Abstract
Pregnancy-mediated sodium (Na) retention is required to provide an increase in plasma volume for the growing fetus. The mechanisms responsible for this Na retention are not clear. We first used a targeted proteomics approach and found that there were no changes in the protein abundance compared with virgin rats of the β or γ ENaC, type 3 Na+/H+ exchanger (NHE3), bumetanide-sensitive cotransporter (NKCC2), or NaCl cotransporter (NCC) in mid- or late pregnancy. In contrast, we observed marked increases in the abundance of the α-ENaC subunit. The plasma volume increased progressively during pregnancy with the greatest plasma volume being evident in late pregnancy. ENaC inhibition abolished the difference in plasma volume status between virgin and pregnant rats. To determine the in vivo activity of ENaC, we conducted in vivo studies of rats in late pregnancy (days 18–20) and virgin rats to measure the natriuretic response to ENaC blockade (with benzamil). The in vivo activity of ENaC (UNaV postbenzamil-UNaV postvehicle) was markedly increased in late pregnancy, and this difference was abolished by pretreatment with the mineralocorticoid receptor antagonist, eplerenone. These findings demonstrate that the increased α-ENaC subunit of pregnancy is associated with an mineralocorticoid-dependent increase in ENaC activity. Further, we show that ENaC activity is a major contributor of plasma volume status in late pregnancy. These changes are likely to contribute to the renal sodium retention and plasma volume expansion required for an optimal pregnancy.
Keywords: collecting duct, plasma volume expansion, sodium retention
in humans and rats, pregnancy is accompanied by marked changes in cardiovascular function, renal function, and fluid homeostasis. These adaptations permit an increase in blood volume that will supply the growing uterus and fetus without the development of maternal hypertension.
A normal healthy pregnancy is associated with a cumulative plasma volume expansion (PVE) (30–50%) and avid sodium retention (2, 26, 27). Failure of this adaptation is associated with maternal morbidity/mortality and intrauterine growth restriction (3, 27). Despite the progressive PVE, there is no increase in maternal blood pressure due to a marked decrease in total peripheral vascular resistance (2, 7).
Renal sodium excretion determines volume homeostasis and the progressive PVE of normal pregnancy must reflect net renal sodium retention. Alexander et al. (1) performed balance studies in the pregnant rat that demonstrated cumulative sodium retention during pregnancy. The reabsorption of sodium along the renal tubule is determined by the regulation of the individual tubular cotransporters and channels (16), such that a change in the activity of any transporter or channel can lead to altered volume status (16). The regulation of transepithelial sodium transport across the renal tubules is mediated by apical sodium transporters. The first aim of this study was to identify any changes that occur in the abundance of specific sodium transporters that would permit the required sodium retention for a healthy pregnancy. We performed renal tubule sodium transporter profiling in virgin, mid pregnant, and late pregnant female Sprague-Dawley rats to determine time-course adaptations of the sodium transporters/channels. The second aim of this study was to examine whether increases in protein abundance of specific sodium transporters/channels correspond to an increase in in vivo activity and to determine its contribution to the PV status of pregnancy.
METHODS
Animals and experiments.
Animals were housed in the Virginia Commonwealth University animal facility in agreement with institutional guidelines. All animal protocols were approved by the Virginia Commonwealth University's Institutional Animal Care and Use Committee and in accord with the National Institutes of Health's (NIH's) Guide for Animal Use. Female Sprague-Dawley rats from Harlan (Indianapolis, IN) were used for these experiments. Timed pregnant and age-matched virgin rats were used for this study. A total of 61 rats, 3–4.5 mo of age were used. Rats destined to become pregnant were placed with a fertile male, and day 1 of pregnancy was designated as the day that sperm was present in vaginal smears. Rat gestation is ∼21 days.
