Abstract
ANG II causes renal injury through hemodynamic and other effects, and pressor doses of ANG II induce heme oxygenase-1 (HO-1) as a protective response. The present studies examined the hemodynamic effects of more clinically relevant, lower doses of ANG II and the role of HO activity in influencing these effects. Under euvolemic conditions, ANG II increased arterial pressure and renal vascular resistance. ANG II did not induce oxidative stress, inflammation/injury-related gene expression, or proteinuria and did not alter extrarenal vascular reactivity. At these doses, ANG II failed to increase HO-1 or HO-2 mRNA expression or HO activity. Inhibiting HO activity in ANG II-treated rats by tin mesoporphyrin further increased renal vascular resistances, decreased renal blood flow, and blunted the rise in arterial pressure without inducing oxidative stress or altering expression of selected vasoactive/injury/inflammation-related genes; tin mesoporphyrin did not alter vasorelaxation of mesenteric resistor vessels. We conclude that in this model renal vasoconstriction occurs without the recognized adverse effects of ANG II on glomerular filtration rate, renal blood flow, oxidative stress, vascular reactivity, proteinuria, and injury-related gene expression; renal HO activity is essential in preserving perfusion of the ANG II-exposed kidney. These findings represent an uncommon example wherein function of a stressed organ (by ANG II), but not that of the unstressed organ, requires intact renal HO activity, even when the imposed stress neither induces HO-1 nor HO activity. These findings may be germane to conditions attended by heightened ANG II levels, ineffective renal perfusion, and susceptibility to acute kidney injury.
Keywords: tin mesoporphyrin, ischemia
ang ii is a major contributor to chronic kidney disease by virtue of its hemodynamic, proinflammatory, and profibrotic effects. Indeed, agents that interrupt the generation of ANG II and the engagement of ANG II with its receptor comprise a fundamental therapeutic approach in the management of patients with chronic kidney disease (12, 20, 22). Such therapies, however, reduce but do not abort the progression of chronic kidney disease, and thus complementary strategies that can synergize with these therapies would be of interest. In this regard, a fundamental basis for the injurious effects of ANG II involves the imposition of oxidant stress via NADPH oxidase (35). Studies by us and others have demonstrated that ANG II induces the antioxidant gene heme oxygenase-1 (HO-1) (3, 4, 11) in the kidney in vivo and in vitro and that such induction of HO-1, by virtue of its antioxidant, anti-inflammatory, and vasorelaxant properties (1, 2, 15, 24), is implicated as an adaptive, protective mechanism that can reduce the severity of ANG II-induced renal injury.
An established approach employed in studying the pathogenetic basis for ANG II-induced renal injury utilizes the chronic administration of ANG II by osmotic minipumps. Such studies demonstrate that ANG II, so administered, provokes markedly increased systemic arterial pressure, reduces glomerular filtration rate (GFR), increases urinary protein excretion, induces the expression of proinflammatory and other damaging genes, and provokes proliferation as well as apoptosis of tubular epithelial cells (3, 4, 11, 36). However, many of these studies, including the initial studies by us and others demonstrating renal induction of HO-1 by ANG II in vivo, have employed pressor dosages/regimens of ANG II that lead to markedly elevated plasma levels of ANG II. To more closely simulate levels of ANG II that are clinically observed, lesser amounts of ANG II (subpressor or low dose) are also chronically employed (27–29). Such delivery of ANG II elicits a delayed pressor response and less elevation in systemic arterial pressure (27–29). However, even subpressor amounts of ANG II may exert adverse effects including oxidative stress and a predisposition to stress-induced systemic hypertension (27–29).
Given the importance of ANG II as a determinant of acute kidney injury and chronic kidney disease, in part, through its hemodynamic effects, the present study analyzed the renal hemodynamic actions of ANG II when relatively low doses of ANG II are chronically administered. Since previous studies indicate that HO-1 is induced by ANG II when given in high doses, the present studies also examined the effect of low-dose ANG II on HO-1 and HO-2 mRNA expression and HO activity and the extent to which HO activity influences the renal hemodynamic effects of low-dose ANG II. To examine the involvement of HO activity, we employed the widely used metalloporphyrin inhibitor, tin mesoporphyrin (SnMP), which has been shown to effectively inhibit HO activity in stressed and control conditions (32, 33).
MATERIALS AND METHODS
All experiments were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) were maintained on standard rat chow with water ad libitum and were used in all protocols.
Administration of ANG II by Osmotic Minipumps
ANG II (50 ng·kg−1·min−1 iv) or saline vehicle was administered for 2 wk to rats via an osmotic minipump (model 2ML2; Durect, Cupertino, CA) as detailed in our prior study (27). Briefly, under intramuscular ketamine and xylazine (100 and 10 mg/kg, respectively) anesthesia, incisions were made in the midscapular region and in the ventral neck. Osmotic minipumps were implanted in a subcutaneous pocket created in the midscapular region, and a catheter connected to the minipumps was tunneled through the subcutaneous space to the ventral neck and implanted into the external jugular vein.
Assessment of Renal Hemodynamics
Two weeks after the start of ANG II or saline vehicle infusion by minipump, renal hemodynamics were assessed in rats using methods described in detail in our prior studies (26, 27). Briefly, rats were anesthetized with Inactin (100 mg/kg ip; BYK-Gudden, Konstanz, Germany) and placed on a heated table to maintain body temperature at 37°C. A tracheotomy was performed using polyethylene (PE)-240 tubing. The femoral artery and vein were cannulated with PE-50 tubing for monitoring blood pressure and for infusions, respectively. Euvolemia was achieved and maintained by infusion of 4% albumin in 0.9% saline solution, initially at 1% of body wt over 30 min and subsequently at the rate of 0.5 ml/h. Additionally, a 1% inulin solution in 0.9% saline was given as a 1-ml bolus infused over 5 min and thereafter at a rate of 1.5 ml/h for clearance studies. The urinary bladder was catheterized with PE-160 tubing for urine collection. Whole kidney blood flow of the left kidney was measured with a 0.7 mm-diameter perivascular flow probe (Transonic Systems, Ithaca, NY) placed around the renal artery. Intrarenal distribution of renal blood flow was measured using a laser Doppler needle flow probe (25 gauge; Transonic Systems) set on a micromanipulator; one probe was placed on the superficial cortex, and a second probe was advanced into the renal medulla (visually verified at the end of the experiment). ANG II-treated and saline vehicle-treated rats were administered an inhibitor of HO activity (40 μmol/kg iv SnMP, given as a bolus) or vehicle, exactly as previously described by Rodriguez and colleagues (32, 33). After 60 min of equilibration, clearance studies were begun during which urine was collected for two consecutive periods with blood samples drawn in the middle of each period.
