Abstract
The rapid diagnosis and quantification of acute kidney injury (AKI) severity remain high clinical priorities. By combining intravital fluorescent ratiometric two-photon kidney imaging and the two-compartment pharmacokinetics model, we demonstrate that rapid quantification of glomerular filtration rate (GFR) can be achieved in physiologic and AKI rat kidney models. Using a bolus infusion of a mixture of FITC-inulin and a 500-kDa Texas Red dextran, a full spectrum of GFR values, ranging from 0.17 to 1.12 ml·min−1·100 g−1, was obtained. The GFR values thus determined correlated well with values obtained by the standard 2-h inulin infusion clearance method with a Pearson's correlation coefficient of 0.85. In addition, postischemia deterioration was studied by measuring GFR using the two-photon approach during 24 h following a 45-min bilateral ischemia clamp model. The GFR was found to decline sharply during the initial 4 h followed by a nadir with little sign of rising over the ensuing 24-h period. Moreover, a FITC-labeled 5-kDa dextran was identified as having nearly identical filtration characteristics as FITC-inulin, but had markedly increased fluorescent intensity, thus minimizing the quantity needed for individual studies. The technique reported allows for very rapid GFR determinations, within 10–15 min, based on plasma clearance of a freely filtered fluorescence probe, instead of a prolonged one-compartment interstitial space reporter molecule clearance employed by other technologies.
Keywords: kidney function, inulin clearance
acute kidney injury (AKI) remains a diagnostic and staging enigma. This has minimized the ability to provide rapid therapy, a fact most believe has severely hampered the translation of promising therapeutic results from preclinical animal models. Numerous investigators are now feverously pursuing “biomarkers” for early identification of injury, and progress is being made (7). For AKI patients, a rapid determination of glomerular filtration rate (GFR) would be an ideal functional biomarker for diagnostic and staging purposes. Unfortunately, the current popular method for evaluating kidney function in AKI, by measuring either serum creatinine or cystatin C, is inaccurate due to several well-known variables (7). Inulin is an ideal GFR reporter since it is freely filtered by glomeruli and unlike creatinine it is neither secreted nor reabsorbed by tubular epithelial cells. Therefore, inulin clearance is regarded as the standard for quantifying GFR. However, the standard procedure for measuring inulin clearance also requires a continuous infusion as well as serum and urine collection for many hours along with tedious laboratory work. As a result, the clinical value of this approach is discounted, especially for AKI patients, where rapid results are essential.
Over the years, alternative techniques for GFR assessment have been developed aiming to make the GFR measurement more efficient and accurate. For these alternative methods, different types of GFR reporter molecules have been used: radioactive materials such as [125I]iothalamate (17), 51Cr-EDTA (2), and 99mTc-DTPA (11); nonradioactive contrast media such as iohexol, iopromide (4, 5, 15), and more recently, fluorescently conjugated inulin (10, 18) and sinistrin (8, 9). A broad range of techniques have been used for detection of the reporter, including colormetric assay, scintillation counting, X-ray fluorescence, HPLC, visible fluorescence, and two-photon intravital microsocpy. Despite the differences between these techniques, the fundamental concept is very similar, that is to monitor the decay of the reporter molecule from the plasma or the extracellular space overtime following a bolus infusion. The mathematical models used for these studies bear similarities as well.
Yu et al. (18) developed a new technique in GFR assessment by using ratiometric two-photon intravital microscopy, a minimally invasive approach, in which two species of fluorescently labeled conjugates were injected simultaneously with a bolus infusion into the subject animals. The large-sized injected conjugates were nonfilterable by the kidneys, and therefore were confined within the vascular space, whereas the small conjugates were freely filterable by the kidneys. It has been demonstrated that using the fluorescence ratio (small/large or reporter/marker), rather than the fluorescence from the filterable reporter in the plasma alone, improved the accuracy of the measurement since the ratio suppressed the fluctuation of the signal induced by the movement of the focal point, mostly caused by the respiration. However, this approach is not applicable to the clinical situation as the nonrenal clearance was obtained by using an anephric rat model.
