, and 0.10 l-arginine (pH 7.4) for 30 min at 37°C. Nuclei were washed twice in HEPES buffer to remove any unbound dye and then incubated with varying concentrations of Ang-(1–7) alone or with 1 μM losartan (LOS), PD123319 (PD), DALA, or 1 mM nonselective NOS inhibitor N-nitro-l-arginine methyl ester (l-NAME). Increases in DAF fluorescence, indicative of nitric oxide (NO) production, were measured using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA) at wavelengths of 488 nm (excitation) and 510 nm (emission) as described (18). DAF has a detection limit of 5 nM and does not react with other stable oxidized forms of NO, such as NO2−, or ROS (21).
Proximal tubule isolation.
A portion of the freshly isolated renal cortex was dissected out on ice for isolation of proximal tubules as described previously (41). Kidney outer cortex was minced into fine pieces and incubated with collagenase (1 mg/ml, CLS 1, Worthington) at 37°C in a water-jacketed flask for 60 min containing 100 ml (in mM) of a Krebs-Henseliet buffer (KHB; 25 HEPES, 118 NaCl, 4.8 KCl, 0.96 KH2PO4, 25 NaHCO3, 0.12 MgSO4, 2.55 CaCl2, pH 7.4) with 100 μl/ml DNase. At the end of the digestion, ice-cold KHB containing 10% fetal calf serum (FCS) was added, and the suspension was filtered through a nylon mesh (70 μm) and centrifuged at 500 g for 5 min at 4°C to pellet the tubules. The pellet was resuspended with 32 ml of ice-cold KHB/5% FCS and gently applied to an isotonic discontinuous Percoll gradient (Pharmacia) of 10–35% (vol/vol) with KHB/5%FCS and centrifuged at 15,000 g for 60 min at 4°C. The cell layer at a density of 1,063 (F4, proximal tubules) as determined by density beads was washed in 3× KHB to remove the Percoll media. The tubules were immediately frozen and stored at −80°C.
Peptidase activities.
Peptidase activities for ACE, ACE2, and neprilysin were determined in the nuclear fraction obtained from isolated proximal tubules as previously described (41). Assays were conducted at 37°C in 10 mM HEPES, 125 mM NaCl, 10 μM ZnCl2, pH 7.4, with 10 μg protein of tubule nuclei in a final volume of 0.5 ml with or without the indicated inhibitors, and 1.0 nM 125I-Ang I (ACE and neprilysin) or 125I-ANG II (ACE2). The following inhibitors, based on previous studies to distinguish ACE2 activity using ANG II as a substrate, comprised the inhibitor cocktail in the assay: amastatin (2 μM), bestatin (10 μM), chymostatin (10 μM), benzyl succinate (10 μM), and para-chloro-mercuribenzoic acid (0.5 mM). We subsequently added lisinopril to block ACE activity, SCH39370 to inhibit neprilysin (ANG I as substrate for both assays), or MLN4760 to block ACE2 conversion of ANG II to Ang-(1–7).
Statistical analysis.
Data are represented as means ± SE. Paired Student's t-test, one-way ANOVA with Newman-Keuls multiple comparison post hoc and nonlinear regression were performed for the data sets using GraphPad Prism 5.0 plotting and statistical software.
RESULTS
125I-Sarthran binding in nuclei.
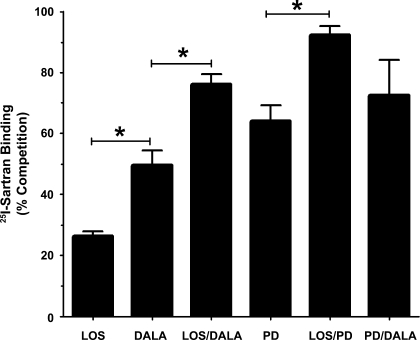
In the isolated cortical nuclei, we initially determined the relative proportion of angiotensin receptor subtypes by competition of 125I-Sarthran binding with selective antagonists. As shown in Fig. 1, the AT1 antagonist LOS competed for 28 ± 3% of Sarthran binding while the Ang-(1–7) antagonist DALA blocked a greater proportion of sites (47 ± 6%; P < 0.05). Addition of LOS and DALA exhibited additivity competing to a greater extent (78 ± 4%; P < 0.05) than either LOS or DALA alone. PD, the AT2 receptor antagonist, competed for the majority of Sarthran binding (63 ± 3%) and exhibited additive effects when combined with LOS (93 ± 3%; P < 0.05; Fig. 1). The extent of competition with the combination of LOS/PD was not significantly different than the constant value set at 100% (P > 0.10; paired t-test). In contrast, the combination of DALA and PD (74 ± 9%) was not additive with respect to PD alone, suggesting a partial overlap of these antagonists to compete for Sarthran binding.
