Abstract
The zebrafish is a valuable vertebrate model for kidney research. The majority of previous studies focused on the zebrafish pronephros, which comprises only two nephrons and is structurally simpler than the mesonephros of adult fish and the metanephros of mammals. To evaluate the zebrafish system for more complex studies of kidney development and regeneration, we investigated the development and postinjury regeneration of the mesonephros in adult zebrafish. Utilizing two transgenic zebrafish lines (wt1b::GFP and pod::NTR-mCherry), we characterized the developmental stages of individual mesonephric nephrons and the temporal-spatial pattern of mesonephrogenesis. We found that mesonephrogenesis continues throughout the life of zebrafish, with a rapid growth phase during the juvenile period and a slower phase in adulthood such that the total nephron number of juvenile and adult fish linearly correlates with body mass. Following gentamicin-induced renal injury, the zebrafish mesonephros can undergo de novo regeneration of mesonephric nephrons, a process known as neonephrogenesis. We found that wt1b expression was induced in individually dispersed cells in the mesonephric interstitium as early as 48 h following injury. These wt1b-expressing cells formed aggregates by 72–96 h following injury which proceeded to form nephrons. This suggests that wt1b may serve as an early marker of fated renal progenitor cells. The synchronous nature of regenerative neonephrogenesis suggests that this process may be useful for studies of nephron development.
Keywords: wt1, nephron, gentamicin, renal injury, renal progenitor
mammals go through three discrete stages of kidney development. The first of these, the pronephros, develops from the intermediate mesoderm ventral to the anterior somites. While the pronephric kidney in mammals serves no excretory function and quickly degenerates, in lower vertebrates, such as teleost fish, the pronephros functions transiently as an essential excretory organ. Later in development, nephrogenic mesenchyme caudal to the pronephros condenses into tubules that will fuse with the pronephric duct and give rise to the mesonephric kidney. In humans, this is the first kidney to serve a known excretory function before giving rise to the reproductive ducts and metanephric kidney. In juxtaposition to its functional transience in mammals, the mesonephros of teleost fish and many other “lower” vertebrates represents the final stage of kidney development; it persists throughout life and can become quite complex (8).
The zebrafish pronephros, because of its simplicity, has been used extensively in genetic studies relating nephron structure and function to human kidney diseases (5, 6). However, this simplicity presents a technical limitation in modeling more complex disorders and in studying the molecular regulation of nephron development. Initially developing ∼12 days postfertilization, the zebrafish mesonephros ultimately acquires a high degree of complexity and potentially offers a more versatile and relevant model for the study of kidney development and human disease. Mesonephric nephrons in teleosts are derived from precursor cells within the “nephrogenic zone” defined by the mesonephric interstitium; studies demonstrating the ability of teleosts to regenerate nephrons de novo (neonephrogenesis) following renal injury imply that the nephrogenic capacity of cells within this field is maintained throughout life (20). Although this regenerative mechanism has not been found in the metanephros, studying neonephrogenesis will lead to new knowledge of in vivo regulation of renal progenitor cells and may provide insights into stem cell-based therapies for kidney diseases.
To date, little is known about the morphological structure, development, and regeneration of the zebrafish mesonephric kidney. Therefore, we studied the development of the mesonephric kidney in normal zebrafish and mesonephric regeneration in a zebrafish model of gentamicin-induced renal injury (11, 22). To facilitate our study, we isolated the kidney-specific promoters of zebrafish podocin and cadherin-17 genes and generated transgenic fish to fluorescently label kidney structures in vivo. Using these transgenics and the previously published wt1b::GFP transgenic fish (16), we defined the developmental process of mesonephric nephrons. We also found that through a mechanism of continuous growth, mesonephric nephron number positively correlates with the body mass of zebrafish. In addition, we demonstrated that wt1b, a zebrafish homolog of Wilm's Tumor 1, may serve as a molecular marker for renal progenitor cells during development and regeneration.
MATERIALS AND METHODS
Fish breeding and maintenance.
Zebrafish (Danio rerio) were reared and maintained as described (28). Embryos were collected after natural spawns, raised at 28.5°C, and staged according to hours postfertilization or days postfertilization (dpf). A lab-inbred wild-type strain and AB* wild-type strain were used for generating transgenic fish.
Based on the growth curve of nephron number, wt1b::GFP fish that are at least 6 mo old were used in regeneration studies to minimize the background rate of nephrogenesis. To induce kidney injury, 75 mg/kg of gentamicin (GIBCO) diluted in PBS (pH 7.4) were injected intraperitoneally into adult zebrafish. PBS alone was used as vehicle control. All procedures have been approved by the University Committee on Use and Care of Animals at the University of Michigan.
