Abstract
The serum bicarbonate in neonates is lower than adults due in large part to a lower rate of proximal tubule acidification. It is unclear if the neonatal proximal tubule is functioning at maximal capacity or if the proximal tubule can respond to metabolic acidosis as has been described in adult proximal tubules. We find that neonatal mouse brush-border membranes have a lower Na+/H+ exchanger (NHE) 3 protein abundance (neonate 0.11 ± 0.05 vs. adult 0.64 ± 0.07; P < 0.05) and a higher NHE8 protein abundance (neonate 1.0 ± 0.01 vs. adult 0.13 ± 0.09; P < 0.001) compared with adults. To examine if neonates can adapt to acidosis, neonatal mice were gavaged with either acid or vehicle for 4 days, resulting in a drop in serum bicarbonate from 19.5 ± 1.0 to 8.9 ± 0.6 meq/l (P < 0.001). Proximal convoluted tubule Na+/H+ exchanger activity (dpHi/dt) was 1.68 ± 0.19 pH units/min in control tubules and 2.49 ± 0.60 pH units/min in acidemic neonatal mice (P < 0.05), indicating that the neonatal proximal tubule can respond to metabolic acidosis with an increase in Na+/H+ exchanger activity. Similarly, brush-border membrane vesicles from neonatal rats had an increase in Na+/H+ exchanger activity with acidemia that was almost totally inhibited by 10−6 M 5-(N-ethyl-n-isopropyl)-amiloride, a dose that has little effect on NHE3 but inhibits NHE8. There was a significant increase in both NHE3 (vehicle 0.35 ± 0.07 vs. acid 0.73 ± 0.07; P < 0.003) and NHE8 brush-border membrane protein abundance (vehicle 0.41 ± 0.05 vs. acid 0.73 ± 0.06; P < 0.001) in acidemic mouse neonates compared with controls. A comparable increase in NHE3 and NHE8 was found in neonatal rats with acidosis. In conclusion, the neonatal proximal tubule can adapt to metabolic acidosis with an increase in Na+/H+ exchanger activity.
Keywords: acidification, sodium/hydrogen exchanger 3, sodium/hydrogen exchanger 8, sodium/hydrogen exchanger, renal development
the proximal tubule reabsorbs the majority of filtered bicarbonate. Two-thirds of luminal proton secretion (6, 36) and virtually all the luminal acidification in the neonate is due to the Na+/H+ exchanger (6). A small fraction of proximal tubule proton secretion in the adult is the result of a luminal H+-ATPase (6, 29, 36). In the adult proximal tubule, luminal Na+/H+ exchange is predominantly mediated by Na+/H+ exchanger (NHE) 3 (12, 37, 43, 44). In adult animals, proximal tubule luminal bicarbonate absorption, Na+/H+ exchange, and NHE3 protein abundance are upregulated in response to metabolic acidosis (3, 19, 35, 41, 44).
Neonates have a lower serum bicarbonate level, which is due to a lower bicarbonate threshold, and are more prone to acid-base disturbances than adults (21). The lower bicarbonate threshold is predominantly due to the lower rate of bicarbonate reabsorption in the neonatal proximal tubule compared with the adult proximal tubule (9, 38). The maturational increase in proximal tubular acidification is largely due to the maturational increase in apical membrane NHE3 (5–7, 10). Examination of neonatal proximal tubular acidification mechanisms demonstrated that, before weaning, there was Na+/H+ exchange activity despite a paucity of NHE3, suggesting another NHE isoform (39). The presence of another proximal tubule Na+/H+ exchanger was confirmed in NHE3 null mice (17).
NHE8 was cloned by Goyal et al. (24) and localized to the apical membrane of all segments of the proximal tubule (11, 23). NHE8 is a Na+-dependent proton exchanger that is sensitive to 5-(N-ethyl-n-isopropyl)-amiloride (EIPA) (46). Our laboratory has shown that NHE8 is a developmental NHE isoform that is highly expressed on the apical brush-border membranes in neonatal rat tubules and that expression decreases with age (11).
Metabolic acidosis has been shown to increase apical brush-border membrane NHE3 protein expression and Na+/H+ exchange activity in adult rats (2, 3, 28, 44). Because renal acidification in the neonate is less than that of the adult, it is unclear if the neonate can adapt to metabolic acidosis, as seen in the adult, with an increase in Na+/H+ exchange activity. The purpose of this study was to examine if metabolic acidosis increases proximal tubule Na+/H+ exchange activity and if NHE8 expression is increased by metabolic acidosis in vivo.
METHODS
Animals and gavage.
C57BL/6 mice were born at our institution. Starting at 5 days of age, mice were gavaged orally with 1 mmol/100 g body wt with a volume of 1 ml/100 g body wt with either ammonium chloride or sodium chloride (control) twice daily for seven doses. This protocol is similar to one that had previously been used to study metabolic acidosis in adult rats (18, 35) and one used in neonatal rats starting as young as 2 days of age to produce metabolic acidosis (27). The age chosen was at a point during postnatal development where there is a paucity of NHE3 and NHE8 is normally highly expressed on the apical membrane of the proximal tubule (11). The kidneys were harvested for brush-border membrane vesicle (BBMV) isolation, renal cortical protein analysis, RNA for cDNA synthesis, or for proximal tubule isolation for in vitro microperfusion. The studies performed were in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Blood collection and analysis.
