Abstract
During ischemia and some types of muscular contractions, oxygen tension (Po2) declines to the point that mitochondrial ATP synthesis becomes limited by oxygen availability. Although this critical Po2 has been determined in animal tissue in vitro and in situ, there remains controversy concerning potential disparities between values measured in vivo and ex vivo. To address this issue, we used concurrent heteronuclear magnetic resonance spectroscopy (MRS) to determine the critical intracellular Po2 in resting human skeletal muscle in vivo. We interleaved measurements of deoxymyoglobin using 1H-MRS with measures of high-energy phosphates and pH using 31P-MRS, during 15 min of ischemia in the tibialis anterior muscles of 6 young men. ATP production and intramyocellular Po2 were quantified throughout ischemia. Critical Po2, determined as the Po2 corresponding to the point where PCr begins to decline (PCrip) in resting muscle during ischemia, was 0.35 ± 0.20 Torr, means ± SD. This in vivo value is consistent with reported values ex vivo and does not support the notion that critical Po2 in resting muscle is higher when measured in vivo. Furthermore, we observed a 4.5-fold range of critical Po2 values among the individuals studied. Regression analyses revealed that time to PCrip was associated with critical Po2 and the rate of myoglobin desaturation (r = 0.83, P = 0.04) but not the rate of ATP consumption during ischemia. The apparent dissociation between ATP demand and myoglobin deoxygenation during ischemia suggests that some degree of uncoupling between intracellular energetics and oxygenation is a potentially important factor that influences critical Po2 in vivo.
Keywords: phosphocreatine, deoxymyoglobin, bioenergetics, oxidative phosphorylation, mitochondria
the relationship between intracellular oxygen tension (Po2) and mitochondrial respiration is complex. As oxygen tension declines during ischemia or exercise, the cell first reaches a state of “metabolic adaptation,” in which oxygen plays a regulatory role in sustaining mitochondrial ATP flux, but there is no overt impairment of ATP synthesis (3). This regulation may be accomplished through coupling of Po2 and the phosphorylation and/or redox potential, and the magnitude of the range is dependent upon the rate of ATP production (33). As Po2 declines within this range of metabolic adaptation, the continued ATP demand of the cell would be expected to increase the driving force for oxidative phosphorylation by increasing [ADP] and the redox state (3, 33). At some point, as Po2 continues to fall, the rate of mitochondrial ATP synthesis becomes limited by oxygen availability (3). When this critical Po2 is reached, the cell must satisfy its energetic needs by increasing ATP synthesis through anaerobic glycolysis and/or hydrolysis of stored high-energy phosphates [i.e., phosphocreatine (PCr) and ATP].
A number of studies have been designed to identify a precise critical Po2, below which mitochondrial ATP flux is impaired. In isolated mitochondria from rat liver and heart tissue, the critical Po2 has been shown to be less than 1 Torr (4, 9, 24). When measured across the intracellular space of dog gracilis muscle, the critical Po2 was found to be ∼ 0.5 Torr (7). The similarity of these values suggests that the Po2 gradient between the cytosol and the mitochondria in whole cells is minimal. Although Gayeski and Honig (8) initially confirmed this notion by cryospectrophotometry, a subsequent reassessment of the methodology revealed that intracellular O2 gradients in exercised muscle may have been underestimated by this method (30). Nonetheless, the cytosolic critical Po2 should be similar to that measured at the cytochrome level, at least in resting muscle (31). It is interesting to note that somewhat higher critical Po2 values (1.25 Torr) were observed in the extracellular space in single rat cardiac myocytes (21) and from the interstitium of intact rat spinotrapezius muscle (2.4–2.9 Torr) (22).
While it is possible that higher interstitial compared with intracellular critical Po2 values are due to a steep oxygen gradient across the sarcolemma (31), controversy remains regarding the lack of consistency between in vitro and in vivo measures of critical Po2 in skeletal muscle. At this time, the data are equivocal, as there is some evidence to suggest, for example, that the oxygen sensitivity of cytochrome c oxidase is higher in vitro than in vivo (28). Higher oxygen sensitivity would allow respiration to continue at a lower Po2 in vitro compared with in vivo, resulting in a lower critical Po2 in vitro. However, other investigators found that in vitro estimates of critical Po2 were similar to those found in surgically exteriorized muscle of anesthetized animals (7, 22).
