Abstract
The Na-K-2Cl cotransporter (NKCC2) mediates NaCl absorption by the thick ascending limb of Henle's loop (THAL). Exocytosis and endocytosis regulates surface expression of most transporters. However, little is known about the mechanism of NKCC2 trafficking in the absence of stimulating hormones and whether this mechanism contributes to regulation of steady-state surface expression of apical NKCC2 in the THAL. We tested whether NKCC2 undergoes constitutive endocytosis that regulates steady-state surface NKCC2 and NaCl reabsorption in THALs. We measured steady-state surface NKCC2 levels and the rate of NKCC2 endocytosis by surface biotinylation and Western blot and confocal microscopy of isolated perfused rat THALs. We observed constitutive NKCC2 endocytosis over 30 min that averaged 21.5 ± 2.7% of the surface pool. We then tested whether methyl-β-cyclodextrin (MβCD), a compound that inhibits endocytosis by chelating membrane cholesterol, blocked NKCC2 endocytic retrieval. We found that 30-min treatment with MβCD (5 mM) blocked NKCC2 endocytosis by 81% (P < 0.01). Blockade of endocytosis by MβCD induced accumulation of NKCC2 at the apical membrane as demonstrated by a 60 ± 16% (P < 0.05) increase in steady-state surface expression and enhanced apical surface NKCC2 immunostaining in isolated, perfused THALs. Acute treatment with MβCD did not change the total pool of NKCC2. MβCD did not affect NKCC2 trafficking when it was complexed with cholesterol before treatment. Inhibition endocytosis with MβCD enhanced NKCC2-dependent NaCl entry by 57 ± 16% (P < 0.05). Finally, we observed that a fraction of retrieved NKCC2 recycles back to the plasma membrane (36 ± 7%) over 30 min. We concluded that constitutive NKCC2 trafficking maintains steady-state surface NKCC2 and regulates NaCl reabsorption in THALs. These are the first data showing an increase in apical membrane NKCC2 in THALs by altering the rates of constitutive NKCC2 trafficking, rather than by stimulation of hormone-dependent signaling.
Keywords: apical trafficking, cholesterol, Na-K-2Cl cotransport
the thick ascending limb of the loop of Henle (THAL) reabsorbs up to 30% of the renal salt load and plays an important role in the maintenance of body salt and fluid homeostasis. This function is achieved by the apical renal Na-K-2Cl cotransporter (NKCC2), a 160-kDa glycosylated protein composed of 12 transmembrane domains. NKCC2 is expressed in the apical membrane and cytoplasmic space of medullary and cortical thick ascending limbs, including macula densa cells (12, 14, 32, 39).
Most of the literature indicates a direct relationship between the amount of apical NaCl transporters in the plasma membrane and their activity (1, 2, 4, 34). We previously studied the effect of second messengers on NaCl transport and steady-state surface NKCC2 levels in THALs. We found that cGMP, the second messenger for nitric oxide (NO) and natriuretic peptides, decreases NaCl reabsorption by reducing steady-state surface NKCC2 levels (1). Opposite to cGMP, vasopressin and the second messenger cAMP enhanced NaCl reabsorption by increasing steady-state surface NKCC2 levels in THALs (4, 34). In addition to increased apical membrane levels, others have shown enhanced phosphorylation of NKCC2 in response to physiological stimuli (11, 16, 17). Despite the importance of NKCC2 in NaCl homeostasis, little is known about the membrane-trafficking pathways controlling its apical membrane levels, whether membrane trafficking occurs in the absence of hormonal stimulation, or the physiological relevance of such mechanisms in the THAL.
The abundance of some transporters in the apical membrane, like sodium-hydrogen exchanger 3 (NHE3) (8), is decreased by stimulated endocytosis whereas others, such as the chloride channel (CLC-5) (20), epithelial Na+ channel (ENaC) (45), and sodium-chloride cotransporter (NCC) (24) undergo constitutive and regulated endocytosis. Recently, we observed constitutive exocytic insertion of NKCC2 in THALs (4), suggesting that NKCC2 cycles between intracellular compartments and the apical membrane. However, it is not known whether NKCC2 undergoes endocytosis and recycling and whether these trafficking mechanisms contribute to the physiological function of the THAL.
Cholesterol is an essential constituent of membranes and plays a crucial role in the endocytic process. A high proportion of total cell cholesterol (70–90%) is located in the plasma membrane of eukaryotic cells (25, 26). Cholesterol depletion increases membrane rigidity and potently inhibits endocytosis in all cells (23, 33, 53). Cyclodextrins are a family of cyclic oligosaccharides produced from starch by an enzymatic reaction complex and chelate cholesterol. Among them, methyl-β-cyclodextrin (MβCD) has been shown to be the most efficient in chelating and extracting cholesterol from the plasma membrane (23, 33) and in blocking clathrin- and lipid raft/caveolae-dependent endocytosis (6, 18, 27, 40, 42, 46). In renal epithelial cells, Lu et al. (28) showed that MβCD induced the accumulation of aquaporin-2 at the plasma membrane, suggesting inhibition of endocytosis. It is not known whether NKCC2 endocytosis is sensitive to cholesterol chelation. In the present study, we tested the hypothesis that endocytosis regulates steady-state surface NKCC2 expression and that this trafficking pathway is blocked by the cholesterol-chelating agent MβCD in THALs. In addition, we studied whether retrieved NKCC2 recycles back to the membrane in a constitutive manner. Our data indicate that MβCD induces NKCC2 accumulation at the apical surface by potently blocking constitutive NKCC2 endocytosis in rat medullary THALs. To our knowledge, this is the first evidence showing that a membrane transporter is regulated by endocytosis and recycling in the THAL.
MATERIALS AND METHODS
Suspensions of Medullary THALs
Suspensions of rat medullary THALs were obtained as previously described (1, 4, 34). Male Sprague-Dawley rats, weighing 200–250 g (Charles River Breeding Laboratories, Wilmington, MA), were maintained on a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) with water ad libitum. Rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). The abdominal cavity was opened, and the kidneys were perfused retrograde via the aorta with physiological solution (PS) containing 0.1% collagenase (Sigma, St. Louis, MO) and 100 U/ml heparin. The composition of the PS was (in mM) 130 NaCl 130, 2.5 NaH2PO4, 4.0 KCl, 1.2 MgSO4, 6 l-alanine, 1.0 Na-citrate, 5.5 glucose, 2.0 Ca-lactate, and 10 HEPES, pH 7.40. The inner stripe of the outer medulla was cut from coronal slices, minced, and incubated at 37°C for 30 min with 0.1% collagenase in PS and gassed every 5 min with 100% O2. Tissue was pelleted by gentle centrifugation, resuspended in PS, and stirred on ice for 30 min to release tubules. The suspension was filtered through a 250-μm nylon mesh, washed, centrifuged again, and resuspended in PS. All animal protocols were approved by the Institutional Animal Care and Use Committee at Henry Ford Hospital.