In the present study, we extended and greatly improved our previously described two-photon microscopy approach (18), by using the two-compartment model for analysis. We studied healthy and AKI rat kidney models to produce a full spectrum of GFR values and possible clinical spectrums of disease. The values we report show a very good correlation with GFR values obtained with the standard inulin clearance analysis. We also quantified, for the first time ever, the early effect of kidney ischemia on kidney function by determining the GFR values at multiple time points within 24 h following a 45-min clamp model of ischemia. The rapid nature of our test allows a diagnostic window not previously possible.
MATERIALS AND METHODS
Animal preparation.
All animal studies were conducted in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines. All protocols and procedures were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Male and female Sprague-Dawley rats 250–350 g (Harlan, Indianapolis, IN) were used for all studies. Animals were anesthetized with 50 mg/kg pentobarbital sodium (Ovation Pharmaceutical, Deerfield, IL). A femoral intravenous access line was placed for delivery of fluorescent molecules. To expose the kidney for intravital imaging, an incision was made on the left back. The rat was placed on the microscope stage with the exposed kidney in contact with the inner surface of the No. 1.5 glass of the 75-mm glass-bottom dish (Willco Wells BV, Netherlands) (3). During imaging, the body of the animal was kept warm by a water-circulating warming blanket. In addition, the microscope stage was warmed using 2 ReptiTherm heating pads (Zoo Med Laboratories, San Luis Obispo, CA) one under the upper torso/head and one under the lower abdomen. The temperature of the animal was maintained between 36.5 and 37.5°C. The ×60 water immersion objective (Nikon Plan Apo, NA 1.2) was heated using an objective heater (Warner Instruments, Hamden, CT). The mean arterial blood pressure was from 80 to 112 mmHg as measured via a femoral arterial line using a direct blood pressure kit (Kent Scientific, Torrington, CT).
Injury models.
To quantify GFR during AKI, three different AKI injury models were used: 1) 45-min bilateral ischemia with 2, 4, 8, and 24 h of recovery; 2) LPS (Sigma, St. Louis, MO) ip injection (7.5 mg/kg) with 4-h recovery; and 3)daily gentamicin (Sigma) ip injections (200 mg/kg).
Fluorescent conjugates.
FITC-inulin was purchased from Sigma, and 500-kDa amino dextran and Texas Red sulfonyl chloride were both from Invitrogen. After conjugation of Texas Red to the large dextran, the unbound dye molecules were removed by dialyzing against 0.9% NaCl in ddH2O using 300-kDa cutoff MWCO filter, Cellulose Ester Membrane from Spectrum Laboratories (Rancho Dominguez, CA). FITC-dextran (5 kDa) was a gift from FAST Diagnostics (Indianapolis, IN).
Two-photon microscopy.
Unless specified otherwise, two-photon microscopy was conducted on the Bio-Rad MRC 1024 confocal/two-photon microscope as described previously (3, 18). The illumination source was a tunable Tsunami Ti:Sapphire laser from Spectra-physics (Mountain View, CA). The microscope was an inverted Nikon Eclipes TE200. The band pass filters used for the red and green channels were 605/90 and 525/50, respectively. The excitation wavelength was set at 800 nm for all experiments. The anesthetized rat was placed on the microscope stage with the exposed kidney in direct contact with the coverglass (see Animal preparation). A ×60 Nikon Plan Apo water immersion objective (NA 1.2) was used for all imaging work. Plasma clearance was monitored by collecting two-dimensional time series over the kidney cortical microvasculature 15–20 μm below the surface. Each time series, with a duration up to 30 min, started seconds before the infusion to obtain the background image. Unless specified, all image series were collected at a constant pixel dwell time, which yielded a 1.1-s frame time for the frame size of 512 × 512. The first 100 time points were acquired at no interval between frames, but the rest of time points in each series were collected with a 1-min interval. The initial high-frequency sampling rate was necessary to capture the rapid intensity change during the initial mixing and distribution phase following the bolus infusion, but the subsequent longer interval between time points was intended for reducing the total laser exposure. All images for a given study were collected from the same focal plane.
Image processing and data analysis.