Group 1 rats were used to acquire baseline physiological data and determine Na retention over the course of pregnancy. A total of 22 rats were used. Metabolic cage studies were performed in virgin, early pregnant (days 6–8), mid-pregnant (days 12–14), and late pregnant (days 19 and 20) rats, and most rats were studied at 2 or more time points. Rats were given ad libitum access to a gelled diet containing water (62.3%), agar (0.3%), and rat chow (37.4%) (Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet; Harlan Teklad, Madison, WI) for 2 days to get rats acclimated to the metabolic cages. On the 3rd day, a 24-h collection was made, from which gel food intake (g), Na intake (mmol/24 h), urine output (V, ml/24 h), urine sodium [(UNa, mmol/l) fecal Na was not determined], sodium excretion (UNaV = V·UNa, mmol/24 h), and Na retention (Na intake − UNaV) were determined. Urine samples were analyzed for sodium by flame photometry.
Group 2 rats were used for Western blot analysis of whole kidney homogenates in age-matched virgin (n = 12), mid-pregnant (days 11–13, n = 4), and late pregnant (days 18–20, n = 7) rats, for a total of 23 rats. Kidneys were harvested and homogenized whole using a tissue homogenizer (Pro Scientific, Oxford, CT). All samples were homogenized in a chilled isolation solution containing 10 mM triethanolamine, 250 mM sucrose (Mallinckrodt Baker, Phillipsburg, NJ), and protease inhibitors, phenylmethyl sulfonyl fluoride (25 mg/ml isopropyl alcohol; Biochemical Corp, Lakewood, NJ) and leupeptin (1 mg/ml ddH2O) (Novabiochem/EMD Chemicals, Gibbstown, NJ). Protein concentrations of the homogenates were determined by the BCA protein assay kit (Pierce Biotechnology, Rockford, IL). All samples were solubilized at 60°C for 15 min in a Laemmli sample buffer and stored at −80°C. SDS-PAGE was performed on Criterion precast 10% Tris·HCl gels (Bio-Rad Laboratories, Hercules, CA). An initial gel was stained with GelCode Blue Reagent (Pierce Biotechnology), as described previously, to confirm equal loading (28).
Whole kidney homogenates were used for semiquantitative immunoblotting. Each sample was loaded into individual lanes of Criterion precast 10% Tris·HCl gels. The proteins were transferred electrophoretically from unstained gels to nitrocellulose membranes. The blots were blocked with 5 g/dl blotting-grade blocker nonfat dry milk (Bio-Rad Laboratories) for 1 h and probed with primary antibody overnight. Blots were incubated with peroxidase-conjugated secondary antibodies (Pierce Biotechnology; no. 31458 and 31452), followed by an enhanced luminol reagent, Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer LAS, Boston, MA). Blots were exposed to X-ray film, and band densities were quantified by VersaDoc Imaging System (VersaDoc Bio-Rad, Bio-Rad Laboratories). To facilitate comparisons, we normalized the densitometry values, defining the mean for the virgin control group as 100%.
We used the following previously characterized rabbit polyclonal antibodies (a kind gift from Mark Knepper, NIH/Laboratory of Kidney and Electrolyte Metabolism, Bethesda, MD) summarized in the following: 1) the Na+/H+ exchanger type 3 (NHE3) (1:1,000 dilution) (7); 2) the bumetanide-sensitive Na+-K+-2Cl− cotransporter (NKCC2) (1:1,000 dilution) (14); 3) the thiazide-sensitive Na-Cl cotransporter (NCC) (1:1,000 dilution) (15); 4) the three subunits of the amiloride-sensitive epithelial sodium channel (α, β, and γ subunits of ENaC) (1:500 dilution) (21).
Group 3 rats (virgin and pregnant) were implanted with catheters for determination of plasma volume status using Evan's Blue Dye technique and examination of in vivo ENaC activity (net natriuretic response to ENaC blockade with benzamil). Five pregnant rats were studied at mid-pregnancy (days 12–14), six were studied at late pregnancy (days 17–21), and seven virgin rats were studied at 13–14 days after catheterization. Catheters were placed using a sterile technique under general anesthesia with 1–4% isoflurane (Baxter Healthcare, Deerfield, IL). The left femoral artery and vein were cannulated with microrenthane tubing (Braintree Scientific, Braintree, MA) and fed forward with the tip of cannulas placed in the abdominal aorta and abdominal vena cava. The catheters were threaded under the skin and exteriorized at the nape of the neck. The catheters were primed with a 1:1 solution of heparin (1,000 IU/ml) and dextrose (50%), and the ends were plugged with stainless-steel pins. Pregnant rats were catheterized 6–8 days after conception, and at least 4 days, were permitted for complete recovery from the surgery before any experiments were carried out. Prior to experiments, rats had been handled and trained to sit quietly in restraining cages to accept the noises and activity of the laboratory. There was no effect of anesthesia/surgery performed on the total number of pups [nonsurgical rats (n = 6) = 12 ± 1; catheterized rats (n = 7) = 14 ± 1, nonsignificant (NS)] or individual pup weights (nonsurgical rats = 2.25 ± 0.24; catheterized rats = 2.69 ± 0.36 g/pup, NS).
