Abstract
The hypothesis that TNF receptor 1-deficient (TNFR1−/−) mice display blood pressure (BP) and renal functional responses that differ from wild-type (WT) mice was tested in an angiotensin II (ANG II)-dependent model of hypertension. Basal systolic BP (SBP), mean arterial pressure, diastolic BP, heart rate (HR), and pulse pressure were similar in WT and TNFR1−/− mice. Infusion of ANG II for 7 days elevated SBP to a greater extent in TNFR1−/− compared with WT mice; pulse pressure was also elevated in TNFR1−/−. HR decreased in TNFR1−/− mice infused with ANG II, an effect prominent on day 1. Basal urinary albumin excretion was similar in WT and TNFR1−/− mice but was higher in TNFR1−/− in response to ANG II infusion. Water intake and urine volume were increased by ANG II infusion; this increase was higher in TNFR1−/− vs. WT mice, whereas body weight and food intake were unaffected. Baseline creatinine clearance (Ccr), urinary sodium excretion, and fractional excretion of sodium (FENa%) were similar in vehicle-treated WT and TNFR1−/− mice. ANG II infusion for 7 days increased Ccr and filtered load of sodium in TNFR1−/− but not WT mice, whereas it elicited an increase in FENa% and urinary sodium excretion in WT but not TNFR1−/− mice. ANG II also inhibited renal TNFR1 mRNA accumulation while increasing that of TNFR2. These findings indicate deletion of TNFR1 is associated with an exacerbated SBP response, decrease in HR, and altered renal function in ANG II-dependent hypertension.
Keywords: TNF, TNF receptors, angiotensin II, hypertension, albuminuria
tumor necrosis factor (TNF)-α plays an integral role in immunity and inflammation and exerts diverse actions on the cardiovascular and renal systems. For instance, TNF can stimulate (6, 15) as well as inhibit (34, 37, 43) the renin-angiotensin system, suggesting it may exhibit both pro- and anti-hypertensive functions. Either pharmacological blockade or genetic deletion of TNF attenuated ANG II-induced hypertension (16, 41) and slowed the progression of blood pressure elevation in rats receiving ANG II and high salt concurrently (11). TNF increased blood pressure in a rat model of pregnancy-induced hypertension, in association with decreases in glomerular filtration rate (GFR) and renal plasma flow and an increase in renal vascular resistance (25, 26). However, TNF does not increase blood pressure in nonpregnant rats (1) or two additional rat models of hypertension; namely, double-transgenic rats (dTGR) and DOCA-salt (10, 33). We previously reported that acute inhibition of TNF by intravenous administration of a neutralizing polyclonal antibody increased mean arterial pressure (MAP) in rats infused with ANG II (13). We also found that ANG II induced TNF production by the thick ascending limb (TAL) that, in turn, induced COX-2 expression and synthesis of PGE2 (12), which has been reported to inhibit activity of the Na+-K+-2Cl− cotransporter (21). Thus, the ability of TNF-neutralizing antibodies to increase blood pressure may be due to loss of the ability of TNF to mitigate the prohypertensive effects of ANG II.
TNF signals through two distinct receptors, a 55-kDa TNFR1 and a 75-kDa TNFR2 containing similar extracellular domains but distinct intracellular sequences, which mediate different functions of TNF. The contribution of TNFR1 to chronic blood pressure regulation and renal function has not been addressed. However, acute administration of human recombinant TNF (0.3 ng·min−1·g−1), which selectively activates mouse TNFR1, to anesthetized C57BL/6 mice decreases renal blood flow and GFR in association with increases in absolute and fractional excretion of sodium, without a concomitant change in blood pressure (39). These data suggest that activation of TNFR1 subserves renal vasoconstrictor and natriuretic functions in mouse kidney. The effects of TNFR1 activation are not limited to changes in renal hemodynamics and excretory function, as this receptor subtype also induces short-term increases in heart rate (HR) and negative inotropic effects (8, 41). Accordingly, we determined the effects of TNFR1 gene deletion on blood pressure and HR responses, as well as renal function in mice with ANG II-dependent hypertension.
MATERIALS AND METHODS
Animals.
Seven- to ten-wk-old male C57BL/6J wild-type (WT; stock 000664) and TNFR1-deficient (TNFR1−/−; B6.129-Tnfrsf1atm1Mak/J; stock 002818) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). TNFR1−/− mice were congenic on the C57BL/6J genetic background. Animals were housed in a room at an ambient temperature of 22 ± 1°C with a 12:12-h light-dark cycle, fed a corn-based 0.6% NaCl diet ad libitum (TD99414, Harlan Teklad), and given free access to tap water throughout the study. Experimental procedures were conducted in accordance with institutional and international guidelines for the welfare of animals (animal welfare assurance number A3362-01, Office of Laboratory Animal Welfare, PHS, National Institutes of Health), and received prior approval by the Institutional Animal Care and Use Committee at New York Medical College.
Measurement of blood pressure and HR by radiotelemetry/ANG II infusion.