Assessment of Renal mRNA Expression
In additional groups of rats, ANG II (50 ng·kg−1·min−1 iv) or saline vehicle was administered by osmotic minipump as described above, and after 2 wk, renal mRNA expression was assessed. Renal mRNA expression was also assessed in studies in which SnMP or vehicle was administered to ANG II-treated rats. Total RNA was extracted from rat kidney tissue using the Trizol method (Invitrogen, Carlsbad, CA) and further purified with an RNeasy Mini Kit (Qiagen, Valencia, CA), according to each manufacturer's protocol. Two hundred nanograms of total RNA were used in reverse transcription reactions (Transcriptor First Strand cDNA Synthesis Kit; Roche Applied Science, Indianapolis, IN) by using random hexamers. The resulting cDNA was used in quantitative real-time PCR analysis as in our earlier study (31). Reactions were performed on an ABI Prism 7900HT (Applied Biosystems, Foster City, CA) using TaqMan Mastermix reagent (part no. 4324020; Applied Biosystems). Probes and primers used for quantification were obtained as assay sets (TaqMan Gene Expression Assays; Applied Biosystems) and used according to the manufacturer's protocol. In addition, the probes and primers for the quantification of HO-1 and 18S expression were designed using Primer Express software (Applied Biosystems) as detailed in our previous study (31). Parameters for quantitative PCR were as follows: 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C and 1 min at 60°C. Values for the expression of each target mRNA were derived from relative quantification against a standard curve constructed for each mRNA target and normalized for the expression of 18S rRNA.
Assessment of Renal HO Activity
HO activity was determined by the generation of bilirubin by microsomes prepared from kidneys as described previously (11, 19). Briefly, microsomes were incubated with mouse liver cytosol (a source of biliverdin reductase), hemin (20 μM), glucose-6-phosphate (2 mM), glucose-6-phosphate dehydrogenase (4 U/ml), and NADPH (0.8 mM) for 90 min at 37°C in the dark. The formed bilirubin was extracted with chloroform, ΔOD 464–530 nm measured (extinction coefficient, 40 mM/cm for bilirubin), and enzyme activity was determined (pmol formed bilirubin·h−1·mg protein−1).
Assessment of Indices of Oxidative Stress
Lipid peroxidation and protein carbonyl content were used as indices of oxidative stress in the kidney, as described in our previous study (11). Lipid peroxidation in whole kidney homogenates was determined by measurement of thiobarbituric acid reactive substances, and protein carbonyl content was determined employing the method based on 2,4-dinitrophenylhydrazine.
Studies of Vascular Reactivity
These studies were performed by methods described in detail in our prior publications (21, 25).
Studies in aortic rings.
Briefly, isolated aortic rings (4 mm length) were connected to a force transducer for recording of isometric force and placed in an organ bath filled with 25 ml modified Krebs-Ringer solution containing (in mM) 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, 0.026 calcium EDTA, and 11.1 glucose (pH 7.4), maintained at 37°C, and bubbled with a gaseous mixture of 94% O2/6% CO2 (25). Aortic rings were stretched progressively to their optimal tension (2.5 g) in response to 80 mM KCl. Endothelium-dependent relaxations in response to acetylcholine (10−9-10−5 M) were cumulatively obtained during submaximal contractions to phenylephrine. In some studies, aortic rings were preincubated with an inhibitor of HO activity, SnMP (10−5 M), for 10 min. Relaxations were expressed as a percentage of maximal relaxations induced by 3 × 10−4 mol/l papaverine. Concentration-response curves to phenylephrine (10−9-10−5 M) were also obtained in similarly prepared and treated aortic rings.
Studies in mesenteric resistor vessels.
The analysis of vascular reactivity in rat mesenteric artery segments was performed using arteriography (21). Briefly, experiments were performed on 5-mm-long tertiary branches of mesenteric artery from rats that had been anesthetized with pentobarbital (60 mg/kg ip). Mesenteric arteries were carefully removed, placed immediately into cold (4°C)-modified Krebs-Ringer bicarbonate solution. Arteries were dissected free from connective tissue and transferred to an arteriograph (Living Systems Instrumentation, Burlington, VT). At the beginning of each experiment, vessels were equilibrated for 45 min at 50 mmHg, and wall thickness and inner diameter were measured. Vessels were contracted with phenylephrine (10−6-10−5 mol/l). When a steady tone was established, acetylcholine (10−9-10−5 mol/l) or diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NONOate; 10−9-10−5 mol/l) were added in a cumulative manner. In some studies, vessels were preincubated with an inhibitor of HO activity, SnMP (10−5 M), for 10 min prior to the phenylephrine incubation.
Statistics
Results are expressed as means ± SE. Comparisons between two groups were performed using the Student's t-test for parametric data and the Mann-Whitney test for nonparametric data. Comparisons among more than two groups were performed using one-way ANOVA followed by a Bonferroni post test for parametric data, and the Kruskal-Wallis one-way ANOVA followed by the Wilcoxon rank-sum test for nonparametric data (7). Results are considered statistically significant for P < 0.05.