Cloning promoter and constructing plasmids.
Promoters of cadherin-17 and podocin were amplified from zebrafish genomic DNA by PCR and cloned into pGEM-T Easy vector (Promega) for sequence verification. Primers used to clone podocin promoter are (forward) 5′-CGGTCACCGGAAGTTTATAAGTATATGGG-3′; (reverse) 5′-AAGAATGTCGAGATGTTTCTGTTTCGGTCC-3′.
The cdh17 promoter was cloned into BglII and SacI sites in pEGFP-1 (Clontech). Subsequently, the cdh17::GFP minigene was cloned into pminiTol2 (1, 24) between the Tol2 transposon left and right arms to facilitate the generation of transgenic fish. The pod::GFP construct was made by replacing the cdh17 promoter with the podocin promoter in the construct described above. The podocin promoter was also cloned into a construct containing the nitroreductase-mCherry gene (4) for generating pod::mCherry transgenic fish.
Generating transgenic fish.
Capped RNA encoding the Tol2 transposase was synthesized from linearized plasmid (24) using mMessage mMachine T3 RNA synthesis kit (Ambion) via in vitro transcription. To generate transgenic fish, the DNA plasmid was coinjected with RNA encoding the Tol2 transposase into fertilized eggs. Embryos expressing the transgene were raised to adulthood and out-crossed to wild-type fish to obtain germ-line transgenics.
Imaging analyses.
Embryonic and larval zebrafish (<20 dpf) were anesthetized with tricaine (Sigma) and imaged directly under a Leica stereoscope. Juvenile (>20 dpf) and adult fish were anesthetized with tricaine before a surgical removal of gut and other visceral organs to expose the mesonephric kidney. After dissection, fish were mounted ventral side up on an agarose plate and imaged with a Leica stereoscope. For confocal imaging, mesonephric kidneys were first fixed with 4% PFA in PBS, washed with PBS, mounted in 70% glycerol, and imaged with a Leica SP5X laser confocal microscope. The confocal images were processed with Leica AF software.
To measure nephron number, pod::GFP fish were anesthetized and dissected as described above and pod::GFP-expressing glomeruli were visually counted at the time of dissection.
Immunohistochemistry.
Zebrafish kidneys were fixed with 4% PFA in PBS overnight and stored in Dent's fixative (80% methanol, 20% DMSO) at −20°C. For immunohistochemical staining, the tissue was rehydrated with PBS and blocked at room temperature for 2 h with incubation buffer (2 mg/ml BSA, 1% DMSO, 0.5% Triton X-100, 10% serum, PBS pH 7.4). After being blocked, the embryos were incubated with mouse anti-PCNA (1:1,000; Sigma) and rabbit anti-GFP antibody (1:500; Santa Cruz Biotechnology) overnight at 4°C. After being washed with incubation buffer, tissue was incubated with Alex594-conjugated anti-mouse IgG and Alex488-conjugated anti-rabbit IgG (1:1,000; Invitrogen) at 4°C overnight. The secondary antibodies were then washed off and the tissue was mounted in VECTASHIELD mounting medium (Vector Laboratories) and imaged with a Leica SP5X laser confocal microscope as described above.
Statistical analysis.
The linear and logarithmic regression coefficients regarding change in mesonephric nephron number throughout our period of observation were calculated from datasets with the statistically identified outliers removed (25).
For the linear regression of the correlation between medial nephron number and body mass, a mean ratio of medial nephron number to body mass was first calculated from the raw dataset (R2 = 0.682). The regression coefficient was calculated from a new mean value weighted for a y-intercept value of 0 with the outlying 10/101 datapoints exceeding 1.5 standard deviations from the original mean value excluded.
To identify outliers for the logarithmic regression of medial nephron number to age, a logarithmic regression was first performed on the raw dataset (R2 = 0.454) and the data were normalized according to predicted values from the original coefficient. The data points within the top and bottom 10 percentiles of variance from the original predicted value were considered to be outliers and the logarithmic regression was repeated.
RESULTS
cadherin-17 and podocin promoters confer kidney-specific expression in transgenic zebrafish.
