Abstract
We investigated water drinking and arterial blood pressure responses to intravenous infusions of ANG II in young (4 mo), middle-aged adult (12 mo), and old (29 mo) male Brown Norway rats. Infusions of ANG II began with arterial blood pressure either at control levels or at reduced levels following injection of the vasodilator minoxidil. Under control conditions, mean arterial pressure (MAP) in response to ANG II rose to the same level for all groups, and middle-aged and old rats drank as much or more water in response to ANG II compared with young rats, depending on whether intakes were analyzed using absolute or body weight-adjusted values. When arterial blood pressure first was reduced with minoxidil, MAP in response to ANG II stabilized at significantly lower levels compared with control conditions for all groups. Young rats drank significantly more water under reduced pressure conditions compared with control conditions, while middle-aged and old rats did not. Urine volume in response to ANG II was lower, while water balance was higher, under conditions of reduced pressure compared with control conditions. Baroreflex control of heart rate was substantially reduced in old rats compared with young and middle-aged animals. In summary, young rats appear to be more sensitive to the inhibitory effects of increased arterial blood pressure on water drinking than are older animals.
Keywords: aging, water intake, urine volume, heart rate, water balance, minoxidil, captopril
elderly people have disordered sensations of thirst, and do not drink enough water in response to dehydration to fully replenish body water (9, 16, 17, 21, 22). Like elderly humans, old animals fail to drink adequately in response to several experimental thirst challenges (18, 19, 24–26, 33, 34). For example, we have noted a striking deficiency in the drinking response to hypotension in old rats (30). Hypotension causes thirst in rats primarily by stimulating the release of renal renin and subsequent formation of ANG II in the circulation (18, 20). Old rats secrete considerably less renin in response to most stimuli than younger animals (4–6, 25) and therefore may not generate dipsogenic levels of ANG II during hypotension. Additionally, it is possible that old rats do not adequately sense the levels of ANG II that are produced during thirst challenges and may have elevated levels of factors such as atrial natriuretic peptide that are known to inhibit drinking (15).
The administration of exogenous ANG II, whether by subcutaneous bolus or intravenous infusion, produces drinking in rats (8, 19, 23–25, 28). In addition, administration of exogenous ANG II produces a marked pressor response that is capable of inhibiting drinking (8, 23, 28). Specifically, the sensing of increased arterial blood pressure by baroreceptor mechanisms attenuates drinking responses (28). Animals drink more during administration of ANG II when levels of arterial pressure are reduced by coadministration of hypotensive drugs (8, 23, 31, 32). Old rats, like elderly humans, have severely impaired baroreflex buffering of heart rate (HR) responses (10, 11, 13). This suggests that sensing of ANG II-induced increases in arterial blood pressure could differ with age and thereby have different effects on the amount of water consumed. Previous studies of age-related drinking in response to ANG II did not record arterial blood pressure (19, 24–26). Therefore, in the present work, we examined age-related drinking responses to ANG II and simultaneously measured arterial blood pressure in the same animals. In addition, we examined whether reducing levels of arterial blood pressure during administration of ANG II resulted in additional drinking by old animals, as has been shown to occur in young animals. We delivered ANG II continuously by intravenous infusion under conditions in which arterial pressure was allowed to rise freely and also under conditions in which arterial pressure was initially reduced by prior administration of the hypotensive drug minoxidil. The vehicle solution included the angiotensin converting enzyme inhibitor captopril to prevent contributions of endogenously formed ANG II to the observed drinking and pressor responses (8, 23, 31).
METHODS
Animals.
Male Brown Norway rats aged 3 mo (young), 11 mo (middle-aged adult), and 28 mo (old) were obtained from Harlan (Indianapolis, IN) through services provided by the NIH's National Institute on Aging. A separate group of male Sprague-Dawley-derived rats (4 mo) also was obtained from Harlan. All rats were housed singly in hanging stainless steel cages in a temperature-controlled room (23°C) on a 12:12-h light-dark cycle. They received ad libitum access to standard Teklad Rodent Diet and tap water. Brown Norway rats were allowed 1 mo for adaptation and were tested at 4, 12, and 29 mo of age, respectively. Sprague-Dawley rats were tested within 2 wk of receipt. All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Drugs.
Captopril (Sigma-Aldrich, St Louis, MO) was dissolved in sterile 0.9% NaCl (2.5 mg/0.6 ml) immediately before each experiment. The infusion rate was 0.6 ml/h, yielding 2.5 mg per 0.6 ml/h. Minoxidil (Sigma-Aldrich) was dissolved in propylene glycol for a stock solution of 20 mg/ml. It was administered as an intravenous bolus in a range of doses (0.5–1.5 mg/kg body wt). ANG II (Sigma-Aldrich) was dissolved in isotonic saline (50 ng/μl) and stored frozen in small aliquots. Fresh samples were thawed before each experimental session and diluted with captopril solution, which served as the vehicle, for intravenous infusion at a dose of 50 ng·kg body wt−1·min−1.
Catheter surgery.