RESULTS
Studies of Renal Hemodynamics
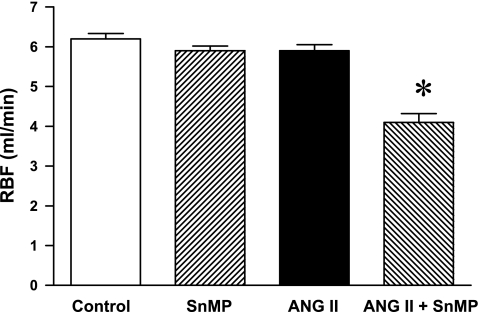
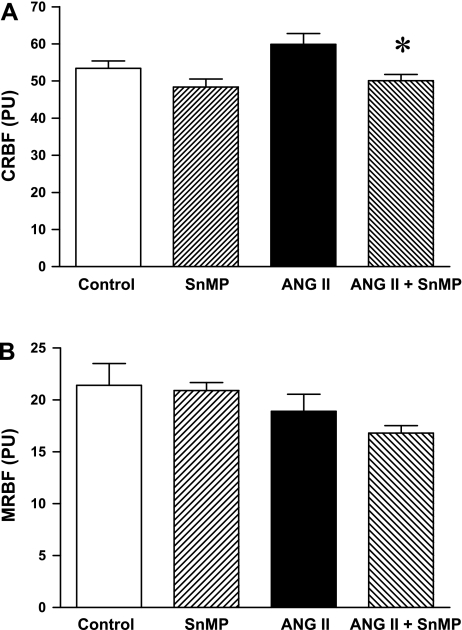
GFRs were not significantly different among the control, SnMP-treated, and ANG II-treated groups (Table 1); the GFR in ANG II/SnMP-treated rats was significantly lower compared with the control but not the other groups (Table 1). Renal blood flow was uninfluenced by the administration of SnMP or ANG II, but was significantly decreased in ANG II-treated rats subjected to SnMP (Fig. 1). To assess the extent to which such reductions in total renal blood flow reflected changes in cortical and medullary blood flow, regional blood flow was determined by laser Doppler methodology. Cortical renal blood flow in ANG II-treated rats also administered SnMP was significantly decreased compared with ANG II-treated rats (Fig. 2); medullary blood flow was not significantly different among the four groups, thereby demonstrating that the reduction in renal blood flow in ANG II-treated rats, attendant upon the administration of SnMP, mainly reflected changes in cortical blood flow.
Table 1.
Hemodynamic studies in vehicle-treated (control, n = 7), tin mesoporphyrin-treated (SnMP, n = 8), angiotensin II-treated (ANG II, n = 7), and ANG II/SnMP-treated (n = 6) rats
| Control | SnMP | ANG II | ANG II/SnMP | |
|---|---|---|---|---|
| Body weight | 322 ± 4 | 331 ± 5 | 335 ± 5 | 338 ± 9 |
| Combined kidney weight | 2.04 ± 0.04 | 2.05 ± 0.04 | 2.01 ± 0.02 | 2.01 ± 0.04 |
| GFR | 2.23 ± 0.04 | 2.13 ± 0.07 | 2.00 ± 0.08 | 1.85 ± 0.09* |
Values are means ± SE. Glomerular filtration rate (GFR) was determined by inulin clearance during renal hemodynamic studies.
P < 0.05 vs. control values.
Fig. 1.
Effect of ANG II and tin mesoporphyrin (SnMP) on renal blood flow (RBF). RBF was determined during renal hemodynamic studies performed under euvolemic conditions in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. *P < 0.05 vs. all other groups.
Fig. 2.
Effect of ANG II and SnMP on cortical RBF (CRBF) and medullary RBF (MRBF). CRBF (A) and MRBF (B) were determined during renal hemodynamic studies performed under euvolemic conditions and expressed as perfusion units (PU) in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. *P < 0.05 vs. the ANG II-treated group.
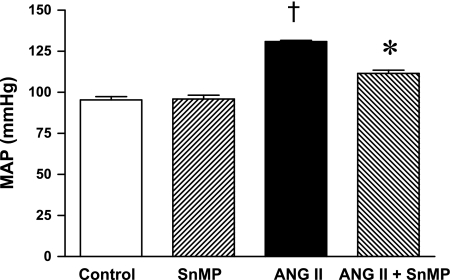
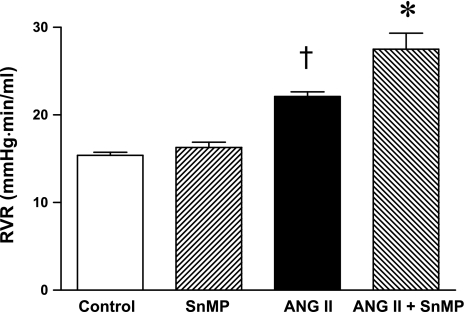
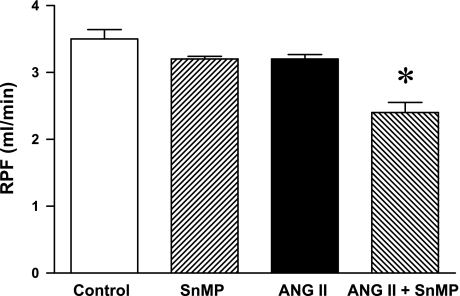
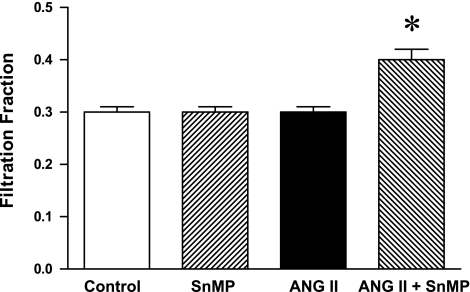
Mean arterial pressure was significantly increased in ANG II-treated rats, but significantly less so in rats concomitantly administered SnMP (Fig. 3). Renal vascular resistances were increased by ANG II, and to a greater degree in ANG II-treated rats also administered SnMP (Fig. 4). Only in the ANG II/SnMP-treated rats was renal plasma flow significantly decreased (Fig. 5) and filtration fraction significantly increased (Fig. 6). Thus, while exerting no effect on renal hemodynamics in vehicle-treated rats, SnMP decreased renal blood flow and renal plasma flow rates in ANG II-treated rats due to an augmentation in renal vascular resistances.
