Abstract
Obesity of women at conception is increasing, a condition associated with offspring obesity. We hypothesized that maternal obesity increases placental fatty acid transporter (FATP) expression, enhancing delivery of fatty acids to their fetuses. Sheep are a commonly utilized biomedical model for pregnancy studies. Nonpregnant ewes were randomly assigned to a control group [100% of National Research Council (NRC) recommendations] or obese group (OB, 150% of NRC) from 60 days before conception to 75 or 135 days of gestation (dG; term = 150 dG), when placental cotyledonary tissue was collected for analysis. Fetuses of OB ewes were markedly heavier (P < 0.05) on 75 dG than fetuses from control ewes, but this difference disappeared by 135 dG. Maternal obesity markedly increased (P < 0.05) cholesterol and triglyceride concentrations of both maternal and fetal blood. There is no difference in lipoprotein lipase mRNA expression between control and OB group at either gestational age. On 75 dG, the mRNA expression of FATP1 (P < 0.05), FATP4 (P = 0.08), and fatty acid translocase CD (cluster of differentiation) 36 (P < 0.05) proteins were more enhanced in cotyledonary tissue from OB than control ewes; consistently, protein expression of FATP1 and FATP4 was increased (P < 0.05). Similarly, on 135 dG, the mRNA levels of FATP1, FATP4, and CD36 were all higher (P < 0.05), but only FATP4 protein content was enhanced (P < 0.05) in OB cotyledonary tissue. Peroxisome proliferator-activated receptor (PPAR)-γ regulates the expression of FATPs. Both the mRNA expression and protein content of PPARγ were increased in OB cotyledonary in the midgestation. In conclusion, maternal obesity enhances the mRNA expression and protein content of FATPs in cotyledonary in the midgestation, which is associated with higher PPARγ content in cotyledonary.
Keywords: placenta, lipid, sheep, PPARγ
in the united states, pre-pregnancy obesity in women increased from 13.0% in 1993–1994 to 22.0% in 2002–2003, a net increase of 69.3% (21). Recent evidence suggests that high pre-pregnancy body mass index is associated with either an enlarged or normal-weight fetus, increased newborn adiposity, and an increased incidence of obesity and metabolic diseases of offspring in later life (2, 5, 13, 28, 30, 33, 42). However, mechanisms linking maternal obesity (MO) to the increased incidence of obesity and metabolic diseases in offspring remain poorly defined.
In our previous studies, we observed that MO induced inflammation in fetal tissue, which was associated with altered fetal development (50, 58, 59). Toll-like receptor (TLR)-4 induces inflammation through promotion of the inflammatory NF-κB (60) and JNK pathways (34). A high level of fatty acids in the fetal circulation due to MO promotes inflammation via the TLR4 receptor (37, 39).
Long-chain fatty acids in fetal circulation are mainly derived from the maternal circulation through transplacental transport (15), which is mediated by a family of transmembrane fatty acid transport proteins including fatty acid transporters (FATPs), fatty acid binding proteins, and fatty acid translocase FAT/CD36. The expression of FATPs including FATP1 and FATP4 exhibited tissue-specific distribution (35, 45). FATP1, a member of the FATP/SLC27A1 solute carrier family 27, is an insulin-sensitive transporter that facilitates the cellular uptake of long-chain fatty acids (56). FATP4 are more effective FATPs compared with FATP1 in rat skeletal muscle (35). FATP1 and FATP4 are known to be expressed in the human term placenta (22); however, the mechanisms regulating FATP1 and FATP4 in the placenta are unclear. CD36 is not the primary FATP (45) and is ubiquitously expressed (35). Peroxisome proliferator-activated receptors (PPARs) are highly expressed in the placenta (53); as transcription factors, they are essential for placental development and fatty acid metabolism and transport (41). PPARγ enhances the expression of FATPs (31), and the activation of p38 is necessary for PPARγ to regulate FATP expression (40). We hypothesized that MO increased FATP expression that was associated with greater PPARγ expression and p38 activation in the placenta of OB ewes.
MATERIALS AND METHODS
Care and use of animals.
