Abstract
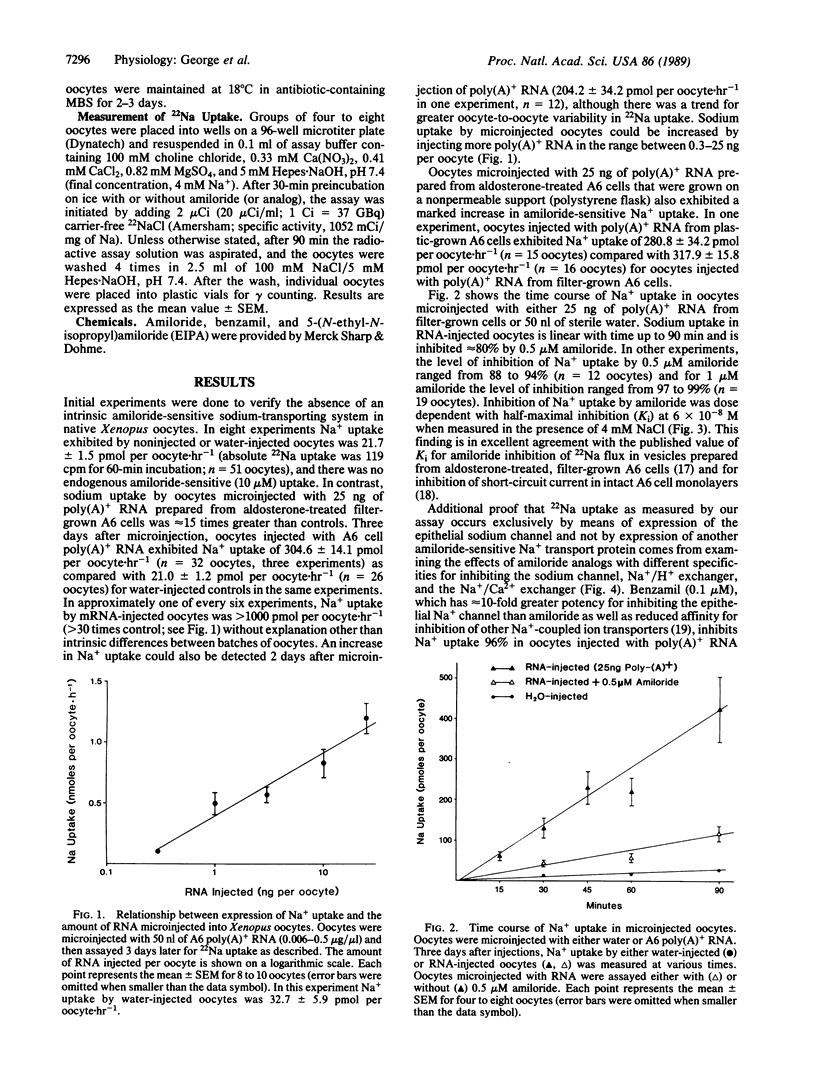
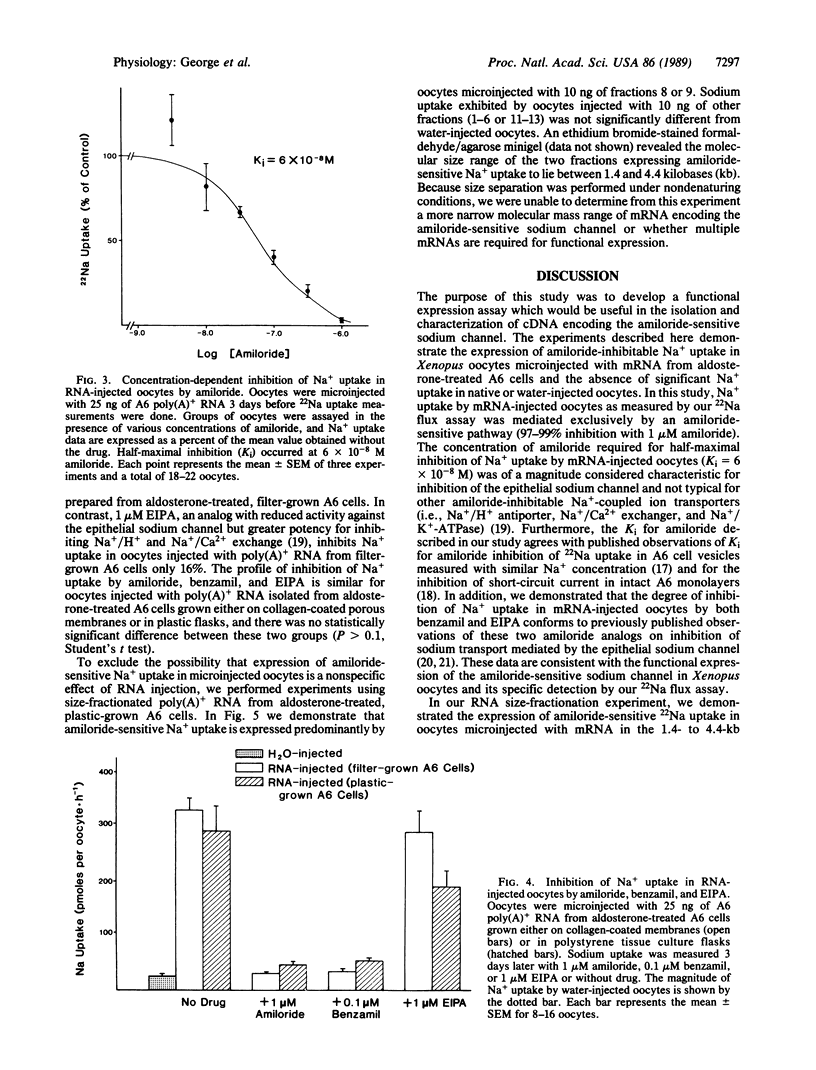
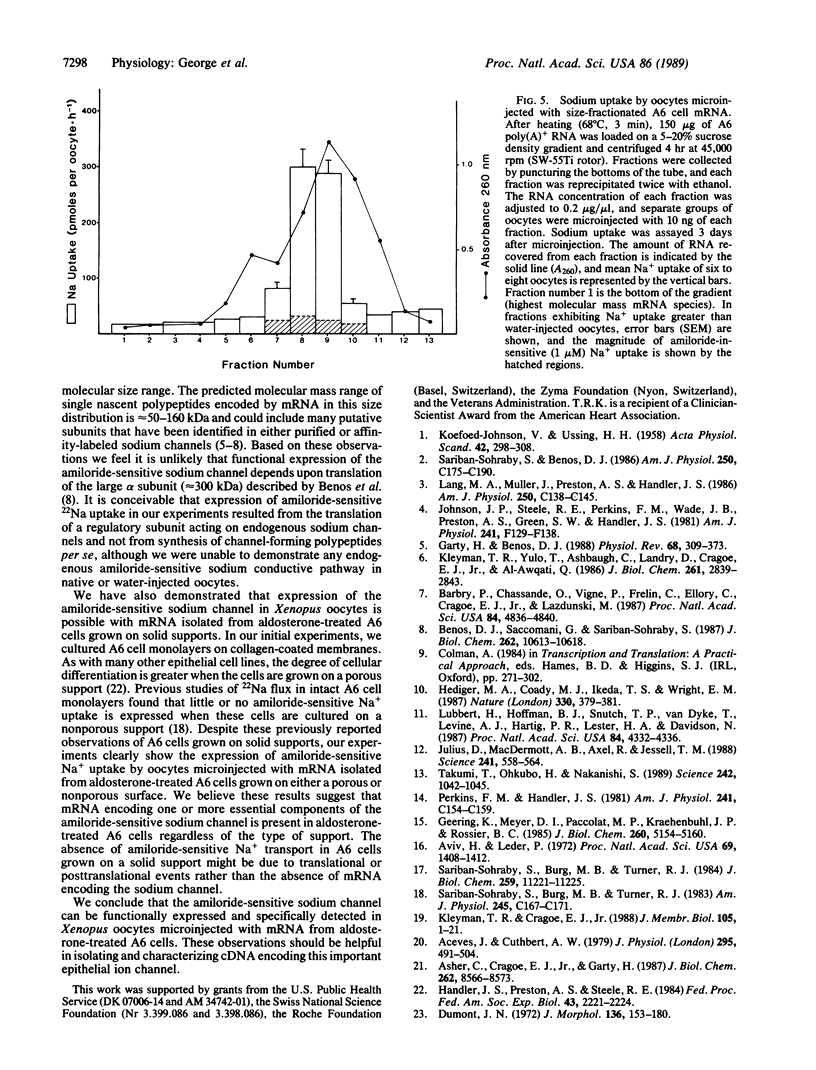
Expression of the amiloride-sensitive sodium channel was examined in Xenopus oocytes that were microinjected with A6 cell mRNA. Amiloride-inhibitable 22Na flux could be measured in intact oocytes 2-3 days after injection with 25 ng of poly(A)+ RNA isolated from aldosterone-treated A6 cells. The rate of 22Na uptake was approximately 15-fold greater in oocytes microinjected with 25 ng of poly(A)+ RNA than in water-injected control oocytes. An increase in 22Na uptake by mRNA-injected oocytes occurred whether the mRNA was isolated from A6 cells grown on a porous or nonporous support. In the presence of 4 mM NaCl, amiloride caused dose-dependent inhibition of 22Na uptake in mRNA-injected oocytes, which was half-maximal at 6 x 10(-8) M. Both 1 microM amiloride and 0.1 microM benzamil inhibited 22Na uptake in mRNA-injected oocytes by greater than 95%, whereas less than 50% inhibition occurred with 1 microM 5-(N-ethyl-N-isopropyl)amiloride. When A6 cell mRNA was size fractionated by sucrose density-gradient centrifugation, amiloride-sensitive 22Na uptake was expressed predominantly by oocytes injected with mRNA from two contiguous fractions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Cuthbert A. W. Uptake of [3H]benzamil at different sodium concentrations. Inferences regarding the regulation of sodium permeability. J Physiol. 