Fig. 3.
Effect of ANG II and SnMP on mean arterial pressure (MAP). MAP was determined during renal hemodynamic studies performed under euvolemic conditions in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. †P < 0.05 vs. control group. *P < 0.05 vs. the ANG II-treated group.
Fig. 4.
Effect of ANG II and SnMP on renal vascular resistance (RVR). RVR was calculated as MAP/RBF from values of these indices determined during renal hemodynamic studies performed under euvolemic conditions in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. *P < 0.05 vs. the ANG II-treated group; †P < 0.05 vs. control group.
Fig. 5.
Effect of ANG II and SnMP on renal plasma flow (RPF). RPF was calculated as RBF × (100-hct)/100 from values of these indices determined during studies of renal hemodynamics performed under euvolemic conditions in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. *P < 0.05 vs. all other groups.
Fig. 6.
Effect of ANG II and SnMP on filtration fraction (FF). FF of the kidney was calculated as single-kidney GFR/RPF from these indices as measured/derived from renal hemodynamic studies performed under euvolemic conditions in vehicle-treated (control, n = 7), SnMP-treated (n = 8), ANG II-treated (n = 7), and ANG II/SnMP-treated (n = 6) rats. *P < 0.05 vs. all other groups.
Studies of Indices of Oxidative Stress
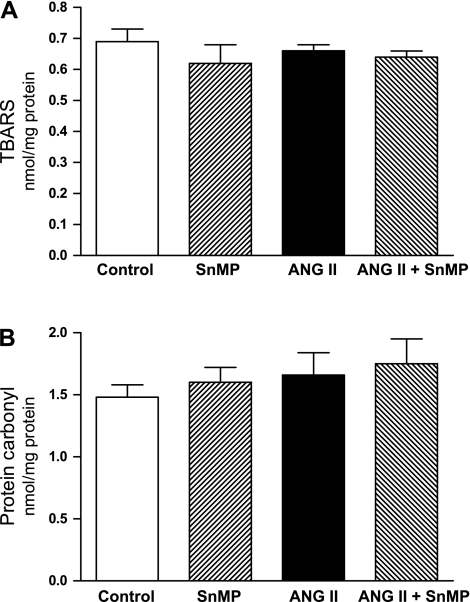
To determine whether the observed renal hemodynamic effects reflected oxidative stress in the kidney, lipid peroxidation and protein carbonyl content (indices of oxidative stress) were measured in the kidney in the control, SnMP-treated, ANG II-treated, and ANG II/SnMP-treated rats. As demonstrated in Fig. 7, oxidative stress, as assessed by either index, was not induced by ANG II, SnMP, or ANG II/SnMP.
Fig. 7.
Effect of ANG II and SnMP on indices of oxidative stress. Lipid peroxidation was measured by the thiobarbituric acid reactive substances (TBARS) assay (A), and protein oxidation was assessed by protein carbonyl content (B) in renal tissues from vehicle-treated (control), SnMP-treated, ANG II-treated, and ANG II/SnMP-treated rats, n = 6 for each group. There were no significant differences among the groups for either oxidative index.
Studies of Vascular Reactivity
Studies in rat aortic rings.
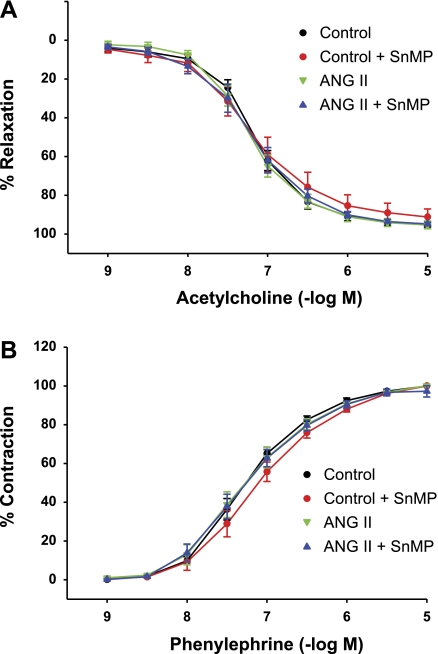
The administration of SnMP in vivo led to a fall in mean arterial pressure in ANG II-treated rats, thereby raising the possibility that SnMP exerted a vasorelaxant effect in ANG II-treated rats. We thus examined the effect of SnMP on vascular reactivity ex vivo in aortas from additional groups of ANG II-treated and vehicle-treated rats. As demonstrated in Fig. 8, the vasorelaxant responses to acetylcholine and the vasoconstrictor responses to phenylephrine were not altered by ANG II compared with vehicle-treated rats, and the exposure of the aortas from ANG II-treated and vehicle-treated rats to SnMP did not alter such vascular reactivity in either group. Thus we could not identify a vasorelaxant effect of SnMP on aortic segments that could account for the blunted increase in mean arterial pressure in ANG II-treated rats concomitantly treated with SnMP.
Fig. 8.
Effect of incubation with SnMP on vascular responses in aortas from ANG II and vehicle-treated (control) rats. Concentration-response curves to acetylcholine (A) and phenylephrine (B) in aortas from control and ANG II-treated rats in the absence and presence of SnMP (10−5 M). Data for the acetylcholine concentration-response curve are expressed as %maximal relaxation induced by 3 × 10−4 mol/l papaverine (n = 4 rats for each group with 2 aortic rings per rat).
Studies in rat mesenteric artery segments.
