Abstract
We have shown that electroacupuncture (EA) inhibits sympathoexcitatory rostral ventrolateral medulla (rVLM) neurons and reflex responses following activation of a long-loop pathway in the arcuate nucleus and ventrolateral periaqueductal gray (vlPAG). Additionally, EA at P 5–6 acupoints (overlying the median nerve) activates serotonin-containing neurons in the nucleus raphé pallidus (NRP), which, in turn, inhibit rVLM neurons. Although direct projections from the vlPAG to the rVLM exist, it is uncertain whether an indirect pathway through the NRP serves an important role in vlPAG-rVLM cardiovascular modulation. Therefore, the splanchnic nerve (SN) was stimulated to induce cardiovascular sympathoexcitatory reflexes, and EA was applied at P 5–6 acupoints in α-chloralose-anesthetized cats. A single-barreled recording electrode was inserted into the NRP or rVLM. Microinjection of dl-homocysteic acid (DLH) into the vlPAG increased the NRP neuronal response to SN stimulation (5 ± 1 to 12 ± 2 spikes/30 stim). Likewise, EA at P 5–6 for 30 min increased the NRP response to SN stimulation (3 ± 1 to 10 ± 2 spikes/30 stim), an effect that could be blocked by microinjection of kynurenic acid (KYN) into the caudal vlPAG. Furthermore, the reflex increase in blood pressure induced by application of bradykinin to the gallbladder and the rVLM cardiovascular presympathetic neuronal response to SN stimulation was inhibited by injection of DLH into the vlPAG, a response that was reversed by injection of KYN into the NRP. These results indicate that EA activates the vlPAG, which excites the NRP to, in turn, inhibit rVLM presympathetic neurons and reflex cardiovascular sympathoexcitatory responses.
Keywords: kynurenic acid, dl-homocysteic acid, splanchnic nerve, bradykinin, gallbladder
we have demonstrated that electroacupuncture (EA) at acupoints P 5–6 (overlying the median nerve) inhibits the reflex increase of blood pressure (BP) and cardiovascular presympathetic neurons in the rostral ventrolateral medulla (rVLM) (10, 21). This somatic inhibition depends upon activation of a long-loop pathway in the hypothalamus and midbrain to inhibit rVLM neurons (14, 15, 23–25). Neurons in the ventrolateral periaqueductal gray (vlPAG) directly project to the rVLM (6, 9, 16, 17, 26). Therefore, the vlPAG might directly inhibit the rVLM neurons. However, the vlPAG also sends dense excitatory projections to the midline medullary nuclei, including the nuclei raphé magnus (NRM) (2), raphé obscurus (NRO), and raphé pallidus (NRP) (9). We have shown recently that the NRP contains a large number of serotonergic and enkephalinergic neurons that are activated by EA (8). Furthermore, stimulation of cell bodies in the NRP decreases sympathoexcitatory cardiovascular responses through a serotonergic mechanism involving 5-HT1A receptors in the rVLM (18). It is unknown, however, if the vlPAG influences the rVLM through the NRP during EA. In light of our previous studies suggesting that the NRP plays a prominent role in EA-cardiovascular modulation, we hypothesized that an indirect pathway from the vlPAG through the NRP plays a major role in EA-related sympathoinhibition in the rVLM.
MATERIALS AND METHODS
Surgical Preparation
The experimental preparations and protocols for this study were reviewed and approved by the Animal Care and Use Committee of the University of California, Irvine CA. The studies conformed to the American Physiological Society's guidelines and principles for research involving animals. Adult cats of either sex (2.4–5.5 kg) were anesthetized by injection of ketamine (40 mg/kg sc) followed by α-chloralose (50 mg/kg iv). Additional injections of α-chloralose (5 mg/kg iv) were given to maintain an adequate depth of anesthesia, as assessed by the lack of a response (including pupil dilatation) to noxious toe pinch, a respiratory pattern that followed the ventilator (i.e., not overbreathing), and a stable blood pressure and heart rate. The magnitudes of responses to splanchnic nerve or gallbladder stimulation and EA at P 5–6 acupoints (P refers to the pericardium meridian along the forelimb over the median nerve, see protocols below) were unchanged by supplemental anesthesia. The trachea was intubated, and respiration was maintained artificially (model 66, Harvard Apparatus, South Natick, MA). Gallamine triethiodide (4 mg/kg) was administered intravenously before recording neuronal activity to avoid muscle movement during stimulation of somatic nerves. Following paralysis, supplemental α-chloralose was administered on a regular basis. Arterial blood gases and pH were measured periodically in all animals with a blood gas analyzer (model ABL3; Radiometer, Copenhagen, Denmark). Arterial Po2 and Pco2 were kept within normal limits (CO2 30–35 mmHg; Po2 > 100 mmHg) by enriching the inspired O2 supply and adjusting the ventilation rate or volume. Arterial pH was maintained between 7.34 and 7.43 and corrected, as necessary, by administering 8% sodium bicarbonate. Body temperature was monitored with a rectal probe connected to a thermometer (model 44TD, Yellow Springs Instrument, Yellow Springs, OH) and was maintained at a range of 36–38°C by a water-heating pad and a heating lamp.
The left femoral vein was cannulated for administration of drugs and fluids. Systemic arterial blood pressure was monitored by a pressure transducer (model 1290, Hewlett-Packard, Waltham, MA) attached to a cannula inserted into the left femoral artery.
A laparotomy provided exposure of the gallbladder and isolation of the splanchnic nerve. The splanchnic nerve was placed on a bipolar stimulating electrode connected to an isolation unit and a stimulator (Grass, model S88). The epoxy glue, vinyl polysiloxane impression material, (VPS Pentron, Wallingford, CT) was used to isolate the electrode and to hold the intact nerve in place. In a few experiments near the end of the experiment, the splanchnic nerve was tied at the distal end, stimulation of its central part showed the same result as with stimulation of the intact nerve. The abdominal wall was closed with clips to maintain moisture in the abdominal cavity and to prevent heat loss. The abdominal cavity was reopened only when filter paper, dipped in bradykinin (BK; 10 μg/ml), was applied to the serosal surface of the gallbladder. Thereafter, the neural axis of the cat was stabilized with a stereotaxic head frame (Kopf). A dorsal craniotomy was performed to expose the midbrain vlPAG, medullary rVLM (bilaterally), and NRP (midline) for microinjection of agonists, antagonists, or vehicle controls and for recording extracellular activity.