Evan's blue dye administration.
Plasma volume was measured by the Evan's blue dye technique. Following a baseline blood collection, Evan's blue dye (0.3 mg/ml) was infused into the venous catheter (tip of catheter in the vena cava via the femoral vein). Blood collections were then taken from the arterial catheter (tip of catheter in the abdominal aorta via the femoral artery) at 5 and 10 min postinfusion. The plasma was collected following centrifugation of whole blood. Erythrocytes were reconstituted in 13.4% Ficoll in saline and returned to rats intravenously. To determine ENaC-mediated actions on plasma volume status, we measured plasma volume before and 8 h after benzamil (0.7 mg/kg) administration.
Evan's blue dye analysis.
The analysis that was used was a modification of the method used by Blair and Mickelsen (5). Briefly, the standards were made with 1% plasma at 0, 1, 5, 10, and 20 μg/ml of Evan's blue dye. A 100-μl plasma sample was read by ThermoSpectronic Helios γ spectrophotometer at 610 nm, to determine the observed concentration of the Evan's blue dye at baseline (before dye administration), as well as concentrations 5 min and 10 min following dye administration. Background correction was made by subtracting the observed concentration at baseline from the observed concentrations of the 5- and 10-min samples. PV was then calculated by dividing the amount of Evan's blue dye given (75 μg) by the background corrected concentrations. Averages were then taken of the 5- and 10-min samples, to give the average plasma volume.
In vivo ENaC activity.
Rats received vehicle (0.33 g/kg ddH2O iv) injections via perfusion pump (0.008 ml/min, 90–120 μl total volume) and were placed in metabolism cages for 8 h with drinking water ad libitum. Urine collections were taken at 0–3 and 3–8 h postinjection, which was used for determination of baseline sodium excretion (UNaV). Benzamil was given intravenously (0.7 mg/kg iv), and postbenzamil UNaV was subtracted from the previous day's postvehicle UNaV to determine the net natriuretic response to ENaC blockade (benzamil), which is used as an index of in vivo ENaC activity.
Group 4 rats were used to study mineralocorticoid receptor inhibition (with eplerenone) in late pregnancy (days 19–20). Late pregnant (days 16–17, n = 4) rats were given ad libitum access to a gelled diet containing water (62.3%), agar (0.3%), and rat chow (37.4%) (Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet) for 2 days. Then, on the basis of intake, ∼60 g of gelled chow, was given overnight with eplerenone to deliver a dose of 100 mg/kg. Only rats that ate >90% of the gelled chow containing eplerenone were included. Rats in the days 18–19 group received vehicle (0.33 g/kg ddH2O iv) injections via perfusion pump and were placed in metabolism cages with water available ad libitum. Urine collections were taken at 0–3 and 3–8 h postinjection and used to determine baseline UNaV. Overnight, rats were given 100 mg/kg eplerenone in gelled rat chow, and the next morning, benzamil was given (0.7 mg/kg iv). Urine collections were taken at 0–3 and 3–8 h postinjection. The net natriuretic response to ENaC blockade was determined as described above. Kidneys were harvested and processed for immunoblotting as described above.
Statistical analysis.
Data are given as means ± SE. An unpaired t-test, a one-way ANOVA with Bonferroni post hoc, or two-way repeated-measures ANOVA with Bonferroni post hoc was performed. The null hypothesis was rejected at P < 0.05.
RESULTS
Physiological data.