Notably, several older studies showed that oxygen consumption in resting skeletal muscle increases in response to increased blood flow and arterial oxygenation (6, 18), suggesting that respiration may be limited by oxygen availability, even at rest. However, more recent work suggests that this scenario is unlikely, as myoglobin is only ∼9% desaturated in resting muscle, which corresponds to a resting intracellular Po2 of ∼34 Torr (20); a number that is well above reported values for intracellular or interstitial critical Po2. Furthermore, other investigators find that changing blood flow or Po2 has no effect on oxygen uptake by resting or contracting skeletal muscle unless Po2 was below a critical threshold (23, 27). One possible explanation for the inconsistencies regarding critical Po2 is that the absence of intact circulatory and regulatory systems in vitro may result in lower critical Po2 values than would be observed under noninvasive conditions in vivo. In particular, the role of myoglobin in intracellular oxygen transport is ignored in measurements of critical Po2 in isolated mitochondria.
The ability to simultaneously measure intracellular oxygenation and high-energy phosphates using concurrent heteronuclear magnetic resonance spectroscopy (MRS) provides an opportunity to determine critical intracellular Po2 in resting human skeletal muscle in vivo and noninvasively. This technique overcomes many of the limitations inherent in more commonly used approaches, such as phosphorescence lifetime decay, NADH fluorescence, and oxygen microelectrodes. First, concurrent MRS allows essentially simultaneous measurement of changes in intracellular oxygenation and energetics, whereas other methods typically require separate, parallel experiments. Second, MRS samples from a relatively large volume of tissue and represents an average response over many potentially heterogeneous regions within the tissue. Compared with techniques that necessitate small tissue samples, MRS is less susceptible to sampling errors due to this type of heterogeneity. As a result, however, MRS lacks the spatial resolution to detect differences in energetics between cells. Finally, MRS is noninvasive and allows rapid, repeated measurements from human skeletal muscle in vivo.
The aim of the present study was to combine proton (1H) MRS measures of the proximal histidyl NδH signal from deoxymyoglobin (dMb) with phosphorous (31P) MRS measures of PCr, ATP, and pH to determine the intracellular critical Po2 of resting human tibialis anterior muscle in vivo. The critical Po2 was established as the Po2 at which, during ischemia, the muscle began to rely on the net breakdown of PCr to satisfy its energy demands. The 31P and 1H variables were used to estimate rates of ATP production and myoglobin (Mb) desaturation, respectively, throughout ischemia.
MATERIALS AND METHODS
Subjects.
Six healthy young men were enrolled in this study (29 ± 3 years). Their physical activity levels ranged from recreationally active to endurance trained. All subjects gave written informed consent, as approved by the University of Massachusetts, Amherst, and the Yale University School of Medicine institutional review boards, and in conformance with the standards set by the Declaration of Helsinki.
Experimental arrangement.
Subjects were positioned supine in the bore of a 4.0-Tesla whole-body, superconducting magnet (Bruker Biospin, Rheinstetten, Germany) with a probe assembly consisting of coplanar 7-cm diameter 1H and 3 cm × 5 cm elliptical 31P surface coils secured over the tibialis anterior muscle. A blood pressure cuff was placed around the thigh and connected to a rapid cuff inflator (Hokanson, Belleview, WA) for occlusion of blood flow to the lower leg. The lower leg was immobilized with Velcro straps across the shank and just distal to the knee. Correct positioning of the leg in the isocenter of the magnet was ensured using transverse gradient-echo scout images. Scout images were also used to select a region of interest within the tibialis anterior muscle for localized shimming on the muscle water peak using the FASTMAP method (26). This shimming procedure yielded a full width at half-maximal height of the unapodized PCr peak of 9.4 ± 1.5 Hz (mean ± SD, all subjects), indicating excellent magnetic field homogeneity in the volume of interest.
Procedures.
Proton scans of dMb were acquired at 170.6 MHz using a 500-μs, frequency-selective Gaussian pulse centered 12.5 kHz downfield of the water peak [repetition time = 25 ms, sweep width = 30 kHz, number of acquisitions = 80, yielding 1 free induction decay (FID) every 2 s]. Phosphorous scans were acquired at 68.98 MHz using a 125-μs hard pulse with a nominal 60 degree flip angle (repetition time = 2 s, 2,048 data points, sweep width = 8 kHz). The 1H and 31P scans were acquired in an interleaved manner by alternating the nucleus of interest every 2 s, using the Bruker Multiscan Tool. Thus, one complete cycle occurred every 4 s.
After initiation of the interleaved pulse sequence, subjects rested for 86 s with intact blood flow before the thigh cuff pressure was increased to 240 Torr to induce ischemia to the lower leg. After 15 min of resting ischemia, the cuff pressure was released, and the interleaved scans continued for a recovery period of 10 min.
Spectral analysis.