Images were processed using Metamorph (Universal Imaging, West Chester, PA). Blood vessels containing regions in each image were outlined manually. The average intensity of all pixels in the outlined regions in each image was measured. The average intensity values were exported to Microsoft Excel spread sheet. Background correction was made for each series by subtracting the average intensity in the vessel containing regions (outlined manually) in images taken before the bolus infusion from the average intensity of the same outlined regions in images taken after the infusion. Background-corrected fluorescence intensity data were fitted to Eq. 4 (with the small molecule only) or Eq. 6 (with the ratio) using Sigmaplot version 11.0 (Systat) to obtain parameters in the equations. The fluorescence intensity vs. time plots were also created using SigmaPlot. A certain amount of fluorescence signal generated by the green fluorophore bled over into the red channel. For each experiment, the crosstalk factor was determined by imaging a solution containing only the green conjugate using a coverslip bottom dish. Since the solution contained no red conjugate, the net intensity in the red channel (Ir), which was proportional to the signal in the green channel (Ig), was solely attributed as a consequence of bleed over from the green fluorescence. The amount of the crosstalk for a given image was determined by multiplying the green channel intensity of that image with the crosstalk factor (Ir/Ig). The product was then subtracted from the red channel for each image in the series. Images containing a substantial number of saturated pixels in the outlined regions were excluded for analysis; however, this was a rare event as injections were given with continuous monitoring of vascular fluorescence.
GFR determination by standard inulin clearance.
Tracheas were intubated using PE-240 tubing. The femoral artery was catheterized using PE-50 tubing to monitor blood pressure and allow blood sampling. The bladder was cannulated with PE-240 for urine collection. The jugular vein was catheterized for delivery of inulin (100 g/kg body wt in 1 ml normal saline). Continuous infusions of inulin were made at 0.04 ml/min of a 6.667 mg/ml solution in normal saline. Three consecutive 15-min periods of urine collection were done, with blood samples drawn at the beginning and end of the collection period. Inulin concentrations were determined using the classic anthrone method and GFR was calculated using a standardized formula.
Two-compartment model.
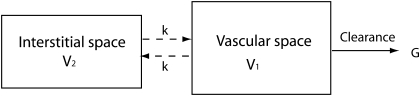
We used the mathematical model described below to calculate the reported GFR values in the current study. Studies demonstrated that GFR and apparent volumes of distribution can be measured by monitoring the plasma disappearance of the fluorescently labeled molecules intravenously administered by a single dose bolus injection (10, 13, 15). Figure 1 illustrates a widely used two-compartment model, also known as three-component model. The two compartments in question are vascular space and interstitial space. The basic assumption for this model is that after the bolus injection, the infused small-sized molecules will distribute from the vascular space into the interstitial space, due to the permeable barriers between the two compartments, and will not move into cells. Also, large-sized molecules will be retained in the vascular space into which the bolus injection was given. Furthermore, to use this model to measure GFR, one assumes clearance only takes place from the vascular place. The sum of the vascular and interstitial fluid volumes is the extracellular fluid volume.
Fig. 1.
Two-compartment model.
The plasma clearance rate and the intercompartment clearance rate are denoted as G and k, respectively. The fluid volumes for the vascular space and interstitial space are V1 andV2, respectively. As demonstrated by Sapirstein et al. (13), the concentration of the infused GFR reporter molecule in the plasma can be expressed as a biexponential equation:
| (1) |
where the amplitudes A, B, and the decay constants can be obtained by fitting the experiment data to the above equation.
Assuming the intercompartment movement is negligible before the intracompartment mixing in V1 is completed, then the GFR value can be expressed as (13):
| (2) |
where D is the dose of the injected reporter molecule. The plasma volume V1 can then be expressed as:
| (3) |
As demonstrated by Lee and Blaufox (6), V1 can also be measured using large-sized molecules, which will stay in the vascular space after bolus infusion.