Mice were sedated with Inactin (1 mg/10 g body wt). Whole blood (100 μl) was collected via intracardiac puncture and placed in heparinized tubes for analysis of serum electrolytes, pH, Pco2, and bicarbonate levels using a Stat Profile Critical Care Express blood gas machine (Nova Biomedical, Waltham, MA).
BBMV isolation.
BBMVs were prepared as previously described (13, 20, 39). Intraperitoneal Inactin (1 mg/10 g) was administered to mice or rats gavaged with saline (control) and NH4Cl before death. Kidneys were removed quickly and placed in 6 ml of isolation buffer containing 300 mM mannitol, 16 mM HEPES, and 5 mM EGTA that was titrated to a pH of 7.4 with Tris. The isolation buffer contained phenylmethylsulfonyl fluoride (100 μg/ml) and 1 μl/ml protease inhibitor cocktail (Sigma, St. Louis, MO). The kidneys were homogenized with 15 strokes of a Teflon-glass homogenizer at 4°C. Briefly, the kidney was homogenized in ice-cold isolation buffer, and crude membranes were obtained by centrifugation at 48,000 g at 4°C for 1 h (Beckman J2–21M; JA-20 rotor; Beckman Coulter, Fullerton, CA). The pellets were resuspended, homogenized in a Dounce glass homogenizer, and subjected to Mg2+ precipitation by addition of MgCl2 at a final concentration of 15 mM at 4°C for 20 min. The precipitated membranes were removed by centrifugation at 3,000 g for 10 min at 4°C, and the supernatant was subjected to two additional rounds of Mg2+ aggregation. This was followed by centrifugation at 48,000 g for 30 min at 4°C, and the resulting pellet enriched in BBMV was used for Na+/H+ exchange activity experiments or immunoblotting. All protein fractions were assayed using the Bradford method with bovine serum albumin as the standard (15). The brush-border membrane enrichment compared with homogenate of leucine amino peptidase in neonatal rats was 10.6 ± 0.6, and that of adults was 9.8 ± 0.7 (P = 0.38), neonatal mice 9.4 ± 0.9, and 8.7 ± 1.0 in adult mice (P = 0.61).
SDS-PAGE and immunoblotting.
Brush-border membrane (15 μg) cortical homogenates were diluted in 2.5× loading buffer [2.5 mM Tris·HCl (pH 6.8), 2.5% β-mercaptoethanol, 25% glycerol, and 2.5% SDS] (15 μg/lane) and heated at 85°C for 5 min (NHE3) or 37°C for 5 min (NHE8). The proteins were then separated on an 8% polyacrylamide gel using SDS-PAGE as previously described (20) and transferred to a polyvinylidene diflouride membrane (Immobilon; Millipore, Billerica, MA) at 400 mA at 4°C for 1 h (8, 14, 39). The blots were blocked with Blotto (1% nonfat milk and 0.05% Tween 20 in PBS, pH 7.4) for at least 1 h before incubation with primary antibodies to either NHE3 or NHE8. The NHE8 antibody was the monoclonal antibody 7A11 at a 1:5 dilution from a hybridoma supernatant, and the NHE3 antibody was a rabbit anti-rat polyclonal antiserum 1568 against amino acids 809–822 of NHE3 at a 1:1,000 dilution (4, 23) (gifts from Orson W. Moe and Peter Aronson, respectively). The blots were washed in Blotto followed by the addition of the secondary antibody, horseradish peroxidase-conjugated donkey anti-mouse antibody at a 1:10,000 dilution. Enhanced chemiluminescence was used to detect bound antibody (Amersham Biosciences, Piscataway, NJ). Equal loading of the samples was verified using an antibody to β-actin at 1:15,000 dilution (Sigma Biochemicals and Reagents, St. Louis, MO). Relative NHE3, NHE8, or β-actin protein abundance was quantitated using densitometry.
cDNA synthesis and real-time PCR.
Total cellular RNA was isolated from whole kidneys of 8-day-old mice using the GenElute Mammalian Total RNA Miniprep Kit per the manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). The quantity and purity of total RNA was assayed using an LKB Ultra-spec III spectrophotometer at 260- and 280-nm wavelengths. Total RNA (2 μg) was first treated with DNase I (Invitrogen, Carlsbad, CA), and the product was used to synthesize cDNA with random hexamer primers and reverse transcriptase (Stratagene, La Jolla, CA) at an annealing temperature of 25°C for 10 min, extension at 42°C for 50 min, and termination at 70°C for 15 min.
Real-time PCR was performed using an iCycler PCR Thermal Cycler (Bio-Rad, Hercules, CA) to quantify relative mRNA abundance. Primers for NHE3 and NHE8 (Table 1) were mixed with cDNA and SYBR green master mix per the manufacturer's instructions (Bio-Rad). The PCR conditions were denaturation at 94°C for 30 s, annealing at 61°C for 20 s, and extension at 72°C for 20 s for 40 cycles. The housekeeping gene 28S was used to normalize the relative expression of NHE3 and NHE8 using the method described by Vandesompele et al. (42).
Table 1.