Prior to analysis, the interleaved FIDs were parsed into 1H and 31P data sets. A final temporal resolution of 8 s was then achieved for each nucleus by averaging 2 successive FIDs. All spectral analyses were performed using NUTS software (Acorn NMR, Livermore, CA). Proton FIDs were first apodized with a mixed Lorentzian/Gaussian multiplier (−300 Hz/0.1), followed by zero-filling and Fourier transform. The resulting spectra were phased manually and baseline-corrected using a fifth-order polynomial fit of the baseline region. The area of the dMb peak was quantified using an interactive, least-squares curve-fitting routine to optimize peak width, chemical shift, height, and fraction lorentzian.
31Phosphorous FIDs were apodized using an exponential multiplier corresponding to 10 Hz of line-broadening, followed by zero-filling and Fourier transform. After manual phasing, the underlying broad peak due to the phosphorous in bone was removed with a polynomial fit to obtain a flat baseline. Peaks corresponding to PCr, inorganic phosphate (Pi) and γATP were then fit with Lorentzian-shaped curves to quantify their respective areas. Two peaks were used to fit γATP.
Calculations.
Concentrations of PCr and Pi were calculated assuming a constant [PCr] + [Cr] = 42.5 mM (12). The change in [Pi] and [Cr] were assumed to be equivalent based on the equilibrium of the creatine kinase reaction and impermeability of the cell to Pi and free creatine (15). [ATP] was assumed to be 8.2 mM at baseline (12), and changes in [ATP] were determined from the γATP peak before and at the end of ischemia. The chemical shift of the Pi peak relative to PCr was used to calculate intracellular pH (13). The rate of ATP consumption was determined from the linear rate of PCr hydrolysis during ischemia (2, 10) based on the stoichiometry of PCr and ATP hydrolysis and the absence of net ATP hydrolysis. Under these conditions, the rates of ATP consumption and production are equivalent.
Our determination of ATP consumption rate assumes that the rate of PCr hydrolysis and ATP demand are equivalent and that anaerobic glycolysis has a negligible contribution to PCr synthesis. This assumption is based on previous reports that, following muscle contractions, PCr resynthesis is absent when the muscle is kept ischemic (2, 11, 19). However, Amara et al. (1) found that anaerobic glycolysis accounts for <8% of total ATP turnover in ischemic human skeletal muscle at rest. In consideration of this study, we point out that our assumptions of negligible glycolytic flux during resting ischemia may underestimate ATP turnover by up to 8%. Although we (16) and others (5) have previously reported transient glycolytic flux persisting for several seconds following vigorous muscle activity, we find no evidence that glycolytic flux has a measurable contribution to ATP supply under the conditions of the present study.
The area of the dMb peak was expressed relative to its level at maximum desaturation, established from an average of the dMb peak area during the final 5 min of ischemia. Recently, we showed that 10 min of ischemia is sufficient to fully desaturate Mb (29). Intracellular Po2 (Torr) was calculated from the oxygen binding curve for myoglobin:
| 1 |
where f is the fractional desaturation of myoglobin, and assuming a P50 for myoglobin of 2.39 Torr (25).
Statistical analyses.
During ischemia, PCr was expected to remain steady initially, followed by an inflection point after which PCr would decrease at a constant rate. Herein, the term inflection point refers to the point in time where the fit of two linear slopes describing PCr or pH intersect. Intracellular pH was expected to remain constant, followed by alkalosis, as protons were consumed by the hydrolysis of PCr. To determine the time point indicating compromised oxidative production of ATP (i.e., critical Po2), [PCr] during ischemia was plotted for each subject and a segmented, bilinear regression model was used to fit the data (PROC NLIN in SAS, Statistical Analysis Software Institute, Cary, NC), according to the following function (32):
| 2 |
where Y is the predicted [PCr], a0 is the intercept of the first line, a1 is the slope of the first line, b1 is the slope of the second line, and ip is the inflection point. For all cases where time < ip, id was assigned a value of 1, indicating that the data point belonged to the first line. When time ≥ ip, id was assigned a value of 0, indicating that the data point belonged to the second line. The NLIN procedure was used to simultaneously estimate a0, a1, b1, and ip, based on a two-stage iterative fitting procedure that minimized the error sums of squares for the above model. To evaluate the onset of alkalosis, changes in pH during ischemia were also fit on an individual basis using the bilinear model, as that model yielded a very good fit of the data along with an ip for pH.
The appearance of dMb (% max) was plotted for each subject and fit using a 5-parameter sigmoidal curve:
| 3 |
where Y is dMb at time x, Y0 is dMb at time 0, a is the amplitude, x is time, x0 is the inflection point of the sigmoid, and b describes the curvature of the sigmoid. The resulting coefficients for each subject were used to predict the relative dMb where x = ip (from Eq. 2) for each subject. Equation 1 was then used to calculate the intracellular Po2 from the predicted dMb at this sample time. For each subject, the rate of Mb desaturation was determined from the slope of a linear fit of all dMb values between 20% desaturation and the dMb inflection point (x0), obtained from the sigmoidal fit. Critical Po2 was operationally defined as the Po2 at the ip for PCr.