Rats received femoral venous and arterial catheters under halothane anesthetic for drug injections and measurement of arterial pressure, respectively. Both catheters were made from polyethylene tubing (PE-50) ∼20 cm in length that was heat-welded to a shorter piece of PE-10. The PE-10 was inserted into the vessel and advanced 3.5 cm for arterial lines and 3.0 cm for venous lines. The catheters were tunneled under the skin, secured between the scapulae, and exited at the base of the neck. When not in use, the catheters were filled with heparinized saline (200 U/ml) and plugged with 23-gauge obturators. Rats were allowed 2–3 days of recovery from surgery before testing.
Blood pressure recording.
The analog signal from a Cobe pressure transducer was processed by a CED 1401 laboratory interface (Cambridge Electronics Design, Cambridge, UK) using Spike 2 software (Cambridge Electronics Design) on a Dell Optiplex GX620 Pentium D computer. The data were analyzed by a script file that used the digitized pulsatile pressure to calculate mean arterial pressure (MAP), heart rate (HR), and systolic and diastolic pressures. The data were averaged over 1, 5, or 10 min bins. Initial, or starting, MAP and HR were determined using the last 30 min of the baseline period, i.e., before the bolus injections of vehicle or minoxidil, and was the average of three 10-min bins.
Urine analysis.
Urine was collected and the volume (UV) was measured. Relative water balances were calculated by subtracting UV from water intake.
Procedure.
The test cages were wooden with aluminum-lined interiors (24 × 29 cm) that extended 31 cm above suspended, stainless steel metabolism cages. On the morning of testing, rats were brought to the experimental room, weighed, connected to arterial and venous lines with lengths of PE-50 tubing, and placed in the test cages. The arterial lines were connected to pressure transducers and arterial blood pressure was recorded continuously. The venous lines were connected to syringes filled with vehicle (captopril solution) that were secured in infusion pumps (model 22; Harvard Apparatus). The pumps were running so infusions (0.6 ml/h) began as soon as the lines were connected. The vehicle contained captopril to prevent endogenous formation of ANG II during testing and thus clamp circulating levels of ANG II to those provided exclusively by infusion (8, 23, 31). Chemical burettes filled with water were attached to the front of the cages.
Rats were allowed 1 h to adjust after being connected to the arterial and venous lines, and then they received bolus intravenous injections over ∼30 s of propylene glycol (100–210 μl, i.e., control condition) or minoxidil solution (0.5–1.5 mg/kg body wt, i.e., reduced pressure condition). (The intravenous line was momentarily disconnected from outside the metabolism cage to allow injection of the bolus and attachment of another line that contained ANG II in captopril solution. This was timed to allow the vehicle or minoxidil bolus and new infusate to clear the dead space at the appropriate time.) Infusions of ANG II began 1 h after the bolus intravenous injections, and water intakes were recorded every 30 min for 3 h. Each rat received intravenous ANG II under both treatment conditions in two tests separated by 2–4 days. The order of treatment conditions was approximately counterbalanced. Water intakes in the hour after the intravenous bolus and before start of intravenous ANG II were small (average all runs: 0.9 ± 0.1 ml/h) and were not considered in the analysis.
Blockade of angiotensin converting enzyme.
A small preliminary study tested the effectiveness of captopril treatment in preventing endogenous formation of ANG II during testing. A mixed group (young, n = 4; middle-aged, n = 2) of Brown Norway rats was instrumented with arterial and venous catheters. On the morning of testing, they were placed in the test cages and attached to arterial and venous lines as described above. They were infused intravenously with isotonic saline (0.6 ml/h) for 1 h and then tested for pressor responses to bolus intravenous injections of ANG I (50, 100, and 200 ng/kg body wt) and ANG II (50 and 100 ng/kg body wt). Then the infusate was switched to captopril solution (2.5 mg/0.6 ml) and the animals were retested for pressor responsiveness 1 h later.
Subcutaneous delivery of ANG II.
In a separate test using Sprague-Dawley-derived rats, we injected ANG II as a subcutaneous bolus to compare the pattern of arterial pressure and HR changes to those obtained in response to continuous intravenous administration. Our dose was 200 μg/kg body wt, which is typical of doses employed in previous work from several laboratories assessing age-related drinking responses to ANG II (19, 24–26).
Statistical analysis.
Data were analyzed by one-way or repeated-measures ANOVA. Analysis of the drinking data was conducted on the raw, noncumulated measures at each time point (12). Planned comparisons were made with Fisher's least-significant difference tests when the global F ratio was significant or with Bonferroni tests when the global F ratio was not significant. Values were considered significant at the P < 0.05 level. The results are expressed as means ± SE.