Primers used for real-time PCR
| Gene | Primers |
|---|---|
| SLC9A3 (NHE3) | F: 5′-TTC AAA TGG CAC CAC GTC CAG G-3′ |
| R: 5′-TGA CCT TGT GGG ACA GGT GAA AG-3′ | |
| SLC9A8 (NHE8) | F: 5′-ACA GTT TCG CAT TTG GCT CCC TG-3′ |
| R: 5′-TGT TGG TGA GGA CGA TGG AGA CTG-3′ | |
| 28S | F: 5′-TTG AAA ATC CGG GGG AGA G-3′ |
| R: 5′-ACA TTG TTC CAT GCC AG-3′ |
NHE, Na+/H+ exchanger; F, forward; R, reverse.
In vitro pHi studies.
Proximal tubules were dissected from 8-day-old mice that were either gavaged for 4 days with normal saline (control) or with ammonium chloride as described above. After death, the kidney was quickly placed in ice-cold Hanks' solution that contained (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 tris(hydroxymethyl)amino methane hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Proximal convoluted tubules were dissected free hand from the midcortical and juxtamedullary region of the kidney. The dissected tubules were transferred in cold solution to a bathing chamber on a Nikon Eclipse TE 2000-U inverted epifluorescent microscope with a Photometrics Cascade model 512F camera (Ottobrunn, Germany) and Lambda DG-4/DG-5 illumination system (Sutter Instrument, Novato, CA) to measure cell pH (pHi). The tubules were perfused using concentric glass pipettes with an ultrafiltrate-like solution that contained (in mM) 115 NaCl, 25 NaHCO3, 4.0 Na2HPO4, 10 sodium acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, 2 lactate, and 2 glutamine and bathed with the same solution preheated to 37°C. The bath solution was changed to one containing 5 × 10−6 M BCECF-AM (Molecular Probes, Eugene, OR) to load the tubule with the pH-sensitive dye BCECF. To estimate cell pHi, a nigericin calibration curve was generated comparing the ratio of fluorescence at 500 nm to 450 nm with pHi as previously described (1, 5, 17, 39).
After the tubular cells were loaded with BCECF for ∼2 min, the bathing solution was changed to solution A containing 1 mM 4-acetamido-4′-isothiocyano-stilbene-2,2′ disulfonic acid (SITS) to inhibit the sodium-dependent bicarbonate cotransporter on the basolateral membrane, the major regulator of cell pH in the proximal tubule (1, 1, 5, 36). The solution had a bicarbonate concentration of 5 mM and a pH of 6.6 to compensate for the cell alkalinization caused by SITS (solution A in Table 2). The sodium-containing luminal solution perfusing the tubule (solution B in Table 2) was rapidly changed to one without sodium (solution C in Table 2), and the rate of change in cell pH was measured. The initial change in cell pHi after sodium removal was used to measure dpHi/dt. The luminal solution was then changed back to the original sodium-containing solution. This protocol has been used previously to study the activity of the luminal Na+/H+ exchanger (1, 17, 20, 36).
Table 2.
Solutions in cell pH studies
| Bath (Solution A) | Luminal Na+ (Solution B) | Luminal 0 Na+ (Solution C) | |
|---|---|---|---|
| NaCl | 140 | 115 | |
| NaHCO3 | 5 | 25 | |
| NMDG-Cl | 115 | ||
| Choline HCO3 | 25 | ||
| KCl | 5 | ||
| K2HPO4 | 2.5 | 2.5 | |
| MgCl2 | 1 | 1 | |
| MgSO4 | 1 | ||
| Na2HPO4 | 1 | ||
| Glucose | 5 | ||
| l-Alanine | 5 | ||
| Urea | 5 | ||
| CaCl2 | 1.8 | 1.8 | 1.8 |
| Heptanoic acid | 2 | ||
| pH | 6.6 | 7.4 | 7.4 |
Salt concentrations are in mM. Solutions had an osmolality of 300 mosmol/kgH2O. NMDG, N-methyl-d-glucamine.
Na+/H+ exchange activity in BBMV.
Because of the small amount of renal cortex available from mice, rat renal cortical BBMV were prepared as above. Neonatal rats were gavaged starting at 10 days with vehicle or acid two times daily as above for mice and killed on day 13 of age. Na+/H+ exchange activity was measured using freshly prepared BBMV using the acridine orange fluorescence quenching technique (13). The BBMV pellet obtained as above was rehomogenized in intravesicular solution containing 280 mM mannitol and 5 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.5, with a Potter-Elvehjem Teflon-glass homogenizer, equilibrated at 4°C for 2 h, centrifuged at 48,000 g, and resuspended with a 27-gauge syringe needle. The final protein concentration was 10 mg/ml. The extravesicular solution contained 120 mM N-methyl-d-glucamine (NMDG)-gluconate and 20 mM HEPES, pH 7.5, with acridine orange (Invitrogen) added to a final concentration of 6 μM. This solution was placed in a cuvette with a magnetic stirrer. Acridine orange fluorescence was monitored with a QM-8/2003, Photon Technology Spectrofluorometer (International, London, ON, Canada) using λexcitation = 493 nm and λemission = 530 nm. Fluorescence was quenched by the addition of acid-loaded BBMV (a volume containing 150 μg protein) in the absence of Na+. Addition of extravesicular Na+ (sodium gluconate to a final concentration of 60 mM) resulted in Na+/H+ exchange and an increase in fluorescence. In some experiments, 10−6 M EIPA, an inhibitor of Na+/H+ activity, was added before sodium. NMDG-gluconate used to monitor Na+-independent quenching, which was < 1/10 of sodium-dependent activity. Na+/H+ exchange activity was estimated as the initial rate of increase in fluorescence on addition of sodium gluconate minus the sodium-independent increase in fluorescence. Adult rat Na+/H+ exchange activity was examined for comparison.