A paired t-test was used to compare [ATP] at rest and at the end of ischemia to confirm the absence of net ATP hydrolysis. Linear regression analyses were used to examine the extent to which the intersubject range in critical Po2 values was associated with ATP production rate, the rate of Mb deoxygenation, and the time required to reach the PCr inflection point. All statistical analyses were performed using SAS software (SAS Institute, Cary, NC, USA). Unless otherwise stated, data are expressed as mean ± SD, and precise P values are given.
RESULTS
Phosphocreatine, pH, and deoxymyoglobin during ischemia.
Intramuscular 31P metabolites and deoxymyoglobin were monitored simultaneously in the tibialis anterior muscle during 15 min of ischemia. The Fig. 1, top, shows a stackplot of 31P MRS spectra from a representative subject, with 8-s temporal resolution. Fig. 1, bottom, shows a stackplot of 1H MRS spectra measured simultaneously from the same subject with the same temporal resolution. As expected, no dMb signal was observed during the rest interval prior to cuff inflation. The dMb peak became evident within 1 min of cuff occlusion. Rapid recovery of PCr and dMb upon release of the cuff is evident.
Fig. 1.
31P and 1H magnetic resonance spectroscopy (MRS) spectra from the tibialis anterior muscle during ischemia in a single subject. Representative stackplots of phosphorous (top) and proton (bottom) spectra at rest (first spectrum, 60-s average) and throughout 15 min of ischemia (8-s temporal resolution), and 10 min of reperfusion (8-s temporal resolution). The first spectrum in each plot shows the baseline in resting muscle, prior to ischemia. Phosphocreatine (PCr) and inorganic phosphate (Pi) remained constant during the initial portion of ischemia (illustrated by the black bars) and then showed linear changes with time. Following cuff release (illustrated by the gray bars), both metabolites rapidly recovered to resting levels. The signal corresponding to deoxymyoglobin (dMb) was not apparent at rest but became visible shortly after ischemia was induced. The dMb signal reached a plateau after ∼5 min of ischemia, and recovered rapidly upon cuff release.
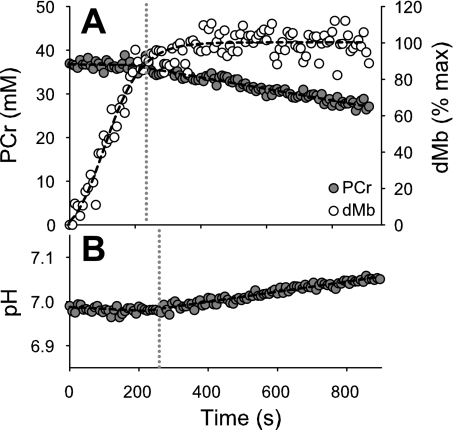
Fig. 2, top, shows [PCr] for the same subject depicted in Fig. 1. The concentration of PCr did not differ from the preocclusion value until 184 s of ischemia, after which it decreased at a linear rate. Deoxymyoglobin increased until a plateau was reached at ∼400 s of ischemia, consistent with complete desaturation of Mb at that point. Fig. 2, bottom, shows the intracellular pH response, which is initially constant, followed by an inflection point after which pH increased linearly with time. The lines resulting from iterative bilinear fits for PCr and pH are shown as black dashed lines, and the inflection points are indicated by the gray vertical dotted lines. At the time of the PCr inflection point, dMb was 77% of maximal, which corresponded to a critical Po2 of 0.72 Torr for this particular subject.
Fig. 2.
[PCr], dMb, and pH during ischemia in a single subject. The bilinear fit of PCr and the sigmoidal fit of dMb are indicated with dashed lines (top). Intracellular pH (bottom) remained at the resting level during the early portion of ischemia, and increased steadily after 219 s, as determined from the bilinear fit (dashed line). The gray dotted vertical lines denote the location of the inflection points for PCr and pH.