RESULTS
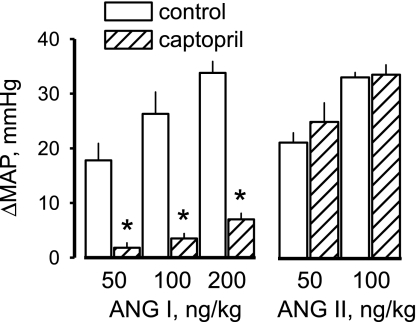
The preliminary study established the effectiveness of captopril treatment in preventing endogenous formation of ANG II. During control infusions of isotonic saline, injections of ANG I caused dose-related increases in MAP (Fig. 1). After 1 h of captopril treatment, pressor responses were nearly abolished at the two lower doses of ANG I and were significantly reduced (80–90%) at the highest dose of ANG I. Captopril treatment did not affect the dose-related pressor responses to injections of ANG II. Therefore, the captopril treatment substantially prevented conversion of ANG I to ANG II within the systemic circulation without impairing vascular reactivity to ANG II.
Fig. 1.
Peak change in mean arterial pressure (MAP) in response to intravenous injections of ANG I and ANG II. Control responses were obtained, then captopril was added to the infusate, and responses were obtained again 1 h later. Values are means ± SE. *Significantly different from control, P < 0.01.
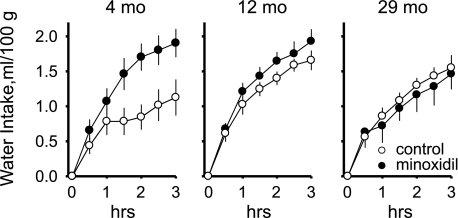
To assess drinking behavior across the ages at comparable differences in levels of MAP between the treatment conditions, we established a criterion that each animal must have at least 20 mmHg differences in the ANG II-induced levels of MAP between the treatments to be included in the analysis. Using this criterion, results from one middle-aged rat and seven old rats were excluded from the analysis. The final group numbers for the main experiment were young, n = 8; middle-aged, n = 8; and old, n = 5 rats. Initial body weight was significantly different between ages (the means ± SE were: 306 ± 9, 402 ± 12, and 460 ± 11 g at 4 mo, 12 mo, and 29 mo, respectively; F2,18 = 38.98, P < 0.001). Therefore, water intake was analyzed as rate of intake, i.e., milliliters per 30 min of testing, both as absolute and as body weight-adjusted (i.e., ml/100 g) measures. We used a two-way mixed-model ANOVA with time and treatment condition (i.e., with or without the minoxidil bolus) as within-subjects repeated-measures and age as the between-subjects variable. Analysis of the absolute intakes revealed that the highest rates of intake occurred in the first 30–60 min of the experimental sessions (time main effect, F5,90 = 15.48, P < 0.001). A main effect of age (F2,18 = 5.49, P < 0.05) revealed that young rats drank significantly less during testing than middle-aged and old rats, which did not differ in the amounts of water they drank. There was no main effect of treatment, and there were no significant interactions. However, planned contrasts revealed that young rats drank more in response to ANG II after pretreatment with minoxidil, when arterial pressure was initially reduced, than under control conditions, while middle-aged and old rats did not. When water intakes were adjusted for body weight, there were significant main effects of time (F5,90 = 16.99, P < 0.001) and treatment (F1,18 = 4.66, P < 0.05) but not of age, and there was a significant treatment times age interaction (F2,18 = 4.14, P < 0.05). The body weight-adjusted drinking data are shown in Fig. 2 as cumulative intakes. As with the prior analysis, the highest rates of intake were in the first 30–60 min of testing. The interaction effect revealed that young rats drank significantly more water in response to infusions of ANG II after pretreatment with minoxidil than without minoxidil, while middle-aged adult and old rats did not.
Fig. 2.
Water drinking in response to intravenous infusions of ANG II (50 ng·kg−1·min−1) in young (4 mo), middle-aged adult (12 mo), and old (29 mo) rats under conditions when initial arterial blood pressure is normal (control) or reduced (minoxidil) by pretreatment with the vasodilator minoxidil. Minoxidil effects were observed only in young rats. Values are means ± SE.