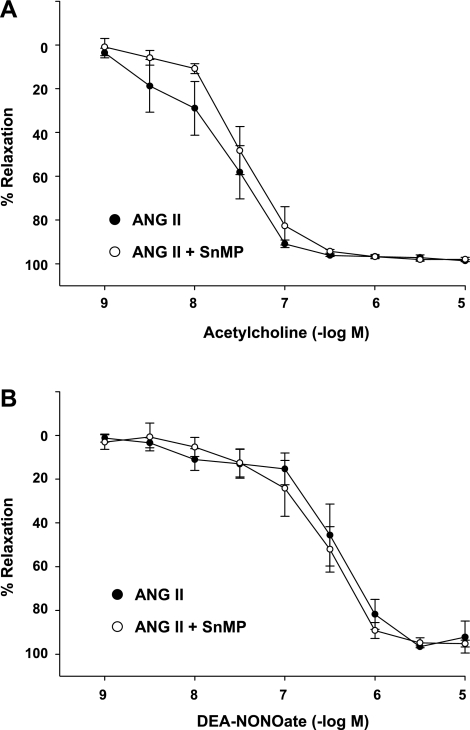
Since aortic segments represent conduit vessels, studies of vascular reactivity were also undertaken in resistor vessels, such as the tertiary branches of mesenteric arteries, to determine whether altered vascular reactivity induced by SnMP may contribute to the lower mean arterial pressure in ANG II-treated rats concomitantly treated with SnMP. Vasorelaxant responses were thus examined in these resistor vessels obtained from ANG II-treated rats exposed to SnMP or vehicle. As demonstrated in Fig. 9, prior incubation with SnMP did not alter the vasorelaxant responses of these resistor vessels to either acetylcholine or DEA-NONOate. Additionally, the mean diameter values in mesenteric vessels in ANG II-treated rats were not significantly different in the absence or presence of SnMP (301.0 ± 18.4 vs. 310.7 ± 12.7 μm, respectively, n = 6).
Fig. 9.
Effect of incubation with SnMP on vascular responses in mesenteric arteries from ANG II rats. Concentration-response curves to acetylcholine (A) and diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NONOate ) (B) in the tertiary branches of mesenteric arteries from ANG II-treated rats in the absence and presence of SnMP (10−5 M). Data are expressed as %maximal relaxation induced by 3 × 10−4 mol/l papaverine (n = 6 rats for each group).
Evaluation of the HO System
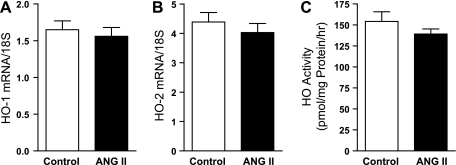
The HO system was evaluated by gene expression and enzyme activity in additional groups of ANG II-treated and vehicle-treated rats, studied 2 wk after the initiation of administration of ANG II or vehicle by osmotic minipump. ANG II did not induce HO-1 or HO-2 mRNA expression in the kidney, nor did ANG II induce renal HO activity (Fig. 10).
Fig. 10.
Effect of ANG II on heme oxygenase (HO)-1 and HO-2 mRNA expression and HO activity. Renal expression of HO-1 mRNA (A) and HO-2 mRNA (B) was determined by quantitative real-time RT-PCR in rats treated with ANG II (n = 9) or vehicle (control, n = 6) for 2 wk. Renal HO activity (C) in similarly treated rats was determined by the rate of generation of bilirubin by microsomes extracted from kidney tissues (n = 6 and n = 10 for control and ANG II rats, respectively).
Evaluation of ANG II and SnMP on Indices of Renal Injury
The absence of an effect of ANG II as administered in the present study on GFR, renal blood flow, the HO system, and vascular reactivity led us to assess the effect of such administration of ANG II on relevant indices that reflect, initiate, or contribute to renal injury. Following the administration of vehicle or ANG II for 2 wk, proteinuria was not significantly (NS) different in the two groups (24 ± 2 vs. 23 ± 3 mg/24 h, n = 7 and n = 6, respectively, P = NS), and mRNA expression of assorted proinflammatory and fibrogenic genes, incriminated in the initiation/progression of renal injury, was not increased (Table 2).
Table 2.
mRNA expression in the kidney of the saline-treated (control) and ANG II-treated rats after 2 wk of treatment
| mRNA | Control | ANG II | P Value |
|---|---|---|---|
| MCP-1 | 1.4 ± 0.2 | 2.1 ± 0.6 | NS |
| IL-6 | 11.2 ± 1.5 | 12.1 ± 1.7 | NS |
| PAI-1 | 4.9 ± 0.8 | 4.5 ± 0.6 | NS |
| TGF-β1 | 4.9 ± 0.3 | 4.8 ± 0.4 | NS |
| SDF-1 | 2.3 ± 0.1 | 2.5 ± 0.1 | NS |
| HIF-1α | 6.6 ± 0.2 | 7.1 ± 0.3 | NS |
| Col I | 10.0 ± 0.1 | 9.7 ± 0.3 | NS |
| Col III | 10.6 ± 1.0 | 11.2 ± 0.8 | NS |
| Col IV | 14.2 ± 0.6 | 14.8 ± 0.8 | NS |
Values are means ± SE; n = 6 for control and n = 9 for the ANG II. mRNA expression was assessed by quantitative real-time RT-PCR. Values are the result of relative quantification performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units.
Effect of SnMP on Renal Gene Expression in ANG II-Treated Rats
We also determined whether administration of SnMP increased inflammation-related gene expression or altered vasoactive gene expression in ANG II-treated rats. Such mRNA expression in ANG II-treated rats and ANG II/SnMP-treated rats was not significantly different for either MCP-1 (6.5 ± 1.7 vs. 3.9 ± 0.9, standardized units, P = NS, n = 6 in each group in this and other analyses of gene expression), TGF-β1 (4.9 ± 0.5 vs. 5.6 ± 0.7, standardized units, P = NS), IL-6 (7.4 ± 2.2 vs. 3.7 ± 1.1, standardized units, P = NS), endothelial NOS (3.0 ± 0.4 vs. 3.2 ± 0.4, standardized units, P = NS), or endothelin-1 (4.5 ± 0.5 vs. 4.9 ± 0.7, standardized units, P = NS).