If the linear relationship between the concentration and fluorescence intensity holds for the GFR reporter molecule, Eq. 1 can then be rewritten as:
| (4) |
where F1 is the fluorescence intensity of the reporter molecule as a function of time. A1 and B1 are new amplitudes in terms of fluorescence intensity. Thus, Eq. 2 can be rewritten as follows:
| (5) |
Most of our GFR measurements have been conducted by a bolus infusion of a mixture of small conjugates (such as FITC-inulin) and large conjugates (such as 500-kDa Texas Red-dextran). Since the small conjugates are freely filterable by the kidneys, they become the GFR reporter. Following a brief mixing period, usually ∼20 s for rats, fluorescence from the small injectate starts a decaying process, whereas the large injectate remains in the vascular space so that its fluorescence will be stable over time. As a result, Eqs. 4 and 5 can be expressed using the fluorescence ratio:
| (6) |
where R(t) is the fluorescence ratio of the small conjugates over the large conjugates. Thus, the GFR can be expressed as:
| (7) |
RESULTS
Kidney imaging by two-photon microscopy and GFR determination.
Initial studies were undertaken to quantify the rate of vascular disappearance of FITC-inulin and document the intravascular stability of the large Texas Red-dextran. Images from the same focal plane were selected from two-dimensional kidney time series images and are displayed in Fig. 2. The rat was infused with a mixture of 500-kDa Texas Red-dextran (4 mg) and 5-kDa FITC-inulin (3.3 mg) over a 5- to 10-s period. It is evident that the fluorescence intensity of large Texas Red-dextran (top panels) in the blood vessels remained relatively stable from 12 s (A) to 15 min (D). Meanwhile, the intensity of 5-kDa FITC-inulin decreased substantially during the same time period (Fig. 2, E–H). Figure 2I shows normalized quantitative ratiometric fluorescent data from an experiment carried out for 20 min.
Fig. 2.
Two-photon images from a living rat infused a mixture of 500-kDa Texas Red-dextran (red; A–D) and FITC-inulin (green; E–H). The overall intensity in the capillaries (CAP) was relatively stable for the red channel, but it decreased drastically for the green channel indicating the loss of labeled inulin from the vascular space. Plasma regions in the capillaries were stained by the probes. The dark objects in the capillaries are red blood cells (RBC) as they exclude staining. FITC-inulin was cleared by the kidney rapidly as it strongly stained the proximal tubule (PT) lumen 80 s after the infusion. Postinfusion times were shown in the bottom panels.
In all studies, the same optical plane was imaged and quantified. As described in methods and materials, the average fluorescence intensity from the vessel-containing regions (outlined manually) in each image of a time series was measured using the Metamorph program. The GFR value was then calculated using the rate of disappearance and the estimated plasma volume based on the body weight of the individual rat (3.13 ml/100 g) (1). In using this approach, actual plasma concentration determinations are not necessary as we can see from Eqs. 5 and 7 that GFR can be determined by using the normalized initial amplitudes A/(A+B) and B/(A+B), the decay rates α and β, and the plasma volume, which are all independent of the concentration. Since the same field and focal plane were imaged for each data set, signal attenuating factors, such as light scattering and movement of red blood cells, would be constant. Therefore, the relative decay of the signal over time would not be affected.
One-compartment vs. two-compartment model.
Presently, techniques used to determine GFR in patients with a stable serum creatinine-like iohexel or iothalamate use a one-compartment model where clearance is only occurring from the equilibrated ECF and vascular volumes. This requires an initial extended equilibration period between these two compartments before sampling, with a minimum of 2 h for iohexol before taking serum samples (15), with later points up to 6 h. Using a two-compartment model allows for rapid secular clearance determinations markedly hastening the test methodology. However, a two-compartment model requires a greater number of data points to fully describe the kinetics of the clearance.
To determine the importance of a one- vs. a two-compartment model, data from rats under physiologic conditions and following ischemia were compared. Two-photon kidney images were obtained following a bolus iv injection of FITC-inulin and 500-kDa Texas Red-Dextran. The normalized fluorescence ratio intensity of FITC-inulin from the blood vessel-containing regions on each image is shown as a single point in Fig. 3. The left column (A and C) shows the data from a rat under physiologic conditions and the right column (B and D) shows the data from a rat that was 24-h postrecovery from a kidney injury induced by 45-min bilateral ischemia (see methods and materials). Experimental data were fit to both one- and two-compartment models. The fitted lines generated by these models are shown in Fig. 3, A and B (top row), as dotted and solid lines, respectively. Even though the one-compartment model fits well to the initial time points, only the two-compartment model fits well to the entire group of experimental data points. In both instances, the relatively rapid initial decay was primarily due to the intercompartment movement of FITC-inulin from the plasma to the interstitial space through the highly permeable capillaries in the vascular system. In contrast, the subsequent slower decaying phase was mainly contributed by kidney clearance of the filterable marker molecule. After early time points, the one-compartment model curve begins to deviate from the experimental data points in each data set (Fig. 3, A and B).