Mice were implanted with radiotelemetry probes for the measurement of blood pressure and HR. Briefly, mice were anesthetized with ketamine (50 mg/kg) and xylazine (20 mg/kg) and under aseptic conditions, a midline incision was made and the left carotid artery was isolated and ligated. Telemetry probes (TA11PA-C10, Data Sciences International) were presoaked in sterile saline to ensure hydration of the device and facilitate removal of the catheter cap. A 25-gauge syringe needle (bent at the bevel) was used to introduce the catheter into the left carotid artery caudal to the ligation, and the tip was advanced ∼12–14 mm so that a 2-mm portion was in the aortic arch. The body of the telemetry probe was inserted into a subcutaneous pocket in the right flank. Mice were allowed to recover for 7 days at which time the probes were turned on by magnetic activation and measurement of blood pressure and HR was started. ANG II (1.6 μg·min−1·kg−1; American Peptides) was dissolved in saline containing 0.01 N acetic acid and infused subcutaneously via osmotic minipumps (model 1002; Alzet, Cupertino, CA) 10 days after telemetry probe implantation. Radio transmission was detected using RA1010 or RLA1020 receivers, converted digitally with Dataquest A.R.T. Silver Acquisition software (version 2.00, Data Sciences International), and analyzed with Dataquest A.R.T. Silver Analysis software (version 2.00, Data Sciences International). Blood pressure and HR data were collected for 5 s from each mouse at 10-min intervals during 12-h light and 12-h dark periods. Pulse pressure was calculated as the difference between systolic and diastolic blood pressure.
Effects of ANG II on albumin excretion, TNF production, and metabolic parameters.
Two mice of the same genotype were housed in metabolic cages (Nalgene) and allowed to acclimatize for 3 days before the study was started. ANG II (1.6 μg·min−1·kg−1) was infused via osmotic minipumps for 7 days. This dose was chosen because it was reported to be the lowest dose of ANG II that induces the development of hypertension in mice in a similar temporal pattern as observed in ANG II-infused rats (7); additional studies used comparable doses (27, 46). Food and water intake, urine output, and body weight were measured before and 7 days after ANG II infusion. Levels of urine albumin were measured using ELISA kits from Bethyl Laboratories, and urine and plasma TNF were measured using ELISA kits purchased from BD Biosciences.
Assessment of renal function.
ANG II (1.6 μg·min−1·kg−1) or vehicle was infused via osmotic minipumps for 7 days. Urine collected from these mice in metabolic cages was used for estimation of 24-h urine volume (V). At the time of death, serum was collected via cardiac puncture and urine was collected from the bladder. Urinary sodium (UNa) was measured with an IL943 Flame Photometer (Instrumentation Laboratories) and urinary creatinine (UCr) was measured using an ELISA kit (Cayman Chemical). Serum parameters were measured with an Olympus Analyzer 640e. Creatinine clearance (Ccr), as an estimation of GFR, was calculated from the formula: (UCr × V)/SCr.. Fractional excretion of sodium (FENa%) was derived from the formula [(UNa × SCr )/(UCr × SNa )]×100, where SCr and SNa denote serum creatinine and sodium, respectively (40).
TNF receptor mRNA determination.
Vehicle or ANG II (1.6 μg·min−1·kg−1) was infused subcutaneously as described above for 1 or 3 days. At death, kidneys were harvested, separated into cortex and outer medulla, and total RNA was isolated.
RNA extraction and real-time quantitative PCR.
Freshly isolated renal cortex and medulla were homogenized in 1 ml of TRIzol reagent and incubated at room temperature for 10 min to permit complete dissociation of nucleoprotein complexes. Following addition of 200 μl of chloroform, the sample was mixed for 15 s and incubated at room temperature for 2 to 3 min. The upper-colorless-aqueous phase containing RNA was collected after centrifugation at 4°C at 12,000 rpm for 15 min. Isopropyl alcohol (0.5 ml/ml TRIzol) was added to precipitate RNA. The mixture was incubated at room temperature for 10 min and centrifuged at 4°C at 12,000 rpm for 15 min. The supernatant was discarded, and the pellet was washed in 1 ml 75% ethanol (1 ml/ml TRIzol), mixed gently, and centrifuged for 5 min at 7,500 rpm at 4°C; the supernatant was removed and the pellet was dried for 5 to 10 min. The RNA pellet was resuspended in 50 μl of RNase-free distilled H2O. After total RNA sample was treated with deoxyribonuclease I for 30 min, a 3-μg aliquot of total RNA was used for cDNA synthesis with the SuperScript preamplification system (Life Technologies) in a 20-μl reaction mixture containing SuperScript II reverse transcriptase (200 U/μl) and random hexamers (50 ng/μl). The reaction was incubated at room temperature for 10 min to allow extension of the primers by reverse transcriptase and then at 42°C for 50 min, 70°C for 15 min, and 4°C for 5 min to obtain cDNA. The cDNA from the total RNA sample was amplified in a mixture, containing 2 mM MgCl2, AmpliTaq Taq DNA polymerase, PCR buffer II, and 2 μmol of specific primers. The primers were as follows: TNFR1: sense, 5′-GAGCGTTGTCAATTGCTG-3′; antisense, 5′-GTGACC CCTGATGGATGTA-3′; TNFR2: sense, 5′-GCTGGTCTTCGAACTGCAG-3′; antisense, 5′-GTATACATGCTTGCCTCACAG-3′. PCR reactions were performed using a GeneAmp System 2400 thermocycler (Applied Biosystems), final volume of 25 μl of dNTPs, PCR buffer, MgCl2, SuperTaq DNA polymerase, and specific primers under standard conditions [10 min of predenaturation at 95°C; 32 cycles of 40 s of denaturation at 95°C; and 90 s of annealing at 55°C, respectively; 2 min of elongation at 72°C; and finally, 7 min at 72°C]. Negative controls included primers that were reverse transcribed in the absence of RNA; contamination was ruled out by including PCR control samples with no DNA template.