Measurement of Steady-State Surface NKCC2 in THALs
To measure steady-state surface NKCC2, THAL suspensions were treated with either agonists or vehicle at 37°C for the indicated time period, rapidly cooled to 4°C, and then surface proteins were labeled with NHS-SS-biotin as previously described (1, 4, 34). Briefly, after THALs were rinsed with buffer [HEPES-Ca2+/Mg2+ (in mM) 150 NaCl, 10 Na2HPO4, 0.1 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.8], tubules were treated with 0.75 ml of 0.9 mg/ml freshly prepared NHS-SS-biotin (Pierce Biotechnology, Rockford, IL) on a rocker platform at 4°C for 15 min. Then, 0.75 ml of freshly prepared NHS-SS-biotin (0.9 mg/ml) was added on top of the previous, and tubules were incubated for an additional 15 min. Excess NHS-SS-biotin was quenched with 100 mM glycine in PS. THAL suspensions were lysed with buffer containing (in mM) 150 NaCl, 50 Tris·HCl, pH 7.4, and 5 EDTA, as well as 2% (vol/vol) Triton X-100, 0.2% (wt/vol) SDS, and protease inhibitors 10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mmol/l benzamidine, 5 μg/ml chymostatin, and 5 μg/ml pepstatin A (Sigma). Total protein content in each sample was measured in triplicate. Equal amounts of protein were incubated with streptavidin-agarose beads (Pierce Biotechnology) at 4°C overnight. Supernatants were separated from the beads by centrifugation and reincubated with streptavidin-coated agarose beads for 2 h at 4°C. The supernatant was saved for determination of intracellular NKCC2. Beads were pooled and washed twice in lysis buffer, twice in high-salt buffer, and twice in no-salt buffer. Biotinylated proteins were extracted from the beads by boiling in 60 μl SDS-loading buffer containing 100 mM dithiothreitol and 5% β-mercaptoethanol. Proteins were resolved by SDS-PAGE (6% gels), and NKCC2 present in the surface fraction was detected by Western blotting. Control experiments showed complete extraction of biotinylated NKCC2 from lysates and complete elution of biotinylated NKCC2 from beads. Absence of the intracellular protein GAPDH in the biotinylated surface fraction was confirmed, and the surface-to-intracellular NKCC2 ratio was determined in every experiment. Given the small percentage of NKCC2 at the surface (3–5%), changes in the surface fraction do not result in detectable changes in the intracellular fraction. Day-to-day variation in surface biotinylation experiments was <25% across data sets. Additional controls were performed to ensure that measurement of surface NKCC2 by Western blotting was within a linear range.
Endocytosis of NKCC2 in THALs
Endocytosis of NKCC2 was measured by a surface biotinylation protocol adapted and modified from Carter et al. (5). Biotinylation of surface proteins was performed as described above. THALs were then warmed to 37°C (with vehicle or treatment) to allow endocytosis to occur for different periods of time (7.5, 15, and 30 min). Samples were then rapidly cooled by adding chilled PS to stop trafficking. THALs were treated for 30 min with the membrane-impermeant reducing agent sodium 2-mercaptoethanesulfonate (MesNa; 50 mM) in a reducing buffer (in mM: 10 HEPES, 1 MgCl2, 125 NaCl, 50 Tris·Cl 50, pH 8.0) to strip biotin from surface proteins by breaking disulfide bridges. Endocytosed biotinylated proteins are protected due to the low membrane permeability of MesNa. Excess MesNa was washed away and quenched with iodoacetamide (25 mM). THALs were lysed with buffer as previously described (1, 4, 34). Equal amounts of protein were incubated with streptavidin-agarose beads, and endocytosed proteins were recovered from the beads as described above and resolved by SDS-PAGE. In each experiment, a control biotinylated sample never incubated at 37°C was treated with MesNa to measure complete stripping of surface biotin. MesNa stripped 97 ± 3% (n = 22) of biotin from surface NKCC2. The remaining signal was considered background and subtracted from the other bands treated with MesNa.
Recycling of NKCC2 in THALs
Recycling of NKCC2 was measured by a surface biotinylation protocol adapted and modified from Damke et al. (10) and also from Refs. 31 and 48. First, surface NKCC2 was biotinylated in THAL suspensions at 4°C as described above. One aliquot was kept at 4°C to measure total surface NKCC2. The rest of the THALs were then warmed to 37°C for 30 min to allow endocytic retrieval while the bath solution was gassed every 5 min with 100% O2. Then, THALs were rapidly cooled to 4°C and maintained on ice. Next, biotinylated NKCC2 remaining in the surface was stripped at 4°C with 50 mM MesNa (as described for endocytosis). After this first round of stripping, only retrieved proteins remain biotinylated. Then, four aliquots were warmed to 37°C for 0 (kept at 4°C), 7.5, 15, or 30 min. Then, THALs were rapidly cooled to 4°C, and biotinylated surface proteins (that recycled back to the membrane) were stripped with 50 mM MesNa (second-round stripping). Finally, THALs were treated with iodoacetamide (a reagent that eliminates the remaining MesNa from the bathing solution) and lysed as described above. In this manner, we monitored the decrease in retrieved NKCC2 that occurs due to recycling. In every experiment, we measured initial surface NKCC2, the efficiency of MesNa, and retrieved NKCC2 over 30 min (0 time point recycling), which was set as 100%. We then calculated the percentage of retrieved NKCC2 that disappears on the second round of stripping (recycling of internalized NKCC2).
Stability of Biotinylated Surface NKCC2 in THALs
Cell surface biotinylation of THAL suspensions was performed as described above. After biotinylation, THALs were incubated for 0, 60, and 120 min at 37°C while the bath solution was gassed every 5 min with 100% O2. Then, THALs were cooled to 4°C and lysed, protein content in each sample was measured, and equal amounts of protein were incubated with streptavidin-agarose beads to recover biotinylated NKCC2, detected by Western blot. Time 0 was set as 100% of initial surface NKCC2.
Western Blotting
Proteins eluted from streptavidin beads or from THAL lysates were centrifuged for 1 min at 10,000 g, loaded into each lane of a 6% SDS-polyacrylamide gel, separated by electrophoresis, and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blocked and blotted as previously described (1, 4, 34). Monoclonal anti-GAPDH (Chemicon) was used at 1:10,000 dilution. Reaction was detected by chemiluminescence and quantified by densitometry. Exposure time and amount of protein loaded were optimized so that optical densities were linear.
Measurement of Net Cl− Flux in Medullary THALs
Isol ation and perfusion of rat THALs were performed as previously described (35, 37, 38). Male Sprague-Dawley rats, weighing 120–150 g (Charles River Breeding Laboratories), were used for THAL perfusion. Medullary THALs were dissected from the medullary rays at 4–10°C. THALs ranging from 0.5 to 1.0 mm were transferred to a temperature-regulated chamber mounted on an inverted microscope and perfused using concentric glass pipettes at 37 ± 1°C. The flow rate of the basolateral bath was 0.5 ml/min. Luminal perfusion rate was set at 5–10 nl·min−1·mm−1. PS gassed with 100% O2 (pH = 7.40) was used for the bath and perfusate. After initial perfusion, THALs were equilibrated for 20 min and four collections of luminal fluid were made to calculate basal Cl− reabsorption rate. Then, MβCD was added to the lumen and the bath. After a 15-min reequilibration period, four additional collections were made. Cl− concentration in the perfusate and collected fluid was measured by microfluorometry as described previously (35–38). Because water is not reabsorbed by the THAL, chloride reabsorption (JCl−) was calculated as follows
where C.R is the collection rate normalized per tubule length, CCo is the Cl concentration in the perfusion solution and CCl is the Cl concentration in the collected fluid.