Fig. 1.
Angiotensin receptor expression of renal cortical nuclei. Competition binding was performed in purified nuclei obtained by OptiPrep density gradient separation using 0.5 nmol/l 125I-Sarthran and angiotensin receptor antagonists alone (n = 8), or in combination (n = 3) at a final concentration of 10 μM. Values are expressed as means ± SE (*P < 0.05). Receptor antagonists are the AT1 antagonist losartan (LOS), the Ang-(1–7) antagonist d-Ala7-Ang-(1–7) (DALA), and the AT2 antagonist PD123319 (PD).
Nuclear NO responses.
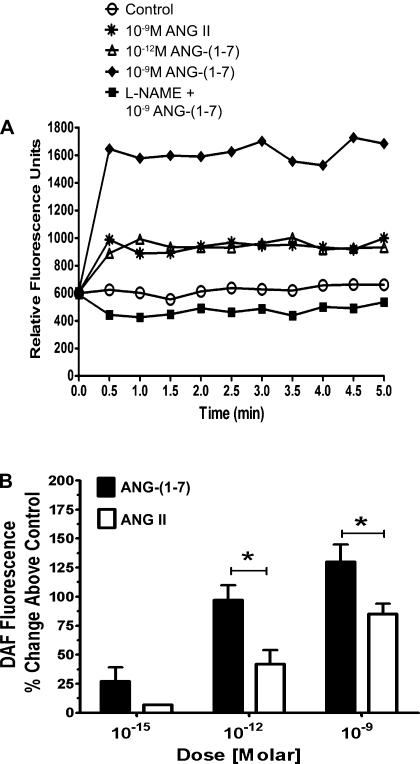
The competition for Sarthran binding by DALA suggested the presence of Ang-(1–7) receptors in the nuclear fraction. To address the potential functional aspects, freshly isolated nuclei were preincubated with the selective NO fluorescence detector DAF and exposed to three concentrations of Ang-(1–7). As shown in the fluorescent tracings of Fig. 2A, Ang-(1–7) at 10−9 and 10−12 M elicited an immediate increase in DAF fluorescence. The NOS inhibitor l-NAME returned the response of the 10−9 M dose of Ang-(1–7) to baseline. ANG II at 10−9 M also increased DAF fluorescence but was less potent than Ang-(1–7) at the same concentration. In Fig. 2B, we compare the mean DAF responses to Ang-(1–7) and ANG II in isolated cortical nuclei. At the lowest dose of 10−15 M, Ang-(1–7) elicited a significant increase in DAF fluorescence (∼25%; P < 0.05) vs. control. Compared with ANG II, Ang-(1–7) was more potent at both 10−12 and 10−9 M peptide concentrations.
Fig. 2.
Ang-(1–7) is a potent stimulator of nuclear nitric oxide production. Freshly isolated cortical nuclei purified by OptiPrep density gradient separation were preincubated with the selective nitric oxide (NO) fluorescence detector DAF. A: representative tracings of DAF fluorescence over a 5-min time course. Nuclei were stimulated with increasing concentrations of Ang-(1–7) or ANG II for a period of 5 min. B: mean fluorescence intensity was measured for each treatment. Values represented are expressed as % increase in DAF fluorescence over control (baseline). Data are expressed as means ± SE; n = 6; *P < 0.01 vs. ANG II.