An incision was made in the left flank region of the cat, and the retroperitoneal renal sympathetic nerve was exposed for renal sympathetic nerve recordings. A dissecting microscope (Zeiss) was used to isolate a branch of the renal nerve from connective tissue. The nerve was covered with warm mineral oil and placed across one pole of the recording electrode, while the other pole of the electrode was grounded with a cotton thread to the animal.
Stimulation, Recording, and Microinjection Methods
The splanchnic nerve was stimulated with 0.2–0.4 mA, 0.5-ms pulse duration at 2 Hz with a Grass stimulator (model S88K) at a level sufficient to induce a reflex increase in blood pressure. EA was applied bilaterally using pulses of 1–4 mA, 0.5-ms duration, and 2 Hz at the P 5–6 acupoints (pericardium meridian, also designated as PC) for 30 min. We have demonstrated previously that EA at these locations (pericardium meridian, Neiguan and Jianshi, referring to P 5 and P 6, respectively, located 1.5–2.0 and 2.5–3.0 cm above the wrist between the ligaments of the flexor carpi radialis and the palmaris longus) stimulates the median nerves that project to the spinal segments between C8 and T1 and modulates sympathoexcitatory cardiovascular responses (5, 8, 11, 13–15, 20, 23, 28, 29).
Using the atlas of Fikova and Marsala (7) as a guide, we positioned stainless-steel tubes (guide tubes 0.8 mm and injection tubes 0.4 mm in diameter and 1 mm longer than the guide tube) perpendicularly to the cortex (0.7–1.0 mm lateral on either side of the midline, 0–2 mm rostral to the tentorium), then lowered 22 mm from the dorsal surface of the midbrain to access the vlPAG. In some animals, guide tubes were positioned using a 77° rostral → caudal angle from the dorsal surface, 5 mm anterior to the tentorium to approach the caudal vlPAG at a depth of 23 mm (14). For microinjection into the NRP stainless-steel tubes were positioned perpendicularly to the surface of the 4th ventricle of the medulla (midline, 3–4 mm rostral to the obex and 5 mm from the dorsal surface). Additional experiments that examined rVLM neuronal responses following microinjection into the NRP used an injection tube inserted at an angle of 37° caudal → rostral from the vertical axis to a depth of 7.7 mm to reach the pallidus. Preliminary assessment of the location of the NRP was identified by injecting 50 nl of 4 nM dl-homocysteic acid (DLH) to evoke a small, but reproducible, transient (1–2 min) decrease in blood pressure of 5 to 10 mmHg. Single-unit extracellular activity in the NRP and rVLM was recorded with a single-barrel glass pipette containing 0.5 M sodium acetate and 2% Chicago sky blue (Sigma Chemical, St. Louis, MO). To record neuronal activity in the rVLM, a glass pipette was positioned perpendicularly to the surface of the 4th ventricle of the medulla (3 mm right or left of the midline, 3 mm rostral to the obex) and was lowered 5.5 mm from the surface to reach the rVLM. Evoked activities of NRP and rVLM neurons were recorded during stimulation of the splanchnic nerve and P 5–6 acupoints. All recorded neurons were identified by observing evoked activity during both splanchnic nerve and P 5–6 acupoint stimulation, thus documenting that they received visceral and somatic convergent input.
We used peristimulus time histogram analysis to assess evoked responses to stimulation of the splanchnic and median nerves and to evaluate the influence of EA on NRP and rVLM neurons. Action potentials were amplified with a preamplifier (Neuroprobe Amplifier Model 1600, A-M Systems) attached to a Nerve Traffic Analysis System 662C-3 (Bioengineering, College of Medicine, University of Iowa), then were filtered (3–10 kHz) and monitored with an oscilloscope (Tektronix 2201). Action potentials, blood pressure, and heart rate were digitized and analyzed online with a Pentium IV computer and a four-channel data acquisition system (Shanghai Medical College of Fudan University, China). A subgroup of rVLM neurons also was characterized by evaluating the relationship between rVLM discharge and renal sympathetic nerve activity to determine whether they could be classified as presympathetic in function. To record renal activity, a recording electrode was attached to a high-impedance probe (model P511k). The signal was amplified and processed through an audio amplifier, monitored with the oscilloscope, then processed with the computer for on- and offline analyses through the four-channel data acquisition system. The electrical noise level in the neural recordings was determined by crushing the nerves at the end of the experiments. A window discriminator was set with a threshold just above the noise level so that only renal nerve discharge signals were counted. The relationship between neural activity, renal sympathetic nerve discharge, and blood pressure was assessed with time domain analysis using arterial pulse or spike-triggered averaging and with frequency domain analysis using coherence (1, 14, 21, 22). The time domain analysis involved either arterial pulse-triggered or spike-triggered averaging, while frequency domain analysis compared autospectra of rVLM activity with either BP or renal nerve activity using a fast Fourier transform (FFT) algorithm (18, 21). A threshold was set at the systolic phase of the arterial pulse and used spike height discrimination and waveform recognition to sort action potentials during the evaluation period of 300 s. Averages of the arterial pulse and histograms of sympathetic discharge and rVLM neuronal activity were constructed as in our previous studies (14, 15, 23).
As described previously (18, 21, 23), we recorded data using a sampling rate of 10,000 Hz. The reconstructed data used every 10th sample, including assessment of the mean and peak amplitudes and the maximum and minimum slopes of the original spike to preserve the action potentials. We sorted and identified spikes with a window discriminator to construct histograms prior to coherence analysis. The number of data sections (15–20 each lasting for 12.8 s) was chosen to determine the average histogram. Autospectra of rVLM discharge and arterial blood pressure or renal sympathetic nerve activity were generated with the FFT. Then, coherence was generated with seven overlapping windows, each with a length of 12.8 s, consisting of 256 bins, with bin widths of 50 ms. The autospectral analysis was adopted from Shin et. al. (19) using contiguous segments of 256 beats with 50% overlap between the segments. The frequency resolution was 1/12 s or 0.08 Hz. Coherence values of ≥0.5 reflected a statistically significant relationship between rVLM spikes and arterial blood pressure. The coherence function provided a measure of the strength of linear correlation between rVLM neuronal activity and blood pressure or renal nerve activity at each frequency. (14, 18, 20).