Confocal Microscopy for Detection of Apical Endocytosis in THALs
To monitor apical endocytosis in THALs, we measured the accumulation of the fluorescent fluid-phase marker FITC-dextran (10 kDa, Invitrogen) added to the lumen (apical side only) of isolated, perfused THALs. Briefly, THALs were perfused and equilibrated for 20 min at 37°C, then incubated with vehicle or MβCD (5 mM) in the lumen and bath for an additional 20 min. After this, FITC-dextran (10 kDa, 5 mg/ml) was added to the apical tubular fluid and incubated for an additional 10 min in the luminal perfusion solution. After labeling, the lumen was washed at 6°C for 5 min, and THAL cells were imaged by confocal microscopy. Fluorescent images were acquired using a laser-scanning confocal system (Visitech International) mounted on a Nikon TE2000-eclipse microscope and visualized at 488-nm excitation. Fluorescence was measured and recorded with a 500-nm LP filter. Identical laser, slit, and acquisition settings were used to obtain cross-sectional (z-axis) images of labeled THALs. Two-dimensional image analysis (Visitech Acquisition software) of original images was used to count the number of punctae per cell after application of a fluorescence threshold to all images to decrease background.
Immunofluorescence and Confocal Microscopy for Detection of Luminal Membrane NKCC2 in Isolated, Perfused THALs
Immunofluorescence and confocal microscopy detection of luminal membrane NKCC2 were performed as described previously (1). Microdissected medullary THALs were perfused and equilibrated for 20 min at 37°C; then, either vehicle (control conditions) or MβCD 5 mM was added to the lumen and bath for 20 min. Then, the flowing bath was rapidly exchanged (3 s) with chilled perfusion solution (4°C), and the chamber temperature was maintained at 6°C to stop protein trafficking for a total period of 40 min. After the initial cooling, the luminal perfusion solution was replaced by perfusion solution containing 2.5% BSA (pH 7.6) and incubated for 5 min to block nonspecific antigenic sites. Then, an anti-NKCC2 antibody (which recognizes amino acids 363–376 located between the predicted transmembrane domains 5 and 6 of NKCC2 facing the extracellular side, 1:100 dilution in 2.5% BSA, pH 7.6) was perfused into the THAL lumen for 30 min while the bath temperature was maintained at 6°C. We previously observed that these temperature conditions (6°C) completely blocked apical endocytosis (1). After labeling of intact THALs, the lumen was washed at 6°C for 5 min by exchanging the luminal solution with physiological saline. THALs were immediately fixed at 6°C with 4% paraformaldehyde in PBS (pH 7.4) for 10 min and then incubated with fixative for an additional 20 min at room temperature. Fixed tubules were blocked for 5 min with 2.5% BSA in perfusion solution added into the lumen and in the bath, followed by 30-min luminal perfusion with Alexa Fluor 488 goat anti-rabbit IgG secondary antibody cross-adsorbed against IgGs from other species (Molecular Probes, Eugene, OR) diluted 1:200 in perfusion solution containing 2.5% BSA. THALs were washed for 10 min with perfusion solution in the lumen and bath. Control experiments showed no detectable background staining when the secondary antibodies were incubated in the absence of antiserum. Fluorescent images were acquired using a laser-scanning confocal system (Visitech International) mounted on a Nikon TE2000-eclipse microscope. Secondary labeling was visualized by exciting at 488 nm, and fluorescence was measured and recorded with a 500-nm LP filter. Identical laser, slit, and acquisition settings were used to obtain cross-sectional (z-axis) images of labeled THALs. To diminish day-to-day variation, fluorescence intensity was regularly calibrated to a linear range using Focal-check slides (Molecular Probes) containing a serial dilution of fluorescent-labeled beads. Two-dimensional image analysis (Visitech Acquisition software) of original images was used to measure mean fluorescent intensity in regions of interest encompassing the apical membrane of labeled THALs. Several cross-sectional images at different depths (z-axis) were obtained from individual THAL cells along the tubule to obtain the best possible focus of apical membranes. For quantification, regions of interest were generated encompassing the apical membranes of several cells in each THAL image (generally 4–8 cells at each side of the tubule wall). Mean fluorescence intensity of apical labeling was obtained and averaged for each tubule. One control and one MβCD treatment experiment were performed each day. Image files were converted to TIFF and minimally deconvolved.
Preparation of MβCD/Cholesterol Complex and Filipin III Staining
Preparation of MβCD/cholesterol complex (MβCD/Chol) was performed as described by Christian et al. (7) with some modifications. Briefly, cholesterol (Sigma) was stored as a stock solution in ethanol (18 mM). Stock solution (235 μl, 2.6 mg cholesterol) was added to a glass tube, and 65 mg of MβCD (powder) was added to the same tube and dissolved. Ethanol was evaporated in a Savant speedvac concentrator, and the resulting powder was dissolved in 10 ml of PS, giving a 5 mM concentration of MβCD. The solution was vortexed, incubated in a bath sonicator for 5 min, and maintained at 37°C. Insoluble material was removed by filtration through a 0.45-μm filter before use. Under these conditions, MβCD/Chol reaches a ratio of 8:1. To determine the efficiency of plasma membrane cholesterol depletion by MβCD, we treated THAL suspensions with vehicle or MβCD (5 mM, 20 min), then fixed the tubules and stained membrane cholesterol with the fluorescent cholesterol-binding agent filipin III (250 μg/ml) as previously described (9, 15). THALs were imaged by fluorescence microscopy (340/25-bp excitation, 400 DCLP, 435-ba emission filters) after filipin III staining. The mean fluorescence intensity for several tubules was averaged. In control THALs, fluorescence intensity was 4,285 ± 286 arbitrary units (AU; n = 22 tubules from 2 preparations) whereas in THALs treated with MβCD (5 mM) for 20 min, fluorescence intensity was 1,966 ± 115 AU (n = 18 tubules from 2 preparations), an approximately 54% decrease (data not shown).
Statistics
Data are expressed as mean ± SE. One-way ANOVA was used to determine statistical differences between means in different treatment groups when surface and total NKCC2 were measured by Western blotting and fluorescent imaging. A paired t-test was used to determine changes in net Cl− reabsorption. P < 0.05 was considered significant.