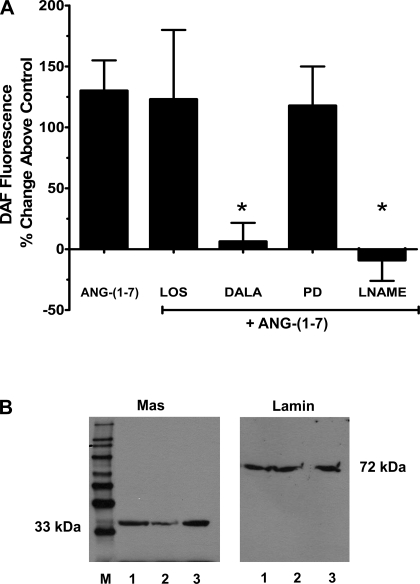
As shown in Fig. 3A, the pretreatment of nuclei with the either DALA or the NOS inhibitor l-NAME abolished the effects of 1 nM Ang-(1–7). In contrast, addition of LOS or PD did not attenuate the Ang-(1–7) response (Fig. 3A). Since the Mas protein may account for the receptor-mediated actions of Ang-(1–7), immunoblots of purified cortical nuclei were probed with the antibody directed against the protein. As shown in Fig. 3B, the full-length gel revealed the presence of a single band at ∼33 kDa in isolated nuclei from the renal cortex. Probing of the nuclear extracts with an antibody against the nuclear protein lamin A/C revealed a single band at ∼72 kDa.
Fig. 3.
Ang-(1–7) stimulates NO through nuclear Ang-(1–7) receptors. A: purified nuclei were stimulated with Ang-(1–7) (1 nM) in the presence of the AT1 antagonist LOS (1 μM), the AT2 antagonist PD (1 μM), DALA (1 μM), or the NOS inhibitor l-NAME (1 mM). Data are expressed as means ± SE; n = 7; *P < 0.05 vs. Ang-(1–7). B: immunodetection of Ang-(1–7)/Mas receptor. Purified nuclear extracts were probed with antibodies against the Mas receptor (1:200) or the nuclear membrane marker lamin A/C (1:500). Lanes 1–3 represent nuclei prepared from 3 different preparations; M is the lane for molecular weight markers.
Mas receptor immunofluorescence.
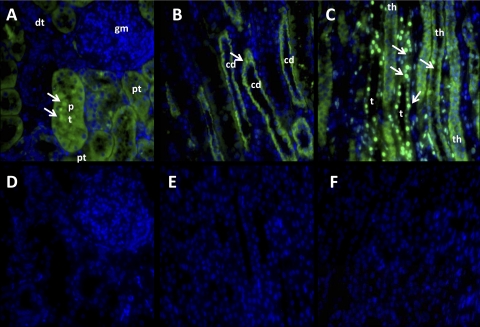
We next assessed the immunofluorescent distribution of the Mas receptor utilizing the same antibody for the immunoblot in the previous figure. As shown in Fig. 4, the immunofluorescent signal for Mas in the cortex was predominantly associated with the proximal tubules but was notably absent within the glomerulus of the sheep kidney (Fig. 4A). In the medullary region, Mas staining was present in the collecting ducts (Fig. 4B) as well as the thick ascending limb of Henle (Fig. 4C). The green fluorescent signal for Mas was not exclusively found on nuclei as denoted by the nuclear marker DAPI in blue, although intensely stained nuclei in the medullary region localized to perhaps the thin loop of Henle or vasa recta capillaries (Fig. 4C). The immunostaining for Mas in all sections was abolished by preabsorption of the antibody with the antigenic peptide for the protein (Fig. 4, D–F).
Fig. 4.
Immunodetection of Mas receptor expression in sheep kidney. A–C: kidney sections were probed with an antibody against the Mas receptor protein followed by the secondary antibody conjugated to Alexa Fluor 488 (green fluorescence) and the nuclear marker stain DAPI (blue). D–F: Mas antibody immunoreactivity was blocked by preincubation with the immunogenic Mas peptide. Mas-stained nuclei are denoted by arrows. c, Collecting ducts; dt, distal tubule; pt, proximal tubule cell; gm, glomerulus; th, thick limb of Henle; t, thin loop. Immunofluorescent studies are representative of the staining in 3 sheep kidneys.