1979 Oct;295:491–504. doi: 10.1113/jphysiol.1979.sp012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher C., Cragoe E. J., Jr, Garty H. Effects of amiloride analogues on Na+ transport in toad bladder membrane vesicles. Evidence for two electrogenic transporters with different affinities toward pyrazinecarboxamides. J Biol Chem. 1987 Jun 25;262(18):8566–8573. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbry P., Chassande O., Vigne P., Frelin C., Ellory C., Cragoe E. J., Jr, Lazdunski M. Purification and subunit structure of the [3H]phenamil receptor associated with the renal apical Na+ channel. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4836–4840. doi: 10.1073/pnas.84.14.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J., Saccomani G., Sariban-Sohraby S. The epithelial sodium channel. Subunit number and location of the amiloride binding site. J Biol Chem. 1987 Aug 5;262(22):10613–10618. [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Geering K., Meyer D. I., Paccolat M. P., Kraehenbühl J. P., Rossier B. C. Membrane insertion of alpha- and beta-subunits of Na+,K+-ATPase. J Biol Chem. 1985 Apr 25;260(8):5154–5160. [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Steele R. E. Factors affecting the differentiation of epithelial transport and responsiveness to hormones. Fed Proc. 1984 May 15;43(8):2221–2224. [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Steele R. E., Perkins F. M., Wade J. B., Preston A. S., Green S. W., Handler J. S. Epithelial organization and hormone sensitivity of toad urinary bladder cells in culture. Am J Physiol. 