DISCUSSION
To the best of our knowledge, a comprehensive in vivo assessment of the effect of inhibition of HO activity on the renal hemodynamic profile of clinically relevant doses of ANG II and one that includes GFR and renal blood flow has, to date, not been undertaken; our present study addressed this issue. Studies that examine the renal hemodynamic effects of ANG II often employ doses/protocols that lead to appreciable reductions in GFR and/or renal blood flow and to marked elevations in systolic/mean arterial pressures. The present study employed a regimen that achieves plasma levels of ANG II that more closely mirror those observed in clinical settings, and such administration of ANG II led to modest elevations in mean arterial pressure, no change in GFR or renal blood flow, and increased renal vascular resistance. However, the maintenance of renal perfusion within the normal range in the kidney exposed to ANG II required the offsetting vasorelaxant effects emanating from HO activity. When HO activity was concomitantly inhibited by SnMP, ANG II caused substantial reductions in renal blood flow and plasma flow rates, effects that arose from an augmentation in renal vascular resistances; GFR was not maintained compared with values observed in the control group. From these findings, we conclude that HO activity is critical in preserving perfusion in the kidney exposed to ANG II and in preventing GFR from declining below control values.
In the ANG II-infused rat, SnMP did not affect GFR but decreased renal plasma flow rate, thereby leading to an increase in filtration fraction. These findings raise the possibility that in the presence of SnMP there is a heightened vasoconstrictive effect of ANG II on the efferent arteriole, a resistor vessel targeted by ANG II. The countervailing vasorelaxant effect arising from renal HO activity when ANG II is administered possibly reflects the products of HO activity, carbon monoxide, and bilirubin. For example, in the isolated perfused kidney, ANG II exerts pressor effects as well as generates carbon monoxide via HO activity; inhibiting HO activity blocks the generation of carbon monoxide and exaggerates the pressor effect of ANG II (18). The exaggerated vasoconstrictive response when HO activity is inhibited may also reflect decreased kidney content of bilirubin. Bilirubin effectively scavenges superoxide anion generated by ANG II (17, 30), and the generation of superoxide anion is incriminated in the vasoconstrictive and other actions of ANG II. However, oxidative stress, as reflected by whole kidney content of lipid peroxidation products and protein carbonyl content, was not increased by ANG II, either in the presence or absence of SnMP. The role of oxidative stress in the renal hemodynamic effects of low-dose ANG II, in general, merits additional study and elucidation. It is possible that nonoxidant mechanisms may contribute to the hemodynamic effects of low-dose ANG II observed in the present study, as well as the exacerbatory effects of inhibiting HO activity. As regards the latter consideration, studies that examine the modulatory effects of carbon monoxide would be of interest.
In the present studies, inhibition of HO activity did not alter the hemodynamic profile in vehicle-treated rats. In prior studies, inhibition of HO activity decreases GFR and/or renal blood flow in vehicle-treated rats with intact kidneys and not concomitantly exposed to vasoactive agents (5, 33, 45). The basis for these differing effects is uncertain at the present time. We speculate that such differences may reflect the specific conditions employed during these studies, including the use of euvolemic conditions, the latter employed in the present study. During studies of glomerular and renal hemodynamics, fluid loss/shifts and alterations in intravascular volume incurred during anesthesia, laparotomy, and surgical preparation may lead to hemodynamic effects; these latter effects may be minimized by euvolemic conditions achieved by the administration of rat plasma or albumin. Euvolemic conditions, we speculate, may reduce the likelihood that pressor systems (such as the renin-angiotensin system) would be recruited during hemodynamic studies; in turn, euvolemic conditions may vitiate the need for offsetting vasorelaxant effects emanating from systems such as HO.
The regimen of administering ANG II employed in the present study did not induce HO-1 or HO-2 mRNA and did not increase HO activity. This absence of an inductive effect of ANG II on the HO system in these studies is notable for at least three reasons. First, it demonstrates that, unlike prior studies by us and others that employ relatively large amounts of ANG II (4, 11, 14), this enzyme system is not induced when ANG II is administered in lower and more clinically relevant amounts. Second, even when HO-1 is not induced and HO activity remains unaltered, HO activity confers a countervailing vasorelaxant effect in the kidney exposed to ANG II. These findings thus differ from prior observations in which inhibition of HO activity accentuated NG-nitro-l-arginine methyl ester (l-NAME)-induced reduction in renal blood flow as, in these prior studies, l-NAME induced HO activity (32, 33). The present findings thus represent a relatively uncommon, if not unique, instance in the HO field wherein the preservation of function of a stressed organ (by ANG II), but not that of the unstressed organ, requires the involvement of intact renal HO activity even when the imposed stress neither induces HO-1 nor increases HO activity (1, 2, 15, 24). Third, recent studies have demonstrated that the genetic deficiency of HO-2 does not accentuate the vasoconstrictive effects of ANG II on renal blood flow (38). These observations would suggest that HO-2, per se, does not exert a vasorelaxant effect in the kidney that offsets the vasoconstrictive effects of ANG II. Integrating these findings with the fact that SnMP inhibits HO activity arising from both HO isoforms, we suggest that it is the cumulative HO activity arising from both HO-1 and HO-2 that is functionally involved in mitigating the vasoconstrictive effects of ANG II.
In the ANG II-infused rat, the administration of SnMP led to a fall in blood pressure, a finding also observed in prior studies in which SnMP was administered to rats rendered hypertensive by l-NAME (33). A number of studies have examined the effect of modulating the HO system on models of systemic hypertension. For example, upregulation of human HO-1 by a retroviral approach reduces the hypertensive effect of ANG II (43). Similarly, systemic hypertension induced by ANG II is lessened when HO-1 is induced by cobalt protoporphyrin in vivo (41, 42), the latter not affecting the vascular reactivity ex vivo (37). In models of renovascular hypertension, induction of HO activity reduces and inhibition of HO activity increases, systemic blood pressure (6). However, a vasodepressor effect of HO activity has not been uniformly observed. Specifically, neither ANG II nor l-NAME elicits a higher blood pressure in HO-2−/− mice (38); in certain models of systemic hypertension, inhibition of HO activity reduces, rather than increases, systemic blood pressure (16, 40), and quite surprisingly, transgenic mice that overexpress HO-1 in smooth muscle cells exhibit an increase, and not a decrease, in systemic blood pressure (13). Thus divergent effects of HO activity on systemic blood pressure have been described.