Nuclear RAS components from proximal tubules.
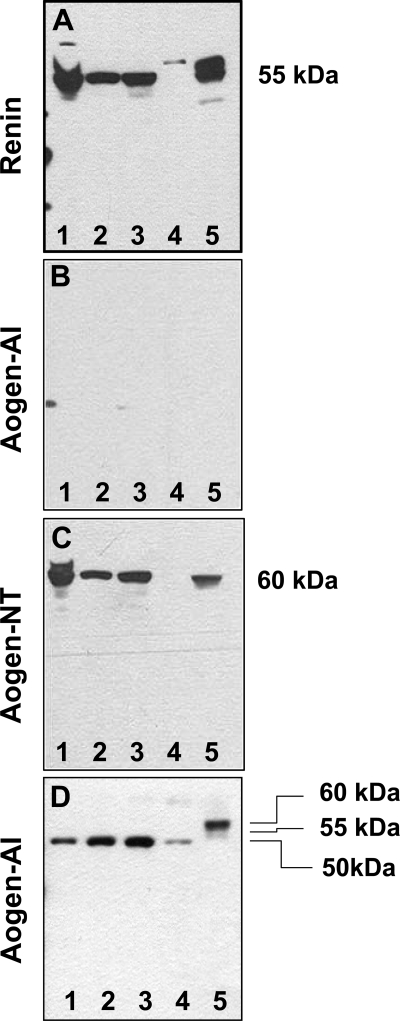
Given the localization of the Mas protein to the proximal tubules within the cortical region, we prepared nuclei from isolated tubules and assessed the RAS components that may contribute to intracellular Ang-(1–7) formation. As shown in the immunoblots of Fig. 5, proximal tubule nuclei (lanes 1–3) and the rat kidney homogenate (lane 5) exhibited an immunoreactive band for renin (Fig. 5A), but not for the precursor angiotensinogen (Fig. 5B) using the antibody against the NH2-terminal ANG I sequence (Aogen-AI). In Fig. 5C, reprobing the same gel with the angiotensinogen antibody [Aogen-NT] raised against the peptide sequence distal to ANG I revealed a band of the appropriate molecular weight (60 kDa) in the nuclear fraction and the kidney homogenate. As shown in Fig. 5D, the Aogen-AI antibody recognized a 50-kDa band for angiotensinogen in sheep nephrectomized (lanes 1–3) and intact plasma (lane 4) collected without a renin inhibitor, as well as 60- and 55-kDa bands in rat plasma collected with a rat renin inhibitor (lane 5).
Fig. 5.
Immunoblots reveal the presence of renin and angiotensinogen in the nuclear fraction from proximal tubules. The nuclear fractions from 3 separate proximal tubule preparations (lanes 1–3) and a rat kidney homogenate as control (lane 5) were separated on 2 SDS gels and probed with antibodies against renin (A) and angiotensinogen (B–C). A: renin antibody revealed a single band of ∼55 kDa in the nuclei of the tubules and kidney homogenate. B: no bands were detected using the angiotensinogen antibody that recognizes the ANG I-containing sequence [Aogen-AI]. C: gel B was stripped and reprobed with a second angiotensinogen antibody against the 42–57 sequence [Aogen-NT] revealing a ∼60-kDa band in the nuclear fraction of the tubules (lanes 1–3) and the kidney homogenate (lane 5). D: Aogen-AI antibody recognized nephrectomized sheep plasma (lanes 1–3: 10, 20, and 30 nl, respectively), intact sheep plasma (lane 4: 30 nl), and rat plasma (lane 5: 380 nl).