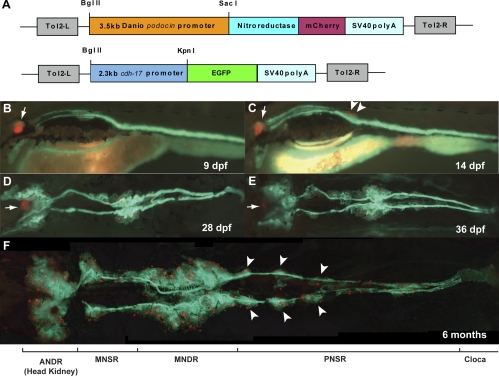
To analyze kidney development in zebrafish using whole mount fluorescence imaging, we sought to generate transgenic fish with fluorescently labeled kidney cells. cadherin-17 (cdh17), the zebrafish homolog of the mammalian kidney-specific cadherin, has been reported to be expressed in the epithelium of the pronephric tubule and ducts as well as in the mesonephric tubules and ducts of juvenile fish (12). Thus, we isolated a ∼2.3-kb upstream sequence to the cdh17 gene and found that this promoter sequence could drive GFP expression specifically in the tubules and ducts of the pronephric and mesonephric kidney (Fig. 1), recapitulating the endogenous expression of cdh17. To fluorescently label renal glomeruli, we isolated a ∼3.5-kb upstream sequence to the zebrafish podocin gene, which we found could specifically drive fluorescent mCherry expression in both pronephric and mesonephric glomerular podocytes (Fig. 1).
Fig. 1.
Mesonephrogenesis in cdh17::GFP/pod::mCherry double-transgenic zebrafish. A: schematic graph illustrating the transgene structures of pod::mCherry and cdh17::GFP. Tol2-L and Tol2-R are Tol2 transposon elements to facillite the transgenesis. B: lateral view of a 9 days postfertilization (dpf) larva. Podocytes in pronephric glomeruli (arrow) are marked with mCherry fluorescence (red) and pronephric tubules and ducts are labeled with GFP fluorescence (green). cdh17::GFP expression is also present in the intestinal epithelia, where the endogenous cdh17 is expressed (12). C: lateral view of a 14 dpf larva that developed the first pair of mesonephric glomeruli (arrowheads) with matured podocytes-expressing mCherry (red). D: ventral view of a 28 dpf juvenile. Multiple glomeruli (red) are visible at the anterior and medial portions of the mesonephric kidney with convoluted mesonephric tubules. E: ventral view of a 36 dpf juvenile. More mesonephric glomeruli and more convolutions of tubules developed in the anterior and medial regions. A few nephrons are present in between the rostral and medial regions as well as in the posterior region. F: ventral view of the whole mesonephric kidney in a 6-mo-old adult. ANDR, anterior nephron-dense region; MNSR, medial nephron-sparse region; MNDR, medial nephron-dense region; PNSR, posterior nephron-sparse region. Note symmetric groups of nephrons (arrowheads) in PNSR are segmentally distributed. Anterior to the left in all the panels.
By generating double-transgenic fish carrying both the cdh17::GFP and pod::mCherry transgenes, we were able to label the renal glomeruli, tubules, and ducts in live zebrafish. We used this line to characterize the process of mesonephric development. The first pod::mCherry-expressing mesonephric glomerular podocytes appear ∼12 dpf (Fig. 1C). However, a thickening and convolution of the pronephric duct within the presumptive region of the first mesonephric glomerulus is visible even before the appearance of pod::mCherry-expressing podocytes. A pair of glomeruli, one on each side of the duct, consistently develops by 14 dpf (Fig. 1C). Mesonephric nephrogenesis then proceeds in an anterior to posterior manner starting from the initial mesonephric nephrons until 18–20 dpf, when nephrons start to form in the rostral region corresponding to the pronephric proximal convoluted tubules (Fig. 1, D–E). Starting at the age of 1 mo, scattered and sparse nephrogenesis occurs in the rostral-medial region and the most caudal region (Fig. 1, D–E). Thus, we may divide the whole mature zebrafish mesonephric kidney into four morphologically distinct regions with alternating densities of nephrons: an anterior nephron dense region (ANDR) contained along the dorsal wall of the thoracic cavity, previously described as the “head kidney” (13); a medial nephron sparse region (MNSR); a medial nephron dense region (MNDR), the tubules which possess secondary branches and are evenly spaced in a manner resembling the mesonephric tubules that give rise to the vas deferens in higher mammals (9), and a posterior nephron sparse region (PNSR) running from the MNDR to the cloaca (Fig. 1F). Interestingly, in the PNSR, the nephrons exist within several distinct symmetric segments (Fig. 1F).
wt1b expression is differentially regulated during the development of mesonephric nephrons.