Statistical analysis.
All data are expressed as means ± SE. Comparisons were made using an unpaired Student's t-test or ANOVA when more than two groups were studied. A P value of ≤0.05 was considered significant.
RESULTS
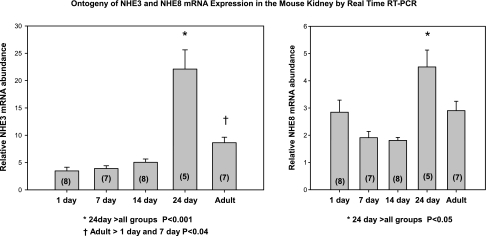
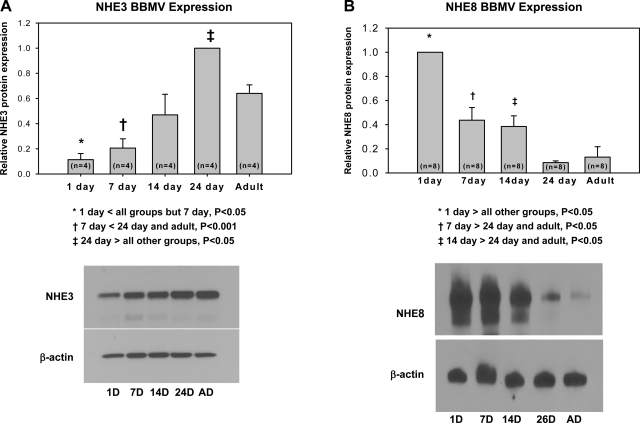
In our first series of experiments, we examined the ontogeny of NHE3 and NHE8 mRNA and brush-border membrane protein abundance in the mouse. In Fig. 1, we find that at 1, 7, and 14 days of age there was less NHE3 mRNA abundance than in 24-day-old and adult mice. The abundance of NHE8 mRNA was comparable at all ages, except NHE8 mRNA abundance was higher at 24 days than at any other age. In Fig. 2, we show the ontogeny of NHE3 and NHE8 brush-border membrane protein abundance. There was a maturational increase in NHE3 protein abundance and decrease in NHE8 protein abundance.
Fig. 1.
Developmental changes in mouse Na+/H+ exchanger (NHE) 8 and NHE3 mRNA expression. cDNA from mouse total kidney was analyzed by real-time RT-PCR normalized to 28S mRNA. NHE3 mRNA expression increased from day 1 to 7 days of age when it reached the level seen in adults. There was no significant change in NHE8 mRNA expression except at 24 days. No. of mice is in parentheses.
Fig. 2.
Developmental changes in mouse brush-border membrane NHE3 (A) and NHE8 (B) protein abundance. Brush-border membrane vesicles (BBMV) were harvested from mice from 1 day of age to adults. The relative protein abundance of NHE3 and NHE8 at the ages shown was compared relative to β-actin. As can be seen, there is a developmental increase in NHE3 and a decrease in NHE8. D, day; AD, adult; n, no. of mice.
We next examined the effects of acid gavage on serum electrolytes, pH, and Pco2. Neonatal mice and rats were gavaged with NH4Cl or vehicle for 4 days. The results of the electrolytes and blood gases are shown in Table 3. Neonates appeared healthy but developed a significant hyperchloremic metabolic acidosis with acid gavage compared with controls as well as higher blood potassium levels and blood urea nitrogen.
Table 3.
Effect of acid gavage on electrolytes and blood gas in neonatal mouse and rat
| Mouse | Rat | P Value | |
|---|---|---|---|
| HCO3−, meq/l | |||
| Control | 19.5 ± 1.0 | 21.3 ± 0.2 | <0.001 |
| Acid | 8.9 ± 0.6 | 11.2 ± 0.4 | |
| pH | |||
| Control | 7.39 ± 0.02 | 7.45 ± 0.01 | <0.001 |
| Acid | 7.15 ± 0.02 | 7.26 ± 0.02 | |
| Pco2, mmHg | |||
| Control | 32.5 ± 2.2 | 30.5 ± 0.4 | 0.005 |
| Acid | 24.8 ± 1.2 | 24.5 ± 0.8 | |
| Na+, meq/l | |||
| Control | 153 ± 1 | 142 ± 1 | NS |
| Acid | 151 ± 2 | 111 ± 1 | |
| K+, meq/l | |||
| Control | 3.1 ± 0.1 | 3.7 ± 0.1 | <0.001 for mouse |
| Acid | 4.6 ± 0.2 | 4.2 ± 0.2 | NS for rat |
| Cl−, meq/l | |||
| Control | 127 ± 2 | 116 ± 1 | <0.001 |
| Acid | 139 ± 2 | 122 ± 1 | |
| BUN, mg/dl | |||
| Control | 26 ± 2 | 15.1 ± 1 | 0.03 for mouse |
| Acid | 35 ± 3 | 23.6 ± 1 | 0.001 for rat |
Values are means ± SE. NS, not significant.