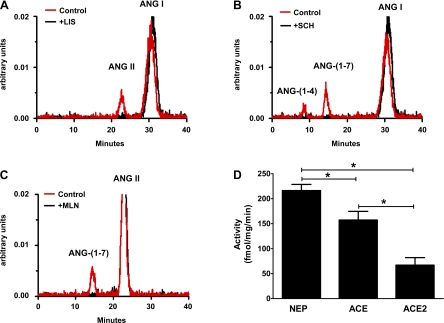
Finally, we determined peptidase activities in the nuclear fraction of the isolated tubules for neprilysin and ACE with the substrate 125I-Ang I and ACE2 with 125I-ANG II. As shown in the chromatographs (Fig. 6, A–C), activities for all three peptidases were evident in the nuclear fraction of the proximal tubules and their selective inhibitors abolished activity. Quantification of the enzyme activities from four separate tubule preparations revealed significant differences with neprilysin activity higher than ACE or ACE2 (Fig. 6D).
Fig. 6.
Peptidase activities in the nuclear fraction from isolated proximal tubules of the sheep cortex. Representative chromatographs for angiotensin-converting enzyme (ACE; A), neprilysin (B), and ACE2 (C) activities in the absence (control) and presence of selective inhibitors for ACE (lisinopril, +LIS), neprilysin (SCH39370, +SCH), and ACE2 (MLN4760, +MLN). Peptidase activities (D) were quantified in the nuclear fraction from 4 separate tubule preparations. Data are expressed as means ± SE; n = 4; *P < 0.05.
DISCUSSION
Emerging evidence clearly supports the localization of functional angiotensin receptors as well as other GPCRs within the nuclear compartment of various tissues including the kidney, heart, vascular smooth muscle cells, and immune cells (6, 12, 15, 17, 18, 20, 21, 23, 24, 32, 45). In the rat kidney, the density of AT1 receptor expressed on the nucleus is equal to or greater than that found on the cell membrane (25, 26, 33, 45). Although the majority of studies assessed the AT1 subtype on nuclei, we recently reported that cortical nuclei of sheep express AT2 binding sites that are functionally linked to NO formation (18). We now extend the characterization of an intracellular angiotensin system within the kidney by providing evidence for functional Ang-(1–7)/DALA-sensitive sites. As revealed by 125I-Sarthran binding in cortical nuclei, the Ang-(1–7) receptor antagonist DALA (A779) competed for a significant portion of the binding sites. Moreover, DALA abolished the Ang-(1–7)-dependent stimulation of NO; however, neither the AT1 nor AT2 receptor antagonists attenuated the response. Isolated nuclei from the renal cortex also expressed a single protein band recognized by an antibody to the Mas protein, suggesting that the nuclear Ang-(1–7) receptor is likely the Mas protein. Utilizing this same antibody, immunofluorescent staining revealed that the Mas protein was primarily localized to proximal tubules but not the glomerulus of the renal cortex consistent with our previous studies in the mouse kidney. Finally, we demonstrate multiple components of the RAS in the nuclear fraction including immunoreactive renin and angiotensinogen, as well as ACE, ACE2, and neprilysin activities that may contribute to the intracellular formation of Ang-(1–7).