To further categorize cardiovascular sympathetic neurons in the rVLM, responses of rVLM neurons were assessed by stimulating or unloading the baroreceptors following intravenous injection of 10 μg phenylephrine or 2.5 mg nitroglycerin, respectively.
Chemicals for microinjection.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). The glutamate agonist DLH (4 nM) and the glutamatergic antagonist kynurenic acid (KYN; 100 nM) were dissolved in normal saline. We microinjected 50 nl of DLH, KYN, or saline into the vlPAG or NRP.
Verification of injection and recording sites.
Animals were euthanized with α-chloralose followed by intravenous saturated KCl at the end of each experiment. Recording sites then were marked by microinjection (50 nl) of 2% Chicago blue dye. The hypothalamus, midbrain, and medulla were removed and fixed in 10% formalin for 4–7 days. Frozen serial 60-μm brain sections were cut with a freezing microtome (Leica CM 1850). Slices were stained with neutral red and examined with a microscope (Nikon Eclipse 6400) to identify recording and microinjection sites. These areas were reconstructed from the dye spots with New Bitmap Image plotted on coronal sections that were separated by 1–2 mm, with respect to the auditory line. Composite coronal caudal and rostral sections were composed from multiple tissue sections. Sections were scanned and traced with Corel suite software. Nuclei were superimposed with nuclear structures identified with the aid of the atlas of Fikova and Marsala for the midbrain (7) and Berman's atlas (3) for the medulla.
Experimental Protocols
Hemodynamic study.
Pledgets of filter paper (1 cm2) soaked in a solution of bradykinin (10 μg/ml) applied to the gallbladder in seven cats induced consistent reflex increases in blood pressure in response to repetitive gallbladder stimulation. Recovery periods of at least 10 min were provided between consecutive stimuli to prevent tachyphylaxis. In nine other animals, bradykinin was applied eight times to the gallbladder to induce repetitive increases in blood pressure over a period of 80 min. After the first two consecutive applications of bradykinin, 50 nl DLH (4 nM) was microinjected in the vlPAG. KYN (100 nM, 50 nl, five cats) or saline (50 nl, four other cats) was administered into the NRP. Thus, these protocols evaluated the magnitude of the gallbladder blood pressure reflex during excitation of the vlPAG following glutamate receptor blockade of the cell bodies in the NRP.
Electrophysiological study.
All NRP and rVLM neurons evaluated in this study received convergent input from the splanchnic and median nerves, as well as the baroreceptors. Each neuron was identified for sympathoexcitatory cardiovascular rhythmicity over a period of 5 min by noting their close correlation with renal sympathetic nerve activity and BP using pulse or spike-triggered averaging, as well as coherence analysis (14, 20, 23). The neurons also were examined for their responsiveness to cardiovascular stimulation by altering baroreceptor input following administration of nitroglycerin or phenylephrine.
To examine the effect of vlPAG excitation on NRP neurons and the electrophysiological relationship between the vlPAG and NRP, microinjection and recording electrodes were positioned in both nuclei. During repeated stimulation of the splanchnic nerve, DLH (n = 5) or normal saline (NS; n = 6) was microinjected into the vlPAG 2 min before the third splanchnic stimulation. Evoked activity was assessed following stimulation of the vlPAG.
EA was applied at P 5–6 for 30 min during assessment of the NRP neuronal responses to splanchnic nerve stimulation. After two consecutive stimulations of the splanchnic nerve, NS (n = 5) or KYN (n = 5) was microinjected into the caudal vlPAG just before the onset of EA. Before, during, and after the termination of EA, the NRP neuronal response to splanchnic nerve stimulation was recorded every 10 min for 60 min.
To examine the role of the NRP in the vlPAG inhibition of rVLM neurons, repeated splanchnic nerve stimulation was applied four times every 10 min. After two control recordings of the rVLM neuronal response, DLH was injected into vlPAG to inhibit the rVLM. Then, either KYN (n = 5) or NS (n = 4) was injected into NRP to examine the role of this nucleus in the vlPAG-induced rVLM inhibition.
Statistical Analysis
Data are presented as means ± SE. The assumption of normal data distribution was evaluated with the Kolmogorov-Smirnov test. Blood pressure responses to gallbladder stimulation (ΔMAP) and neural activity (imp/30 stimuli) in response to splanchnic nerve stimulation before, during, and after EA, and after delivery of saline, DLH, or KYN were assessed using a one-way repeated-measures ANOVA, followed by the Student-Newman-Keuls test post hoc. We selected the control immediately preceding the onset of EA as the control against which subsequent values were compared. These tests represent a pairwise multiple-comparisons procedure. We also used a one-way ANOVA to compare the data between different groups following injection of DLH, KYN, or NS. We used SigmaStat and SigmaPlot software (Jandel Scientific, San Rafael, CA) for statistical analysis and graphing. The 0.05 probability level was used to discern statistically significant differences.
RESULTS
Hemodynamic Study
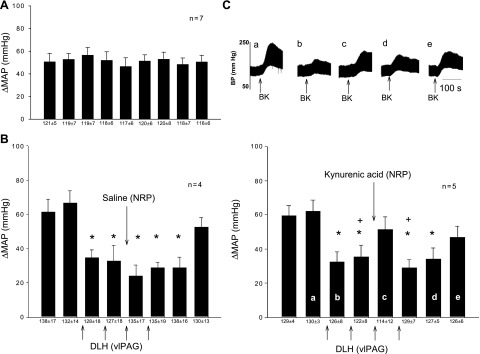
BK applied to the gallbladder every 10–15 min induced consistent increases in BP in seven animals (Fig. 1A). Following two control stimulations of the gallbladder, DLH injected into the vlPAG in five other animals reduced the reflex response from 62 ± 6 to 32 ± 6 mmHg (P < 0.05). This inhibition was reversed transiently to 51 ± 7 mmHg immediately following microinjection of KYN into the NRP (Fig. 1C); the same volume of NS in the NRP did not influence the vlPAG-induced inhibition (Fig. 1B).
Fig. 1.