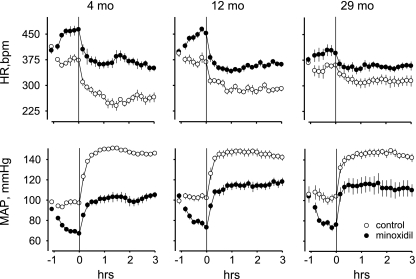
The MAP and HR responses, averaged over 10 min bins, are presented in Fig. 3 and Table 1. For MAP, there were significant main effects of treatment (F1,18 = 85.07, P < 0.001) and time (F2,36 = 446.57, P < 0.001) and a significant time × treatment interaction (F2,36 = 94.08, P < 0.001). Initial levels of MAP were equivalent across the ages and between the treatments (Fig. 3). Levels of MAP averaged over the last 30 min before starting the infusions of ANG II were also equivalent across the ages, but significantly different between the treatments. Under both treatment conditions, MAP in response to ANG II stabilized at equivalent levels across the groups. The differences in ANG II-induced levels of MAP between the treatment conditions were also equivalent for all ages (Table 1). In addition, the differences between ANG II-induced levels of MAP and the starting, initial values were significantly less after treatment with minoxidil compared with after control treatment (main effect, F1,18 = 115.78, P < 0.001; Table 1). During reduced pressure conditions, treatment with minoxidil caused similar reductions in MAP across the groups (F2,18 = 0.87, P > 0.05, Table 1). However, old rats required slightly lower doses of minoxidil to produce similar decreases in blood pressure than young and middle-aged rats, probably because of diminished baroreflex buffering of minoxidil-induced hypotension (30). The means ± SE for minoxidil doses were: 1.2 ± 0.1, 1.3 ± 0.1, and 0.9 ± 0.1 mg/kg at 4 mo, 12 mo, and 29 mo, respectively; F2,18 = 5.42, P < 0.05. A main effect of age (F2,18 = 3.68, P < 0.05) indicated that MAP, when averaged over time and treatment, was lower for young rats compared with the other groups. For HR, all main effects and most interactions were significant, including a three-way interaction of time × treatment × age (F4,36 = 4.73, P < 0.01). Both the initial HR and the HR averaged over the last 30 min before the start of the ANG II infusions were significantly less in old rats compared with in young and middle-aged rats under both treatment conditions (Fig. 3). Infusion of ANG II significantly reduced HR for all ages. ANG II-induced levels of HR were significantly higher after minoxidil treatment than after control treatment, and the difference in ANG II-induced HR responses between the treatment conditions was significantly greater for young rats compared with both other groups (Table 1). The differences between ANG II-induced levels of HR and the initial values were significantly smaller after minoxidil treatment than after control treatment, and decreased significantly with age under control conditions (time × age interaction, F2,18 = 6.72, P < 0.01; Table 1).
Fig. 3.
MAP and heart rate (HR) responses to intravenous infusions of ANG II (50 ng·kg−1·min−1) in young (4 mo), middle-aged adult (12 mo), and old (29 mo) rats. The initial, or starting values, are averages of the last 30 min before bolus injections of vehicle or minoxidil at −1 h. Lines indicate start of ANG II infusions at 0 h. Values are means ± SE.
Table 1.
Levels of mean arterial pressure (MAP) and heart rate (HR) achieved in response to intravenous infusions of ANG II under control conditions and reduced pressure conditions and differences in these measures from initial values in young (4 mo), middle-aged adult (12 mo), and old (29 mo) rats
|
MAP, mmHg |
HR, beats/min |
|||||
|---|---|---|---|---|---|---|
| Age, mo | 4 | 12 | 29 | 4 | 12 | 29 |
| MAP and HR in Last 2.5 h of iv Infusion of ANG II | ||||||
| ANG II + vehiclea | 148 ± 1 | 146 ± 4 | 145 ± 3 | 260 ± 7 | 288 ± 7 | 313 ± 13 |
| ANG II + minoxidilb | 102 ± 3‡ | 115 ± 4‡ | 113 ± 8‡ | 369 ± 12‡ | 354 ± 6‡ | 355 ± 7‡ |
| Treatment differencec | 46 ± 4 | 31 ± 6 | 32 ± 6 | −109 ± 16† | −66 ± 13 | −42 ± 15 |
| Change in MAP and HR from Initial Values | ||||||
| ANG II + vehiclea | 49 ± 2 | 46 ± 3 | 40 ± 5 | −154 ± 6 | −105 ± 9* | −54 ± 14† |
| ANG II + minoxidilb | 12 ± 4‡ | 11 ± 4‡ | 10 ± 4‡ | −33 ± 17‡ | −44 ± 13‡ | −22 ± 11‡ |
| Minoxidild | −23 ± 2 | −28 ± 4 | −28 ± 2 | 62 ± 13 | 56 ± 9 | 24 ± 9 |
Values are means ± SE. Change scores are the difference between the initial values obtained over the last 30 min before bolus injection of vehicle or minoxodil and values averaged over the last 2.5 h of ANG II infusion when arterial pressure appeared stable.
Measures in response to ANG II infused intravenously under control conditions;
measures in response to ANG II infused intravenously after pretreatment with minoxidil;
differences in the measures between the treatment conditions;
differences between values in response to minoxidil averaged over the last 30 min before infusion of ANG II and initial values.
Significantly different from young rats, P <0.05;
significantly different from both other groups, P <0.05;
significantly different from control values, P <0.05.
Urine was collected at the end of testing and measured for volume (Table 2). Absolute measures of UV were significantly reduced after pretreatment with minoxidil compared with control conditions (treatment main effect, F1,18 = 124.53, P < 0.001), and increased significantly with age (age main effect, F2,18 = 7.57, P < 0.01). Water balance, calculated as the difference between UV and water intake, was significantly greater for all groups after minoxidil pretreatment (treatment main effect, F1,18 = 101.37, P < 0.001) and was higher, overall, in middle-aged rats compared with the other groups (age main effect, F2,18 = 5.84, P < 0.05). When adjusted for body weight, UV was reduced after treatment with minoxidil for all groups (treatment main effect, F1,18 = 116.83, P < 0.001) but was no longer age-related. Analysis of body weight-adjusted water balances revealed that all effects were significant. All groups had greater body weight-adjusted water balances after pretreatment with minoxidil compared with control conditions. Additionally, water balance was significantly greater after minoxidil pretreatment in young rats compared with old rats (treatment × age interaction, F2,18 = 4.39, P < 0.05).