Systemic blood pressure represents the product of cardiac output and peripheral vascular resistance, and thus the reduction in mean arterial pressure by SnMP in ANG II-treated rats may reflect either a fall in cardiac output or in peripheral vascular resistance, or a decrement in both hemodynamic indices. Our findings suggest that a reduction in peripheral vascular resistance may not be readily implicated in the reduction in systemic blood pressure exhibited by ANG II-treated rats when SnMP is administered, at least as judged from studies of vascular resistance/reactivity in different arterial segments. In aortic segments from ANG II-treated rats, SnMP exerted no discernible effects on vascular reactivity, thereby demonstrating that vasomotor tone in this segment in ANG II-treated rats appears impervious to SnMP. However, since this arterial segment is a conduit vessel, studies were also undertaken in resistor vessels, and in the mesenteric circulation, SnMP exerted no overt effects on vasorelaxant responses in the mesenteric resistor vessels from ANG II-treated rats. Finally, in the renal circulation, SnMP substantially and significantly increased vascular resistances. These findings would suggest that reduction in peripheral vascular resistance may not be readily implicated in the fall in blood pressure in ANG II-treated rats following the administration of SnMP; rather, a fall in cardiac output seems a more likely explanation for the reduction in systemic blood pressure observed in ANG II-treated rats attendant upon the administration of SnMP. Indeed, a number of studies have demonstrated that metalloporphyrin inhibitors of HO activity adversely affect cardiac function (10, 23, 39). Such HO inhibitors reduce the cardiac output in rats exposed to chronic hypoxia (10), prevent the rise in cardiac output induced by estrogen in a model of hemorrhage (39), and vitiate the improvement in ventricular function achieved by upregulation of HO-1 in a model of pressure overload (23). Indeed, HO activity protects against ANG II-induced apoptosis in cardiac myocytes (9). Since metalloporphyrin inhibitors of HO activity can adversely affect cardiac output and as we observed that SnMP either increased vascular resistances (kidney) or failed to augment vasorelaxant responses (mesenteric resistors), we suggest that the reduction in mean arterial pressure by SnMP in ANG II-treated rats reflects the involvement of HO activity in maintaining cardiac function. HO activity may thus be required to preserve the function of the ANG II-stressed myocardium as it is for the ANG II-stressed kidney.
SnMP, like other protoporphyrin inhibitors of HO activity, such as tin protoporphyrin and zinc protoporphyrin, is widely used in the study of HO. SnMP was specifically chosen in this study since the potent inhibitory effect on HO activity by SnMP has been confirmed in prior studies of renal hemodynamics that assessed a different pressor, namely, l-NAME. As is true for most, if not all, analyses based on the use of pharmacologic inhibitors of a target enzyme, specificity of action is always a concern. Thus the present findings need to be considered with the circumspection that SnMP may influence other vasoactive systems that may be germane to the renal and other hemodynamic effects of low-dose ANG II. In this regard, studies with specific HO knockouts would be of interest, and along these lines, important findings regarding the behavior of HO-2−/− mice in response to ANG II have quite recently been published (38). However, it should be noted that HO activity, regardless of its origin from either HO-1 or HO-2, exerts vasoactive effects. Genetic approaches that negate both HO-1 and HO-2 have not been described to date, and indeed, the issue of viability may vitiate the feasibility of such an approach. SnMP effectively inhibits HO activity arising from both isoforms and thus allows the evaluation of the net effect of combined HO activity; indeed, protoporphyrin-based HO inhibitors represent the only currently available experimental approach that effectively inhibits HO activity regardless of the HO isoform from which such activity emanates. We also suggest that, given the widespread usage of SnMP in examining the involvement of HO in pathophysiologic studies, analyses that explore the hemodynamic and other renal effects of SnMP would add to the understanding of the biologic effects and utility of these compounds. Finally, we wish to underscore that SnMP exerted a differential hemodynamic effect, substantially impairing the perfusion of the ANG II-stressed kidney, but leaving unaltered the hemodynamic profile of the control unstressed kidney.
Perspective and Significance
Our findings demonstrate that low-dose ANG II induces renal vasoconstriction in the absence of effects on GFR, renal blood flow, oxidative stress, reactivity of the extrarenal vasculature, proteinuria, and selected vasoactive and injury-related gene expression. Preservation of blood flow to the kidney exposed to low-dose ANG II necessitates intact endogenous renal HO activity, even when ANG II induces neither HO-1 mRNA, HO-2 mRNA, nor HO activity. Intact, endogenous HO activity may also be required to preserve cardiac output in the ANG II-stressed heart. Because polymorphisms in the HO-1 gene leading to reduced levels of HO activity are clinically recognized (8), patients with such polymorphisms may be particularly prone to impairment in renal hemodynamics in clinical conditions attended by increased ANG II levels and ineffective renal perfusion (e.g., congestive heart failure, liver disease, nephrosis, third-spacing conditions) wherein GFR is marginally maintained in the vasoconstricted kidney. Such vasoconstriction and the attendant lack of induction of HO activity may render the kidney vulnerable to any additional ischemic and nephrotoxic insults, a vulnerability underscored by the heightened risk for acute kidney injury in such prerenal states of ineffective renal perfusion (34, 44).
GRANTS
These studies were funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-47060 (to K. A. Nath and Z. S. Katusic) and DK-73401 (to L. A. Juncos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the technical expertise of Leslie Smith and the secretarial expertise of Tammy Engel in the preparation of this work.