RESULTS
NKCC2 Undergoes Constitutive Endocytosis in THALs
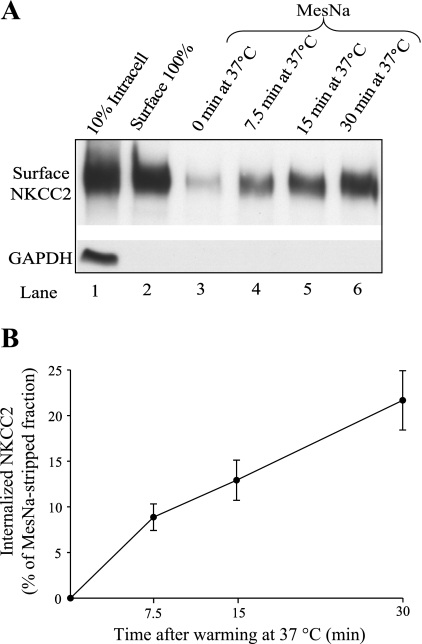
Steady-state surface NKCC2 levels regulate NaCl reabsorption by the THAL. However, little is known about the trafficking mechanisms involved in maintenance of steady-state surface NKCC2 in renal epithelial cells. We tested whether NKCC2 undergoes endocytic internalization in the absence of hormonal stimuli (herein defined as constitutive endocytosis). For this, we first studied the rate of NKCC2 endocytosis in native THALs using a modification of a surface biotinylation protocol previously described by Carter et al. (5) and detailed in materials and methods. Data are expressed as a percentage of surface NKCC2 in control THALs. We found constitutive endocytic internalization of NKCC2 over time. Endocytic internalization of NKCC2 averaged 8.9 ± 1.5% at 7.5 min, 12.9 ± 2.2% at 15 min, and 21.7 ± 3.3% at 30 min (n = 6, Fig. 1, A and B). These data indicate that NKCC2 undergoes constitutive endocytosis in native THALs. In control experiments, no differences in the total pool of NKCC2 were observed over time, and the intracellular protein GAPDH was not detected in surface fractions. These data show for the first time that NKCC2 undergoes endocytosis.
Fig. 1.
Na-K-2Cl cotransporter (NKCC2) undergoes constitutive endocytosis in thick ascending limbs of Henle's loop (THALs). A: representative Western blot of the endocytic protocol for NKCC2 in THALs. Expression of steady-state surface NKCC2 before (lane 2; basal surface NKCC2) and after (lanes 3–6) treatment with sodium 2-mercaptoethanesulfonate (MesNa) to strip surface biotinylated proteins. After stripping, THALs were incubated for 0 (lane 3), 7.5 (lane 4), 15 (lane 5), and 30 min (lane 6) at 37°C to allow protein trafficking. THALs were cooled, and endocytosed NKCC2 (protected from MesNa) was precipitated, detected by Western blotting, and measured by densitometry. As a control, intracellular GAPDH was not detected in the surface fraction, indicating the absence of intracellular protein biotinylation with NHS-SS-biotin as described in materials and methods. B: cumulative data for constitutive NKCC2 endocytosis in THAL suspensions. Constitutive NKCC2 endocytosis over time averaged 8.9 ± 1.5% at 7.5 min, 12.9 ± 2.2% at 15 min, and 21.7 ± 3.3% at 30 min (n = 6). The remaining signal from the stripped sample (lane 3) was always subtracted from the experimental samples, and thus data were expressed as percentage of the MesNa-stripped fraction (see results). Error bars represent SE.
MβCD Blocks Endocytosis of a Fluid-Phase Marker in THALs
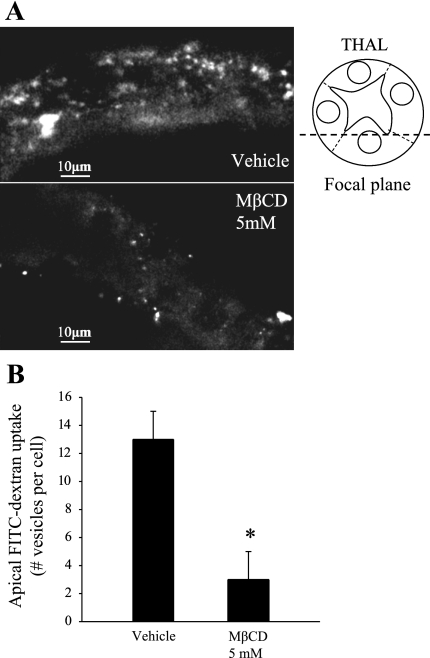
To our knowledge, the apical endocytic pathway of THALs has not been studied. Cholesterol-depleting agents such as MβCD block most forms of endocytosis from the plasma membrane. To test our hypothesis that NKCC2 endocytosis maintains steady-state surface levels, we first studied whether MβCD was effective in blocking apical THAL endocytosis. For this, we examined the effect of MβCD on the internalization of a fluorescent fluid-phase marker (FITC-dextran, 10 kDa). Isolated, perfused THALs were incubated at 37°C for 20 min in the absence or presence of MβCD (5 mM), and then FITC-dextran, 10 kDa (5 mg/ml) was added only to the apical side (tubule lumen) and incubated for 10 min at 37°C to allow endocytosis (Fig. 2A). Then, THALs were cooled and FITC-dextran was washed from the lumen. Confocal imaging of THALs was used to count the number of vesicles internalized during the 10-min period, as described in materials and methods. The number of FITC-dextran-labeled punctae were reduced by 77% in THALs treated with MβCD (13 ± 2 to 3 ± 2 vesicles/cell, *P < 0.05, Fig. 2B). These data indicate that MβCD blocks endocytosis from the apical membrane in THALs.
Fig. 2.
Effect of methyl-β-cyclodextrin (MβCD) on apical endocytosis of a fluid-phase marker in isolated. perfused THALs. A: representative confocal images of isolated and perfused THALs. THALs were incubated with apical FITC-dextran (10-kDa marker, 5 mg/ml) for 10 min, after treatment with vehicle (top) or MβCD (5 mM, bottom) for 20 min (z-section shows only cells at the bottom of the tubule, closer to the coverslip). B: cumulative data for fluid-phase endocytic marker FITC-dextran (10 kDa) in THALs. The number of vesicles (punctae) per cell were decreased from 13 ± 2 to 3 ± 2 (*P < 0.05) in MβCD-treated THALs (see results). Error bars represent SE.
MβCD Blocks Constitutive NKCC2 Endocytosis in THALs
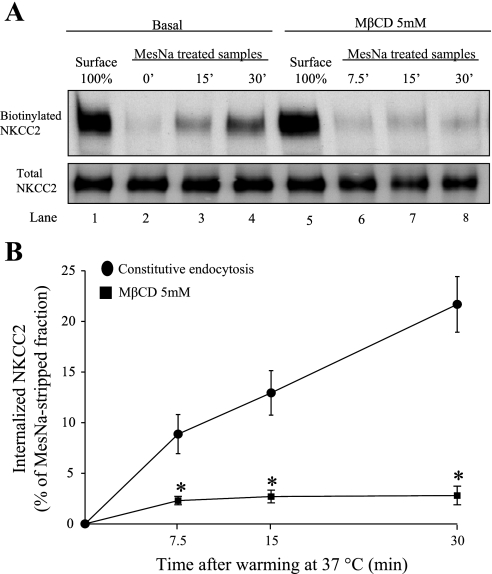
Because MβCD blocked apical endocytosis of a fluorescent marker, we then tested whether MβCD specifically decreases the rate of NKCC2 endocytosis in THALs. We chose 20-min preincubation of the THAL suspension with MβCD (5 mM) because this time was sufficient to decrease cholesterol in cell suspensions (29, 50). Surface proteins in THALs were labeled with NHS-SS-biotin at 4°C as described in materials and methods, and then tubules were warmed to 37°C for 7.5, 15, or 30 min in the absence or presence of MβCD (5 mM). In THALs treated with MβCD, the rate of NKCC2 endocytosis was blocked by 86.4 ± 4.0% at 30 min (n = 6, *P < 0.05) compared with vehicle-treated tubules (Fig. 3, A and B). These data indicate that NKCC2 endocytosis is blocked by MβCD.