Role of nucleus raphé pallidus (NRP) in ventrolateral periaqueductal gray (vlPAG) inhibition of reflex-induced increases in blood pressure (BP). A: control mean arterial pressure (MAP) response to application of repeated bradykinin (BK) to the gallbladder. B: microinjection of dl-homocysteic acid (DLH) (4 nM, 50 nl) into the vlPAG inhibited the reflex increase in BP, a response that was not altered by injection of the saline vehicle into the NRP. C: microinjection of kynurenic acid (KYN; 100 nM, 50 nl) to block glutamate receptors in the NRP reversed inhibition of the BP response induced by injection of DLH into the vlPAG. Upper tracings: original recording of BP. Bottom: times of tracings a–e are shown. *P < 0.05 compared with control 2; +P < 0.05 compared with the response after microinjection of KYN into NRP. Between KYN and saline groups, the responses were significantly different after microinjections (P < 0.05). Numbers below bars show baseline blood pressures before reflex stimulation. These data show that inhibition of the reflex induced increase in BP could be reversed by inactivation of the NRP.
Electrophysiological Study
Excitatory vlPAG-NRP projection.
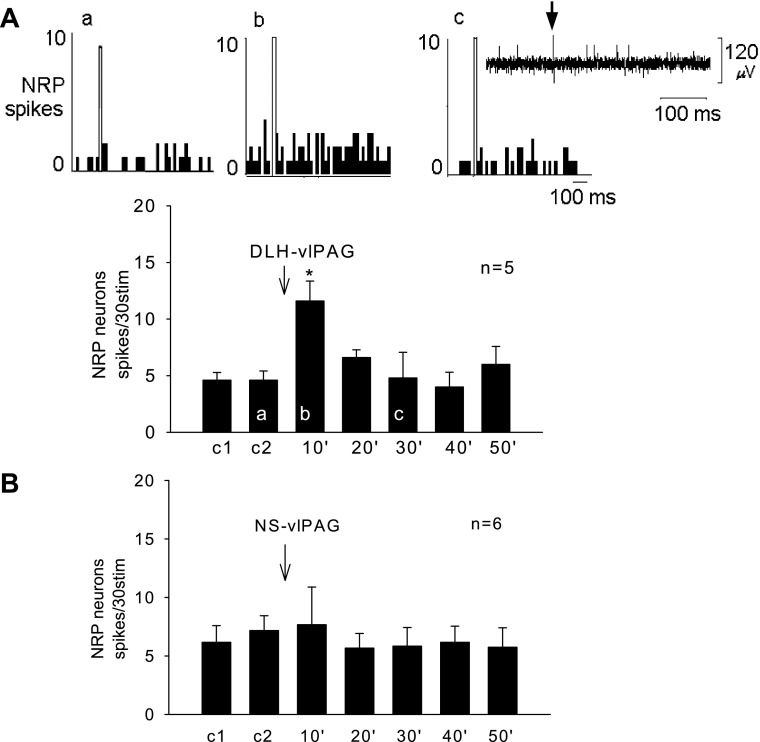
The spontaneous firing rate in five NRP neurons averaged 2 ± 1 spikes/s. Stimulation of the vlPAG with DLH did not change the spontaneous firing rate (4 ± 2 spikes/s, P > 0.05). Splanchnic nerve stimulation (stim) evoked a response of 5 ± 1 spikes/30 stim. Following stimulation of the vlPAG, the NRP response to splanchnic nerve stimulation was increased to 12 ± 2 spikes/30 stim (P < 0.05) (Fig. 2A); conversely, saline in the vlPAG did not affect the evoked NRP response (Fig. 2B).
Fig. 2.
A: microinjection of DLH into the vlPAG facilitated the NRP neuronal response to splanchnic nerve stimulation. Top: peristimulus time histograms demonstrating NRP neuronal responses to repeated splanchnic stimulation before (a), during (b), and after (c) vlPAG activation. Open bars in top panels are stimulation artifacts. Letters a, b, and c displayed in the bar histogram represent the times of recordings. A, c, inset: original recording of an NRP neuron that responded to splanchnic nerve stimulation with the stimulus artifact (arrow) displayed. B: microinjection of normal saline (NS) into the vlPAG did not influence the NRP neuronal response. Data show that DLH into the vlPAG facilitated the NRP response. *P < 0.05 compared with control 2 (c2). Between the DLH and NS groups, the responses were significantly different after microinjections (P < 0.05).
EA action on NRP neuronal activity.
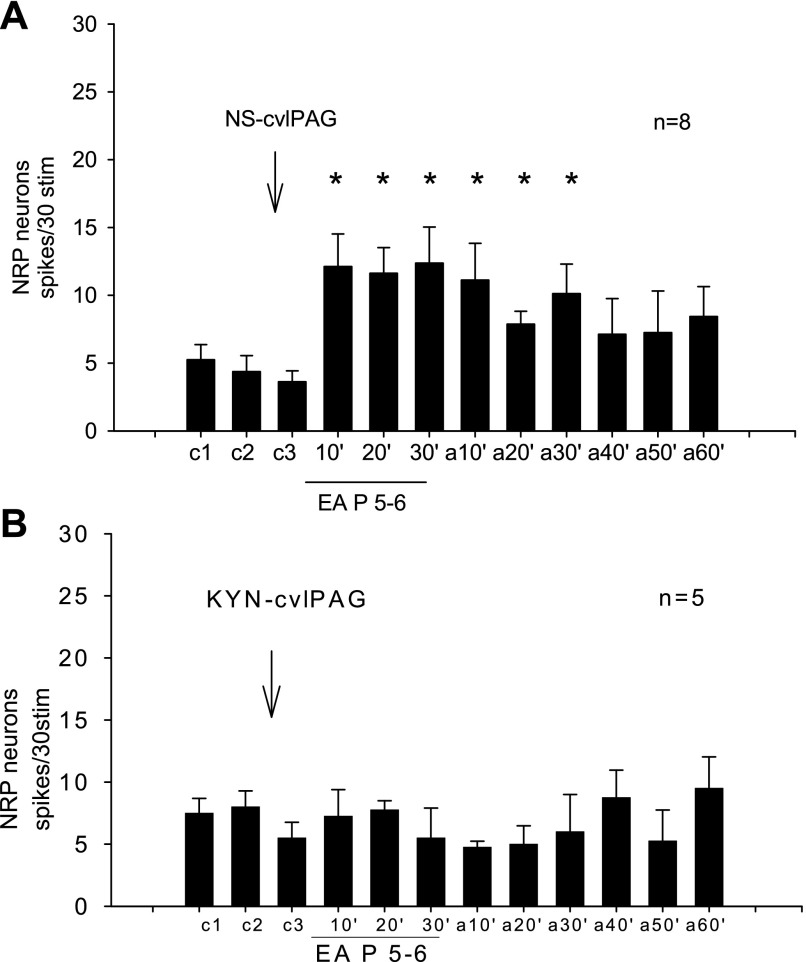
The NRP neuronal responses to repeated splanchnic nerve stimulation were used to examine EA influence. Normal saline injected into the vlPAG after two repeated stimulations of the splanchnic nerve did not change the neuronal response (n = 5, Fig. 3A). Stimulation at P 5–6 for 30 min increased the evoked response of NRP neurons from 3 ± 1 to 10 ± 2 spikes/30 stim (P < 0.05) (Fig. 3A). The facilitation continued after termination of EA and thereafter slowly returned toward the original pre-EA level (Fig. 3A). Glutmatergic blockade in the vlPAG with KYN in five animals prevented the EA-related facilitation of the splanchnic nerve-evoked NRP response (Fig. 3B).