A 0.5 μg aliquot of total RNA was converted to cDNA using random primers and PowerScript RT (Clontech) according to the manufacturer's protocol. The cDNA was placed in a 20 μl RT-PCR mixture using the FastStart DNA Master SYBR Green I kit (Roche) supplemented with 3 mM MgCl2 and Platinum Taq polymerase (Invitrogen). Quantitative real-time PCR was used to determine TNF receptor mRNA accumulation. Input cDNAs were normalized using β-actin, and the efficiency of primer pair amplification was determined using a standard curve generated as described previously (17). Relative TNF receptor mRNA accumulation was calculated using the 2(−ΔΔCT) method (28).
Statistical analysis.
Data are presented as means ± SE. Statistical analyses were performed using two-way ANOVA, by Tukey's multiple comparisons test, or unpaired t-tests as indicated. Differences with a P value of <0.05 were considered statistically significant.
RESULTS
Blood pressure and HR responses in WT and TNFR1−/− mice infused with ANG II.
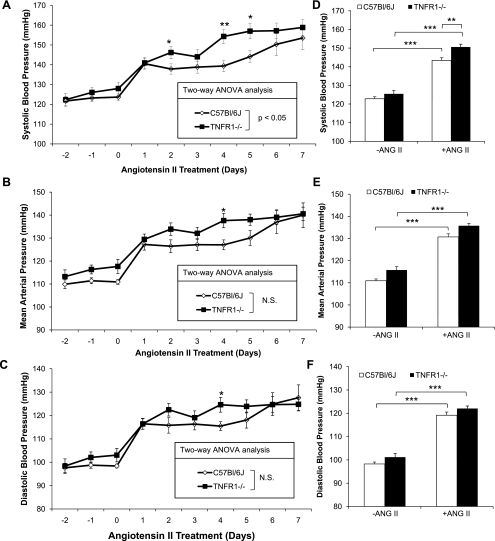
The effects of TNFR1 deletion on blood pressure and HR in response to ANG II infusion were evaluated by radiotelemetry. Baseline systolic blood pressure (SBP), mean arterial pressure (MAP), and diastolic blood pressure (DBP) from days −2 to day 0 were similar in WT and TNFR1−/− mice during both 12-h dark (Fig. 1, A, B, and C, respectively) and 12-h light periods (not shown); SBP, MAP, and DBP were higher in both genotypes during 12-h dark periods compared with 12-h light periods (not shown). Increases in SBP, MAP, and DBP, during both light and dark periods, were evident in WT and TNFR1−/− mice after administration of ANG II for 1 day and were sustained for the duration of the 7-day infusion protocol. SBP, but not MAP or DBP, was higher in TNFR1−/− compared with WT mice during 12-h dark periods, as determined by two-way ANOVA (Fig. 1, A-C). In addition, SBP, MAP, and DBP were significantly higher by ∼15, 10, and 9 mmHg, respectively, in TNFR1−/− compared with WT mice on day 4 of ANG II infusion. SBP also was higher in TNFR1−/− on days 2 and 5 compared with WT mice (Fig. 1A). Significant increases in SBP, MAP, and DBP, averaged over the 7-day infusion period, were observed in WT and TNFR1−/− (Fig. 1, D, E, and F, respectively). Moreover, SBP was higher in TNFR1−/− compared with WT mice (Fig. 1D), whereas MAP and DBP were not different between strains (Fig. 1, E and F, respectively). SBP, MAP, and DBP, during 12-h light periods, were similar in WT and TNFR1−/− mice infused with ANG II (not shown).
Fig. 1.
Effects of TNF receptor 1-deficient (TNFR1) deletion on systolic blood pressure (SBP), mean arterial pressure (MAP), and diastolic blood pressure (DBP) in response to ANG II infusion. SBP (A), MAP (B), DBP (C), average SBP (D), average MAP (E), and average DBP (F) from ANG II (1.6 μg·min−1·kg−1)-infused mice measured during 12-h dark periods by radiotelemetry. Average pressure values were acquired on days −2 to day 0 for baseline and days 1–7 for ANG II infusion. SBP was higher in TNFR1−/− on days 2, 4, and 5 compared with wild-type (WT) mice; average SBP was also higher in TNFR1−/−. MAP and DBP in TNFR1−/− were higher on day 4 compared with WT. A, B, C: *P < 0.05; **P < 0.01, TNFR1−/− vs. WT. D, E, F: **P < 0.01; ***P < 0.001. WT (n = 9) and TNFR1−/− (n = 11).
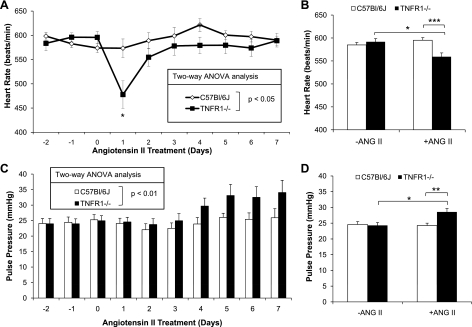
Baseline HR from days −2 to day 0 was similar in WT and TNFR1−/− mice during both 12-h dark (Fig. 2A) and 12-h light periods (not shown); HR was higher in both genotypes during 12-h dark periods compared with 12-h light periods (not shown). HR was lower in TNFR1−/− compared with WT mice in response to ANG II infusion (Fig. 2A). Average HR from days −2 to day 0 was similar between strains and lower in TNFR1−/− compared with WT mice over 7 days of ANG II infusion (Fig. 2B); a prominent decrease was observed 1 day after administration of ANG II (Fig. 2A). As this decrease in HR on day 1 after ANG II infusion may affect data interpretation, data were reanalyzed with exclusion of HR on day 1. HR in TNFR1−/− still remained lower compared with WT over 6 days of ANG II infusion, both analyzed by two-way ANOVA (P < 0.05) and as 6-day averages (P < 0.01). Baseline pulse pressure was similar between WT and TNFR1−/− mice (Fig. 2C). However, pulse pressure during 12-h dark periods was higher in TNFR1−/− compared with WT mice during ANG II infusion, as analyzed by two-way ANOVA (Fig. 2C) or 7-day averages (Fig. 2D).