Fig. 3.
Effect of MβCD on constitutive NKCC2 endocytosis in THALs. A: representative Western blot for a single experiment showing constitutive NKCC2 endocytosis in basal condition or in the presence of MβCD. B: THALs were pretreated with vehicle or MβCD for 20 min before biotinylation. After biotinylation, samples were incubated at 37°C for 0, 7.5, 15, and 30 min in the presence of vehicle (●) or MβCD (■). Treatment of THALs with MβCD (5 mM) blocked constitutive NKCC2 endocytosis (basal = 8.95 ± 1.58% at 7.5 min, 11.0 ± 1.9% at 15 min, 21.5 ± 2.7% at 30 min; MβCD = 2.4 ± 0.4% at 7.5 min, 2.7 ± 0.6% at 15 min, 2.9 ± 0.9% at 30 min, n = 6, *P < 0.05). Error bars represent SE. Note: the vehicle experiments at time point 7.5 min, were performed in a separate group of animals.
Cholesterol-Saturated MβCD Does Not Block NKCC2 Endocytosis in THALs
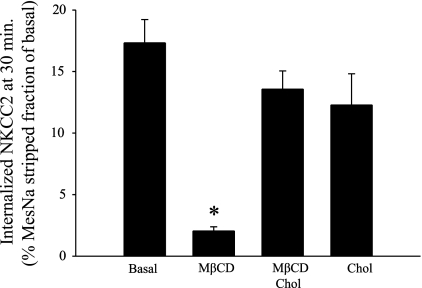
To determine whether the effect of MβCD was primarily due to cholesterol depletion rather than a nonspecific effect of MβCD, we measured NKCC2 endocytosis during treatment of THALs with MβCD saturated with cholesterol (MβCD/Chol). In a different set of experiments, constitutive NKCC2 endocytosis (measured at 30 min) was blocked by 87.7 ± 1.4% in THALs treated with MβCD (Fig. 4). However, in THALs treated with MβCD/Chol the rate of endocytosis was not significantly different from control conditions. In addition, treating THALs with perfusion solution saturated with cholesterol alone (2 μg/ml) did not significantly affect NKCC2 endocytosis by 30 min. These data indicate that NKCC2 endocytosis is highly sensitive to the cholesterol-chelating effect of MβCD in THALs.
Fig. 4.
Effect of cholesterol-saturated MβCD on NKCC2 endocytosis in THALs. Effect of MβCD (5 mM), MβCD/cholesterol (Chol), or Chol alone on constitutive NKCC2 endocytosis measured at 30 min. MβCD/Chol does not significantly affect NKCC2 endocytosis. Basal = 17.3 ± 1.8%, MβCD = 2.1 ± 0.3%, MβCD/Chol = 13.5 ± 1.4%, and Chol (2 μg/ml) = 12.3 ± 2.3%. Data are expressed as percentage of the MesNa-stripped fraction after 30 min of endocytosis (n = 6, *P < 0.05).
MβCD Increases Steady-State Surface NKCC2 Levels in THALs
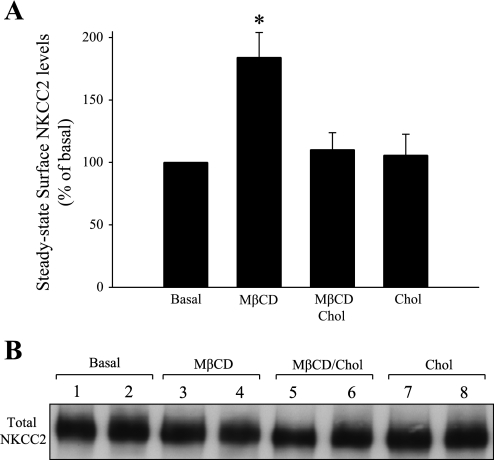
We found that MβCD almost completely blocked NKCC2 endocytosis. If the rate of exocytic insertion of NKCC2 is not significantly inhibited by MβCD, then we would expect an increase in steady-state surface NKCC2 expression due to its accumulation at the plasma membrane. To test this, we studied the effect of MβCD on steady-state surface NKCC2 levels in suspensions of THALs. Suspensions of THALs were incubated for 20 min at 37°C with vehicle (basal), MβCD (5 mM), MβCD/Chol, or cholesterol alone (2 μg/ml). After this, THALs were cooled and surface proteins were biotinylated, extracted, and quantified as described in materials and methods. We found that MβCD increased steady-state surface NKCC2 by 84% (basal: 100%, MβCD: 184 ± 20%, *P < 0.05, Fig. 5), whereas MβCD/Chol or cholesterol alone had no effect on steady-state surface NKCC2. Importantly, the total pool of NKCC2 was not affected after treatment with MβCD for 20 min (basal = 100%, MβCD 5 mM = 110 ± 13%, Fig. 5B), and the intracellular protein GAPDH was not detected in the surface fraction. Taken together, these data indicate that MβCD induces NKCC2 accumulation at the cell surface of THALs by blocking endocytosis and this effect is dependent on its ability to extract cholesterol from the plasma membrane.
Fig. 5.
Effect of Chol-saturated MβCD on steady-state surface NKCC2 in THALs. A: cumulative data showing the effect of MβCD (5 mM), MβCD/Chol, or Chol alone on steady-state surface NKCC2 levels in THALs. Samples were treated for 20 min at 37°C, then biotinylated as described in materials and methods, and NKCC2 was detected by Western blotting and quantified by densitometry. Basal = 100%, MβCD = 184 ± 20%, MβCD/Chol = 110 ± 13%, and Chol = 106 ± 16% (n = 5, *P < 0.05). Data are expressed as a percent of baseline surface NKCC2. Error bars represent SE. B: representative blot showing total NKCC2 expression in THALs treated with vehicle (lanes 1 and 2), MβCD (lanes 3 and 4), MβCD/Chol (lanes 5 and 6), or Chol (lanes 7 and 8).
MβCD Increases Apical Steady-State Surface NKCC2 in Isolated and Perfused THALs
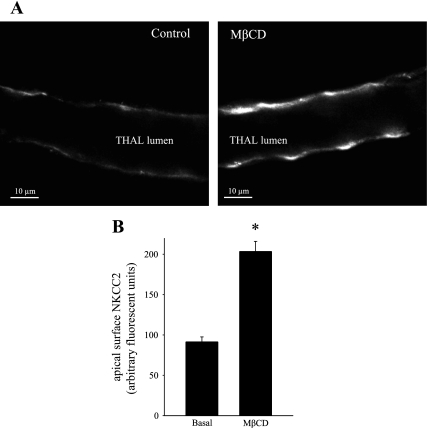
To directly visualize whether blockade of apical endocytosis induces the accumulation of NKCC2 at the apical surface of THALs, we isolated and perfused THALs and labeled NKCC2 with an antibody against the extracellular loop of NKCC2 (located between transmembrane domains 5 and 6). Under control conditions, apical surface NKCC2 was detected as a thin band of fluorescence along the apical membrane in most THAL cells. However, treatment of THALs with 5 mM MβCD for 20 min enhanced the fluorescent intensity of NKCC2 in THALs (Fig. 6A). We quantified mean apical membrane intensity in control and in THALs treated with MβCD and observed an increase in NKCC2 apical surface labeling from 91.5 ± 6.0 to 203.5 ± 12.5 AU (*P < 0.05, n = 74 cells from 4 control THALs, and n = 82 cells from 4 MβCD-treated THALs, Fig. 6B). These data directly demonstrate that cholesterol chelation induces an increase in apical membrane NKCC2 levels.