Some of the physiological adaptations of pregnancy are summarized in Table 1. Pregnant rats demonstrated a progressive increase in body weight (BW), reaching significance in mid- (∼8%) and late pregnancy (∼25%) compared with age-matched virgin rats. Likewise, the intake of the gelled diet was significantly increased in early (∼12.5%), mid- (∼18%), and late pregnant (∼32%) rats compared with virgin rats. Urine output (V), urine sodium concentration, and sodium excretion (UNaV) were similar between pregnant and virgin rats.
Table 1.
Summary of physiological data in pregnant and virgin rats
| Virgin (n = 6) | Early-Pregnant (n = 9) | Mid-Pregnant (n = 9) | Late-Pregnant (n = 6) | |
|---|---|---|---|---|
| BW, g | 272 ± 6 | 272 ± 2‡§ | 296 ± 3*†§ | 362 ± 6*†‡ |
| Gel food intake, g/24 h | 44.38 ± 1.24 | 54.36 ± 1.16*§ | 58.15 ± 1.60*§ | 69.77 ± 1.23*†‡ |
| Na+ intake, mmol/24 h | 2.31 ± 0.06 | 2.83 ± 0.06*§ | 3.03 ± 0.08*§ | 3.63 ± 0.06*†‡ |
| V, ml/24 h | 17.69 ± 1.34 | 24.3 ± 1.7 | 25.8 ± 2.00* | 23.6 ± 2.4 |
| Urine Na+, mmol/l | 129 ± 11 | 106 ± 8 | 98 ± 8 | 111 ± 9 |
| UNaV, mmol/24 h | 2.21 ± 0.09 | 2.48 ± 0.07 | 2.44 ± 0.13 | 2.55 ± 0.17 |
| Na+ retention, mmol/24 h | 0.10 ± 0.08 | 0.35 ± 0.05§ | 0.59 ± 0.10*§ | 1.08 ± 0.18*†‡ |
Summary of physiological data from metabolic cage analysis of virgin, early pregnant (days 6–8), mid-pregnant (days 12–14), and late-pregnant rats (days 19–20). Body weight (BW), gel food intake, sodium intake (Na+ intake), urine output (V), urine sodium concentration (fecal sodium was not determined), and sodium excretion (UNaV). A one-way ANOVA with Bonferroni post hoc was performed. Means ± SE; P < 0.05. Symbols (*, †, ‡, and §) are based on results from a Bonferroni test following a statistically significant finding using a one-way ANOVA. Significance is denoted as follows:
vs. virgin,
vs. early-pregnant,
vs. mid-pregnant,
vs. late-pregnant.
Because of the increased sodium intake and increased renal sodium retention, pregnant rats were in positive sodium balance (intake of sodium > sodium excretion) compared with virgin rats, with the sodium retention increasing progressively during pregnancy (Table 1).
Semiquantitative immunoblotting of tubular sodium transporters.
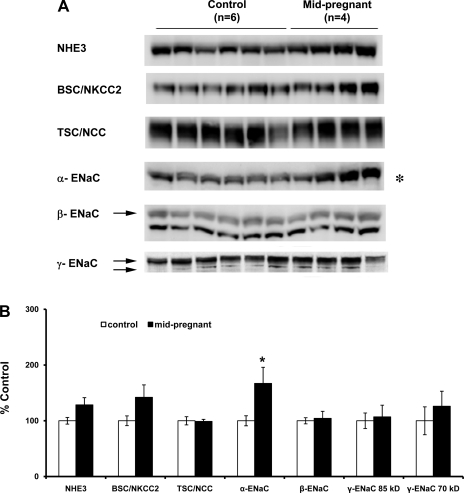
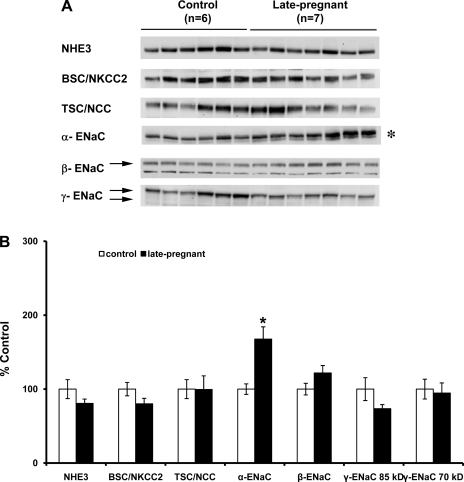
Protein abundance of the three subunits of ENaC (α, β, γ subunits), as well as the other major apical sodium transporters along the renal tubule (NHE3, NKCC2, NCC), in pregnant vs. virgin rats are summarized in Figs. 1 and 2. The only protein that increased in abundance in both mid- (Fig. 1, A and B) and late pregnancy (Fig. 2, A and B) was α-ENaC (midpregnant = 167 ± 29%; late pregnant = 168 ± 17% compared with virgin control, P < 0.05). Since the α-subunit of ENaC is the rate-limiting subunit for channel formation (22), this is consistent with an increased activity of ENaC due to an increase in channel number. There was no difference among groups in the protein abundance of β-ENaC or of γ-ENac (85-kDa or 70-kDa forms).