Fig. 2.
Effects of TNFR1 deletion on heart rate (HR) and pulse pressure in response to ANG II infusion. Assessment by radiotelemetry of HR (A), average HR (B), pulse pressure (C), and average pulse pressure (D) from ANG II (1.6 μg·min−1·kg−1)-infused mice. Days −2 to 0 were used to calculate average values for baseline; days 1 to 7 were averaged for ANG II infusion. HR was lower in TNFR1−/− compared with WT mice after ANG II infusion and markedly reduced on day 1. Pulse pressure was calculated from the formula: SBP − DBP. Pulse pressure was significantly elevated in TNFR1−/− mice after ANG II infusion compared with WT. A: *P < 0.05 vs. WT. B and D: *P < 0.05; **P < 0.01; ***P < 0.001. WT (n = 9) and TNFR1−/− (n = 11).
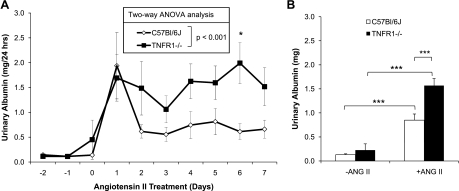
ANG II-induced albuminuria is exacerbated in TNFR1−/− mice.
Urinary albumin excretion was measured to determine whether deletion of TNFR1 affects renal damage in association with ANG II-induced hypertension. Basal levels of albumin excretion were similar in WT and TNFR1−/− mice (Fig. 3, A and B). Infusion of ANG II for 1 day increased urinary albumin excretion in both WT and TNFR1−/− mice (Fig. 3, A and B). Peak excretion of albumin in WT mice was observed 1 day after infusion of ANG II (Fig. 3A). On the second day of infusion, albuminuria in WT mice was diminished and subsequently stabilized over the course of the 7-day infusion. The maximum albumin excretion level in TNFR1−/− was similar to WT mice and also was observed after 1 day of ANG II infusion (Fig. 3A). However, albuminuria remained near peak levels throughout the course of the experiment in TNFR1−/− mice. These data suggest that the absence of TNFR1 is associated with persistent susceptibility to ANG II-induced renal damage.
Fig. 3.
Effects of TNFR1 deletion on urinary albumin excretion in response to ANG II infusion. Daily urinary albumin excretion (A) and average urinary albumin excretion (B) by ANG II (1.6 μg·min−1·kg−1)-infused mice. Days −2 to 0 were used to calculate average values for baseline; days 1 to 7 were averaged for ANG II infusion. Urinary albumin excretion in response to ANG II infusion was significantly elevated in TNFR1−/− compared with WT mice. WT (n = 7) and TNFR1−/− (n = 9); each n reflects pooled urine from 2 mice. A: *P < 0.05 vs. WT. B: ***P < 0.001.
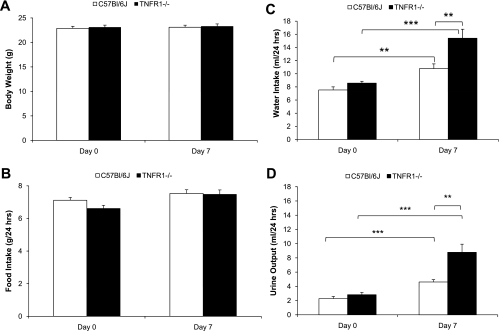
Comparison of metabolic responses to ANG II infusion in WT and TNFR1−/− mice.
Metabolic studies were performed to permit evaluation of TNFR1 gene deletion on renal function. Body weight and food intake were similar in WT and TNFR1−/− mice before (day 0) or 7 days after ANG II infusion (Fig. 4, A and B, respectively). Water intake was similar in WT and TNFR1−/− mice before ANG II infusion and increased in both genotypes after infusion of ANG II for 7 days (Fig. 4C). However, water intake increased to a greater extent in TNFR1−/− (15.4 ± 1.4 ml/day) compared with WT mice (10.8 ± 0.7 ml/day; P < 0.01) in response to ANG II infusion for 7 days (Fig. 4C). Baseline urine volume (UV) was similar in WT and TNFR1−/− mice (2.3 ± 0.3 vs. 2.8 ± 0.3 ml/day, respectively; Fig. 4D). Similar to the pattern seen with water intake, UV in TNFR1−/− (8.7 ± 1.2 ml/day; P < 0.01) was higher compared with WT mice (4.6 ± 0.3 ml/day; Fig. 4D) after 7 days of ANG II infusion.
Fig. 4.
Effects of TNFR1 deletion on body weight, food intake, water intake, and urine output in response to ANG II infusion. Body weight (A), food intake (B), water intake (C), and urine output (D) of mice on day 0 before and day 7 after ANG II (1.6 μg·min−1·kg−1) infusion. Basal levels of body weight, food intake, water intake, and urine output were not different between WT and TNFR1−/−. On day 7, body weight and food intake were not different but water intake and urine output were increased in both strains. Water intake and urine output were higher in TNFR1−/− on day 7 compared with WT. WT (n = 20) and TNFR1−/− (n = 18); each n for B-D reflects pooled data from 2 mice/cage/24-h period. **P < 0.01; ***P < 0.001.