Fig. 6.
MβCD increases apical surface NKCC2 staining in isolated, perfused medullary THALs. A: representative confocal micrograph showing apical surface NKCC2 staining in isolated, perfused medullary THALs under control (unstimulated) conditions (left) and in a THAL treated with MβCD (5 mM) for 20 min (right). B: cumulative data for apical NKCC2 immunofluorescence staining under basal conditions and in THALs treated with MβCD (5 mM). The mean fluorescent intensity in the apical membrane was measured in 15–20 THAL cells in each tubule and then averaged (basal: 91.5 + 6.0%, MβCD: 203.5 ± 12.5%, P < 0.05, n = 4). Error bars represent SE.
MβCD Increases Net Cl− Reabsorption in THALs
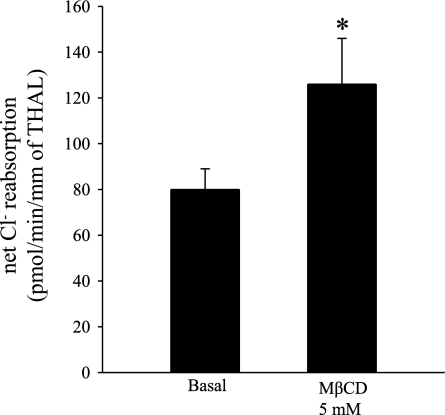
NKCC2 provides the only apical entry pathway for Cl− in the THAL. The physiological relevance of NKCC2 endocytosis is unknown. We previously found that an increase in steady-state surface NKCC2 is associated with an increase in net Cl− reabsorption by the THAL. Therefore, we tested whether blocking NKCC2 endocytosis would increase net Cl− reabsorption. We found that 20-min treatment of THALs with MβCD (5 mM) enhanced net Cl− reabsorption from 80 ± 9 to 126 ± 20 pmol·min−1·mm−1 in THAL (n = 5, *P < 0.04), a 57% increase (Fig. 7). These data are consistent with enhanced apical membrane NKCC2 levels and suggest that NKCC2 endocytosis is a physiologically relevant mechanism that controls the absorptive capacity of the THAL.
Fig. 7.
Effect of MβCD on net Cl− reabsorption. Cumulative data showing the effect of MβCD (5 mM) on net Cl− reabsorption. THALs were isolated and perfused as described in materials and methods. THALs were treated with either vehicle or MβCD (5 mM) in the luminal and basolateral side for 20 min at 37°C. Net Cl− reabsorption was measured as described in materials and methods. MβCD (5 mM) enhanced net Cl− reabsorption from 80 ± 9 to 126 ± 20 pmol·min−1·mm−1 of THAL (n = 5, *P < 0.04). Error bars represent SE.
Retrieved NKCC2 Recycles Back to the Plasma Membrane
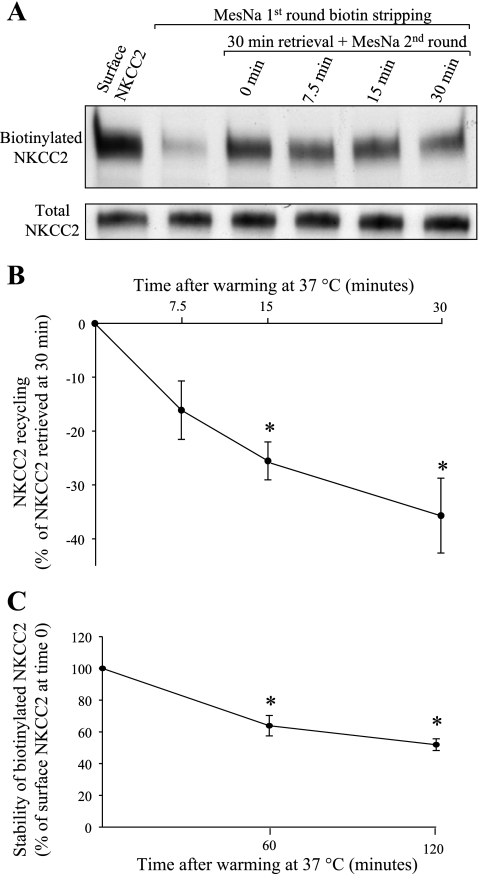
Some apical transporters recycle back to the membrane after endocytosis. The fate of the endocytosed NKCC2 has not been studied. We modified our endocytic retrieval protocol to measure recycling of internalized NKCC2 back to the surface. For this we internalized biotinylated surface proteins for 30 min at 37°C and then measured the decrease in this pool due to recycling (as detailed in materials and methods). We observed a decrease in retrieved NKCC2 that averaged 16.1 ± 5.4% at 7.5 min, 25.5 ± 3.5% at 15 min, and 35.7 ± 7.0% at 30 min (n = 5, Fig. 8, A and B). These data indicate that NKCC2 recycles back to the plasma membrane in a constitutive manner. No differences in the total pool of NKCC2 were observed over time, and the intracellular protein GAPDH was not detected in surface fractions. These data show for the first time that NKCC2 undergoes constitutive recycling.
Fig. 8.
Retrieved NKCC2 recycles back to the plasma membrane. A: representative Western blot showing recycling of NKCC2 in THALs. Lane 1, baseline steady-state surface NKCC2; lane 2, surface NKCC2 after stripping of surface biotin with MesNa; lane 3, 30-min retrieval with one and two rounds of MesNa stripping; lanes 4–6, 30-min retrieval plus incubation at 37°C for 7.5, 15, or 30 min and second round of MesNa stripping (the decrease in the NKCC2 retrieved for 30 min is due to second round stripping of proteins recycling back to the surface). The total pool of NKCC2 did not change over time (bottom). B: cumulative data for NKCC2 recycling in THAL suspensions. THAL surface proteins were biotinylated, incubated at 37°C for 30 min to allow endocytosis, and then the remaining biotin in surface proteins was stripped (first-round stripping). Then, THALs were warmed to 37°C to allow recycling of retrieved biotinylated proteins back to the cell surface, and surface biotin was stripped for a second time. Biotinylated proteins were isolated, and NKCC2 was measured by Western blotting. The fraction of NKCC2 recycled was expressed as a percentage of endocytosed NKCC2 at 30 min (n = 5). C: cumulative data for stability of biotinylated surface NKCC2 in THALs. THAL surface proteins were biotinylated as described in materials and methods and incubated at 37°C for 60 or 120 min. Biotinylated proteins were isolated, and NKCC2 was detected by Western blotting and quantified by densitometry (0 min: 100%, 60 min: 63.9 ± 6.4%, 120 min: 51.9 ± 3.7%, n = 4, *P < 0.05). Data are expressed as a percentage of baseline surface NKCC2. Error bars represent SE.