Fig. 3.
Comparison of 1- and 2-compartment models. Data of fluorescence ratio of FITC-inulin/500-kDa Texas Red-dextran (●) from 2-photon images of a rat under physiologic conditions (left) and from an ischemic model (right) were fitted to 1-compartment (dotted line) and 2-compartment (solid line) models (A and B). The 1-compartment model only fits the initial time points well, but the 2-compartment model fits well to the entire data points for both animal models. The same 2 experimental data sets shown in the top row were replotted with separated fast (solid line) and slow (dotted line) exponentials (bottom row) in C and D, respectively. For clarity purpose, the fast exponential curves have been shifted up. The fast exponential seen on both panels were similar indicating a similar intercompartment movement of FITC-inulin on both animals, but the difference on the slow exponential between the rats was substantial. A more rapid decay seen with the rat under physiologic conditions indicates that this animal had a higher glomerular filtration rate (GFR) than the ischemic rat.
The fast and slow exponential components generated from the same data sets, shown in Fig. 3, A and B, were plotted separately in Fig. 3, C and D, respectively. For clarity purposes, the fast exponential curve is shifted up. Comparing the fast exponential in Fig. 3, C and D, shows they are quite similar as expected, but the slow exponentials in the two panels are different with respect to their decay rates. The difference in GFR between the control and ischemic rat is demonstrated by this slow component. The slow portion of the decay curve is the standard used in studies to measure GFR (17). As few as two data points are necessary for GFR determination, thus minimizing blood draws, sample preparation, and analysis times.
Comparing single- and two-compartment model determinations.
We next compared the GFR values obtained with the two-compartment model GFR2 (using all data points) and single-compartment model GFR1 (using only the slow tail of the decay curve). Using the fluorescent ratiometric technique for one- and two-compartment models described above for four different rat kidney models including one under physiologic conditions and three AKI injury models induced by LPS, gentamicin, or ischemia (see methods and materials). The results are shown in Fig. 4A. The GFR obtained using all time points fit to the two-compartment model was denoted as GFR2 (2 compartment) and the GFR estimated using only the time points (a duration of up to 20 min following the infusion) from the tail of the decay curve, starting approximately from 300 s onward, fit to the single exponential equation was denoted as GFR1. GFR2 was calculated as described in the methods and materials, but GFR1 was calculated using the following equation:
| (8) |
Fig. 4.
Comparison between GFR1 and GFR2 and determination of minimal sampling time. GFR1 was obtained by fitting the time points from 300 s onward to a single exponential equation and using Eq. 8. GFR2 was obtained by fitting all data points to the 2-compartment model. The solid line was obtained by fitting the 2 sets of GFR values to a quadratic equation. At high values, GFR1 deviates from the diagonal line (dotted) on which the 2 sets of GFR would be identical (A). The correlation between GFR2 and GFR2b is shown in B. GFR2b was determined using a 15-min postinfusion internal. Pearson coefficients between GFR2 and the GFR obtained with reduced time intervals of 15, 10, and 7 min are also shown.
where A, B, α, and β are defined above in Eq. 1. A′ and γ are the amplitude and the decay constant of the single exponential decay, respectively. As demonstrated in other studies (15, 17), GFR1 and GFR2 values agree well; however, GFR1 overestimates the GFR2 at high clearance rates as demonstrated by an upward deviation of the data points from the diagonal line (dotted line) at high GFR values. This overestimate by GFR1 is previously well-documented and known to be caused by the underestimate of the total concentration area using only the slow component, which becomes more prominent at high clearance rates. Therefore, it is better to use all of the data and a two-compartment model.
Determination of minimal sampling time.