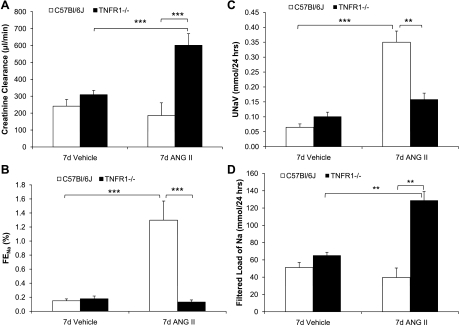
CCr, as an estimation of GFR, and sodium excretion were evaluated to establish whether TNFR1 deletion causes alterations in renal function in response to ANG II infusion. Serum creatinine levels (not shown) and CCr were similar in WT and TNFR1−/− mice infused with vehicle for 7 days (Fig. 5A). CCr in WT mice was not changed after ANG II infusion for 7 days (Fig. 5A). However, ANG II infusion increased CCr approximately twofold in TNFR1−/− mice, suggesting that the absence of TNFR1 affects regulatory mechanisms required for the maintenance of GFR (Fig. 5A). FENa% and UNaV were similar in WT and TNFR1−/− mice after infusion of vehicle for 7 days (Fig. 5, B and C, respectively). However, infusion of ANG II for 7 days increased FENa% in WT mice from ∼0.2 to 1.3% (Fig. 5B); enhanced UNaV was also observed in response to administration of ANG II (Fig. 5C). In contrast, FENa% and UNaV in TNFR1−/− were unchanged after 7 days of ANG II infusion (Fig. 5, B and C, respectively). Serum sodium levels (WT: 147.1 ± 0.6; TNFR1−/−: 146.4 ± 0.4 mmol/l) and filtered load of sodium were similar in WT and TNFR1−/− mice infused with vehicle for 7 days (Fig. 5D). After 7 days of ANG II infusion, serum sodium levels were similar in WT (148.2 ± 0.6 mmol/l) and TNFR1−/− (148.5 ± 0.7 mmol/l); filtered load of sodium did not change in WT but increased approximately twofold in TNFR1−/− mice (Fig. 5D). Collectively, these findings suggest that in response to ANG II infusion, deletion of TNFR1 was associated with an increase in GFR and a concomitant increase in the filtered load of sodium.
Fig. 5.
Deletion of TNFR1 alters renal responses to ANG II. Creatinine clearance (CCr; A), fractional excretion of sodium (FENa%; B), urinary sodium (UNav; C), and filtered load of sodium (D) were determined in WT and TNFR1−/− mice infused with vehicle or ANG II (1.6 μg·min−1·kg−1) for 7 days. Neither CCr nor FENa% was different between genotypes infused with vehicle. After ANG II infusion, CCr and filtered load of sodium increased in TNFR1−/− but not WT mice. Conversely, FENa% and UNaV increased in WT but not TNFR1−/− mice after ANG II infusion. **P < 0.01; ***P < 0.001; n = 4–12.
ANG II increases renal production of TNF.
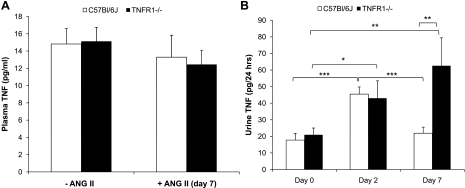
As ANG II increases TNF production by several cell types (2, 12, 22), plasma and urinary levels of TNF were determined before and after infusion of ANG II. Basal levels of plasma TNF were similar in WT and TNFR1−/− mice, as were plasma levels after infusion of ANG II for 7 days (Fig. 6A). Basal levels of urinary TNF were similar in WT and TNFR1−/− mice (Fig. 6B). Urinary levels of TNF increased approximately twofold in both WT and TNFR1−/− mice after infusion of ANG II for 2 days (Fig. 6B). However, 7 days after ANG II infusion, urinary TNF levels were similar to baseline levels in WT but remained elevated in TNFR1−/− mice (Fig. 6B). These data suggest that deletion of TNFR1 is associated with sustained levels of TNF in the kidney during ANG II infusion.
Fig. 6.
Effects of TNFR1 deletion on plasma and urinary TNF excretion before and after ANG II infusion. Plasma TNF (A) and urinary TNF (B) levels were determined in WT and TNFR1−/− mice before and after infusion with ANG II (1.6 μg·min−1·kg−1). Plasma TNF was measured at baseline and 7 days after ANG II infusion. Baseline plasma TNF was not different between WT and TNFR1−/− and was unaffected by ANG II infusion for 7 days. Urinary TNF was measured on days 0, 2, and 7. On day 0, urinary TNF was similar in WT and TNFR1−/− mice, but it increased in both WT and TNFR1−/− 2 days after ANG II infusion. However, 7 days after ANG II infusion, urinary TNF in WT was reduced to basal levels but remained elevated in TNFR1−/−. For plasma TNF, n = 6–19. For urine TNF, WT (n = 8) and TNFR1−/− (n = 7); each n reflects pooled data from 2 mice/cage/24-h period. *P < 0.05; **P < 0.01; ***P < 0.001.
Effects of ANG II on TNF receptor mRNA accumulation.