Fig. 1.
Semiquantitative immunoblots probed for the Na+/H+ exchanger type 3 (NHE3), bumetanide-sensitive Na+-K+-2Cl− cotransporter 2 (NKCC2), thiazide-sensitive cotransporter (NCC), and the three subunits of the epithelial sodium channel (ENaC) (α, β, and γ; 70-kDa and 85-kDa forms). A: immunoblots compare whole kidney homogenates from six virgin and four mid-pregnant rats (days 11–13 of pregnancy). B: band densities were normalized to virgin controls with virgin rats set at 100% and summarized as bar graphs. An unpaired t-test was performed. Values are expressed as means ± SE; *P < 0.05.
Fig. 2.
Semiquantitative immunoblots probed for the NHE3, bumetanide-sensitive NKCC2, NCC, and the three subunits of ENaC (α, β, and γ: 70-kDa and 85-kDa forms). A: immunoblots compare whole kidney homogenates from six virgin and seven late-pregnant rats (days 18–20 of pregnancy). B: band densities were normalized to virgin controls with virgin rats set at 100% and summarized as bar graphs. An unpaired t-test was performed. Values are expressed as means ± SE; *P < 0.05.
Pregnancy-mediated plasma volume expansion: contribution of ENaC.
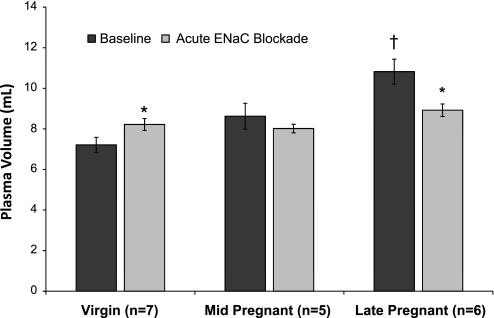
Plasma volume was determined in virgin and pregnant (mid and late) rats before and after ENaC blockade with acute administration of benzamil. A progressive plasma volume expansion was seen with significant increases compared with virgin rats noted at late pregnancy (Fig. 3). ENaC blockade abolished the significant increase in plasma volume in late pregnant rats.
Fig. 3.
Plasma volume, measured as Evans blue dye space, in virgin (n = 7), mid-pregnant (n = 5), and late-pregnant (n = 6) rats before and after ENaC blockade with benzamil (0.7 mg/kg). A two-way repeated-measures ANOVA with Bonferroni post hoc was performed. Values are expressed as means ± SE. Significance is denoted as follows: *P < 0.05 vs. corresponding baseline; †P < 0.05 vs. all groups.