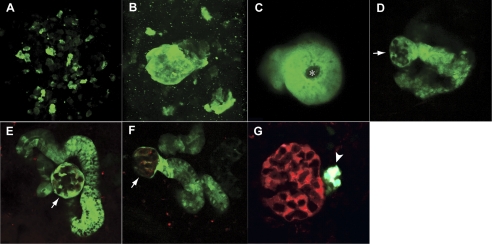
We examined the process of mesonephric nephrogenesis by imaging the mesonephric kidney in wt1b::GFP/pod::mCherry double-transgenic fish. Previously, wt1b::GFP has been found to be expressed by the proximal tubule and the glomerular primordium in the pronephros (2, 16). Mesonephric nephrogenesis initiated with the appearance and aggregation of wt1b::GFP-expressing cells within the nephrogenic region (Fig. 2, A–B). The cells in these aggregates acquired an epithelial shape and formed a tubular structure with a clearly defined lumen (Fig. 2C). As the tube elongated, its proximal end then enlarged to form a glomerulus with the pod::mCherry-expressing podocytes (Fig. 2, D–F). Concurrently, the distal portion of the developing nephron connected either with the duct to create a primary nephron or with an as-yet-undefined segment boundary within a primary nephron. Intriguingly, as the nephron development approached its completion, wt1b::GFP expression became restricted to a ring of cells at the urinary pole of the glomerulus (Fig. 3G; Supplemental Movie S1-S2; the online version of this article contains supplemental data). Thus, wt1b::GFP expression is precisely regulated during mesonephric nephron development and its differential expression, along with that of pod::mCherry, can be used to determine the developmental stage of individual mesonephric nephrons.
Fig. 2.
Staging the development of a mesonephric nephron in wt1b::GFP/pod::mCherry double-transgenic zebrafish. A: dispersed wt1b::GFP-expressing cells in the nephrogenic region before nephron starts to develop. B: early aggregate (stage I): aggregate of wt1b::GFP+ cells with no defined lumen. C: tubular body (stage II): cluster of wt1b::GFP+ cells with a defined lumen (*) but no glomerulus. D, E: protoglomerular nephron (stage III): wt1b::GFP+ tubule with a defined glomerulus (arrow) but no pod::mCherry expression within the glomerulus. F: immature nephron (stage IV): mesonephric glomerulus (arrow) forms at the distal end of the tubule and wt1b::GFP and pod::mCherry (red) are both expressed in the podocytes within the glomerulus. G: mature nephron (stage V): wt1b::GFP expression is restricted to a cluster of cells at the urinary pole of the nephron (arrowhead) and pod::mCherry (red) is expressed in all the podocytes.
Fig. 3.
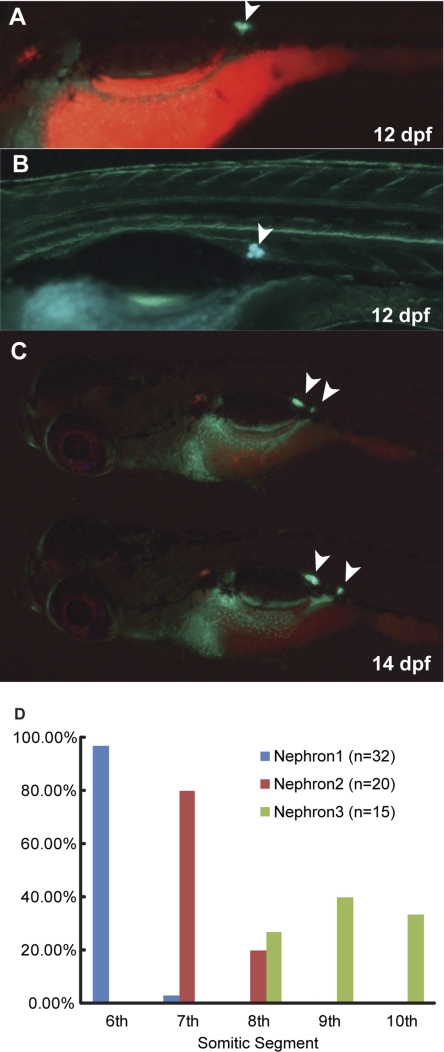
Temporal and spatial patterns of the initial mesonephrogenesis. A: lateral view of a 12-dpf larva of wt1b::GFP/pod::mCherry double-transgenic fish. wt1b::GFP expression (arrowhead) is visible at the location where the first mesonephric nephron is developing. B: lateral view of a 14-dpf larva at higher magnification showing the first wt1b::GFP-expressing aggregates in the mesonephric kidney located ventral to the 6th myotome. C: lateral view of two 20-dpf larvae showing that the locations of the first 2 mesonephric nephrons are consistent. D: histogram of the segmental distribution of the initial mesonephric nephrons indicates that the positioning of the initial mesonephric nephrons is conserved.