In addition, we monitored the stability of biotinylated surface NKCC2 over time. We then studied whether the signal for biotinylated surface NKCC2 is decreased over a period of 2 h. THALs were biotinylated at 4°C, washed in PS, and then warmed to 37°C for 0, 60, or 120 min. Then, THALs were lysed and biotinylated proteins were recovered. We found that the signal for biotinylated NKCC2 decreases over time by 48% after 2 h (0 min: 100%, 60 min: 63.9 ± 6.4%, 120 min: 51.9 ± 3.7%, n = 4, P < 0.05, Fig. 8C). No differences in the total pool of NKCC2 were observed over 2 h, and the intracellular protein GAPDH was not detected in surface fractions. These data are the first indication that surface NKCC2 is slowly degraded over time.
DISCUSSION
We previously reported that NKCC2-dependent Na+ entry and net Cl− reabsorption are modulated by changes in steady-state surface NKCC2 levels in THALs. Our previous data and those from others suggest that trafficking of NKCC2 is critical in determining steady-state surface NKCC2 in the plasma membrane as well as the capacity of the THAL to reabsorb NaCl (1, 4, 34). However, NKCC2 trafficking in those studies was examined after treatment of THALs with agonists or antagonists. The dynamic nature of NKCC2 trafficking into and out of the plasma membrane in the absence of hormonal stimulation has not been studied, and the physiological relevance of these trafficking pathways in THAL function is unclear. We hypothesized that NKCC2 undergoes constitutive endocytosis and that this process is sensitive to acute cholesterol chelation by MβCD.
In the present study, we modified the surface biotinylation technique to measure the endocytic retrieval of NKCC2 in intact THALs. We consistently found that ∼25% of surface NKCC2 is internalized by 30 min in the absence of hormonal stimulation. These results are consistent with our previous data showing exocytic insertion of NKCC2 in the absence of hormonal stimuli that averaged ∼25% over 30 min (4). These rates of trafficking into and out of the cell surface suggest an approximate half-life for NKCC2 in the plasma membrane of 1 h. The rate of constitutive endocytosis for renal transporters in general is highly variable, dependent on the cell type studied and technique used. It is difficult to compare our results to other apical transporters because this is the first study examining the rate of NKCC2 endocytosis in renal THALs. For ENaC, in polarized cultured cells, the apical membrane half-life is ∼20–30 min (2), suggesting faster rates of endocytosis than NKCC2 in THALs. However, for organic anion transporter 1 expressed in COS-7 cells, ∼40% of the surface pool is internalized by 30 min (52). The K+ channel ROMK, also present in the apical membrane of THALs, is rapidly internalized (∼80% in 30 min) when expressed in COS-7 cells (13). Overall, our measurements of NKCC2 endocytosis and exocytic insertion suggest that constitutive apical turnover occurs in THALs. To our knowledge, these are the first data to show constitutive NKCC2 endocytosis in any cell type.
The integrity and composition of the plasma membrane play an important role in the trafficking process. Extraction of plasma membrane cholesterol blocks most forms of endocytosis (41–43, 46). We studied whether acute treatment with the cholesterol-chelating agent MβCD influenced NKCC2 endocytosis. MβCD has been shown to be efficient in extracting membrane cholesterol (7, 22, 29), is not incorporated into the plasma membrane, and does not affect cell viability or plasma membrane permeability in other cells (21) when used at 5 mM and for short periods of time (30 min) (7, 23, 33, 50). We found that acute treatment with MβCD blocked constitutive NKCC2 endocytosis by ∼ 81% at 30 min. In addition, MβCD increased steady-state surface NKCC2 levels by 60–80% without affecting the total pool of NKCC2. Thus these data are consistent with inhibition of endocytosis and accumulation of NKCC2 in the apical membrane. Our data provide the first evidence that NKCC2 membrane accumulation can occur independently of cAMP or vasopressin stimulation. Our data on NKCC2 are consistent with previous reports for other renal apical proteins. Russo et al. (44) and Lu et al. (28) found that 30-min treatment with MβCD increased plasma membrane aquaporin-2 levels in cultured renal epithelial cells, suggesting accumulation of surface channels due to inhibition of endocytosis.
The incubation time with MβCD used in this study (20–30 min) has been previously validated to significantly decrease cholesterol in cell suspensions (29, 50). We think that acute treatment with MβCD at least partially decreased membrane cholesterol levels to effectively block endocytosis. Two lines of evidence indicate that the effect of MβCD on NKCC2 endocytosis is due to a decrease in plasma membrane cholesterol. First, adding MβCD previously complexed and saturated with cholesterol did not block NKCC2 endocytosis or affect steady-state surface NKCC2. Second, MβCD decreased filipin III binding to THALs, as evidenced by a 50% decrease in fluorescence (see materials and methods), similar to what has been reported by other studies in epithelial cells (19, 29, 30). Taken together, our data indicate that the inhibitory effect of MβCD on constitutive NKCC2 endocytosis is likely due to a decrease in plasma membrane cholesterol rather than a nonspecific effect of MβCD.
NaCl reabsorption by the THAL is influenced by steady-state surface NKCC2 levels (1, 34). Here, we found that MβCD blocked endocytosis and increased apical membrane surface NKCC2 levels. As expected from this accumulation of NKCC2 in the plasma membrane, MβCD enhanced THAL net Cl− reabsorption by ∼55%. These data suggest that constitutive NKCC2 endocytosis is an important trafficking step in the regulation of steady-state surface NKCC2 and NaCl reabsorption in the THALs. While we found that in THALs acute treatment with MβCD enhanced steady-state surface NKCC2 as well as net Cl− reabsorption, others found that prolonged treatment (2 h) with MβCD decreased NKCC2-dependent 86Rb+ uptake after heterologous expression in Xenopus laevis oocytes (49). While we have no explanation for these disparate results, there are several differences from our study. First, a higher dose (10 mM) of MβCD and a longer incubation period (2 h) were used for oocytes, suggesting complete extraction of cholesterol from the oocyte membrane. Second, the rates of NKCC2 trafficking in oocytes or nonpolarized cells may be very different from those in fully polarized THALs and thus inhibition of activity may reflect changes in NKCC2 conformation due to cholesterol requirement at the membrane rather than an effect on trafficking. In addition, the aforementioned authors subjected immortalized rabbit THAL cells to chronic cholesterol depletion by adding 10 mM MβCD for 2 h after overnight incubation of cells with mevalonate and lovastatin. These combined treatments decreased the total pool of NKCC2 by 60% (49), suggesting that cholesterol in intracellular membranes may be required for correct NKCC2 processing or folding. It is important to note that we did not observe any change in the total pool of NKCC2 during our incubation period, indicating that differences in surface NKCC2 expression are primarily due to an effect on NKCC2 trafficking. Together, these data point to an important role of membrane cholesterol levels in controlling NKCC2 trafficking and under certain conditions, its correct processing.