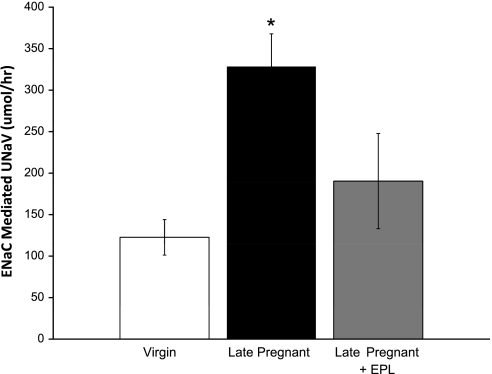
In vivo ENaC activity (natriuretic response to benzamil). To determine whether there is a functional increase in ENaC activity in late pregnant rats, we examined the natriuretic response to ENaC blockade with acute administration of benzamil (0.7 mg/kg iv), an index for in vivo ENaC activity. Most of this natriuretic response occurred within 3 h of benzamil administration. As shown in Fig. 4, the net natriuretic response to ENaC blockade was markedly increased in late pregnant compared with virgin rats at 0–3 h postbenzamil (virgin = 123 ± 21; late pregnant = 328 ± 39 μmol/h, P < 0.05). There were no differences in the net natriuretic response to ENaC blockade at 3–8 h between virgin and late pregnant rats (virgin = 32 ± 15; late pregnant = 19 ± 35 μmol/h, NS).
Fig. 4.
In vivo ENaC activity: net natriuretic response to ENaC blockade with benzamil (0.7 mg/kg). Each rat had vehicle and benzamil injected intravenously on consecutive days. Five virgin, seven late-pregnant, and four late-pregnant rats with eplerenone (EPL; 100 mg/kg) were studied. Urine was collected 0–3 h and 3–8 h following injection for determination of sodium excretion (UNaV). Data from the 0–3-h period are shown here; there were no significant differences among groups in the natriuretic response 3–8 h after ENaC blockade. Net UNaV was calculated as UNaV following benzamil minus UNaV following vehicle. A one-way ANOVA was performed. Values are expressed as means ± SE; *P < 0.05 vs. Virgin.
Effect of mineralocorticoid receptor blockade (eplerenone 100 mg/kg 12 h) on in vivo ENaC activity.
Figure 4 demonstrates that in late pregnant rats, the natriuretic response to ENaC blockade was not elevated if the rats were pretreated with eplerenone, indicating that the increased ENaC activity in late pregnancy is mediated by the mineralocorticoid receptor (MR).
Effect of MR blockade (100 mg/kg eplerenone for 12 h) on α-ENaC protein abundance.
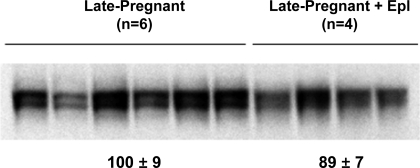
Eplerenone administration for 12 h did not significantly change α-ENaC protein abundance in late pregnant rats (Fig. 5).
Fig. 5.
Semiquantitative immunoblots probed for the α-subunit of ENaC. Immunoblots compare whole kidney homogenates from six late-pregnant rats and four late-pregnant rats treated with eplerenone (Epl; 100 mg/kg). Band densities were normalized to late-pregnant controls. An unpaired t-test was performed. Values are expressed as means ± SE; values were not statistically significant.
DISCUSSION
The main finding from this study is that the α-ENaC protein abundance, which is the rate-limiting subunit for channel formation, is increased in rats during mid- and late pregnancy. In late pregnancy, when the plasma volume expansion and, therefore, the renal sodium retention, are maximal, we observed that in vivo ENaC activity was maximal and ENaC inhibition with benzamil abolished the expanded plasma volume state in late pregnancy. This increased ENaC activity in late pregnancy was prevented by MR blockade with eplerenone, demonstrating that the increased ENaC activity was the result of the increased MR activity of normal pregnancy (10).
The sodium retention that occurs over the course of pregnancy mediates the increase in plasma volume that is needed for the developing uterus and fetus. It is well established that at the whole kidney level, sodium is retained over the course of pregnancy, reaching its peak in late pregnancy (1). Sodium balance is determined by the amount of sodium filtered by the glomerulus and the sodium reabsorbed by each of the renal tubule segments. Glomerular filtration rate (GFR) increases in pregnancy, peaking at mid-pregnancy and returning toward nonpregnant levels near term (2). Thus, this enhanced GFR would favor an increase in sodium excretion. Additionally, pregnancy is associated with increases in other natriuretic factors such as nitric oxide (NO), atrial natriuretic peptide (ANP), and progesterone (2, 10, 18). However, there is also activation of the sodium-retaining renin-angiotensin-aldosterone system (RAAS) in normal pregnancy (10).