Table 2.
Water balance measures during intravenous infusions of ANG II under control and reduced pressure conditions in young (4 mo), middle-aged adult (12 mo), and old (29 mo) rats
| Control Pressure Condition |
Reduced Pressure Condition |
|||||
|---|---|---|---|---|---|---|
| Age, mo | 4 | 12 | 29 | 4 | 12 | 29 |
| Absolute Measures, ml | ||||||
| Intake | 3.5 ± 0.8 | 6.6 ± 0.6* | 7.1 ± 0.7* | 5.8 ± 0.6‡ | 7.7 ± 0.7* | 6.8 ± 1.1* |
| Urine | 6.2 ± 0.5 | 6.9 ± 0.8 | 10.6 ± 1.1† | 0.9 ± 0.4‡ | 2.2 ± 0.7‡ | 2.6 ± 0.7†‡ |
| Balance | −2.7 ± 0.6 | −0.3 ± 0.4† | −3.5 ± 1.5 | 4.9 ± 0.7‡ | 5.5 ± 0.6†‡ | 4.4 ± 1.0‡ |
| Body Weight-Adjusted Measures, ml/100 g | ||||||
| Intake | 1.1 ± 0.3 | 1.8 ± 0.2 | 1.6 ± 0.2 | 1.9 ± 0.2‡ | 1.9 ± 0.2 | 1.5 ± 0.2 |
| Urine | 2.0 ± 0.1 | 1.7 ± 0.2 | 2.3 ± 0.2 | 0.3 ± 0.1‡ | 0.5 ± 0.2‡ | 0.6 ± 0.2‡ |
| Balance | −0.9 ± 0.2 | −0.0 ± 0.1 | −0.7 ± 0.3 | 1.6 ± 0.2‡ | 1.4 ± 0.2‡ | 0.9 ± 0.2‡* |
Values are means ± SE. Water intake, urine volume, and water balance measures over 3 h of infusion with ANG II. Balance measures do not include ∼2.4 ml of infusion volume. There were main effects of age for all absolute measures, but not for body weight-adjusted measures.
Significantly different from young rats, P <0.05;
significantly different from both the other groups, P <0.05;
significantly different from control values, P <0.05.
To assess the sensitivity of the water drinking, UV, and water balance responses to changes in arterial pressure, we calculated slopes of these responses in relation to changes in arterial pressure by the rise overrun method. For each animal, we used the 3-h cumulative measures of these responses (y values) and changes from initial MAP during intravenous ANG II (x values) under both treatment conditions. We analyzed the resulting slopes by two-way ANOVA with response (i.e., water intake, urine volume, and water balance) as the within-subjects variable and age as the between-subjects variable. The means ± SE of the slopes for body weight-adjusted water intake, urine volume, and water balance measures were −0.01 ± 0.01, 0.05 ± 0.01, and −0.06 ± 0.01 (ml/100 g)/mmHg, respectively. There were no effects of age. However, the slopes for the responses differed significantly from one another, revealing that both urine volume and water balance change significantly more as a function of change in arterial pressure than does water drinking (main effect, F2,36 = 75.27, P < 0.001).
As a measure of baroreflex sensitivity, we used the rise overrun method to calculate slopes of the relationship between changes in HR (ΔHR; y values) and ΔMAP (x values) using data obtained at 30 min postinjection of minoxidil and after 30 min of infusion of ANG II for each animal. The slopes for both young and middle-aged rats were significantly different from those of old rats (age main effect, F2,18 = 4.72, P < 0.05), but were not different from each other. The differences in slopes indicate that baroreflex-mediated changes in HR in old rats were less than half as sensitive to changes in arterial blood pressure as were those in young and middle-aged rats. The means ± SE for the slopes were: −2.55 ± 0.36, −2.08 ± 0.30, and −1.01 ± 0.29 beats·min−1·mmHg−1 at 4 mo, 12 mo, and 29 mo, respectively.
In a separate test, we injected ANG II as a subcutaneous bolus and compared the pattern of arterial pressure and HR changes to those obtained in response to continuous intravenous administration in the young Brown Norway rats under control conditions (Fig. 4). In response to bolus subcutaneous administration of ANG II (200 μg/kg body wt), arterial pressure increased 70 mmHg within 5 min and then rapidly recovered, approaching basal levels by 90 min postinjection.
Fig. 4.
Changes in MAP (ΔMAP) and HR (ΔHR) in response to bolus (200 μg/kg sc) or continuous (50 ng·kg−1·min−1 iv) administration of ANG II. Data for subcutaneous administration were obtained using Sprague-Dawley-derived rats (4 mo, n = 8) and are compared with data from intravenous administration in young Brown Norway rats (4 mo, n = 8) under control testing conditions of the main experiment.