We next determined the minimal sampling time that could be utilized with the two-compartment model to obtain an accurate GFR. To do this, we began with all the data collected up until 20 min postinfusion and compared this with reduced time intervals of 15, 10, and 7 min. As can be seen in Fig. 4B, as the interval was shortened, there was a decrease in the correlation coefficient and the greatest error was always seen in the low GFR range. However, the correlation coefficient was still acceptable at 0.968 even with just 10 min of data recording postmarker injection.
Correlation with standard inulin clearance.
The GFR values obtained by two-photon microscopy were validated for all individual rats in the four model groups using the standard 2-h inulin clearance approach (see methods and materials). The correlation plot between the GFR values from the two different methods is shown in Fig. 5. The Pearson correlation coefficient was 0.85. The mean GFR value from the normal animals (the control group) was among the highest of all four groups, whereas the rats in the ischemia group had the lowest mean GFR value. The LPS- and gentamicin-treated groups produced GFR values in the middle range. A full range of GFR values was thus presented by these models. It is likely the discrepancy between the two sets of GFR values was determined by different methods primarily introduced by the error in plasma volume, which was estimated based on the body weight (3.13 ml/100 g body wt; see methods and materials). Variation in plasma volume exits between individuals even under the same physiological conditions, however, the different experiment procedures used in this study would likely introduce more errors in the estimate. We expect the accuracy of the measured GFR by the two-compartment model to be improved when the measured plasma volume can be accurately calculated.
Fig. 5.
Correlation with the standard inulin clearance. The GFR values (ml·min−1·100 g−1) obtained by 2-photon microscopy from all 4 animal models are plotted against the values from the gold standard, the inulin clearance with continuous infusion. The Pearson coefficient is 0.85. The model under physiologic conditions (●) produced the highest GFR, whereas the ischemic model (▵) showed the lowest GFR. The other 2 models, gentamicin (▴)-treated and LPS (○)-treated, had GFR in the middle range.
Postischemia GFR.
The change in GFR within 24-h period after 45-min bilateral ischemia is shown in Fig. 6. Three animals were used for each time point. The average GFR value declined to ∼50% from normal baseline values 2 h after ischemia. This rapid decline in GFR continued up to 4-h postinjury. There was little sign of recovery in terms GFR even at 24 h after ischemia. This represents the first time the early time course of a fall in GFR postischemic injury has been documented.
Fig. 6.
Postischemia effect. The postischemia GFR values obtained using 2-photon microscopy are shown with the recovery time following a 45-min bilateral ischemia treatment.
Use of fluorescent dextran to quantify GFR.
We next asked whether a fluorescently labeled dextran, with a similar molecular weight to FITC-inulin, would be cleared from the vascular compartment in the same fashion as inulin which has been proven as a reliable GFR marker. The relatively inefficient labeling of the FITC-inulin currently available on the market, and the relatively low solubility of inulin, make it highly desirable to find a more efficient substitute. A brighter fluorescent label would help reduce the amount of injectate required for each study. To this end, we used a 5-kDa FITC-dextran and compared it with FITC-inulin in the same rat under physiologic conditions. We found that the two conjugates gave virtually identical disappearance curves when used as GFR reporters. As shown in Fig. 7, the normalized fluorescence decay curves of the two overlapped very well. The two-compartment GFR values determined by the labeled 5-kDa dextran and inulin were virtually identical, 0.99 and 1.01 ml·min−1·100 mg−1, respectively. This experiment was conducted on an Olympus FV1000 two-photon microscope with a ×60, NA 1.2 water immersion objective and external GaAsP photon multiplier tubes. The filter settings were comparable with the settings on the Biorad MRC 1024 two-photon. The single-photon spectroscopy analysis using the Molecular Device SpectraMax M5 indicated that the 5-kDa FITC-dextran was much brighter (4 times higher in absorbance at 488 nm and 5 times brighter in emission at 520 nm) than the FITC-inulin. The emission intensity comparison of the two reporters is shown as an inset in Fig. 7. This result was also supported by an in vivo experiment (Fig. 7) in which the amount (in mg) of 5-kDa FITC-dextran used was four- to fivefold less than the FITC-inulin.
Fig. 7.