Fig. 3.
A: Electroacupuncture (EA) facilitated the NRP neuronal response to repeated splanchnic nerve stimulation during the control period, EA at P 5–6 (P refers to the pericardium meridian along the forelimb over the median nerve) acupoints and after acupuncture. *P < 0.05 compared with control (c3). B: microinjection of KYN into the vlPAG blocked EA facilitation of the NRP response. Between groups EA, 10′ 20′ 30′ and after EA10 (aEA10′) in A and B were significantly different (P < 0.05). These data show that EA excitation of NRP neurons requires a functioning vlPAG.
Identification of rVLM neurons.
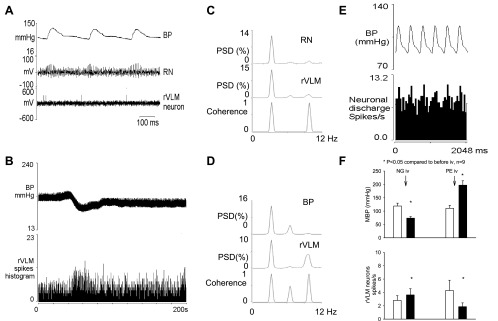
All nine recorded rVLM neurons received convergent input from splanchnic afferents, median nerves (underneath P 5–6 acupoints) and baroreceptors. We observed strong coherence (0.9 ± 0.02) between rVLM discharge and BP. A similar strong correlation was found to exist between rVLM activity and renal nerve discharge (0.9 ± 0.04) in a subset of three animals (Fig. 4, C and D). Pulse-triggered averaging showed that all of the neurons were highly correlated with pulse (Fig. 4E). Furthermore, intravenous injection of nitroglycerin decreased the BP from 119 ± 11 to 74 ± 6 mmHg (P < 0.05) and increased rVLM activity (P < 0.05). On the other hand, phenylephrine, increased BP from 111 ± 11 to 197 ± 16 mmHg (P < 0.05) and decreased the rVLM discharge (P < 0.05, Fig. 4, B and F).
Fig. 4.
Methods used to classify rostral ventrolateral medulla (rVLM) neurons. A: original recordings of BP, renal nerve activity (RNA), and rVLM neuronal discharge. B: Recording of BP and an rVLM neuron after intravenous nitroglycerin (NG). C: coherence (0.88) between renal nerve and rVLM neuronal discharge. D: coherence (0.89) between BP and rVLM neuronal discharge. E: pulse triggered averaging showing a close relationship between the rVLM neuron and BP. F: Bar histograms showing rVLM activity and BP responses to intravenous phenylephrine (PE) and nitroglycerin. Open bars denote controls, and solid bars denote after administration of intravenous NG or PE. PSD, power spectral density. These data show that this is a sympathoexcitatory presympathetic cardiovascular neuron that receives input from the baroreceptors.
vlPAG inhibition of rVLM activity.
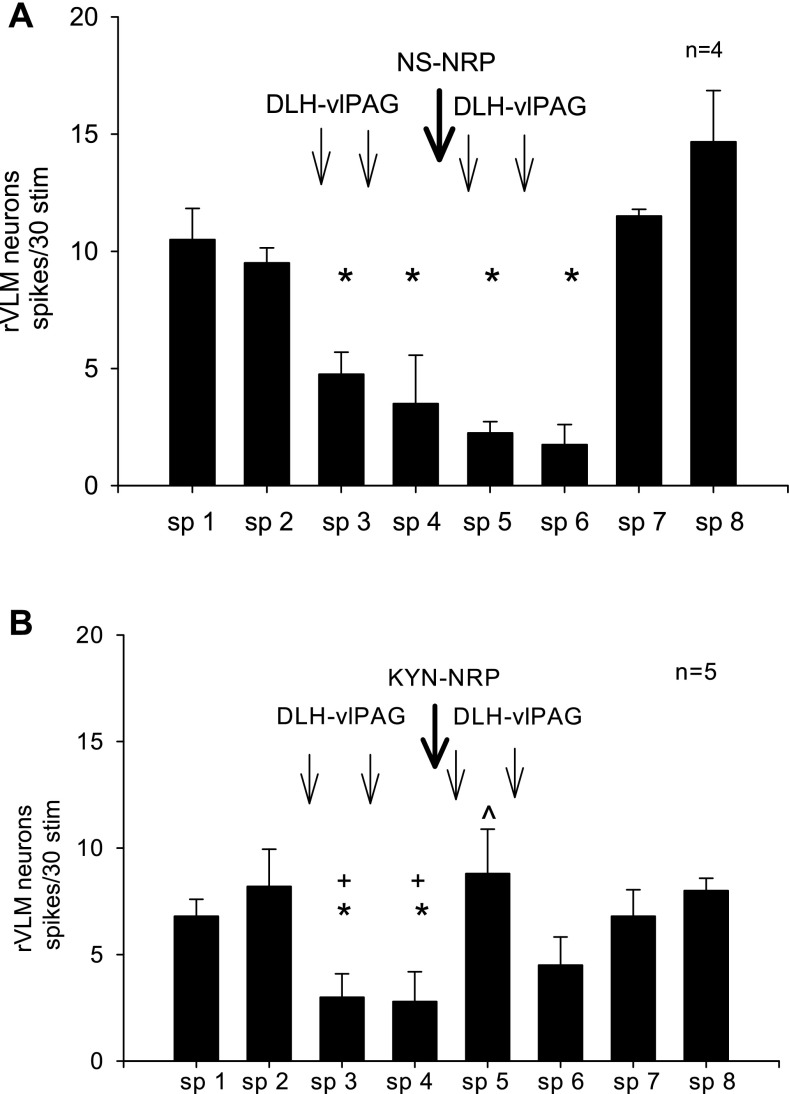
Spontaneous discharge activity of the rVLM neurons averaged 5 ± 2 spikes/s. Splanchnic nerve evoked rVLM activity was decreased from 11 ± 1 to 2 ± 1 spikes/30 stim after microinjection of DLH into the vlPAG (P < 0.05). Injection of NS into the NRP did not influence vlPAG inhibition of the rVLM neuronal response (n = 4, Fig. 5A). Conversely, KYN microinjected into the NRP reversed the vlPAG-induced rVLM inhibition to 9 ± 2 spikes/30 stim (n = 5, Fig. 5B).