There exists a wealth of data suggesting that Ang-(1–7), an alternative product of the RAS, is functional, particularly within the kidney (7, 13, 14, 21, 31, 37). However, it was not until the development of the selective Ang-(1–7) antagonist DALA by Khosla and colleagues (1, 38) and the subsequent demonstration by Santos et al. (39) that Mas knockout mice lack Ang-(1–7) responsiveness that a unique receptor for Ang-(1–7) became evident. Studies to date characterized the Ang-(1–7)/Mas receptor on intact cells or tissues, and functional studies using Ang-(1–7) or the DALA peptide likely reflect the interaction with the cell surface receptor as these peptides should not readily cross the cell membrane to interact with intracellular targets. Reudelhuber and colleagues (28) employed a novel “biological pump” to generate Ang-(1–7); however, the expressed peptide is linked to the constitutive pathway for immediate secretion into the interstitial space rather than the intracellular accumulation of the peptide within the heart. Nevertheless, low concentrations of Ang-(1–7) (10−15 to 10−12 M) stimulate the nuclear formation of NO strongly implicating a functional role for the peptide within the cell. Moreover, the Ang-(1–7) receptor antagonist DALA and the ACE2 inhibitor MLN4760 exacerbate nuclear ROS formation to ANG II (10−9 M), suggesting that processing to Ang-(1–7) may counterbalance the intracellular ANG II-AT1 receptor axis (17). Although it is not known whether Ang-(1–7)-dependent formation of NO influences ROS production by ANG II, the present results confirm previous studies in intact bovine and human endothelial cells, as well as Mas-transfected CHO cells that Ang-(1–7) at higher doses (0.1 to 10 μM) directly stimulates NO (19, 36). It is not clear whether differences in sensitivity of the Ang-(1–7) response between the isolated nuclei and intact cells reflect the method of NO detection, the particular cell type, or possibly altered affinity states of the receptor. Ang-(1–7) stimulated the phosphorylation of both eNOS and Akt which was blocked by a PI3 kinase inhibitor suggesting that PI3 kinase/Akt lies upstream of eNOS (36). Moreover, Savard et al. (40) demonstrated the phosphorylation of Akt in response to the bradykinin-dependent stimulation of iNOS gene expression in isolated liver nuclei. Additional studies are required to elucidate the intranuclear pathways for the Ang-(1–7)-dependent formation of NO within the kidney.
In support for the intracellular processing of angiotensins, particularly Ang-(1–7), we demonstrate enzyme activities for neprilysin, ACE, and ACE2 in the nuclear fraction from isolated proximal tubules. These data confirm recent findings by Casarini and colleagues (3) in cultured rat mesangial cells that reveal the nuclear localization of ANG II and Ang-(1–7), as well as the active forms of ACE and ACE2. We detected immunoreactive evidence for angiotensinogen in the nuclear fraction using an antibody raised to the peptide sequence distal to ANG I, but not with the antibody directed against the NH2-terminal ANG I sequence. During the extended time to isolate the proximal tubules and the subsequent purification of the nuclei, the localization of intracellular renin and angiotensinogen may lead to the enzymatic release of ANG I. Angiotensinogen may be cleaved by renin in the extranuclear space of the cell and the des-ANG I form subsequently traffics to the nucleus. Inclusion of a renin inhibitor during the tubule isolation and subcellular fractionation process will be necessary to resolve this issue. The current data are consistent with the report by Sherrod et al. (42) that localized angiotensinogen exclusively to the nuclei of human glial cells. This report also identified a COOH-terminal region of human angiotensinogen that when expressed in a GFP fusion, protein resulted in the nuclear accumulation of the construct. However, angiotensinogen was not found in nuclei of human HepG2 hepatocytes, suggesting that the trafficking of the protein to the nucleus is likely cell specific (40). Renin is primarily localized to the juxatglomerular cells of renal cortex; however, the enzyme is also found in the proximal tubules and the cortical collecting ducts of the kidney while angiotensinogen is predominantly synthesized by the proximal tubules and may be internalized via high-affinity receptors localized to the tubular epithelium (5, 11, 21, 29, 30). We did not directly access renin activity and other enzymes may cleave ANG I or an ANG I-containing sequence from angiotensinogen, but the presence of peptidases that process ANG I to ANG II or ANG-(1–7) provides additional evidence for the intracellular generation of angiotensins.