Some reports have indicated that NKCC2 partitions in the cholesterol- and sphingolipid-rich domains, Triton X-100-insoluble membrane fraction (51), usually defined as membrane lipid rafts. MβCD extracts cholesterol from both Triton X-100-soluble as well as Triton X-100-insoluble membrane fractions (29, 50, 53). Thus we do not think our study provides additional evidence for NKCC2 localization to these domains. Importantly, MβCD treatment blocks most forms of endocytosis including clathrin-dependent (42, 46) and lipid raft/caveolin-dependent endocytosis (41, 43) in most cells studied, in addition to its ascribed role in disrupting lipid rafts (29, 50). Although our data suggest an essential role of endocytosis in the regulation of steady-state surface NKCC2, we think it is not possible at this time to speculate whether NKCC2 endocytosis occurs via a clathrin-dependent or lipid raft/caveolin-dependent mechanism. Additional specific experiments are required to directly address this question in THALs, since the existence of membrane domains at the apical surface has not been accurately defined and examined in native renal tubules.
Internalized membrane proteins may be retained in an intracellular compartment, may be degraded, or may recycle back to the membrane. We studied whether internalized NKCC2 is recycled to the membrane in a constitutive manner and found that ∼40% of retrieved NKCC2 is recycled by 30 min. These data suggest that endocytic recycling contributes to steady-state surface NKCC2, and its regulation (either inhibition or stimulation) is likely to affect surface NKCC2 levels and activity. This trafficking pathway is very important for the regulation of other apical transporters such as CFTR and ENaC (3, 47). It is difficult to compare the rate of NKCC2 recycling with that of other apical transporters (in cell culture models) because to our knowledge, these are the first data measuring transporter recycling in native renal tubules. We also examined the stability of biotinylated surface NKCC2 for a period of 2 h and found that biotinylated surface NKCC2 is decreased by ∼45% after 2 h. The loss of biotinylated NKCC2 may represent degradation of the full-length protein. However, these data must be interpreted cautiously because it may also be influenced by the loss of the biotin tag over prolonged incubation. The actual mechanism and the rate at which surface NKCC2 is degraded will likely require additional experiments using radioactive metabolic labeling and chase of surface NKCC2.
Overall, our data provide the first evidence that NKCC2 undergoes endocytosis in THALs in the absence of hormonal stimulation. Our data also point to an essential role of membrane endocytosis and its potential regulation in controlling steady-state surface NKCC2 levels and the reabsorptive capacity of the THAL.
GRANTS
This work was supported in part by American Heart Association Grant 0850126Z and National Heart, Lung, and Blood Institute Grant RO-1 HL080409 to P. A Ortiz. G. Ares was supported in part by American Heart Association Fellowship Grant 0715579Z.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Paulo Caceres, Mariela Mendez, and Rebecca Alviani for a thorough reading of the manuscript and comments. We also thank Tasnim Hoda for secretarial assistance.
REFERENCES
- 1.Ares GR, Caceres P, varez-Leefmans FJ, Ortiz PA. cGMP decreases surface NKCC2 levels in the thick ascending limb: role of phosphodiesterase 2 (PDE2). Am J Physiol Renal Physiol 295: F877–F887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125: 81–101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na+-K+-2Cl− cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol 120: 37–45, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Chen L, Wang G, Tang H. Cholesterol-dependent and -independent CD40 internalization and signaling activation in cardiovascular endothelial cells. Arterioscler Thromb Vasc Biol 27: 2005–2013, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res 38: 2264–2272, 1997 [PubMed] [Google Scholar]
- 8.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem 275: 31601–31608, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Covey SD, Brunet RH, Gandhi SG, McFarlane N, Boreham DR, Gerber GE, Trigatti BL. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem Biophys Res Commun 355: 67–71, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimke H, Flyvbjerg A, Bourgeois S, Thomsen K, Frøkiær J, Houillier P, Nielsen S, Frische S. Acute growth hormone administration induces antidiuretic and antinatriuretic effects and increases phosphorylation of NKCC2. Am J Physiol Renal Physiol 292: F723–F735, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na+-K+-2Cl− cotransporter, BSC-1. Am J Physiol Renal Fluid Electrolyte Physiol 271: F619–F628, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 119: 3278–3289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Marcos M, Tandel S, Pochet S, Genin J, De LM, Gomez F, Kumps A, Marino A, Dehaye JP. Cholesterol depletion perturbs calcium handling by rat submandibular glands. J Cell Physiol 203: 429–438, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res 39: 369–379, 1998 [PubMed] [Google Scholar]
- 19.Hassall DG, Graham A. Changes in free cholesterol content, measured by filipin fluorescence and flow cytometry, correlate with changes in cholesterol biosynthesis in THP-1 macrophages. Cytometry 21: 352–362, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, Cook DI, Pollock CA, Poronnik P. Nedd4–2 functionally interacts with ClC-5: involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem 279: 54996–55007, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 140: 1357–1367, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270: 17250–17256, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34: 13784–13793, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RASGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299: F300–F309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange Y, Ramos BV. Analysis of the distribution of cholesterol in the intact cell. J Biol Chem 258: 15130–15134, 1983 [PubMed] [Google Scholar]
- 26.Lange Y, Swaisgood MH, Ramos BV, Steck TL. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem 264: 3786–3793, 1989 [PubMed] [Google Scholar]
- 27.Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 277: 3371–3379, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Mahammad S, Parmryd I. Cholesterol homeostasis in T cells. Methyl-beta-cyclodextrin treatment results in equal loss of cholesterol from Triton X-100 soluble and insoluble fractions. Biochim Biophys Acta 1778: 1251–1258, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Marwali MR, Rey-Ladino J, Dreolini L, Shaw D, Takei F. Membrane cholesterol regulates LFA-1 function and lipid raft heterogeneity. Blood 102: 215–222, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem 280: 2220–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol 275: F885–F893, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem 186: 17–22, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ortiz PA, Garvin JL. NO Inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ortiz PA, Garvin JL. Nitric oxide (NO) modulation of Cl-dependent transporters in the kidney. Adv Exp Med Biol 559: 147–156, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+-K+-2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl− transport. Am J Physiol Renal Physiol 290: F1094–F1102, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 276: 9670–9678, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol 154: 535–547, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodal SK, Skretting G, Garred O, Vilhardt F, van DB, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10: 961–974, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Russo LM, McKee M, Brown D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272: 25537–25541, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96: 6775–6780, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 277: 40099–40105, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Terai T, Nishimura N, Kanda I, Yasui N, Sasaki T. JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin. Mol Biol Cell 17: 2465–2475, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welker P, Bohlick A, Mutig K, Salanova M, Kahl T, Schluter H, Blottner D, Ponce-Coria J, Gamba G, Bachmann S. Renal Na+-K+-Cl− cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am J Physiol Renal Physiol 295: F789–F802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yancey PG, Rodrigueza WV, Kilsdonk EP, Stoudt GW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J Biol Chem 271: 16026–16034, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Yu MJ, Pisitkun T, Wang G, Aranda JF, Gonzales PA, Tchapyjnikov D, Shen RF, Alonso MA, Knepper MA. Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am J Physiol Cell Physiol 295: C661–C678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem 283: 32570–32579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 1768: 1311–1324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]