1981 Aug;241(2):F129–F138. doi: 10.1152/ajprenal.1981.241.2.F129. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Yulo T., Ashbaugh C., Landry D., Cragoe E., Jr, Karlin A., Al-Awqati Q. Photoaffinity labeling of the epithelial sodium channel. J Biol Chem. 1986 Feb 25;261(6):2839–2843. [PubMed] [Google Scholar]
- Lang M. A., Muller J., Preston A. S., Handler J. S. Complete response to vasopressin requires epithelial organization in A6 cells in culture. Am J Physiol. 1986 Jan;250(1 Pt 1):C138–C145. doi: 10.1152/ajpcell.1986.250.1.C138. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins F. M., Handler J. S. Transport properties of toad kidney epithelia in culture. Am J Physiol. 1981 Sep;241(3):C154–C159. doi: 10.1152/ajpcell.1981.241.3.C154. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Benos D. J. The amiloride-sensitive sodium channel. Am J Physiol. 1986 Feb;250(2 Pt 1):C175–C190. doi: 10.1152/ajpcell.1986.250.2.C175. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Burg M. B., Turner R. J. Aldosterone-stimulated sodium uptake by apical membrane vesicles from A6 cells. J Biol Chem. 1984 Sep 25;259(18):11221–11225. [PubMed] [Google Scholar]
- Sariban-Sohraby S., Burg M. B., Turner R. J. Apical sodium uptake in toad kidney epithelial cell line A6. Am J Physiol. 1983 Sep;245(3):C167–C171. doi: 10.1152/ajpcell.1983.245.3.C167. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]