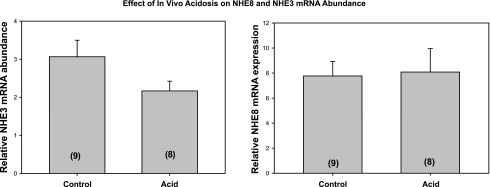
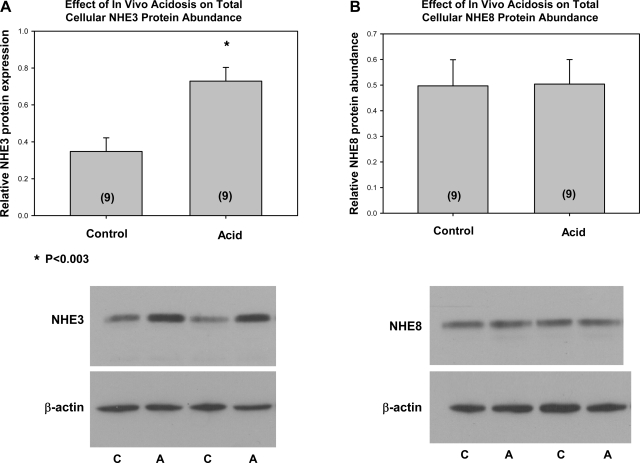
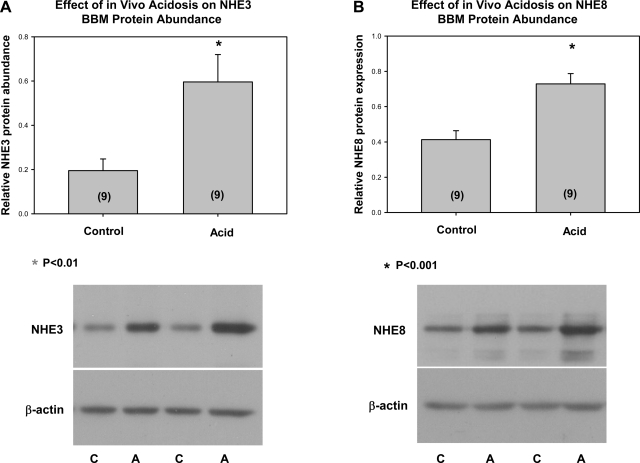
We next examined whether the acidemic neonatal mice had changes in NHE3 and NHE8 mRNA and protein abundance. As shown in Fig. 3, metabolic acidosis did not have an effect on NHE3 or NHE8 mRNA abundance in neonatal mice. In neonatal rats, there was no change in NHE8 mRNA abundance/28S [6.8 ± 1.0 control vs. 4.8 ± 0.9 acid (n = 6), P = 0.16] and a decrease in NHE3 mRNA abundance/28S [3.9 ± 0.6 control (n = 11) vs. 2.0 ± 0.2 acid (n = 10), P = 0.009]. In mice, there was an increase in NHE3 total protein abundance but no change in NHE8 total protein abundance with metabolic acidosis, as shown in Fig. 4. Similarly, in rats, total cellular NHE8/β-actin was 0.58 ± 0.16 in controls, which was not different from 0.70 ± 0.09 measured in acidemic neonatal rats (P = 0.53), whereas total cellular NHE3 was higher in acidemic rats [0.33 ± 0.03 vs. 0.73 ± 0.09 (n = 6), P = 0.002]. Finally, we examined the effect of metabolic acidosis on brush-border membrane NHE3 and NHE8 protein abundance in mice and rats. As shown in Fig. 5, there was an increase in both NHE3 and NHE8 brush-border membrane protein abundance in mice. Similarly, metabolic acidosis in neonatal rats resulted in an increase in both NHE3 [0.70 ± 0.04 vs. 0.90 ± 0.04 (n = 5), P = 0.002] and NHE8 [0.59 ± 0.04 vs. 0.78 ± 0.05 (n = 11), P = 0.009] brush-border membrane protein abundance (data not shown). Thus the neonatal proximal tubule can respond to metabolic acidosis with an increase in brush-border membrane NHE8 protein abundance in mice and rats, demonstrating that NHE8 may play a role in the adaptation to metabolic acidosis.
Fig. 3.
Effect of metabolic acidosis on NHE3 and NHE8 mRNA abundance. NHE3 and NHE8 cDNA from total mice kidney was analyzed by real-time PCR normalized to 28S mRNA. There was no significant difference in the control vs. acid groups for either NHE3 or NHE8 mRNA expression. No. of mice is in parentheses.
Fig. 4.
Effect of metabolic acidosis on total cellular NHE3 (A) and NHE8 (B) protein abundance. Total protein was assayed for NHE3 and NHE8 using immunoblot. Metabolic acidosis resulted in an increase in NHE3 protein abundance, but there was no difference in NHE8 total protein abundance normalized to β-actin. NHE3 and NHE8 had the expected size of ∼80 kDa. C, control; A, acid. No. of mice is in parentheses.
Fig. 5.