The results from more than half (n = 7) of the old rats did not meet the criterion for inclusion in the analysis. Minoxidil treatment did not produce levels of ANG II-induced arterial pressure that were sufficiently different from corresponding levels obtained during control conditions in these nonresponding old rats. The average treatment differences in MAP and HR during intravenous ANG II for the excluded old rats and for the old rats that were included in the analysis were 9 ± 2 vs. 32 ± 6 mmHg and −5 ± 10 vs. −42 ± 15 beats/min, respectively. Unlike the responders, the nonresponders as a group had a complete lack of reflex tachycardia following injection of minoxidil, and their urine volume was unaffected by minoxidil treatment. However, there were no significant differences between the responders and nonresponders on any other measure, including drinking. That is, old rats drank similar amounts of water in response to intravenous ANG II whether there were significant (i.e., >20 mmHg) differences in levels of arterial pressure between the treatment conditions (responders) or not (nonresponders). Together, the results from both groups of old rats emphasize how little drinking by old rats is affected by levels of arterial pressure and HR.
DISCUSSION
The main results of this study were the following: under control conditions, middle-aged adult (12 mo) and old (29 mo) rats drank more water in response to intravenous infusions of ANG II than young (4 mo) rats on an absolute basis and drank as much water as young rats on a body weight-adjusted basis. Young rats increased their drinking response to ANG II under conditions in which arterial blood pressure was initially reduced, so that levels of MAP in response to ANG II stabilized at significantly lower levels than under control conditions, but middle-aged and old rats did not. Urine volume during testing increased significantly with age on an absolute basis and decreased significantly across age on an absolute or body weight basis when the levels of arterial pressure in response to ANG II were reduced by prior injections of minoxidil. Water balance at the end of testing was highest for middle-aged rats, and was significantly greater after minoxidil pretreatment than after control treatment. As with previous studies, analysis of HR indicated that old rats had severely impaired baroreflex responses compared with young and middle-aged animals (1, 2, 10, 14, 27, 30).
Previous work has examined age-related drinking in response to ANG II in rats. Silver et. al (26) conducted a dose-response analysis of age-related drinking to ANG II. They found that when water intake was adjusted for body weight, young (3 mo) rats drank significantly more than all other age groups (12, 20, and 24 mo) at most doses, indicating age-related declines in drinking to ANG II. Other groups did not find consistent age-related differences in drinking to ANG II in rats when water intakes were analyzed either as absolute or body weight-adjusted measures (19, 24, 25). In those studies, the ANG II tests were part of a series of diverse thirst challenges. In all of these studies, ANG II was administered in a subcutaneous bolus in tests lasting 1–2 h using similar age ranges (∼3–24 mo). The design of our experiment differed from all previous work in that we administered much lower total amounts of ANG II directly into the vasculature using constant intravenous delivery for 3 h. Our young rats drank as much in response to ANG II as middle-aged and old rats in the first hour of testing, but drank significantly less in the remaining 2 h. Notably, the pattern of cardiovascular changes during acute bolus and constant intravenous administration are very different. As seen in Fig. 4, bolus subcutaneous administration of ANG II causes an immediate spike in arterial pressure and correspondingly sharp reduction in HR that lasts only a few minutes before returning towards baseline. On the other hand, continuous intravenous administration of ANG II requires nearly an hour to produce maximal changes in arterial pressure and HR, and the levels of MAP and HR remain fairly constant for the remainder of testing (Figs. 3 and 4). These contrasting patterns of cardiovascular changes between the two modes of administration suggest that pressure-related mechanisms may exert inhibitory influences on drinking behavior with distinctly different time courses during testing: 1) immediately, but perhaps briefly, upon subcutaneous delivery of ANG II, and 2) with some delay, but persistently, upon intravenous delivery of ANG II.
Old animals have deficient drinking responses to procedures involving hypovolemia and hypotension that depend on generation of endogenous ANG II to stimulate thirst (19, 24, 25, 30, 33). Deficient drinking by old animals in response to hypovolemia/hypotension may be due to impaired renin release and insufficient generation of circulating ANG II, to impaired ANG II receptor function or to postreceptor signaling impairments. Our finding that old rats drink as much, or more, water in response to intravenous infusion of ANG II as young rats indicates that old rats are as able as young rats to sense and to respond to circulating ANG II. Therefore, the present work supports the idea that old rats drink less in response to hypovolemia/hypotension because they fail to secrete enough renin to generate dipsogenic levels of ANG II (24, 25, 30). That is, impaired thirst in response to hypovolemia/hypotension in old rats is less a problem of signal transduction and more a problem of generating sufficient signal.