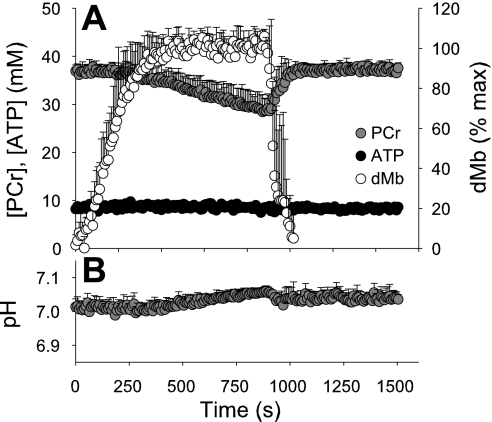
The average response of all six subjects (Fig. 3) was qualitatively similar to the single subject shown in Figs. 1 and 2. During the initial portion of ischemia, [PCr] was constant, followed by an inflection point after which [PCr] decreased linearly. Intramuscular pH was initially stable, followed by a steady alkalosis that coincided temporally with the linear decline in PCr. The correspondence of these changes in PCr and pH, along with the modest drop in PCr and increase in Pi during the short bout of ischemia, indicate that anaerobic glycolysis was not likely to have increased above basal levels. On average, dMb began to increase immediately upon cuff inflation and reached a plateau after several minutes The mean inflection points for PCr and pH are provided in Table 1. No change in [ATP] was observed during the protocol in any subject (P = 0.42, Fig. 3).
Fig. 3.
Mean [PCr], [ATP], dMb, and pH during ischemia in all subjects. Data are shown as group means with standard deviations. Release of the blood pressure cuff is denoted by the vertical dashed line. [PCr] remained at baseline level during the initial minutes of ischemia, followed by a steady decline and subsequent recovery upon release of cuff occlusion. dMb increased immediately upon cuff inflation, plateaued during the final few minutes of ischemia, and rapidly recovered following cuff release. Similar to the response of PCr, pH was initially stable, then progressively increased after an inflection point.
Table 1.
Inflection points, myoglobin desaturation, critical Po2, and ATP production during ischemia in resting muscle
| Variable | Mean | SD | Range | 95% CI |
|---|---|---|---|---|
| PCr inflection point, s | 276 | 102 | 184–428 | 195, 357 |
| pH inflection point, s | 305 | 86 | 219–422 | 229, 380 |
| Myoglobin desaturation at PCr inflection point, % | 87.6 | 6.0 | 85.3–93.8 | 81, 94 |
| Critical Po2, Torr | 0.35 | 0.20 | 0.16–0.72 | 0.14, 0.56 |
| ATP production from PCr, mM/s | 0.013 | 0.003 | 0.011–0.018 | 0.010, 0.015 |
The phosphocreatine (PCr) and pH inflection points were determined from iterative bilinear fits for each subject. Critical Po2, defined here as the Po2 at which oxidative phosphorylation became compromised, was calculated from the relative desaturation of myoglobin (%max) observed at the PCr inflection point. ATP production in the resting tibialis anterior was calculated based on the 1:1 stoichiometry of PCr and ATP in the creatine kinase reaction. CI, confidence interval. See text for details.
Myoglobin deoxygenation, ATP production, and critical oxygen tension.
Means, variance, ranges, and confidence intervals are given in Table 1 for time to PCr and pH inflection points, dMb at the PCr inflection point, critical Po2 and ATP production rate. Notably, we observed a 4.5-fold range in critical Po2 values. Linear regression analyses revealed that the time to the PCr inflection point was the best single correlate with critical Po2 (r = 0.69, P = 0.040, Fig. 4A). In addition, the time required to reach the PCr inflection point was not associated with the rate of ATP consumption (r = 0.004, P = 0.909, Fig. 4B) but was strongly related to the rate of Mb deoxygenation (r = 0.875, P = 0.022, Fig. 4C) and to the time to the inflection point for intracellular pH (r = 0.98, P = 0.002, Fig. 4D). There was no association between ATP demand and the rate of myoglobin deoxygenation during ischemia (r = 0.21, data not shown).
Fig. 4.
Associations between energetic variables. The time required to reach the PCr inflection point during ischemia was significantly associated with critical Po2 (A). The time to reach the PCr inflection point was not associated with the rate of ATP production (B), but it was associated with the rate of Mb desaturation (C). For each subject, the rate of Mb desaturation was determined from the slope of a linear fit of all dMb values between 20% desaturation and the dMb inflection point (x0), obtained from the sigmoidal fit (see text for details). D: PCr and pH inflection points were strongly and significantly correlated, and they were close to the line of identity (Y = X), indicating similarity in the two inflection point values for each subject.
DISCUSSION
Although critical Po2 values have been determined previously in animal tissue in vitro and in situ (4, 7–9, 21, 22, 24), we sought to measure critical Po2 in intact, resting human skeletal muscle to address the question of whether this key mitochondrial characteristic is different from existing literature values when measured in vivo. The results of this study show that the minimum intracellular Po2 required to sustain adequate mitochondrial ATP production to meet the energy needs of resting human skeletal muscle averaged 0.35 Torr in vivo, with a range of 0.16–0.72 Torr. Below this critical Po2, net breakdown of PCr was required to augment oxidative ATP production and maintain [ATP]. This range is similar to the values of <1 Torr measured from mitochondrial preparations from rodent liver and heart tissue (4, 9, 24). The mean value of 0.35 Torr obtained here is also in excellent agreement with our own recent estimate of an in vivo critical Po2 of 0.37 Torr, derived from the dMb inflection point during ischemia in resting human tibialis muscle (29), and only slightly lower than that of Gayeski et al. (7), who reported an intracellular critical Po2 of ∼0.5 Torr in dog gracilis muscle.