Effect of metabolic acidosis on brush-border membrane (BBM) NHE3 (A) and NHE8 (B) protein abundance. Brush-border membranes were harvested from 8-day-old neonatal mice that were gavaged with vehicle or ammonium chloride. The acidemic mice had an increase in brush-border membrane NHE8 and NHE3 protein abundance compared with control mice. NHE3 and NHE8 had the expected size of ∼80 kDa, and the results shown were normalized to β-actin. No. of mice is in parentheses.
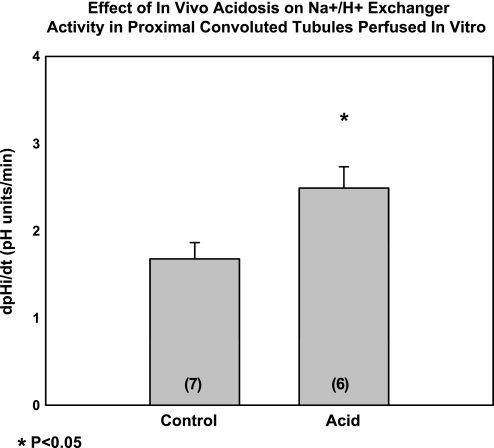
In the next experiments, we examined if neonatal mice can respond to metabolic acidosis with an increase in Na+/H+ exchange activity. Na+/H+ exchange was assayed in control and acidemic neonatal mice in vitro as the rate of change in pHi with luminal sodium removal. The steady-state pH in these experiments is shown in Table 4. Figure 6 shows that Na+/H+ exchange activity was faster in neonatal mice proximal convoluted tubules that received acid gavage. Thus the neonatal proximal tubule can adapt comparable to the adult segment with an increase in Na+/H+ exchange in response to acidosis (35, 41, 44).
Table 4.
Effect of luminal Na+removal on cell pHi
| pHi | Luminal Na+ | ΔpH | 0 Na+ | ΔpH | Luminal Na+ |
|---|---|---|---|---|---|
| Control | 7.39 ± 0.07 | 0.18 ± 0.10 | 7.03 ± 0.05* | 0.22 ± 0.12 | 7.37 ± 0.07 |
| Acid | 7.26 ± 0.13 | 0.41 ± 0.08 | 6.82 ± 0.11† | 0.30 ± 0.06 | 7.25 ± 0.07 |
Values are means ± SE.
P < 0.003 vs. luminal Na+.
P < 0.02 vs. luminal Na+.
Fig. 6.
Effect of in vivo acidosis on Na+/H+ exchanger activity in mouse proximal convoluted tubules perfused in vitro. Proximal convoluted tubules were perfused in vitro from 8-day-old mice that were gavaged with vehicle or ammonium chloride for 4 days. Na+/H+ activity was assessed by measuring the rate of change in cell pHi after luminal sodium removal. Na+/H+ activity was significantly faster in the neonatal mice that were acidemic compared with control. No. of tubules is in parentheses.
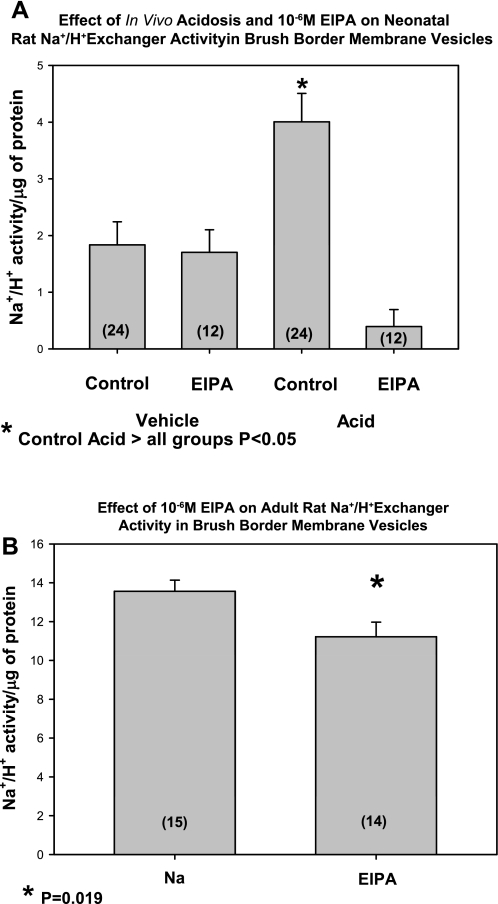
In the last series of experiments, we examined the effect of metabolic acidosis on brush-border membrane Na+/H+ exchange activity. Because of the paucity of tissue from neonatal mice, we performed these studies using 13-day-old neonatal rats that were gavaged with vehicle or acid. As shown in Fig. 7, Na+/H+ exchange was increased in brush-border membranes from acid-gavaged rats compared with control rats. There was a significant reduction in Na+/H+ exchange activity with 10−6 M EIPA in the acidemic rats. As a comparison, we examined Na+/H+ exchange activity in adult animals. Na+/H+ exchange was sevenfold higher in the adults compared with neonates 10−6 M EIPA caused a very small decrease in Na+/H+ in the adult as shown by others (44).
Fig. 7.