The dose of intravenous ANG II we employed (50 ng·kg body wt−1·min−1) produces large increases in arterial pressure and modest water intakes (8). Reductions in MAP have been shown to increase drinking in previous studies (8, 23). We have shown that a similar effect is present in young rats, but not in middle-aged or old rats. Our experimental strategy was similar to one used previously by us (31, 32) and others (8, 23). We injected minoxidil intranvenously and allowed arterial pressure to fall for 1 h before starting intravenous infusions of ANG II. By blocking endogenous formation of ANG II with captopril, relatively low doses of minoxidil reduced MAP by similar amounts across the ages. Under control conditions, blood pressure and HR results were consistent with previous work. Initial MAP was similar across the groups (30), although it is often slightly higher in older animals (3, 7, 35). Baseline HR was considerably lower in old rats (3, 7, 30, 35). In response to intravenous infusion of ANG II, arterial pressure increased similarly across the age groups, as a function of change from initial values (3), and stabilized at equivalently high levels. When MAP first was reduced by minoxidil treatment, arterial pressure increased less from initial values in response to intravenous ANG II for all groups and stabilized at significantly lower levels than under control conditions (Fig. 3). HR was reduced substantially from initial values during infusion of ANG II under both treatment conditions, but was reduced less in older animals under control conditions, probably as a function of their impaired HR baroreflex responses (10, 14).
It seems reasonable to speculate that the greater drinking responses of old rats compared with young rats under control conditions can be explained by diminished baroreflex-mediated inhibition of drinking in the older animals. Similarly, the impaired baroreflex mechanisms of old rats should make them less sensitive to the facilitatory effects of reduced arterial pressure on drinking. For young rats, reductions in arterial pressure remove baroreflex inhibition of drinking behavior. For old rats, the underlying baroreflex inhibitory mechanism is defective, so removing or reducing its residual influence adds little to the drinking response. However, middle-aged rats also failed to significantly increase drinking in response to lowered levels of arterial pressure, and they have relatively normal baroreflex responses. Therefore, there may be age-related levels of other factors (e.g., atrial natriuretic peptide, oxytocin) in addition to baroreflex mechanisms to explain why young rats drank different amounts of water between the treatment conditions and middle-aged and old rats did not. However, it is worth noting that, although there were no significant age differences in absolute levels of ANG II-induced HR under control or reduced pressure conditions, the difference in HR between the treatment conditions was significantly greater in young rats compared with both middle-aged and old animals as was the reduction in HR from initial values during control conditions (Table 1). This may be evidence that young rats experienced significantly greater differences in baroreflex inhibition between the treatments and during control conditions compared with both of the other groups, which could serve as the basis for the young rats drinking different amounts of water between treatment conditions while both of the other groups did not.
We observed effects of arterial pressure on UV and water balance at all ages. Under control conditions in which arterial pressure rose to high levels in response to ANG II, nearly every animal excreted more water than it ingested, and the resulting water balances were uniformly negative across groups. However, when arterial pressure was initially reduced so that maximal levels of arterial pressure in response to ANG II were lower, urine volumes were substantially less, most animals ingested more than they excreted, and the resulting water balances were uniformly positive across groups. Urinary excretion and water balance were more sensitive to changes in MAP than was drinking behavior at all ages.
In summary, based on measures of water consumption, middle-aged adult and old rats appear as capable as young rats of sensing and responding to ANG II that is delivered to them by intravenous infusion. However, there are age-related differences in how the drinking responses to ANG II are influenced by prevailing levels of arterial pressure or to some other factor that varies with arterial pressure.
Perspectives and Significance
With an increasingly aging population, it is critical to understand how impairments in thirst sensation in the elderly contribute to their problems of maintaining adequate hydration. Like the elderly, old rats have diminished thirst and impaired kidney function (4, 19, 24, 30, 33) making them a useful model and valuable tool for exploring diminished water ingestion and impaired excretion in aging animals. Exploring the reasons for these impaired responses is likely to lead to better understanding of why there are similar deficits in the elderly. The current literature suggests that old rats do not generate sufficient endogenous ANG II to elicit thirst in response to hypovolemia and hypotension, yet sense and drink in response to ANG II when it is provided by subcutaneous or intravenous administration. These results suggest that central neural mechanisms that mediate the drinking response to ANG II must be relatively intact in old animals. However, there is a caveat: the impaired baroreflex responses of old rats may actually permit them to ingest more than young rats by providing less inhibition from the increase in arterial pressure. Thus, reduced inhibition from baroreceptors in old rats may mask impaired sensing of ANG II by old rats, thereby permitting seemingly normal drinking responses.