Taken together, adaptations must occur in the renal tubules to permit sodium retention to predominate. Consistent with this, we and others have shown that the pregnant rat is refractory to the renal tubular response to ANP (18, 24) and acute pressure-natriuresis, which requires an intact renal NO system (12, 19). Recent studies suggest that pregnancy is associated with a loss of responsiveness to cGMP (second messenger for both ANP and NO) in the inner medulla, due to increased cGMP breakdown by phosphodiesterase-5 (23). Overall, there is evidence that adaptations occur locally in the inner medullary collecting duct, leading to a loss of responsiveness to natriuretic agents that act via cGMP. However, the details regarding possible pregnancy-mediated adaptations of the individual sodium transporters and channels are unknown and are addressed in the current study.
We found no change at any stage of pregnancy in the protein abundance of 1) the NHE3 present in proximal tubule and thick ascending limb, 2) the NKCC2 present in the thick ascending limb, and 3) the thiazide-sensitive NCC present in the distal convoluted tubule. Although there were no differences in expression of other Na transporters, there is a possibility that their activity may be changed with pregnancy, as increases in sodium delivery occur in the tubules. The only other study to report on apical sodium transporters in pregnancy reported a fall in the renal cortical NHE3 protein abundance in mid- and late pregnancy (13). These workers used the same rat strain and vendor as used in the present study, and the only apparent difference was that they looked at a homogenate of cortex, whereas in the present study, the entire kidney was homogenized. Because NHE3 is present in cortex and medulla, we may have missed regional differences, although it is difficult to reconcile falls in NHE3 with net sodium retention.
In contrast, we observed a marked increase in the α-ENaC subunit, which is present in the collecting duct. Of the three ENaC subunits, the rate of α-ENaC production is rate limiting for assembly of the functional ENaC complex (22). Sodium transport by ENaC can be controlled by complex mechanisms that include regulating channel number, trafficking to the apical membrane, channel open probability, and rate of degradation or recycling (6, 11, 25). However, the goal of the present study was not to determine the molecular mechanisms of ENaC activity in pregnancy, but rather to establish whether the increased α-ENaC subunit protein abundance translated into increased ENaC activity in pregnancy.
In the present study, we confirmed earlier work showing that plasma volume expansion increase in a cumulative manner during pregnancy (2, 7, 26, 27). Unexpectedly, we found that ENaC inhibition resulted in an increase in plasma volume in virgin rats, which we attribute to a rebound effect since this is the opposite of what would be expected with ENaC inhibition. We also found that a major component of the plasma volume status in late pregnancy is mediated by ENaC activity since benzamil administration abolished the differences in plasma volume status in virgin and pregnant rats (Fig. 3); we, therefore, selected late pregnancy for our functional ENaC activity studies.
Using the selective ENaC inhibitor benzamil, we found that a much greater natriuretic response was seen to ENaC inhibition over a 3-h period, in the late pregnant compared with the virgin rat (Fig. 4). This indicates that basal ENaC-dependent sodium retention was much greater in the late pregnant rat, supporting a functional effect of the increased α-ENaC subunit's protein abundance. There is marked activation of the RAAS in normal pregnancy (10), and we have previously reported that both ANG II and aldosterone stimulate the α-ENaC subunit (4, 20). In the present study, we found that the mineralocorticoid receptor antagonist eplerenone reversed the enhanced, acute natriuretic response to benzamil. This demonstrates that the late pregnancy-mediated increase in ENaC-dependent sodium retention is the result of increased stimulation of the MR. In pregnancy, we did not observe an increase in NCC or the 70-kDa form of γ-ENaC, which is associated with the protein profile of aldosterone-mediated stimulation of the MR. This finding is not surprising since pregnancy is a state in which many hormone systems and hemodynamic factors (systemic vasodilation and increased glomerular filtration rate), which are known to influence sodium transport and sodium transporter profiles, change over the course of pregnancy. This is unlike our previous protein profile studies, in which only one variable was being changed—either placing rats on a low-Na diet or aldosterone administration (20). Thus, the protein profile at the stages of pregnancy examined in this study likely represents the culmination of these actions. Further, although we did see eplerenone administration reverse the increase in ENaC activity in late pregnancy, we did not see a decrease in the protein abundance of α-ENaC. We attribute this finding to the fact that administration of eplerenone over 12 h was not an adequate time duration for changes in protein abundance to be observed. We have previously shown that although ENaC activity can change in as little as 15 h, α-ENaC protein abundance changes are not seen 1 day following increases in plasma aldosterone levels (9, 21).