Fig. 5.
A: DLH injected into the vlPAG repeatedly inhibited the rVLM neuronal response to splanchnic (sp) stimulation. B: microinjection of KYN into the NRP blocked the vlPAG-induced inhibition of the rVLM response. *P < 0.05 compared with sp 2, the second control. +P < 0.05 compared with sp 5. Between the NS and KYN treated groups the responses after microinjections at sp 5 were significantly different (P < 0.05). These data suggest that inhibition of rVLM neurons by excitation of vlPAG neurons is mediated by the NRP.
Anatomical locations of microinjection and recording sites.
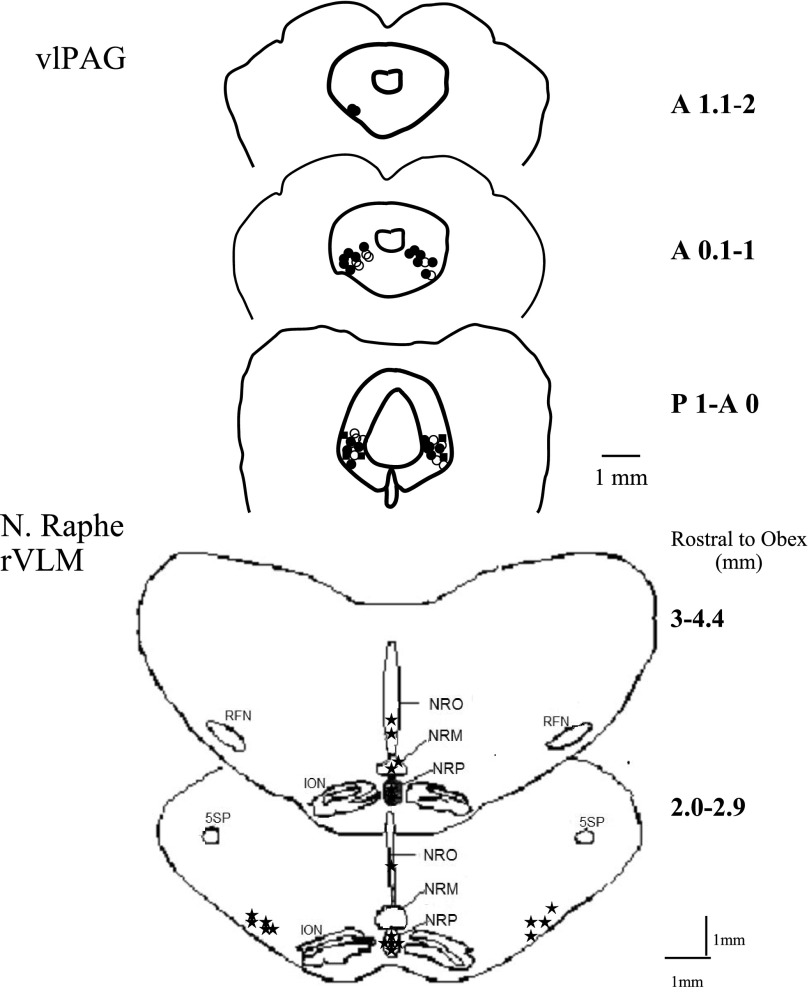
Microinjection sites in the rostral and caudal vlPAG and NRP are shown in Fig. 6. Fourteen neurons recorded in the NRP (3–4.4 mm rostral to obex, 0–1 mm lateral to the midline, and 4.5–5.5 mm depth from the surface of 4th ventricle) were excited by injection of DLH into the vlPAG. Extracellular recordings also were examined in three NRO neurons (2.0–2.9 mm rostral to obex, 0–1 mm lateral to midline, and 3 mm depth) and two neurons in NRM (3.6 mm rostral to obex, on the midline, 3–4 mm depth), all of which showed similar results as the 14 cells in the NRP. However, only the data from the NRP were used in the statistical calculations in this study. Eight neurons were recorded in the rVLM (3–4.6 mm rostral to obex, 3–4 mm lateral to midline, and 5–6 mm depth). One neuron located outside the NRP (2.8 mm rostral to obex, on the midline, 3-mm depth) did not demonstrate facilitation of splanchnic nerve-evoked activity following stimulation of the vlPAG with DLH.
Fig. 6.
Composite map of brain sections showing locations of microinjection and recording sites in the vlPAG, NRP, and rVLM. ●, DLH, n = 23 vlPAG; ○ NS, n = 15 vlPAG, n = 8 NRP; ■, KYN, n = 5 vlPAG, n = 10 NRP; *, neuronal recording, n = 21 NRP, n = 3 nucleus raphé obscurus (NRO), n = 2 nucleus raphé magnus (NRM), n = 9 rVLM. Numbers on right side of the vlPAG slices are millimeters anterior (A) or posterior (P) to the tentorium. Numbers on the right side of the medulla are millimeters rostral to the obex.