Effect of metabolic acidosis on neonatal rat brush-border membrane vesicle Na+/H+ exchanger activity. A: Na+/H+ activity from brush-border membrane vesicles from neonatal rats was measured using the acridine orange technique. Brush-border membrane vesicles from neonatal acidotic rats had a higher Na+/H+ activity than control. The Na+/H+ activity was inhibited by 10−6 M EIPA. B: effect of EIPA on adult rat brush-border membrane vesicle Na+/H+ exchanger activity. Na+/H+ exchanger activity was measured in adults in the absence and presence of EIPA. The activity was severalfold greater than that in neonates and was relatively resistant to EIPA. No. of experiments is in parentheses.
DISCUSSION
The present study examined if neonates could respond to metabolic acidosis with an increase in proximal tubule acidification. We demonstrate that neonates can respond to metabolic acidosis by an increase in proximal tubule sodium-dependent proton secretion. This increase in Na+/H+ exchange is accompanied by an increase in both brush-border membrane NHE3 and NHE8 protein abundance. The increase in NHE8 protein abundance with acidosis and the effect of 10−6 M EIPA to inhibit the increase in BBMV Na+/H+ exchange in neonates demonstrates that NHE8 isoform is regulated and likely plays an important physiological role in neonates.
There have been 10 sodium-hydrogen exchangers identified in humans to this date (16, 30, 33). These NHEs differ in cellular location and function. NHE8 has its phylogenic origin in slime mold (16). From its gene sequence, NHE8 is predicted to be an intracellular NHE isoform, and it shares relatively little sequence similarity with other mammalian NHEs (16, 32, 33). Indeed, NHE8 is an intracellular NHE in the mosquito Malpighian tubule (34). In mammals, NHE8 has been localized to the Golgi where it likely plays a role in pH regulation and ionic composition (32). NHE8 mRNA has a ubiquitous distribution, with the highest expression in the kidney, skeletal muscle, testes, and liver (24, 32). In addition to its intracellular location, NHE8 has also been localized to the apical membrane of the proximal tubule and intestine (11, 22–24, 45). Colocalization studies with megalin suggest that NHE8 may also be present in subapical coated pits and potentially be involved in recycling (23). The function of NHE8 has only recently been examined (22).
There is a maturational increase in serum glucocorticoids and thyroid hormone levels. We have shown that the increase in NHE3 parallels the maturational increase in these levels and that we could prematurely increase NHE3 protein abundance and Na+/H+ exchanger activity by administering glucocorticoids or thyroid hormone to neonates (7, 8). Furthermore, we could prevent the maturational increase in NHE3 by preventing the maturational increase in glucocorticoids or thyroid hormone (8, 25, 26). We have recently shown that high levels of apical membrane NHE8 persist into adulthood in rats made hypothyroid from birth (22). Administration of thyroid hormone to neonates before the normal increase that occurs at the time of weaning resulted in a premature decrease in brush-border membrane NHE8 protein abundance (22).
We have previously shown that there was a maturational increase in NHE3 mRNA and brush-border membrane protein abundance and a maturational decrease in NHE8 protein abundance in the rat (11). NHE8 mRNA abundance did not change during postnatal development in the rat (11). In the present study examining the ontogeny of neonatal mouse kidney NHE3 and NHE8, we found a maturational increase in NHE3 mRNA, with the highest expression at 24 days and no significant difference in NHE8 mRNA at any age except at 24 days where it was higher than at all other ages. The reason for the higher expression of NHE3 and NHE8 mRNA at 24 days is unknown. In the mouse, there was a maturational increase in NHE3 protein expression and decrease in NHE8 protein expression that was comparable to that previously described in the rat (11).
The current study addressed a different and physiologically important question, i.e., if it is possible to alter neonatal proximal tubular acidification in response to metabolic acidosis, a common clinical problem. We have shown that, when neonatal mice are exposed to an acidic environment by acid gavage, they adapt by increasing the brush-border membrane expression of both NHE3 and NHE8. Because there was no increase in NHE8 or NHE3 mRNA in response to acidosis, it is assumed that the increase in Na+/H+ exchangers was secondary to posttranslational mechanisms. Similar findings have been shown for the effect of chronic metabolic acidosis on NHE3 mRNA and brush-border protein abundance in adult rats (3). The regulation of NHE8 appears somewhat different from NHE3. Although there was an increase in both total cellular and brush-border membrane NHE3, there was an increase in brush-border membrane NHE8 but no change in total cellular NHE8 abundance. This suggests that NHE8 is regulated by acid solely by trafficking. This will have to be studied further using in vitro techniques.
We demonstrate that neonatal mice can increase proximal tubule acidification with metabolic acidosis. This study shows that there can be an increase in proximal tubule Na+/H+ exchange activity with a concomitant increase in NHE3 as has been show in adults. Most importantly, there was an increase in Na+/H+ exchanger activity that was responsive to 10−6 M EIPA, a dose that we confirm to have a minimal effect in adult Na+/H+ activity, which is due primarily to NHE3, a relatively EIPA-insensitive exchanger (44). It was somewhat surprising that the control Na+/H+ exchange activity was not inhibited by 10−6 M EIPA. This may be due to the fact that NHE3 has a significant contribution to basal activity or that the level of Na+/H+ exchange was so low that we could not detect a change in activity.