Mesonephric development continues throughout the life of zebrafish.
To track the formation of the initial mesonephric nephrons, we imaged intact double-transgenic fish (wt1b::GFP/pod::mCherry). wt1b::GFP expression could be detected by 12 dpf within defined aggregates at the site of initial mesonephric nephrogenesis (Fig. 3A). This site was highly conserved in each larval fish (Fig. 3B). Among 32 larvae followed from 8 to 14 dpf, 97% had the first nephron primordium located ventral to the sixth myotome. More interestingly, the position of the second pair of mesonephric nephrons was similarly segmentally consistent (Fig. 3C), developing ventral to the seventh myotome in 80%, and ventral to the eighth myotome in the remaining 20% of larvae followed to 14 dpf from above (Fig. 3D). These results suggest that the patterning of mesonephric kidney may be under the regulation of positional cues within the nephrogenic region.
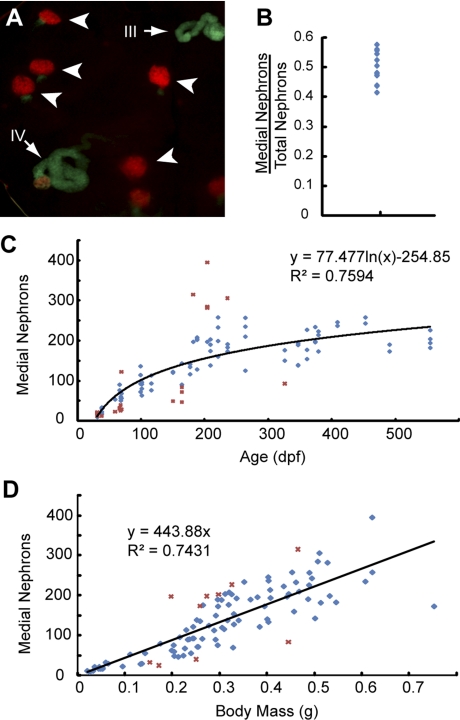
To determine the growth rate of the mesonephros, we sought to construct a growth curve tracking change in nephron number throughout the life cycle of the fish. We sexed, massed, and dissected 101 pod::GFP transgenic fish ranging in age from 28 dpf to 18 mo. We first measured in 13 fish the number of GFP-expressing glomeruli in the whole kidney (including all 4 regions) and found that the nephron counts from the medial portion (MNSR and MNDR) represent 48 ± 5% of the total number of nephron (Fig. 4B). Because the ANDR and the PNSR are likely to be damaged during dissection, precise nephron counts within these regions were technically inefficient to obtain. Therefore, we focused on the nephron counts of the MSNR and MNDR (hereafter referred as “medial nephron number”) in relation to the age of zebrafish. We found that nephron number logarithmically increased throughout the life cycle of the fish. The most rapid growth occurred during the first 3 mo of life (larval to juvenile period), when the medial nephron number increased dramatically until it reached ∼150 (Fig. 4C). After 3 mo of age, when fish were sexually mature, the rate of increase in nephron number sharply declined, but remained positive throughout our period of observation (up to 18 mo; Fig. 4C). Around this same time, pod::GFP expression disappeared from the podocytes of the pronephric glomeruli of the fish in our observation group, suggesting that once an “adult” mesonephric nephron complement is achieved, the pronephric glomeruli cease to serve an excretory function. The continuous rate of kidney development indicated that the zebrafish mesonephros maintained its nephrogenic capacity throughout adult life. Intriguingly, we found medial nephron number to be directly proportional to the body mass of the fish. A linear regression estimated ∼445 medial nephrons per gram body mass (R2 = 0.743; Fig. 4D). This finding suggests that such continuity of mesonephric nephrogenesis may function to keep renal capacity dynamic with the metabolic needs of the fish.
Fig. 4.