DISCUSSION
The present study shows that the vlPAG provides an excitatory projection to the NRP that is involved in the long-loop EA P 5–6-related inhibition of sympathoexcitatory cardiovascular reflex responses. Previous studies have shown that raphé pallidus neurons are activated during EA and that they project to presympathetic rVLM neurons to inhibit excitatory blood pressure responses (8, 18). The current hemodynamic study demonstrates that vlPAG inhibition of the cardiovascular pressor response could be reversed by injection of KYN into the NRP. We also provide electrophysiological evidence showing that stimulation of the median nerves for 30 min facilitates splanchnic nerve-evoked activity in the NRP, which is reversed by blockade of glutamate receptors in the vlPAG (Fig. 3). Some neurons in the rVLM can be classified as sympathoexcitatory cardiovascular neurons, and a subset was shown to be presympathetic in function. In aggregate, the NRP and glutamate systems in the vlPAG and the NRP are essential for vlPAG inhibition of sympathoexcitatory reflex responses during EA.
We have shown in the current study that an excitatory projection from the vlPAG to the NRP is important in the EA-cardiovascular modulatory response. Electrophysiological recordings demonstrated that chemical excitation of vlPAG neurons facilitate the NRP neuronal response to splanchnic nerve stimulation. We also showed that EA facilitates the NRP response to splanchnic nerve stimulation. The facilitated NRP response could be blocked with KYN microinjected into the caudal vlPAG. Thus, the glutamate system in the caudal vlPAG and excitatory projections from the caudal vlPAG to the NRP contribute to the EA response.
We have shown previously that the influence of EA on sympathoexcitatory reflexes is mediated through a long-loop pathway, involving the hypothalamic arcuate nucleus that projects to and receives information from the vlPAG in the midbrain (12). The vlPAG, in turn, inhibits neuronal activity in the rVLM to ultimately modulate the cardiovascular responses during EA (23). We also have reported that the vlPAG, in particular, the caudal vlPAG is important in the arcuate-vlPAG-rVLM long-loop modulatory pathway in EA cardiovascular inhibition (14). The present study expands our understanding of the long-loop pathway and confirms the importance of the caudal vlPAG. Furthermore, since excitation of the caudal vlPAG inhibited rVLM cardiovascular sympathoexcitatory neurons and since this inhibitory response was blocked with microinjection of KYN into the NRP, this midline medullary nucleus through a glutamatergic mechanism could play an important role in EA-related modulation of cardiovascular sympathoexcitatory reflex activation. In this regard, the NRP may simply serve as a modulatory input (e.g., presynaptic facilitatory role) to those vlPAG neurons projecting to the rVLM. The vlPAG to rVLM connection could simply require the integrity of the NRP to function. However, since our previous study has shown that there is an inhibitory serotonergic connection between the NRP and the rVLM, the indirect connection from the vlPAG to the rVLM through the NRP very likely is an important part of the long-loop pathway that modulates bulbospinal presympathetic outflow.
Zhang et al. (27) suggested that the vlPAG inhibits the rVLM through a pathway that involves the NRO. The present study confirmed that neurons in the NRO and NRM, display similar characteristics as the neurons in the NRP. Thus, these raphé nuclei may share similar functions. However, Guo et al. (8) have shown previously that a much greater number of NRP neurons express c-Fos in response to EA stimulation relative to both the obscurus and the magnus. Hence, in the present study we focused on the pallidus region of the nucleus raphé.
Several anatomical studies have suggested that the vlPAG provides direct projections to the rVLM (6, 9, 16, 17, 26). Although we have shown an important role for an indirect pathway from the vlPAG to the rVLM in the EA response, we cannot rule out the possibility that a direct pathway also is involved, which may have lesser inhibitory effect. Such a possibility deserves further investigation.
We have identified rVLM neurons that were correlated significantly with BP and renal sympathetic activity. The neurons not only received convergent input from splanchnic and median nerves and baroreceptor afferents but also were influenced by the NRP. We have shown in our previous studies that premotor rVLM neurons with the specific convergent and cardiovascular characteristics are modified by 30 min of low-frequency, low-current somatic stimulation (21–23). We also have shown strong correlations between rVLM and renal sympathetic activity, thus allowing us to classify a subset of these neurons as presympathetic in function (14, 18). During EA, the NRP exerts its action on presympathetic rVLM neurons through serotonin1A receptors (18). The rVLM neurons that are inhibited by serotonergic projections from the NRP during EA likely are premotor sympathoexcitatory cardiovascular neurons that directly modify sympathetic outflow since as many as 85% of neurons in the rVLM that receive input during EA stimulation can be antidromically driven from the intermediolateral column of the thoracic spinal cord (21). Thus, although we classified only a small subset of neurons that received vlPAG-NRP input as presympathetic, it is likely that many rVLM neurons that receive NRP input and which are modulated by EA either directly or indirectly regulate sympathetic function.
Perspectives and Significance
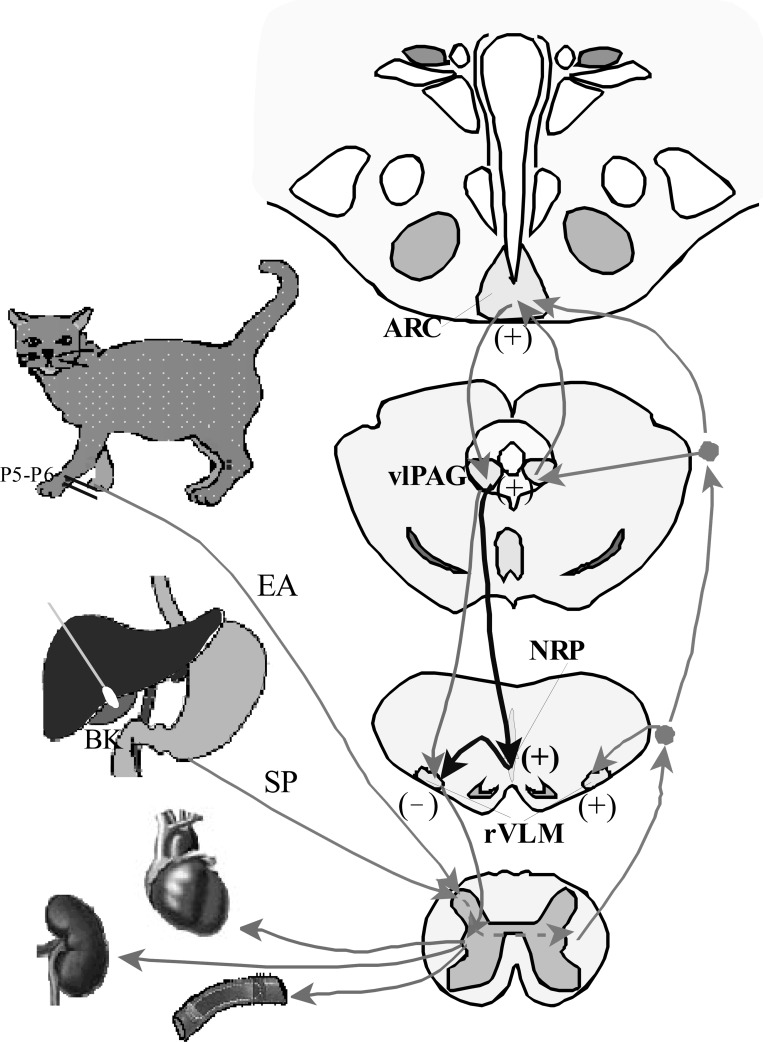
We have identified an important midline medullary region, the NRP that contributes to the long-loop pathway essential to the inhibitory cardiovascular action of acupuncture at certain acupoints (P 5–6). Excitation of vlPAG occurs following activation of the arcuate nucleus that, in turn, through a glutamatergic mechanism activates the NRP, which subsequently inhibits activity of cardiovascular sympathoexcitatory rVLM neurons (Fig. 7). The present study together with our previous work completes the neural circuitry of EA inhibition on rVLM sympathetic cardiovascular neurons and reflex increase of BP. This neural pathway contributes to the long-lasting inhibitory effect of EA on cardiovascular disease, such as hypertension. Our future studies will concentrate on the EA effect on hypotension and analyze how EA lowers BP during hypertension and raises BP during hypotension. Most likely, the EA effect depends on the status of excitation of these neurons and different neurotransmitters released.