The Ang-(1–7) receptor antagonist DALA (A779) abolished the NO response to Ang-(1–7) in isolated nuclei which was not attenuated by either the AT1 or AT2 receptor antagonists. This contrasts to the binding studies with 125I-Sarthran ligand where PD and DALA (but not losartan) exhibit some degree of overlap for competition of binding. It is possible that DALA is not selective for the AT2 receptor at the 10-μM dose and that Sarthran does not recognize Ang-(1–7) sites in the sheep kidney. Whether lower doses of DALA would demonstrate greater selectivity remains to be investigated albeit the current dose of the antagonist is reportedly selective for the Ang-(1–7) site in rat (1, 38). Further studies utilizing radiolabeled or fluorescently tagged Ang-(1–7) as the ligand are necessary to establish the binding characteristics of the heptapeptide for the nuclear site. Immunoblotting revealed a ∼33-kDa protein that was recognized by the antibody to Mas receptor and the molecular size is identical to that reported in intact human endothelia cells and Mas-transfected cells that utilized antibodies raised to different peptide sequences of the protein (2, 35, 36, 39). To our knowledge, the cellular trafficking of the Mas receptor has not been investigated in the kidney or other tissues; however, the human protein contains a canonical nuclear localization sequence suggesting that mature receptor may transport from the post-Golgi complex to the nucleus (24). Based on the immunofluorescent staining of the Mas receptor throughout the tubules, the receptor may internalize to intracellular compartments following binding to the Ang-(1–7) ligand. The current study did not identify the localization of the receptor within the nuclear architecture. In lieu of the rapid NO response to Ang-(1–7) in the isolated nuclei, at least a portion of the Ang-(1–7) receptors may localize to the outer nuclear envelope with the peptide-binding domain oriented to the cytoplasmic face and COOH terminus of receptor extending into the nuclear envelope space.
The immunofluorescent staining of the Mas protein revealed predominant expression in the cortical tubules, collecting ducts, and the limb of Henle with little or no signal evident within the glomerulus. Our previous study of Mas expression in the mouse kidney revealed a comparable distribution to that of the sheep kidney, although protein staining was evident on the afferent glomerular vessels of the mouse kidney (8). In the rat kidney, Da Silveira et al. (10) reported abundant Mas staining in proximal tubules and the collecting duct with minimal immunoreactive signal in the glomerulus. The proximal tubule is considered to be an important site for the renal actions of Ang-(1–7) that includes the peptide's influence on sodium and water regulation (7, 13, 37). Evidence for the Mas protein on the thick limb ascending limb of Henle may reflect another potential site for the natriuretic actions of Ang-(1–7). Indeed, acute infusion of Ang-(1–7) in the sheep increases urinary volume and sodium excretion; however, it is not known whether an acute increase in circulating Ang-(1–7) will stimulate nuclear Ang-(1–7) receptors to influence sodium reabsorption (43). Indeed, we speculate that the Ang-(1–7) intracellular system may be involved in the long-term regulation of NO within the proximal tubules to influence the expression of sodium transporters, as well as provide a physiological buffer to the intracellular actions of the ANG II-AT1 receptor axis (6, 7, 44). The localization of the Mas receptor on the collecting duct cells supports the functional effects of Ang-(1–7) reported by Santos and colleagues (27) on this cell type in the rat kidney. The intense staining of nuclei with the Mas antibody on what appears to be either the thin loop or vasa recta elements may portend additional actions of Ang-(1–7) within the medullary region. Clearly, further studies are required to elucidate how the nuclear receptors for Ang-(1–7) contribute to tubular function, as well as the pathways that may lead to intracellular expression of the peptide.
The present findings reveal the intracellular localization of distinct receptors for Ang-(1–7) within the kidney that respond to very low concentrations of the peptide to stimulate NO formation. The current therapeutic regimens for blockade of the RAS that include ACE inhibitors and AT1 receptor antagonists are generally thought to increase the levels of Ang-(1–7), thus constituting an additional mechanism to their therapeutic value. Whether the nuclear actions of an activated Ang-(1–7) axis within the cell construe significant cardiovascular protection is not known, but the demonstration of functional Ang-(1–7) receptors and the components to generate the peptide may provide an important target of the tissue RAS.
GRANTS
This work was supported by National Institutes of Health Grants HD-17644, HD-47584, HL-51952, and HL-56973.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the generous gift of the rat renin antibody by T. Inagami (Stanford Moore Professor of Biochemistry, Vanderbilt University, Nashville, TN).