Other investigators (21, 22) who have measured extracellular or interstitial Po2 reported critical Po2 values that are consistently higher (1.25–2.9 Torr) than our in vivo, intracellular values. This discrepancy is likely explained by the steep oxygen gradient across the plasma membrane (31). In addition, the choice of the assumed value for myoglobin P50 is also an influential factor in estimating critical Po2. The value of 2.39 Torr used in the present study is based on multiple-wavelength spectroscopy measurements at physiological temperature and pH, with simultaneous correction for met-myoglobin contamination (25). The value for percent myoglobin desaturation is given in Table 1 to allow recalculation of critical Po2 using alternative P50 values. To date, critical Po2 measurements have been performed almost exclusively in animal tissues ex vivo or in situ. The novelty of the present study is the concurrent, noninvasive measurement of energetics and oxygenation to determine the intracellular critical Po2 in resting human skeletal muscle.
Measurement of energetics and critical Po2 in vivo.
We observed a 4.5-fold range of critical Po2 values in the six subjects that we studied. For this range of values, critical Po2 was significantly associated with the time required to reach an inflection point for PCr during ischemia, such that individuals with the longest inflection point times had the lowest critical Po2 values and those with the highest critical Po2 values reached the inflection point more rapidly (Fig. 4A). We expected to find a significant negative association between the time to the PCr inflection point and the rate of ATP consumption (i.e., the slope of PCr following the inflection point) in resting muscle, based on the assumptions that 1) [Mb] is similar among individuals, 2) oxygen consumption is proportional to ATP demand, and 3) critical Po2 is a constant mitochondrial characteristic in resting muscle. However, we found no association between PCrip and the slope of PCr depletion (Fig. 4B), suggesting that the ATP demand of resting muscle was not a primary determinant of the time required to reach the point where the mitochondria failed to satisfy the demand for ATP, i.e., critical Po2. Additional factors such as capillarity, muscle blood volume, and myoglobin concentration may also differ across individuals, and we cannot exclude the possibility that these factors may explain a portion of the range of critical Po2 values observed in this study.
Regression analyses also revealed that PCrip, which identified the critical Po2, was significantly associated with the rate of myoglobin desaturation (Fig. 4C), but not the rate of ATP consumption during ischemia (Fig. 4B). This dissociation between ATP demand and myoglobin deoxygenation during ischemia (r = 0.21) suggests that some degree of uncoupling between intracellular energetics and oxygenation is a potentially important factor that influences critical Po2 in vivo. The source of this variation in critical Po2 is not clear at present, but it appears that variation in this characteristic may be accompanied by differences in the time spent in the adaptive phase of energy metabolism proposed by Connett et al. (3), as discussed below. It is important to emphasize that the measurements of critical Po2 in the present study are specific to resting skeletal muscle, and values during contractions are likely to be quite different (3, 14, 24).
As observed in Fig. 2, myoglobin continued to desaturate for a brief period of time (∼40–56 s) following the PCr inflection point. Because we define critical Po2 as the point where the rate of oxidative phosphorylation becomes limited by oxygen availability, rather than the point where oxygen consumption ceases, a portion of the continued myoglobin desaturation may reflect residual oxygen consumption by the mitochondria. During this brief period, therefore, both oxidative phosphorylation and the breakdown of PCr contribute to total ATP production. A small portion of this continued oxygen consumption may be attributed to nonmitochondrial oxygen consumption in muscle tissue (23). We might also speculate that this represents the metabolic adaptation phase proposed by Connett et al. (3), but resolving this phase in resting muscle is problematic (see discussion below). Overall, the close approximation of the PCr data by the bilinear model (Fig. 2) suggest that our estimates of critical Po2 are accurate.
While the measure of Mb desaturation used here is relatively straightforward, the measurement of in vivo ATP production is somewhat more complex (2, 10). When blood flow to resting muscle is occluded, intracellular oxygen stores will be used to support mitochondrial ATP synthesis. When the oxygen concentration becomes insufficient to maintain respiration, PCr stores will be consumed to meet the ATP requirements of the cell. The rate of PCr hydrolysis upon depletion of intracellular oxygen provides a convenient measure of the rate of ATP consumption at rest, assuming that the metabolic rate of the muscle does not change during the 15 min of ischemia (2, 10). The data from the current study support this assumption, as we observed a linear decline in PCr concentration following the inflection point.