REFERENCES
- 1.Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol 297: F1137–F1152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Aizawa T, Ishizaka N, Kurokawa K, Nagai R, Nakajima H, Taguchi J, Ohno M. Different effects of angiotensin II and catecholamine on renal cell apoptosis and proliferation in rats. Kidney Int 59: 645–653, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, Tang SS, Ingelfinger JR, Ohno M. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension 35: 800–806, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Arregui B, Lopez B, Garcia Salom M, Valero F, Navarro C, Fenoy FJ. Acute renal hemodynamic effects of dimanganese decacarbonyl and cobalt protoporphyrin. Kidney Int 65: 564–574, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Botros FT, Schwartzman ML, Stier CT, Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int 68: 2745–2755, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Saunders B, Trapp RG. Comparing three or more means. In: Basic and Clinical Biostatistics. Norwalk, CT: Appleton & Lange, 1990, p. 124–141 [Google Scholar]
- 8.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 37: 1097–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Foo RS, Siow RC, Brown MJ, Bennett MR. Heme oxygenase-1 gene transfer inhibits angiotensin II-mediated rat cardiac myocyte apoptosis but not hypertrophy. J Cell Physiol 209: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hartsfield CL, McMurtry IF, Ivy DD, Morris KG, Vidmar S, Rodman DM, Fagan KA. Cardioprotective and vasomotor effects of HO activity during acute and chronic hypoxia. Am J Physiol Heart Circ Physiol 287: H2009–H2015, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Haugen EN, Croatt AJ, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int 58: 144–152, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hostetter TH, Rosenberg ME, Ibrahim HN, Juknevicius I. Aldosterone in progressive renal disease. Semin Nephrol 21: 573–579, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Imai T, Morita T, Shindo T, Nagai R, Yazaki Y, Kurihara H, Suematsu M, Katayama S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ Res 89: 55–62, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka N, Aizawa T, Mori I, Taguchi J, Yazaki Y, Nagai R, Ohno M. Heme oxygenase-1 is upregulated in the rat heart in response to chronic administration of angiotensin II. Am J Physiol Heart Circ Physiol 279: H672–H678, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep 11: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Johnson FK, Johnson RA, Durante W, Jackson KE, Stevenson BK, Peyton KJ. Metabolic syndrome increases endogenous carbon monoxide production to promote hypertension and endothelial dysfunction in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 290: R601–R608, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kelsen S, Patel BJ, Parker LB, Vera T, Rimoldi JM, Gadepalli RS, Drummond HA, Stec DE. Heme oxygenase attenuates angiotensin II-mediated superoxide production in cultured mouse thick ascending loop of Henle cells. Am J Physiol Renal Physiol 295: F1158–F1165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Jiang H, Yang L, Quan S, Dinocca S, Rodriguez F, Abraham NG, Nasjletti A. Angiotensin II induces carbon monoxide production in the perfused kidney: relationship to protein kinase C activation. Am J Physiol Renal Physiol 287: F914–F920, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Liang M, Croatt AJ, Nath KA. Mechanisms underlying induction of heme oxygenase-1 by nitric oxide in renal tubular epithelial cells. Am J Physiol Renal Physiol 279: F728–F735, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Del Vecchio L, Cavalli A. Inhibition of the renin-angiotensin system in chronic kidney disease: a critical look to single and dual blockade. Nephron Clin Pract 113: c286–e293, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, d'Uscio LV, Eguchi D, Akiyama M, Smith LA, Katusic ZS. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. J Pharmacol Exp Ther 306: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Meyer TW. Why we block angiotensin II. Kidney Int 58: 458–459, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Mito S, Ozono R, Oshima T, Yano Y, Watari Y, Yamamoto Y, Brydun A, Igarashi K, Yoshizumi M. Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension 51: 1570–1577, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Nath KA, d'Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am J Physiol Heart Circ Physiol 293: H333–H342, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Nath KA, Salahudeen AK. Induction of renal growth and injury in the intact rat kidney by dietary deficiency of antioxidants. J Clin Invest 86: 1179–1192, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 38: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Ortiz MC, Sanabria E, Manriquez MC, Romero JC, Juncos LA. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II. Hypertension 37: 505–510, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Pelaez LI, Manriquez MC, Nath KA, Romero JC, Juncos LA. Low-dose angiotensin II enhances pressor responses without causing sustained hypertension. Hypertension 42: 798–801, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Pflueger A, Croatt AJ, Peterson TE, Smith LA, d'Uscio LV, Katusic ZS, Nath KA. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol 288: F552–F558, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez F, Lamon BD, Gong W, Kemp R, Nasjletti A. Nitric oxide synthesis inhibition promotes renal production of carbon monoxide. Hypertension 43: 347–351, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez F, Zhang F, Dinocca S, Nasjletti A. Nitric oxide synthesis influences the renal vascular response to heme oxygenase inhibition. Am J Physiol Renal Physiol 284: F1255–F1262, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ronco C, Chionh CY, Haapio M, Anavekar NS, House A, Bellomo R. The cardiorenal syndrome. Blood Purif 27: 114–126, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Saito K, Ishizaka N, Aizawa T, Sata M, Iso ON, Noiri E, Ohno M, Nagai R. Role of aberrant iron homeostasis in the upregulation of transforming growth factor-β1 in the kidney of angiotensin II-induced hypertensive rats. Hypertens Res 27: 599–607, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Stec DE, Vera T, McLemore GR, Jr, Kelsen S, Rimoldi JM, Gadepalli RS, Ryan MJ. Heme oxygenase-1 induction does not improve vascular relaxation in angiotensin II hypertensive mice. Am J Hypertens 21: 189–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stec DE, Vera T, Storm MV, McLemore GR, Jr, Ryan MJ. Blood pressure and renal blow flow responses in heme oxygenase-2 knockout mice. Am J Physiol Regul Integr Comp Physiol 297: R1822–R1828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szalay L, Shimizu T, Schwacha MG, Choudhry MA, Rue LW, 3rd, Bland KI, Chaudry IH. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol 289: H92–H98, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Teran FJ, Johnson RA, Stevenson BK, Peyton KJ, Jackson KE, Appleton SD, Durante W, Johnson FK. Heme oxygenase-derived carbon monoxide promotes arteriolar endothelial dysfunction and contributes to salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 288: R615–R622, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Vera T, Kelsen S, Stec DE. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension 52: 660–665, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol 292: R1472–R1478, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension 43: 1221–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689–700, 1984 [DOI] [PubMed] [Google Scholar]
- 45.Zou AP, Billington H, Su N, Cowley AW., Jr Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension 35: 342–347, 2000 [DOI] [PubMed] [Google Scholar]