Comparison of FITC-inulin and 5-kDa FITC-dextran. Data were collected on an Olympus FV 1000 home-built 2-photon system. Fluorescence intensity decay curves from the same rat that was first injected with 0.68 mg of 5k FITC dextran (●) followed by a second infusion with 3.3 mg of FITC inulin (○). The second infusion was given after all 5k FITC dextran from the first infusion had been completely cleared. Inset: result of single-photon fluorescence analysis conducted on the Molecular Device SpectraMaxM5. An identical concentration (1 μg/ml) was used for both 5K FITC-dextran and FITC-inuin. The excitation and emission wavelengths were 488 and 520 nm, respectively.
DISCUSSION
AKI remains a difficult diagnostic and staging dilemma. Functional serum markers of GFR such as creatinine are slow to rise postinjury, insensitive, and affected by many other variables. Biomakrers such as KIM-1, NGAL, and IL-18 are presently under intense evaluation as potential early indicators of injury. However, even if these prove to be of diagnostic value to the individual patient, a functional evaluation of GFR would be extremely important in determining the severity of injury, rapid reversibility, and response to therapy. For instance, if one knew the GFR was <10 ml/min on day 1, then the use of renal replacement therapy may occur earlier in the patient's course. Rapidity, sensitivity, specificity, safety, convenience, cost, and repeatability are all concerns that must be dealt with in this regard. For AKI, the situation is made worse by the lack of equilibrium of serum creatinine and the changing GFR that may be occurring over time secondary to rapid functional deterioration or improvement. Thus, the diagnosis and quantification of severity of AKI are targets for those attempting to conquer this high goal. The presently used techniques to quantify GFR, such as iohexol and iothalamate, are only reliable in patients with a stable GFR setting and need a prolonged measurement time as they require an initial distribution into the interstitial space and subsequent clearance from this space. Therefore, given we want to have a rapid test, we continue to believe quantifying disappearance from the vascular compartment is a superior and attainable goal.
The present studies markedly advanced our previous attempt utilizing a one-compartment model with the fluorescent ratiometric approach (18). As pointed out previously, the ratiometric approach markedly reduces the signal fluctuation, thus it improves the accuracy of the measurements (18). Studies using rat (16) and mouse (10) models demonstrated that a two-compartment model can substantially shorten the length of time for data collection and improve accuracy. However, this requires rapid and numerous blood draws during the initial portion of the decay curve immediately following the bolus infusion. Rapid and repeated sampling, something easily accomplished using two-photon microscopy, is difficult with repeated blood draws.
The two-compartment model markedly limited the total data collection time necessary for accurate determinations. In the rat we were able, with a high degree of confidence, to show that we could limit the collection time to 10–15 min postinjection. We were able to quantify GFR throughout the entire range of GFRs in rats with and without AKI. Furthermore, we were able to determine the rapid rate of decline in GFR following a moderate to severe ischemic injury, something previously not possible. Finally, we developed and reported that a FITC-dextran, with a molecular weight similar to that of FITC-inulin, had nearly identical distribution and elimination characteristics as FITC-inulin. As inulin has poor aqueous solubility and poor labeling characteristics for FITC, the use of a highly water-soluble and highly substituted FITC-labeled dextran is very important in minimizing the dose of fluorescent marker and injectate volume required.
One- and two-compartment models.
Sapirsterin and co-workers (13) introduced the two-compartment pharmacokinetics model to conduct physiological studies in dogs, and variants of this model have been investigated by Sturgeon et al. (16). When measuring the plasma decay of the reporter the best result is usually achieved with the two-compartment model (15). However, using the tail portion of the reporter plasma concentration decay curve (one-compartment model) may produce a good approximation of GFR values (15, 17). When the clearance of the reporter molecule is measured for the entire extracellular space rather than for the vascular space alone, the result is not affected by the intercompartment movement of the reporter. Rabito et al. (11, 12) applied the one-compartment model to measure GFR on patients using a noninvasive radioactive monitor. By measuring [125I]iothalamate plasma clearance in peritoneal dialysis patients, Thomaseth and Amici (17) found that given the low level of plasma clearance among these patients the one-compartment approximation can be used. More recently, Kchock-Kusch and co-workers (14) determined GFR in rats using the one-compartment model with a noninvasive approach by following the interstitial disappearance of the injected FITC-sinistrin. Although this was an important step toward noninvasive determination of GFR using fluorescence technologies, extremely high doses of FITC-sinistrin used in the study could be problematic and the duration of the study was prolonged. In addition, due to the noninvasive nature of the method, the calculation was based on a semiempirical equation.