GRANTS
This research was supported by National Institutes of Health Grants MH-59239 and AG-25465 (to R. L. Thunhorst) and HL-57472, DK-66086, and HL-14388 (to A. K. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Barringer DL, Buñag RD. Differential age-dependent attenuation of reflex tachycardia by verapamil in rats. Mech Ageing Dev 58: 111–125, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Barringer DL, Buñag RD. Autonomic regulation of reflex bradycardia in rats declines with age. Exp Gerontol 26: 65–75, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis 22: 842–850, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol 9: 699–709, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Engels K, Beierwaltes WH. β-Adrenoceptor-stimulated renin release is blunted in old rats. J Am Soc Nephrol 9: 1318–1320, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Baylis C, Engels K, Hymel A, Navar LG. Plasma renin activity and metabolic clearance rate of angiotensin II in the unstressed rat. Mech Aging Devel, 97: 163–171, 1997 [DOI] [PubMed] [Google Scholar]
- 7.da Silva Lemos M, Braga ANG, da Silva JR, dos Santos RAS. Altered cardiovascular responses to chronic angiotensin II infusion in aged rats. Regul Pept 132: 67–73, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Evered MD, Robinson MM, Rose PA. Effect of arterial pressure on drinking and urinary responses to angiotensin II. Am J Physiol Regul Integr Comp Physiol 254: R69–R74, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Farrell MJ, Zamarripa F, Shade R, Phillips PA, McKinley M, Fox PT, Blair-West J, Denton DA, Egan GF. Effect of aging on regional cerebral blood flow response associated with osmotic thirst and its satiation by water drinking: a PET study. Proc Natl Acad Sci USA 105: 382–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari AU, Daffonchio A, Albergati F, Manci G. Differential effects of aging on the heart rate and blood pressure influences of arterial baroreceptors in awake rats. J Hypertens 9: 615–621, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Ferrari AU, Radaelli A, Centola M. Aging and the cardiovascular system. J Appl Physiol 95: 2591–2597, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fitts DA. Misuse of ANOVA with cumulative intakes. Appetite 46: 100–102, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hajduczok G, Chapleau MW, Johnson SL, Abboud FM. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol Heart Circ Physiol 260: H1113–H1120, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Irigoyen MC, Moreira ED, Werner A, Ida F, Pires MD, Cestari IA, Krieger EM. Aging and baroreflex control of RSNA and heart rate in rats. Am J Physiol Regul Integr Comp Physiol 279: R1865–R1871, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol 18: 292–353, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc 33: 1524–1532, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol 76: 1615–1623, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Martin JR, Fuchs A, Harting J. Drinking by senescent and adult rats in response to regulatory challenges. Neurobiol Aging 6: 57–59, 1985 [DOI] [PubMed] [Google Scholar]
- 19.McKinley MJ, Denton DA, Thomas CJ, Woods RL, Mathai ML. Differential effects of aging on fluid intake in response to hypovolemia, hypertonicity, and hormonal stimuli in Munich Wistar rats. Proc Natl Acad Sci USA 103: 3450–3455, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer DK, Rauscher W, Peskar B, Hertting G. The mechanism of the drinking response to some hypotensive drugs: activation of the renin-angiotensin system by direct or reflex-mediated stimulation of β-receptors. Arch Pharmacol 276: 13–24, 1973 [DOI] [PubMed] [Google Scholar]
- 21.Phillips PA, Bretherton M, Johnston CI, Gray L. Reduced osmotic thirst in healthy elderly men. Am J Physiol Regul Integr Comp Physiol 261: R166–R171, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Phillips PA, Rolls BJ, Ledingham JGG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med 311: 753–759, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Robinson MM, Evered MD. Pressor action of intravenous angiotensin II reduces drinking response in rats. Am J Physiol Regul Integr Comp Physiol 252: R754–R759, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Rowland NE, Morien A, Garcea M, Fregly MJ. Aging and fluid homeostasis in rats. Am J Physiol Regul Integr Comp Physiol 273: R1441–R1450, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Rowland NE, Del Bianco A, Fregly MJ. Age-related decline in thirst and sodium appetite in rats related to kininase II inhibition. Regul Pept 66: 163–167, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Silver AJ, Morley JE, Ishimaru-Tseng TV, Morley PMK. Angiotensin II and fluid ingestion in old rats. Neurobiol Age 14: 519–522, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Simpkins JW, Field FP, Ress RJ. Age-related decline in adrenergic responsiveness of the kidney, heart and aorta of male rats. Neurobiol Aging 4: 233–238, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Stocker SD, Stricker EM, Sved AF. Arterial baroreceptors mediate the inhibitory effect of acute increases in arterial blood pressure on thirst. Am J Physiol Regul Integr Comp Physiol 282: R1718–R1729, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Tank JE, Vora JP, Houghton DC, Anderson S. Altered renal vascular responses in the aging rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F942–F948, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Thunhorst RL, Beltz TG, Johnson AK. Hypotension- and osmotically induced thirst in old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol 297: R149–R157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thunhorst RL, Johnson AK. Effects of arterial pressure on drinking and urinary responses to intracerebroventricular angiotensin II. Am J Physiol Regul Integr Comp Physiol 264: R211–R217, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Thunhorst RL, Johnson AK. Effects of hypotension and fluid depletion on central angiotensin-induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 281: R1726–R1733, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Thunhorst RL, Johnson AK. Thirst and salt appetite responses in young and old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol 284: R317–R327, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Whyte DG, Thunhorst RL, Johnson AK. Reduced thirst in old, thermally dehydrated rats. Physiol Behav 81: 569–576, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Zhang XZ, Qiu C, Baylis C. Sensitivity of the segmental renal arterioles to angiotensin II in the aging rat. Mech Ageing Dev 97: 183–192, 1997 [DOI] [PubMed] [Google Scholar]