Another key assumption for the estimation of ATP production in vivo is that, under these conditions, there is no increased synthesis of ATP via anaerobic glycolysis. If a compensatory increase in anaerobic glycolysis occurs, the change in PCr during ischemia would be tempered somewhat by the shift in the source of ATP, and we would thus underestimate the true rate of ATP production or consumption. Two pieces of evidence argue against this possibility. First, while we and others have shown previously that a small amount of glycolytic PCr resynthesis occurs in the first few seconds of an ischemic recovery period following intense muscle contractions (5, 16), the measurements in the present study were conducted on resting muscle, where the levels of cytosolic calcium, Pi, or AMP would be unlikely to reach levels sufficient to elicit this effect. Second, the pH data acquired during the present study do not support the notion that anaerobic glycolysis was contributing to the supply of ATP during ischemia. As shown in Figs. 2 and 3, the decline in PCr during ischemia was accompanied by steady alkalosis, consistent with consumption of protons in the creatine kinase reaction. The linear rate of alkalosis suggests that anaerobic glycolysis, which would result in acidosis or a blunted alkalosis, was not buffering the changes in PCr during ischemic rest. Fig. 4D indicates close agreement between the PCr and pH inflection points, which adds support to the notion that the two inflection points are nearly coincident, and the proton stoichiometry of the creatine kinase reaction is a major determinant of the changes to intracellular pH during ischemia. Finally, our values for resting ATPase rates in the tibialis anterior muscle (Table 1) were similar to those reported from the quadriceps or forearm muscles [0.007–0.010 μM ATP/s, (2, 10, 12)]. Overall, the 31P MRS measure of ATP production used here is considered to be a robust measure of intracellular energetics in vivo.
Integrative model of intracellular energetics and Po2.
Our reported range of critical Po2 can also be examined in the context of the model for four ranges of Po2 within skeletal muscle proposed by Connett et al. (3). Within range 1, oxygen is present at saturating levels, and falling Po2 will have no effect on the metabolic state. Range 2 is defined as metabolic adaptation, in which decreasing Po2 does not change mitochondrial respiration, although there is some compensation to maintain ATP production through greater perturbations in the redox state and/or phophorylation state. Within range 3, oxygen tension becomes rate-limiting to mitochondrial ATP production, and range 4 is characterized by impaired cell function. In our study, we are defining the critical Po2 as the threshold between ranges 2 and 3. The range between thresholds 1 and 2 (i.e., the metabolic adaptation phase) is difficult to define under our experimental conditions. In resting muscle, when ATP flux is a small percentage of maximum, the Po2 range that defines this adaptation phase is quite narrow compared with exercise (3). Consequently, under these experimental conditions, in which the resting skeletal muscle is rapidly deoxygenated, it is likely that the transition from range 1 to range 3 occurs very quickly, perhaps on a time scale that is undetectable by in vivo MRS. In a similar study of mouse hindlimb muscle, Marcinek et al. (17) found that intracellular Po2 declined to less than 2 Torr in the absence of any change in PCr levels. The authors reject the idea that the cell adapts to lower oxygen tensions through changes in phosphorylation state. While we find no evidence for a metabolic adaptation phase like that defined by Connett et al. (3) under the conditions of this study, we do not reject the concept, but emphasize the need for experiments employing steady-state titrations in intracellular oxygen with simultaneous monitoring of ATP production, Po2, and phosphorylation state. Although speculative, the 4.5-fold range of critical Po2 observed in the present study could be explained by individual variation in the range of metabolic adaptation, such that the muscle of some subjects is better able to compensate for decreasing Po2, and thus maintain mitochondrial ATP synthesis in the face of falling oxygen tension. As proposed by Connett et al. (3), the effects of increased mitochondrial redox state on increasing the driving force for ATP synthesis may explain the range of critical Po2 values observed in the present study.
Perspectives and Significance
The experiment and analyses reported here provide in vivo evidence of critical Po2 values less than 1 Torr in resting human skeletal muscle. Our critical Po2 values are consistent with reported values ex vivo and thus do not support the notion that critical Po2 is higher when measured in vivo. It is important to consider that critical Po2 of exercising muscle may be higher than the resting values reported in this study. The range of critical Po2 values among the 6 individuals studied seems to be predicted by the kinetics of myoglobin desaturation, but not the rate of ATP consumption in resting, ischemic human skeletal muscle. This apparent dissociation between ATP demand and myoglobin deoxygenation during ischemia suggests that some degree of uncoupling between intracellular energetics and oxygenation is a potentially important factor that influences critical Po2 in vivo. Furthermore, the observed range of critical Po2 values may be explained by individual variation in the range of metabolic adaptation (3), such that the muscle of some subjects compensates more readily to decreasing Po2, and thus maintains mitochondrial ATP synthesis despite falling oxygen tension, possibly by increasing the redox state of the mitochondria.