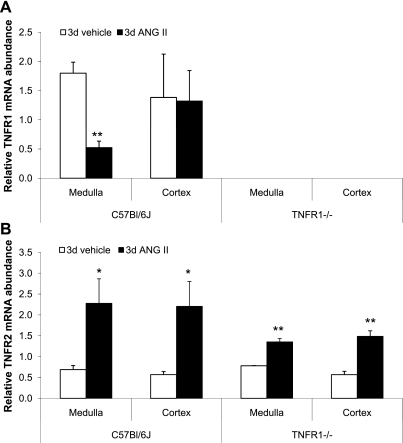
ANG II has been shown to modulate TNF receptor mRNA accumulation in the heart (41); however, its effect in the kidney has not been investigated. The relative accumulation of TNFR1 and TNFR2 mRNA was measured in renal cortex and outer medulla 1 and 3 days after ANG II infusion to determine whether changes occurred early during the course of infusion. ANG II infusion for 3 days significantly reduced TNFR1 mRNA accumulation in the outer medulla but not in the cortex of WT mice (Fig. 7A); similar results were observed on day 1 (not shown). TNFR1 was undetectable in TNFR1−/− mice under all conditions, consistent with the known genotype of these animals. TNFR2 mRNA accumulation in the cortex and outer medulla was increased in WT and TNFR1−/− mice in response to infusion of ANG II for 3 days (Fig. 7B); similar results were observed on day 1 (not shown). Thus, ANG II differentially regulates TNFR1 mRNA in the renal cortex and outer medulla of WT mice, whereas TNFR2 mRNA accumulation is similarly upregulated in both strains.
Fig. 7.
Effects of ANG II on TNF receptor mRNA accumulation in the kidney. TNFR1 (A) and TNFR2 mRNA accumulation (B) was determined by quantitative RT-PCR in renal cortex and outer medulla after infusion of vehicle or ANG II (1.6 μg·min−1·kg−1) for 3 days. There was a reduction in TNFR1 mRNA in the outer medulla of WT mice infused with ANG II. In contrast, ANG II infusion increased TNFR2 mRNA in renal medulla and cortex in WT and TNFR1−/−. *P < 0.05; **P < 0.01 vs. vehicle; n = 3–6.
DISCUSSION
The present study demonstrates that genetic deletion of TNFR1 predisposes mice to exacerbated increases in SBP, renal damage, and altered renal function in an ANG II-dependent model of hypertension. Higher SBP in TNFR1-deficient mice infused with ANG II was associated with a reduction in HR and increase in pulse pressure, changes not observed in WT mice. At the level of the kidney, sustained albuminuria in TNFR1−/− mice in association with elevated urinary TNF levels suggests that TNF may normally modulate renal functional responses to ANG II in WT mice expressing TNFR1. Moreover, Ccr and filtered load of sodium were increased while absolute and fractional excretion of sodium were less in TNFR1−/− compared with WT mice infused with ANG II, suggesting that tubular reabsorption of sodium was enhanced in mice lacking TNFR1. Collectively, these results demonstrate that genetic deletion of TNFR1 alters SBP, cardiac, renal hemodynamic, and tubular reabsorptive responses to ANG II infusion.
Interventions to prevent the responses to TNF, either by neutralization with etanercept or genetic deletion of TNF, have been reported to lower blood pressure in ANG II-induced hypertension (16, 41). Accordingly, the pressor response to ANG II may be negated in instances where activation of TNFR1 and TNFR2 by TNF is prevented. The present study showed that deletion of TNFR1 is associated with a greater increase in blood pressure under conditions where renal TNF levels are increased in response to ANG II infusion. The higher SBP response in TNFR1−/−, compared with WT mice over the course of the 7-day ANG II infusion protocol, is consistent with the finding in anesthetized mice that acute infusion of human recombinant TNF, which only binds to TNFR1 in mice, caused a significant reduction in MAP (24). Accordingly, blood pressure-reducing effects via TNFR1 may be eliminated in mice lacking this receptor, thereby contributing to higher SBP in response to ANG II infusion. T lymphocytes and TNF contribute to ANG II-induced hypertension (16). However, in dTGR where hypertension is also dependent on ANG II, neither blockade of TNF with etanercept nor immunosuppression, which reduced T-cell numbers, alleviated the increase in blood pressure (33). Thus, the contribution of TNF to the development of hypertension may be subject to additional factors that govern the regulation of TNF function. For instance, it is possible that TNF-mediated blood pressure elevation may be affected by the shedding of TNFR. Proteolytic cleavage of TNFR2 has been associated with reduced TNF activity (44), yet this proteolytic process is dependent on TNFR1 as anti-TNFR1 antibodies blocked this shedding process (18). Accordingly, in mice lacking TNFR1, shedding of TNFR2 is inhibited, thus contributing to enhanced actions of TNF, which may contribute to higher SBP in response to ANG II. Collectively, these findings suggest that selective activation or deletion of TNFR1 contributes to a mechanism that may lower or raise blood pressure, respectively.