The present studies had limitations. For calculation of GFR, we utilized standard formulas to determine vascular volumes, which were based on rats under physiologic conditions and this may not be true for rats with AKI and the associated inflammation and endothelial dysfunction. In future studies, we will utilize the dilution of the large fluorescent nonpermeable marker to determine vascular volume.
In summary, we showed that rapid determination of GFR can be achieved by combining ratiometric two-photon microscopy and the two-compartment model to quantify vascular clearance components. Our study represents another step forward in our ultimate goal of using a two-compartment ratio-metric method to rapidly quantify GFRs in humans.
GRANTS
This work was made possible by funding from the National Institutes of Health Grants P30-DK-079312, R01-DK-069408, R41-DK-079477, and from FAST Diagnostics, BioCrossroads, and the Indiana Economic Development's 21st Century Fund.
DISCLOSURES
Dr. Molitoris receives grant funding from, is a founding member of, and is the Medical Director for FAST Diagnostics. R. Sandoval and E. Wang are research consultants for FAST Diagnostics.
REFERENCES
- 1.Altman PL. Blood and other body fluids (ed. DS Dittmer) 1961. Federation of American Societies for Experimental Biology, Washington, DC [Google Scholar]
- 2.Brandstrom E, Grzegorczyk A, Jacobsson L, Friberg P, Lindahl A, Aurell M. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe Nephrology Dialysis. Transplantation 13: 1176–1182, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Erley CM, Bader BD, Berger ED, Vochazer A, Jorzik JJ, Dietz K, Risler T. Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 29: 1544–1550, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Hackstein N, Bauer J, Hauck EW, Ludwig M, Kramer HJ, Rau WS. Measuring single-kidney glomerular filtration rate on single-detector helical CT using a two-point Patlak plot technique in patients with increased interstitial space. AJR Am J Roentgenol 181: 147–156, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 26: 72–76, 1985 [PubMed] [Google Scholar]
- 7.Molitoris BA, Melnikov VY, Okusa MD, Himmelfarb J. Technology insight: biomarker development in acute kidney injury–what can we anticipate? Nat Clin Pract Nephrol 4: 154–165, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Pill J, Issaeva O, Woderer S, Sadick M, Kranzlin B, Fiedler F, Klotzer HM, Kramer U, Gretz N. Pharmacological profile and toxicity of fluorescein-labelled sinistrin, a novel marker for GFR measurements. Naunyn Schmiedebergs Arch Pharmacol 373: 204–211, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Pill J, Kloetzer HM, Issaeva O, Kraenzlin B, Deus C, Kraemer U, Sadick M, Fiedler F, Gretz N. Direct fluorometric analysis of a newly synthesised fluorescein-labelled marker for glomerular filtration rate. Anal Bioanal Chem 382: 59–64, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Rabito CA, Moore RH, Bougas C, Dragotakes SC. Noninvasive, real-time monitoring of renal function: the ambulatory renal monitor. J Nucl Med 34: 199–207, 1993 [PubMed] [Google Scholar]
- 12.Rabito CA, Panico F, Rubin R, Tolkoff-Rubin N, Teplick R. Noninvasive, real-time monitoring of renal function during critical care. J Am Soc Nephrol 4: 1421–1428, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Sapirstein LA, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearances of intravenously injected creatinine in the dog. Am J Physiol 181: 330–336, 1955 [DOI] [PubMed] [Google Scholar]
- 14.Schock-Kusch D, Sadick M, Henninger N, Kraenzlin B, Claus G, Kloetzer HM, Weiss C, Pill J, Gretz N. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant 24: 2997–3001, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Sturgeon C, Sam AD, 2nd, Law WR. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J Appl Physiol 84: 2154–2162, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Thomaseth K, Amici G. Optimal design of a two-sample test for assessing [125I]iothalamate plasma clearance in peritoneal dialysis. Nephrol Dial Transplant 13: 2265–2270, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol 292: F1873–F1880, 2007 [DOI] [PubMed] [Google Scholar]