Fig. 7.
Neural pathways of EA inhibition of cardiovascular sympathetic excitation. Light gray lines and arrows denote afferent inputs from splanchnic organs and EA to different brain areas and efferent output from the ARC to the vlPAG and rVLM. Dark gray lines and arrows denote efferent pathway from the vlPAG to the NRP that, in turn, projects to the rVLM.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (Bethesda, MD) Grants HL-72125 and HL-63313, and the Larry K. Dodge and Susan-Samueli Endowed Chairs (to J. C. Longhurst).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Yinxiang Cao, Department of Physiology, Shanghai Medical College, Fudan University, China, for the development of a four-channel data-acquisition system. We also thank Jesse Ho for his technical assistance.
REFERENCES
- 1. Barman S, Gebber G. Sequence of activation of ventrolateral and dorsal medullary sympathetic neurons. Am J Physiol Regul Integr Comp Physiol 245: R438–R447, 1983. [DOI] [PubMed] [Google Scholar]
- 2. Beitz AL. The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience 7: 2753–2768, 1982. [DOI] [PubMed] [Google Scholar]
- 3. Berman AL. The Brainstem of the Cat: a Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison: The University of Wisconsin Press, 1968. [Google Scholar]
- 5. Chao DM, Shen LL, Tjen-ALooi S, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Farkas E, Jansen ASP, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res 792: 179–192, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Fikova E, Marsala J. Stereotaxic atlases for the cat, rabbit, and rat. In: Electrophysiological Methods In Biological Research, edited by Bures J, Petran M, Zachar J. Prague: Academia Publishing House of the Czechoslavak Academy of Sciences, 1967, p. 653–731. [Google Scholar]
- 8. Guo ZL, Moazzami A, Tjen-A-Looi S, Longhurst J. Responses of opioid and serotonin containing medullary raphe neurons to electroacupuncture. Brain Res 1229: 125–136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huangfu DH, Huang Q, Li P. Afferent connections of the ventrolateral medulla in the rabbit-studied with HRP techniques. Chin J Physiol Sci 3: 86–95, 1987. [Google Scholar]
- 10. Li P, Pitsillides K, Rendig S, Pan HL, Longhurst J. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Li P, Rowshan K, Crisostomo M, Tjen-A-Looi S, Longhurst J. Effect of electroacupuncture on pressor reflex during gastric distention. Am J Physiol Regul Integr Comp Physiol 283: R1335–R1345, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Li P, Tjen-A-Looi SC, Longhurst JC. An arcuate-ventrolateral periaqueductal gray reciprocal circuitin electroacupuncture cardiovascular inhibition. Auton Neurosci In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li P, Tjen-A-Looi S, Longhurst J. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci 89: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Li P, Tjen-A-Looi S, Guo Z, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Tjen-A-Looi S, Longhurst J. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Li P, Lovick TA. Excitatory projections from hypothalamic and midbrain defense regions to nucleus paragigantocellularis lateralis in the rat. Exp Neurol 89: 543–553, 1985. [DOI] [PubMed] [Google Scholar]
- 17. Meller ST, Dennist BJ. Efferent projections of the periaqueductal gray in the rabbit. Neuroscience 40: 191–216. 1991. [DOI] [PubMed] [Google Scholar]
- 18. Moazzami A, Tjen-A-Looi SC, Longhurst JC. Serotonergic projection from nucleus raphe pallidus to rostral ventrolateral medulla modulates cardiovascular reflex responses during acupuncture. J Appl Physiol 108: 1336–1346, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Jpn J Physiol 45: 1053–1069, 1995. [DOI] [PubMed] [Google Scholar]
- 20. Tjen-A-Looi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627–H3635, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119–131, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852–R862, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Tjen-A-Looi SC, Li P, Longhurst JC. Midbrain vIPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol Heart Circ Physiol 290: H2543–H2553, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Tjen-A-Looi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: role of endocannabinoi ds and GABA. J Appl Physiol 106: 1793–1799, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. VanBockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol 309: 305–327, 1991. [DOI] [PubMed] [Google Scholar]
- 27. Zhang YM, Li P, Lovick TA. Role of the nucleus raphe obscurus in the inhibition of rostral ventrolateral medullary neurones induced by stimulation in the ventrolateral periaqueductal grey matter of the rabbit. Neurosci Lett 176: 231–234, 1994. [DOI] [PubMed] [Google Scholar]
- 28. Zhou W, Fu LW, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol 98: 872–880, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Zhou W, Tjen-A-Looi S, Longhurst JC. Brain stem mechanisms underlying acupuncture modality-related modulation of cardiovascular responses in rats. J Appl Physiol 99: 851–860, 2005. [DOI] [PubMed] [Google Scholar]