Increases in peripheral vascular resistance, central arterial stiffness, and cardiac contractility have been proposed to increase SBP (20). It is unlikely that differences in SBP between TNFR1−/− and WT mice were due to differences in peripheral vascular resistance or central arterial stiffness, given the age, C57BL/6J genetic background, and duration of the experiments. Rather, elevated SBP but not DBP in conscious TNFR1−/− mice infused with ANG II may reflect changes in cardiac contractility. TNF exerts negative inotropic effects on the heart (23, 47), possibly via TNFR1-mediated induction of reactive oxygen species that depresses calcium handling and cell fractional shortening (8). Alternatively, TNFR2 has been shown to exert positive inotropic effects in cardiac myocytes via ERK/MSK1/cPLA2 pathway, which enhances calcium handling and cell fractional shortening (8). These findings are consistent with those of the present study showing that TNFR1−/− display higher pulse pressures compared with WT mice in response to ANG II, which in conjunction with slower HR in TNFR1−/− may result in increased ventricular filling time, and therefore increased contractility according to Starling's law. The mechanism underlying the negative chronotropic effect in these experiments is unclear, although activation of TNFR1 increases HR in a dose-dependent manner (39). Thus, in the absence of TNFR1, infusion of ANG II may unmask this fundamental effect. The elevation in pulse pressure in TNFR1−/− mice in response to ANG II, which in the context of decreased HR, suggests a positive inotropic response to ANG II in these mice to maintain cardiac outflow. It is presently unclear whether this HR response is due to the lack of TNFR1, activation of TNFR2, or a combination of both scenarios.
Exacerbated urinary albumin excretion in TNFR1−/− compared with WT mice during ANG II infusion suggests that the absence of TNFR1 is associated with increased susceptibility to renal damage. Consistent with the phenotype of TNFR1-deficient mice, which do not exhibit abnormal renal morphology or function (14, 36), blood pressure and albumin excretion in this study were similar in untreated WT and TNFR1−/− mice. Presently, it is unclear whether worsening albuminuria in TNFR1-deficient mice is linked to changes in blood pressure, reflects kidneys that are more prone to ANG II-mediated damage, or occurs subsequent to changes unrelated to the cardiovascular system (10, 33). Higher levels of water intake, a reflection of the dipsogenic response associated with ANG II (19), and urine output in TNFR1−/− mice 7 days after ANG II infusion compared with WT mice also suggest that TNFR1-deficient mice exhibit a heightened sensitivity to ANG II. Urinary TNF levels were greater in TNFR1−/− than WT mice on day 7 of ANG II infusion. As plasma levels of TNF were not elevated in either genotype, urinary TNF levels most likely reflect renal production of this cytokine. While most proinflammatory effects of TNF are associated with activation of TNFR1 (3), TNFR2 has been linked to vascular inflammation (29), apoptosis, and necrosis in cisplatin-induced acute renal failure (36), and glomerulonephritis (45), as well as having an allelic association with essential hypertension in humans (4, 9). These findings and our observation that TNFR2 is increased in TNFR1−/− mice infused with ANG II suggest that TNFR2 may play a role in the increased susceptibility to albuminuria.
Human recombinant TNF infusion has been shown to reduce renal blood flow and GFR in mice (39). As human recombinant TNF only binds to TNFR1 in mice, activation of TNFR1 may mediate effects of TNF on GFR. In the present study, Ccr was evaluated as an estimate of GFR to determine renal responses in TNFR1-deficient mice to ANG II infusion for 7 days. Ccr was similar between WT and TNFR1−/− mice in response to vehicle treatment for 7 days and fell within the physiological range as reported by others (32, 35). A twofold increase in Ccr was seen in TNFR1−/− compared with WT mice during ANG II infusion and resulted in an approximately twofold increase in the filtered load of sodium in TNFR1−/− mice. However, FENa% was unaffected in TNFR1−/− but increased in WT mice. The differences between TNFR1−/− and WT mice in terms of changes in FENa%, particularly the failure of TNFR1−/− mice to show an increase in FENa%, may be explained primarily on the basis of reduced renal tubular effects of TNF in mice with genetic deletion of TNFR1. This was confirmed by the observation that TNFR1−/− mice excreted less urinary sodium in response to ANG II infusion compared with WT mice. Stimulation of mouse TNFR1 by human TNF infusion produced natriuresis and increased FENa%, an effect occurring despite reductions in renal blood flow and GFR in the absence of blood pressure alteration, and is indicative of renal tubular effect mediated by that receptor (39). This is consistent with the presence of TNFR1 mRNA in the renal outer medulla and cortex of WT but not in the TNFR1-deficient animals. That is, TNFR1 is present in WT kidneys and may contribute to a natriuretic response, consistent with increased FENa% and UNaV, which could secondarily contribute to lower SBP compared with TNFR1−/−. Furthermore, increased GFR in TNFR1−/− mice in response to 7-day ANG II infusion may be explained by the elimination of TNFR1-associated renal vasoconstriction together with an increase in glomerular capillary pressure subsequent to elevated systemic pressure. The failure of FENa% to increase in TNFR1−/− mice is consistent with increased renal tubular sodium reabsorption as a consequence of altered responses of the TAL to TNF. Interestingly, infusion of ANG II for 7 days in mice was associated with decreased expression of NKCC2 (42). As ANG II infusion for 7 days increased urinary, and therefore renal, TNF levels in TNFR1−/− mice, and TNF injection could inhibit NKCC2 mRNA accumulation (38), it can be postulated that inhibition of NKCC2 by ANG II may be mediated through effects of TNF. Thus, TNFR1−/− mice display enhanced tubular sodium reabsorption as exhibited by the failure to elevate FENa%. Interestingly, ANG II infusion to WT and TNFR1−/− mice was associated with increased TNFR2 mRNA accumulation in both outer medulla and cortex, suggesting that upregulation of TNFR2 could also contribute to renal responses seen in the present study. However, renal functions of TNF via TNFR2 remain to be elucidated.