REFERENCES
- 1.Ambühl P, Felix D, Khosla MC. [7-d-ALA]-angiotensin-(1–7): selective antagonism of angiotensin-(1–7) in the rat paraventricular nucleus. Brain Res Bull 35: 289–291, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol 293: H1416–H1424, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Camargo de Andrade MC, Di Marco GS, de Paulo Castro Teixeira V, Mortara RA, Sabatini RA, Pesquero JB, Boim MA, Carmona AK, Schor N, Casarini DE. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol 290: F364–F375, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor. The AT2 receptor comes of age. Hypertension 45: 840–844, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Castrop H, Hocerhl K, Kurtaz A, Schweda F, Tordorov V, Wagner C. Physiology of kidney renin. Physiol Rev 90: 607–673, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC. Angiotensins: endocrine to intracrine functions. In: Bioactive Peptides, eds. Howl J, Jones S. London: CRC, 2009, p. 3–20 [Google Scholar]
- 7.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7) mas receptor axis; more than regulation of blood pressure? Hypertension 50: 596–599, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1–7), ACE2 and blood pressure regulation. In: Kidney and Blood Pressure Regulation, eds. Suzuki H, Saruta T. Basel: Karger, 2004, p. 77–89 [DOI] [PubMed] [Google Scholar]
- 9.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2) Lewis strain. Am J Physiol Renal Physiol 299: F35–F42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romeros O, De Sousa LP, Teixeira MM, Santos RA, Simoes e, Silva AC, RibeiroVieira MA. ACE-Angiotensin-(1–7)-Mas axis in renal ischemia/reperfusion injury in rats. Clin Sci (Colch) 119: 385–394, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Darby IA, Congiu M, Fernley RT, Sarnia C, Coughlin JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int 46: 1557–1560, 1994 [DOI] [PubMed] [Google Scholar]
- 12.DeMello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol 23: 640–646, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Dilauro M, Burns KD. Angiotensin-(1–7) and its effects in the kidney. Scientific World J 9: 522–535, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension 47: 515–521, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Filipeanu CM, Henning RH, Nelemans SA, de Zeeuw D. Intracellular angiotensin II: from myth to reality? J Renin Angiotensin Aldosterone Syst 2: 219–226, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gobeil F, Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, Orleans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem 281: 16058–16067, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gwathmey-Williams T, Pendergrass KD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-ACE2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 55: 166–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 37: 72–76, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18: 208–214, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 279: 7901–7908, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Li XC, Zhuo JL. Intracellular angiotensin II induces in vitro transcription of TGF-β1, MCP-1 and NHE3 mRNAs in rat renal cortical nuclei via activation of nuclear AT1 receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Licea H, Walters MR, Navar G. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Magaldi AJ, Cesar KR, de Araújo M, Simões e, Silva AC, Santos RA. Angiotensin-(1–7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflügers Arch 447: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin-(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 103: 1319–1326, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Olson AL, Perlman S, Robillard JE. Developmental regulation of angiotensinogen gene expression in sheep. Pediatr Res 28: 183–195, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan N, Luo J, Kaiser SJ, Frome WL, Dart RA, Tewksbury DA. Specific receptor for angiotensinogen on human renal cells. Clin Chim Acta 373: 32–36, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2 Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e, Silva AC. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 75: 1184–1193, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Re R. Intracellular renin-angiotensin system: the tip of the intracrine physiology iceberg. Am J Physiol Heart Circ Physiol 293: H905–H906, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sampaio WO, dos Santos RA, Faria-Silva R, de Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 1885–1892, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Santos RA, Ferreira AJ, Simoes e, Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol 93: 519–527, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen JC, Gropen C, Jr, Carvalho WS, Simões e, Silva AC, Khosla MC. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Santos RA, Silva ACS, Maric C, Speth R, Machado RP, Pinheiro SV, Lopes MT, Mendes EP, Bader M, Schultheiss HP, Campagnole-Santos MJ, Walther T. Evidence that angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savard M, Barbaz D, Belanger S, Muller-Esterl W, Bkaily G, Orleans-Juste P, Cote J, Bovenzi V, Gobeil F. Expression of endogenous nuclear bradykinin B2 receptors mediates signaling in immediate early gene activation. J Cell Physiol 216: 234–244, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol 288: R539–R546, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Tang L, Carey LC, Bi J, Valego NK, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuo JL, Li XC. Novel roles of intracrine angiotensin II and signaling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst 8: 23–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]