Mesonephrogenesis continues throughout the life of zebrafish. A: representative image of mesonephric tissue from an adult fish (age <6 mo) showing that nephrons at different developmental stages are present in adult fish. Although the majority of the nephrons are fully matured at stage V (arrowheads), 2 developing nephrons (arrows) are at stage III and stage IV with significant expression of wt1b::GFP. B: ratio of medial nephron number to total nephron number is 48 ± 5%. C: medial nephron number is logarithmically correlated to age, indicating a continuous increase of nephron number in the life of zebrafish. D: medial nephron number is positively correlated to the body mass of zebrafish. Red crosses in (C and D) represent the outliers not included in the regression calculations as described in materials and methods.
wt1b expression demarcates the early renal progenitor cells during de novo regeneration in the mesonephric kidney.
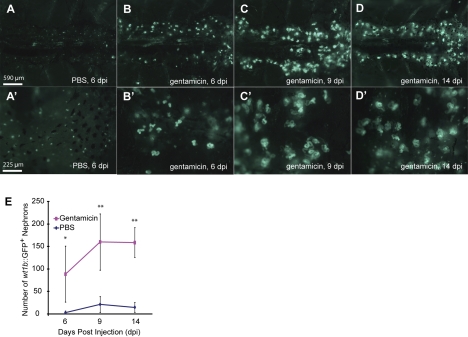
To further investigate this apparent persistent nephrogenic capacity, we then sought to determine whether the zebrafish mesonephros is capable of regenerative neonephrogenesis following renal injury like other teleosts (20, 22, 27). Having defined wt1b::GFP as a developmental marker for mesonephric nephrons, we used it to monitor mesonephric regeneration in transgenic zebrafish following gentamicin-induced renal injury. Twenty-four hours following injection, tubule debris was observed in the water of the gentamicin-injected fish but not in that of the PBS-injected fish. The 24-h survival rate of gentamicin-injected fish was ∼90% (13/15). At 5 days postinjection (dpi), we observed a dramatic increase of wt1b::GFP+ nephrons in the gentamicin-injected fish (Fig. 5, B and B') relative to the controls (Fig. 5, A and A'); additionally, the morphology of these nephrons (Fig. 5B') indicated that they were newly developing (Fig. 3). The number of wt1b::GFP+ nephrons in the gentamicin-injected group had further increased by 9 dpi and the morphology of these nephrons suggested that they were more mature than those seen at 5 dpi. The number of newly developing nephrons had plateaued by 14 dpi (Fig. 5, D–E). Based on the wt1b::GFP expression pattern, regenerative mesonephric nephrogenesis appears to recapitulate the normal stages of mesonephric nephron development.
Fig. 5.
Gentamicin-induced renal injury triggers de novo regeneration of mesonephric nephrons. A, A': injection of PBS does not increase mesonephrogenesis at 5 days postinjection (dpi). The green puncta are wt1b::GFP-expressing cells at the neck of matured nephrons. B, B': by 5 dpi of gentamicin, there is an increased number of developing nephrons. C, C': at 9 dpi of gentamicin, wt1b::GFP expression indicates the progression of these newly made nephrons. D, D': level of nephrogenesis is still elevated at 14 dpi of gentamicin. E: quantification of wt1b-GFP+ nephron numbers postgentamicin-induced renal injury. *P < 0.05, **P < 0.01.
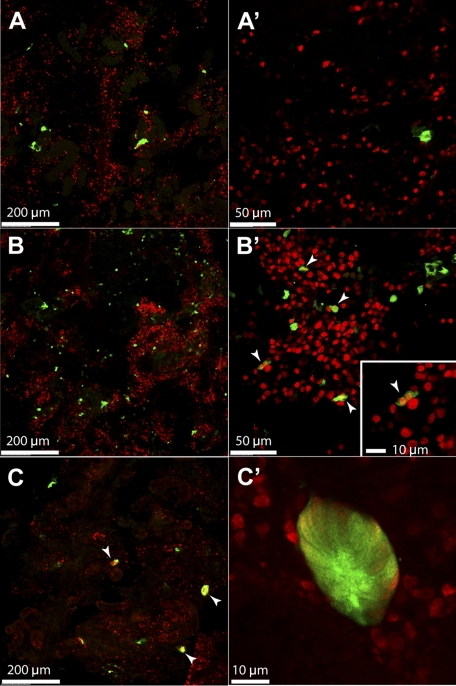
To better understand the mechanism of this regenerative mesonephrogenesis, we examined the early response following gentamicin-induced injury. At 2 dpi, the increase of wt1b::GFP expression was already evident, although no aggregates or nephron-like structure was observed. Instead, there was a drastic increase of wt1b::GFP+ cells scattered throughout the kidney (Fig. 6, B and B') compared with PBS-injected control (Fig. 6, A and A'). Costaining with an antibody against PCNA, a proliferative marker, revealed that many of these wt1b::GFP+ cells were indeed proliferative, By 4 dpi, wt1b::GFP expression was found in stage I nephron primordia. Additionally, all of the cells within the examined nephron primordia were PCNA positive, indicating that they were actively proliferating (Fig. 6, C and C'). Taken together, these results demonstrate that wt1b is expressed in the nephron precursor cells during regenerative neonephrogenesis just as it is during the normal nephron development.