In conclusion, our findings show that α-ENaC protein abundance and ENaC activity are increased in the late pregnant rat, thereby contributing to the positive sodium balance of pregnancy. Furthermore, this increased ENaC activity is due to the stimulation of the MR in normal pregnancy. Thus, in spite of pregnancy-mediated increases in both natriuretic and antinatriuretic factors, the important modifications to renal sodium transport that occur in pregnancy and permit net renal sodium retention and volume expansion are localized to the collecting duct. Specifically, enhanced MR-mediated, ENaC-dependent sodium reabsorption occurs in the collecting duct in combination with blunted inner medullary collecting duct responsiveness to natriuretic stimuli.
GRANTS
This research was supported by grants from the National Heart, Lung, and Blood Institute (K22HL66994).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The author's thank Collin Berry and Erin McGuire for their technical assistance.
REFERENCES
- 1.Alexander EA, Churchill S, Bengele HH. Renal hemodynamics and volume homeostasis during pregnancy in the rat. Kidney Int 18: 173–178, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 1: 789–813, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Bedard S, Sicotte B, St-Louis J, Brochu M. Modulation of body fluids and angiotensin II receptors in a rat model of intra-uterine growth restriction. J Physiol 562: 937–950, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Blair ML, Mickelsen D. Activation of lateral parabrachial nucleus neurons restores blood pressure and sympathetic vasomotor drive after hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol 291: R742–R750, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp Clin Pharmacol 19: 613–625, 1997 [PubMed] [Google Scholar]
- 8.Fernandez-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper M. Concentrating defect in experimental nephrotic syndrone: altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int 54: 170–179, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM. Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113: 435–444, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Khraibi AA. Renal interstitial hydrostatic pressure and pressure natriuresis in pregnant rats. Am J Physiol Renal Physiol 279: F353–F357, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Khraibi AA, Dobrian AD, Yu T, Solhaug MJ, Billiar RB. Role of RIHP and renal tubular sodium transporters in volume retention of pregnant rats. Am J Hypertens 18: 1375–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na+-K+-2Cl− cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 276: F96–F103, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knepper MA, Kim GH, Masilamani S. Renal tubule sodium transporter abundance profiling in rat kidney: response to aldosterone and variations in NaCl intake. Ann NY Acad Sci 986: 562–569, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Masilamani S, Castro L, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol Regul Integr Comp Physiol 267: R1611–R1616, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Masilamani S, Hobbs GR, Baylis C. The acute pressure natriuresis response blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol 179: 486–491, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol 283: F648–F657, 2002 [DOI] [PubMed] [Google Scholar]
- 22.May A, Puoti A, Gaeggeler HP, Horisberger JD, Rossier BC. Early effect of aldosterone on the rate of synthesis of the epithelial sodium channel alpha subunit in A6 renal cells. J Am Soc Nephrol 8: 1813–1822, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Ni XN, Safai M, Rishi R, Baylis C, Humphreys MH. Increased activity of cGMP-specific phosphodiesterase (PDE5) contributes to renal resistance to atrial natriuretic peptide in the pregnant rat. J Am Soc Nephrol 15: 1254–1260, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omer S, Mulay S, Cernacek P, Varma DR. Attenuation of renal effects of atrial natriuretic factor during rat pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 268: F416–F422, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Salas SP, Giacaman A, Vio CP. Renal and hormonal effects of water deprivation in late-term pregnant rats. Hypertension 44: 334–339, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol Renal Fluid Electrolyte Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]