Conclusions.
It is likely that critical Po2 is influenced by many factors such as age, physical activity, and the metabolic phenotype of the tissue studied. The observed range of critical Po2 values reported here underscores the need for a better understanding of the factors that regulate mitochondrial bioenergetics under conditions when oxygen availability to the cytochrome chain may become limiting.
GRANTS
Support was provided by National Institutes of Health/National Institute on Aging K02 AG023582 and the Keck Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank the participants for their time, and Dr. Douglas Rothman, for assistance with theoretical aspects of the MRS acquisition.
Present addresses: I. R. Lanza, Division of Endocrinology, Endocrine Research Unit, Mayo Clinic College of Medicine, Rochester, MN 55905; M. A. Tevald, Department of Rehabilitation Sciences, The University of Toledo, Toledo, OH 43606.
REFERENCES
- 1.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 104: 1057–1062, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465: 203–222, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connett RJ, Honig CR, Gayeski TE, Brooks GA. Defining hypoxia: a systems view of V̇o2, glycolysis, energetics, and intracellular Po2. J Appl Physiol 68: 833–842, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Costa LE, Mendez G, Boveris A. Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am J Physiol Cell Physiol 273: C852–C858, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II. Turning it off. Am J Physiol Endocrinol Metab 282: E74–E79, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Duran WN, Renkin EM. Oxygen consumption and blood flow in resting mammalian skeletal muscle. Am J Physiol 226: 173–177, 1974 [DOI] [PubMed] [Google Scholar]
- 7.Gayeski TE, Connett RJ, Honig CR. Minimum intracellular Po2 for maximum cytochrome turnover in red muscle in situ. Am J Physiol Heart Circ Physiol 252: H906–H915, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Gayeski TE, Honig CR. O2 gradients from sarcolemma to cell interior in red muscle at maximal V̇o2. Am J Physiol Heart Circ Physiol 251: H789–H799, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Gnaiger E, Mendez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci USA 97: 11080–11085, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol 81: 1410–1417, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Harris RC, Hultman E, Kaijser L, Nordesjo LO. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Lab Invest 35: 87–95, 1975 [PubMed] [Google Scholar]
- 12.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974 [PubMed] [Google Scholar]
- 13.Hoult DI, Busby SJ, Gadian DG, Radda GK, Richards RE, Seeley PJ. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 252: 285–287, 1974 [DOI] [PubMed] [Google Scholar]
- 14.Jones DP, Kennedy FG. Analysis of intracellular oxygenation of isolated adult cardiac myocytes. Am J Physiol Cell Physiol 250: C384–C390, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994 [PubMed] [Google Scholar]
- 16.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcinek DJ, Ciesielski WA, Conley KE, Schenkman KA. Oxygen regulation and limitation to cellular respiration in mouse skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 285: H1900–H1908, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Pappenheimer JR. Blood flow, arterial oxygen saturation, and oxygen consumption in the isolated perfused hindlimb of the dog. J Physiol 99: 283–303, 1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 291: 681–686, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571: 415–424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richmond KN, Burnite S, Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol Cell Physiol 273: C1613–C1622, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical Po2 of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277: H1831–H1840, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Rolfe DF, Newman JM, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. Am J Physiol Cell Physiol 276: C692–C699, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem 265: 15392–15402, 1990 [PubMed] [Google Scholar]
- 25.Schenkman KA, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82: 86–92, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP]. Magn Reson Med 38: 834–839, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Stainsby WN, Otis AB. Blood flow, blood oxygen tension, oxygen uptake, and oxygen transport in skeletal muscle. Am J Physiol 206: 858–866, 1964 [DOI] [PubMed] [Google Scholar]
- 28.Tamura M, Oshino N, Chance B, Silver IA. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys 191: 8–22, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Tevald MA, Lanza IR, Befroy DE, Kent-Braun JA. Intramyocellular oxygenation during ischemic muscle contractions in vivo. Eur J Appl Physiol 106: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voter WA, Gayeski TE. Determination of myoglobin saturation of frozen specimens using a reflecting cryospectrophotometer. Am J Physiol Heart Circ Physiol 269: H1328–H1341, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Wagner PD. Diffusive resistance to O2 transport in muscle. Acta Physiol Scand 168: 609–614, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol 97: 2385–2394, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Wilson DF, Owen CS, Erecinska M. Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration: a mathematical model. Arch Biochem Biophys 195: 494–504, 1979 [DOI] [PubMed] [Google Scholar]