Fig. 6.
Renal injury induces wt1b::GFP expression in dispersed cells in the mesonephric kidney. A, A': 2 dpi of PBS does not induce wt1b:GFP expression. Most wt1b::GFP+ cells are in cluster at the urinary neck of intact mature nephrons in PBS-injected fish. B, B': at 2 dpi of gentamicin, wt1b:GFP expression (green) is induced in dispersed cells in the mesonephric kidney. A subset of these cells (arrowheads) are proliferative as shown with PCNA-staining (red). Inset: higher-magnification image showing a pair of wt1b:GFP-expressing cells are PCNA+. C, C': at 4 dpi of gentamicin, wt1b::GFP+ cells have formed aggregates that are PCNA+ (red).
DISCUSSION
The zebrafish pronephros has been extensively studied in the past few decades and is well-established as a valid vertebrate model system for kidney development and human kidney diseases. Unfortunately, the very simplicity that makes the pronephros a useful model organ system imposes a real technical limitation; its impairment in the fish usually leads to lethality within the first few days of life. In contrast, the mesonephros, as the functional renal organ in juvenile and adult fish, possesses a significantly higher degree of structural and functional complexity. To evaluate the viability of the zebrafish mesonephros as a system for kidney research, we systematically observed and documented its development and morphology using transgenic lines we developed. These transgenics fluorescently label the majority, if not all, of the renal epithelial cells in live zebrafish allowing for convenient imaging and analysis of renal structures. The demonstrated utility of these lines implies that they can be used as effective tools in future studies.
Building from the known regenerative capacity of the zebrafish in several organ systems (15, 17–20), we demonstrated that the mesonephros is capable of de novo regeneration of nephrons following renal injury. While this process has been previously described in other teleost species, that the zebrafish is already a well-established genetic system makes it favorable for use in kidney regeneration studies. Indeed, we found that a zebrafish-specific wt1 homolog, wt1b, displays a unique expression pattern in developing and regenerating nephrons, providing a convenient marker for tracking mesonephric development and regeneration in vivo. In addition, the induction of its expression during regeneration will be useful to isolate and characterize the specification and regulation of the presumptive renal progenitor population within the zebrafish mesonephros. The molecular mechanisms governing mesonephric regeneration and development remain elusive; however, given our finding that mesonephric growth is continuous throughout life, it is probable that the regeneration of the mesonephros may simply be a fast synchronous initiation of normal mesonephrogenesis. Although the function of wt1b in pronephric development has been studied by morpholino oligo-mediated knockdown (16), the precise role played by wt1b in the specification and differentiation of mesonephric nephron progenitors remains unknown. While several possible mechanisms underlying the specification, activation, and differentiation of these progenitors have been hypothesized, the specific mechanism and its molecular regulators have yet to be revealed. As a renal progenitor marker, wt1b::GFP reporter lines will be useful in characterizing the molecular regulation of nephron development and experiments involving manipulation of wt1b expression during mesonephric nephrogenesis and regeneration will be critical in determining the renal progenitor developmental pathway.
That the total mesonephric nephron number directly correlates to the body mass of the fish suggests the existence of a mechanism to keep kidney development and renal capacity dynamic with the needs of the fish. Thus, the activation of normal and regenerative mesonephrogenesis may be well-controlled by potential endocrine and paracrine regulators. Among the candidate molecular regulators of mesonephric nephron development and progenitor specification, it is worth specifically noting both the renin-angiotensin system (RAS) and retinoid signaling.
In vertebrates, the RAS plays an integral role in regulating blood pressure, blood osmolarity, and kidney function. De novo intrarenal ANG II production is known to increase because of renal insufficiency following experimental nephrectomy (7) and during renal disease (14) or in response to chronic peripheral ANG II infusion (23). Additionally, RAS signaling, partially mediated by Wnt4 induction of ACE in the metanephric cap mesenchyme (21, 26), is required for normal metanephric nephron development (10) and ANG II has been demonstrated to lie upstream of many growth factors and transcription factors required for normal kidney development (30, 31). Finally, it is noteworthy that ANG II is able to induce the proliferation of hematopoietic progenitor cells (HPC) isolated from murine bone marrow or human cord blood (21), suggesting its role in regulating stem cells. Thus, experiments involving the manipulation of RAS components during zebrafish nephron regeneration will be particularly useful to determine whether the RAS plays a role in the molecular regulation of renal progenitor cells during mesonephrogenesis and regeneration in zebrafish.