A critical renal protective role for TNFR1 is postulated since deletion of this receptor is associated with adverse effects, including higher SBP and urinary albumin excretion, as well as altered GFR and FENa% in response to infusion of ANG II. The absence of an overt renal phenotype in untreated TNFR1−/− compared with WT mice in the present study is in agreement with others (14, 30, 36, 45) and suggests that deletion of TNFR1 sensitizes mice to varied effects of ANG II. In conclusion, the present study demonstrates that selective deletion of TNF receptor 1 can cause alterations to blood pressure and renal function in response to ANG II. Elevated SBP is now known to be highly associated with cardiovascular risks and mortality, including coronary artery and heart diseases, heart failure, stroke, and end-stage renal disease, especially in middle-aged and elderly adults (5, 31, 48). The findings of this study are important in understanding possible mechanisms underlying higher pulse pressure, especially in those with systolic hypertension, and may suggest selective targeting of individual TNFRs as a therapeutic approach to improve cardiovascular and renal function.
GRANTS
This work was supported by National Institutes of Health Grants HL-085439 and HL-34300.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. M. A. Inchiosa, Jr., for valuable advice.
REFERENCES
- 1.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-α. Am J Physiol Cell Physiol 286: C779–C784, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Barbara JA, Smith WB, Gamble JR, Van Ostade X, Vandenabeele P, Tavernier J, Fiers W, Vadas MA, Lopez AF. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. EMBO J 13: 843–850, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjafield AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med 79: 109–115, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 160: 1085–1089, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brasier AR, Li J, Wimbish KA. Tumor necrosis factor activates angiotensinogen gene expression by the Rel A transactivator. Hypertension 27: 1009–1017, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Cervenka L, Maly J, Karasova L, Simova M, Vitko S, Hellerova S, Heller J, El-Dahr SS. Angiotensin II-induced hypertension in bradykinin B2 receptor knockout mice. Hypertension 37: 967–973, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Defer N, Azroyan A, Pecker F, Pavoine C. TNFR1 and TNFR2 signaling interplay in cardiac myocytes. J Biol Chem 282: 35564–35573, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Eguchi T, Maruyama T, Ohno Y, Morii T, Hirao K, Hirose H, Kawabe H, Saito I, Hayashi M, Saruta T. Possible association of tumor necrosis factor receptor 2 gene polymorphism with severe hypertension using the extreme discordant phenotype design. Hypertens Res 32: 775–779, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47: 557–562, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ferreri NR, Escalante BA, Zhao Y, An SJ, McGiff JC. Angiotensin II induces TNF production by the thick ascending limb: functional implications. Am J Physiol Renal Physiol 274: F148–F155, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ferreri NR, Zhao Y, Takizawa H, McGiff JC. Tumor necrosis factor-alpha-angiotensin interactions and regulation of blood pressure. J Hypertens 15: 1481–1484, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol Renal Physiol 277: F766–F772, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Gurantz D, Cowling RT, Varki N, Frikovsky E, Moore CD, Greenberg BH. IL-1beta and TNF-alpha upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol 38: 505–515, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Expression and function of NFAT5 in medullary thick ascending limb (mTAL) cells. Am J Physiol Renal Physiol 296: F1494–F1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol 152: 3550–3558, 1994 [PubMed] [Google Scholar]
- 19.Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res 586: 289–294, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Izzo JL., Jr Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol 19: 341–352, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kaji DM, Chase HS, Jr, Eng JP, Diaz J. Prostaglandin E2 inhibits Na-K-2Cl cotransport in medullary thick ascending limb cells. Am J Physiol Cell Physiol 271: C354–C361, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Kalra D, Sivasubramanian N, Mann DL. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation 105: 2198–2205, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kapadia S, Torre-Amione G, Yokoyama T, Mann DL. Soluble TNF binding proteins modulate the negative inotropic properties of TNF-α in vitro. Am J Physiol Heart Circ Physiol 268: H517–H525, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Kramer SM, Aggarwal BB, Eessalu TE, McCabe SM, Ferraiolo BL, Figari IS, Palladino MA., Jr Characterization of the in vitro and in vivo species preference of human and murine tumor necrosis factor-alpha. Cancer Res 48: 920–925, 1988 [PubMed] [Google Scholar]
- 25.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 26.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Buurman WA, Moore MW, Dayer JM, Fiers W, Bluethmann H, Grau GE. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol 27: 1719–1725, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol 169: 1886–1898, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann SJ. Systolic hypertension in the elderly. Pathophysiology and management. Arch Intern Med 152: 1977–1984, 1992 [PubMed] [Google Scholar]
- 32.Meneton P, Ichikawa I, Inagami T, Schnermann J. Renal physiology of the mouse. Am J Physiol Renal Physiol 278: F339–F351, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natarajan R, Ploszaj S, Horton R, Nadler J. Tumor necrosis factor and interleukin-1 are potent inhibitors of angiotensin II-induced aldosterone synthesis. Endocrinology 125: 3084–3089, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: F610–F618, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Saijonmaa O, Nyman T, Fyhrquist F. Downregulation of angiotensin-converting enzyme by tumor necrosis factor-alpha and interleukin-1beta in cultured human endothelial cells. J Vasc Res 38: 370–378, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Shahid M, Francis J, Majid DS. Tumor necrosis factor-α induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol 295: F1836–F1844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol 287: F1204–F1212, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51: 1345–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex differences in adaptive downregulation of premacula densa sodium transporters with ANG II infusion in mice. Am J Physiol Renal Physiol 298: F187–F195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-α inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol 283: R1046–R1051, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol 180: 2747–2751, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest 115: 1199–1209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 88: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest 92: 2303–2312, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol 13: 2776–2782, 2002 [DOI] [PubMed] [Google Scholar]