In conclusion, we confirm that there is a maturational increase in brush-border membrane NHE3 and maturational decrease in NHE8 protein abundance. This study shows that, in neonates, NHE8 can also play an adaptive role in response to acidosis. The cellular and molecular mechanisms for the regulation of NHE8 by acidosis are unclear at present and will require further in vitro studies.
Perspectives and Significance
The study of postnatal development has brought about surprising findings. While initial studies looking at protein and mRNA abundance of transporters characterized in adult animals showed that there were changes in expression during postnatal development, theses studies did not explain all of the transport data (17, 39, 40). Thus far, developmental isoform changes have been found for both the Na+/H+ exchanger (11) and the sodium-dependent phosphate transporter (31). Whether there are other isoform changes that occur during the course of pre- and postnatal development is unknown. In addition, NHE8 is predominantly an intracellular transporter, and the total abundance of the transporter does not change in the cell during development; however, there are marked changes in abundance on the apical membrane (11). The reason for the isoform change, what is mediating this change from NHE8 to NHE3, as well as the other factors that regulate NHE8 will be of interest and physiological importance. In addition, there may be circumstances in adults where NHE8 plays an important role in acidification outside the neonatal period. We are only beginning to understand the importance of this developmentally regulated Na+/H+ exchanger.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612 and DK-078596 to M. Baum, T32 DK-07257, and the O'Brien Center Grant P30DK-079328.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alpern RJ, Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest 78: 502–510, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpern RJ, Horie S, Moe O, Tejedor A, Miller RT, Preisig PA. Chronic adaptations in proximal tubular H/HCO3 transporters. Kidney Int Suppl 33: S29–S32, 1991 [PubMed] [Google Scholar]
- 3.Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F917–F925, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest 85: 499–506, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule acidification. Pediatr Res 31: 411–414, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: effect of glucocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol 268: F815–F820, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on the neonatal renal cortical Na+/H+ antiporter. Kidney Int 53: 1254–1258, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum M, Quigley R. Prenatal glucocorticoids stimulate neonatal juxtamedullary proximal convoluted tubule acidification. Am J Physiol Renal Fluid Electrolyte Physiol 261: F746–F752, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Baum M, Quigley R. Ontogeny of proximal tubule acidification. Kidney Int 48: 1697–1704, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Becker AM, Zhang J, Goyal S, Dwarakanath V, Aronson PS, Moe OW, Baum M. Ontogeny of NHE8 in the rat proximal tubule. Am J Physiol Renal Physiol 293: F255–F261, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Fluid Electrolyte Physiol 265: F736–F742, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297: F1419–F1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol 289: F685–F691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288: C223–C239, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest 105: 1141–1146, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogan MG, Maddox DA, Lucci MS, Rector FC., Jr Control of proximal bicarbonate reabsorption in normal and acidotic rats. J Clin Invest 64: 1168–1180, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogan MG, Rector FC., Jr Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 242: F499–F507, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelmann CMJ, Soriano JR, Boichis H, Gruskin AB, Acosta M. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest 46: 1309–1317, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattineni J, Sas D, Dagan A, Dwarakanath V, Baum M. Effect of thyroid hormone on the postnatal renal expression of NHE8. Am J Physiol Renal Physiol 294: F198–F204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467–F473, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Gupta N, Dwarakanath V, Baum M. Maturation of the Na/H antiporter (NHE3) in the proximal tubule of the hypothroid adrenalectomized rat. Am J Physiol Renal Physiol 287: F521–F527, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta N, Tarif SR, Seikaly M, Baum M. Role of glucocorticoids in the maturation of the rat renal Na+/H+ antiporter (NHE3). Kidney Int 60: 173–181, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes JM, Zhang S, Leske DA, Lanier WL. Metabolic acidosis-induced retinopathy in the neonatal rat. Invest Ophthalmol Vis Sci 40: 804–809, 1999 [PubMed] [Google Scholar]
- 28.Kinsella J, Cujdik T, Sacktor B. Na+-H+ exchange in isolated renal brush-border membrane vesicles in response to metabolic acidosis. Kinetic effects. J Biol Chem 259: 13224–13227, 1984 [PubMed] [Google Scholar]
- 29.Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest 80: 928–935, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Kim T, Park ES, Yang S, Jeong D, Choi Y, Rho J. NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival. Biochem Biophys Res Commun 369: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27: 503–515, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol 296: F730–F750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest 82: 1445–1453, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC., Jr Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest 80: 970–978, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GH, Evan AP. Development of solute transport in rabbit proximal tubule. I. HCO3 and glucose absorption. Am J Physiol Renal Fluid Electrolyte Physiol 245: F382–F390, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Shah M, Gupta N, Dwarakanath V, Moe OW, Baum M. Ontogeny of Na+/H+ antiporter activity in rat proximal convoluted tubules. Pediatr Res 48: 206–210, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer A, Barac-Nieto M. Ontogeny of renal phosphate transport and the process of growth. Pediatr Nephrol 16: 763–771, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Tsai CJ, Ives HE, Alpern RJ, Yee VJ, Warnock DG, Rector FC., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 247: F339–F343, 1984 [DOI] [PubMed] [Google Scholar]
- 42.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Wu MS, Biemesderfer D, Giebisch G, Aronson PS. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem 271: 32749–32752, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol 293: F761–F766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]