As a known transcriptional activator of zebrafish wt1b (3) and essential to the proper development of zebrafish pronephros (29), retinoic acid and retinoid signaling are likely required for the activation of nephrogenic precursor cells as well as the later patterning of mesonephric nephron segment identity. Experiments using pharmacological and genetic manipulation of retinoid signaling and subsequently wt1b expression will be useful to uncover the molecular mechanisms of mesonephric nephrogenesis and regeneration.
The zebrafish mesonephros is a promising model system for the study of kidney development, structure, and function. Future studies using this organ system will hopefully be able to provide a valuable supplement for studies of complex kidney diseases that are not fulfilled using previously established models. Additionally, studies of the mechanisms of mesonephric regeneration could ultimately pave the way for the development of future therapeutic strategies to address human kidney disease.
GRANTS
F. Hildebrandt is the Frederick G. L. Huetwell professor, Investigator of Howard Hughes Medical Institute, and Doris Duke Distinguished Clinical Scientist and is supported by National Institutes of Health Grants R01-DK-088767 and P30-DK-079312.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Present address of F. Bollig: Hannover Medical School, Institute for Clinical Chemistry, OE 8110, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany.
Supplementary Material
ACKNOWLEDGMENTS
The authors appreciate Drs. R. C. Wiggins and I. A. Drummond for enlightening discussions and Drs. K. Kawakami, S. C. Ekker, and D. Stainier for sharing plasmids.
REFERENCES
- 1.Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, Wang X, Hackett PB, Largaespada DA, McIvor RS, Ekker SC. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet 2: e169, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn 235: 554–561, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bollig F, Perner B, Besenbeck B, Kothe S, Ebert C, Taudien S, Englert C. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development 136: 2883–2892, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236: 1025–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol 16: 299–304, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Drummond IA. The zebrafish pronephros: a genetic system for studies of kidney development. Pediatr Nephrol 14: 428–435, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RE, Wu LL, Kelly DJ, Cox A, Wilkinson-Berka JL, Johnston CI, Cooper ME. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol 155: 429–440, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert SF. Developmental Biology. Sunderland, MA: Sinauer Associates, 2000 [Google Scholar]
- 9.Gilbert SF. Developmental Biology. Sunderland, MA: Sinauer Associates, 2006 [Google Scholar]
- 10.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288: F923–F929, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Horsfield J, Ramachandran A, Reuter K, LaVallie E, Collins-Racie L, Crosier K, Crosier P. Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development. Mech Dev 115: 15–26, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Marvalee H. Hyman's Comparative Vertebrate Anatomy. Chicago, IL: Univ. of Chicago Press, 1992 [Google Scholar]
- 14.Metzger R, Bohle RM, Pauls K, Eichner G, Alhenc-Gelas F, Danilov SM, Franke FE. Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int 56: 1442–1454, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes 58: 1844–1851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perner B, Englert C, Bollig F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309: 87–96, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev 124: 218–229, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science 298: 2188–2190, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci USA 106: 9310–9315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimschuessel R. A fish model of renal regeneration and development. ILAR J 42: 285–291, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 18: 287–294, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med 51: 56–59, 2001 [PubMed] [Google Scholar]
- 23.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174: 639–649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Utts JM, Heckard RF. Mind on Statistics. Florence, KY: Cengage Learning, 2002 [Google Scholar]
- 26.Valerius MT, McMahon AP. Transcriptional profiling of Wnt4 mutant mouse kidneys identifies genes expressed during nephron formation. Gene Expr Patterns 8: 297–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N, Kato M, Suzuki N, Inoue C, Fedorova S, Hashimoto H, Maruyama S, Matsuo S, Wakamatsu Y. Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ 51: 135–143, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: Univ. of Oregon, 2000 [Google Scholar]
- 29.Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 3: 1922–1938, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yosypiv IV. A new role for the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Keio J Med 57: 184–189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yosypiv IV. Renin-angiotensin system-growth factor cross-talk: a novel mechanism for ureteric bud morphogenesis. Pediatr Nephrol 24: 1113–1120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.