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia ewes were studied. Ewes were all mated to a single ram. From 60 days before conception to day 135 of gestation (first day of mating = day 0), ewes were individually fed either a highly palatable diet at 100% (control) of National Research Council recommendations for energy (36) (n = 20), or 150% (OB group) of the recommended energy requirements for early gestation (n = 20) as previously reported (50, 65). Ewes were housed in individual pens within a temperature-controlled room (∼20°C). Ewes were weighed at weekly intervals, and rations were adjusted for weekly changes in metabolic body weight (BW0.75) (12). The body condition of each ewe was scored at monthly intervals to evaluate changes in fatness as previously described (11).
Tissue collection.
Immediately before necropsy on 75 or 135 days of gestation (dG; gestation length ∼150 days), ewes were weighed and sedated with intravenous ketamine (22.2 mg/kg), and anesthesia was maintained by isofluorane inhalation (1.0–2.5%). Maternal (jugular vein) and fetal (umbilical vein) blood samples were collected from five twin-bearing ewes in each dietary group while they were under anesthesia. Maternal blood was collected into a chilled nonheparinized vacutainer tube (no additives; Sigma, St. Louis, MO), and serum was collected and frozen at −80°C for leptin assay. Blood was collected into a separate chilled tube (heparin, Sigma), and plasma was frozen at −80°C until it was utilized for lipid analysis. Following midventral laparotomy, the gravid uterus was located and the umbilical cord to each fetus was isolated. Umbilical venous blood was collected from each fetus via venipuncture, and serum and plasma were collected and stored as described for maternal blood. Ewes were then exsanguinated while remaining under general anesthesia, and the gravid uterus was immediately recovered and opened from base to tip. For each conceptus, cotyledonary tissue was obtained from type A placentomes (n = 2) of similar size located within 10 cm of the umbilical attachment site, frozen in liquid nitrogen, and stored at −80°C for Western blot and real-time RT-PCR analysis. All placentomes for both gestations used in this study are type A placentomes, using the criteria previously described (51).
Antibodies.
Rabbit anti-FATP1 (cat. no. sc-25541), FATP4 (cat. no. sc-25670), and CD36 (H-300) (cat. no. sc-9154) antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Phospho-p38MAPK (Thr180/Tyr182) (cat. no. 9215), p38MAP kinase (cat. no. 9212), and PPARγ (81B8) (cat. no. 2443) were purchased from Cell Signaling (Danvers, MA). Anti-β-tubulin (cat. no. T4026) antibody was purchased from Sigma. These antibodies have been previously used in sheep studies (16, 49, 52).
Western blot analysis.
Western blot analyses were conducted by procedures previously published from our laboratory (61, 62). Briefly, protein extractions were separated by 5–15% SDS-PAGE gels and transferred to nitrocellulose membranes for immunoblotting analyses. The primary antibodies were diluted 1:1,000. Band density was normalized according to the β-tubulin content (61, 62).
Immunohistochemical staining.
A single placentome was dissected from the surrounding tissue. A cross-section of the placentome containing caruncular and cotyledonary tissue was placed in a tissue cassette (Tissue Tek; Miles Labs, Elkhart, IN) and fixed with 4% (wt/vol) paraformaldehyde in a phosphate buffer (0.12 M; pH 7.4), and embedded in paraffin. Five micrometer, paraffin-embedded, fixed sections were prepared from the paraffin-embedded placentomal tissues. For each antibody, two sections of a control ewe placentome at each gestation were deparaffinized and hydrated by routine methods before the antigen retrieval procedure. Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 solution for 30 min at room temperature. Nonspecific antigenic sites were blocked by a 30-min incubation in diluted normal serum (Vector Laboratories, Burlingame, CA) before the sections were incubated for 2 h at room temperature with any of the following primary antibodies (1:50 dilution): anti-FATP1, anti-FATP4, or anti-CD36 antibody. The sections were then incubated at room temperature with a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 30 min and followed with the peroxidase-conjugated biotin-avidin complex (Vectastain ABC kit; Vector Laboratories) for 30 min. Finally, the peroxidase was revealed by immersion in diaminobenzidine tetrahydrochloride per manufacturer's instruction (Vector Laboratories). For a negative control, the isotype-matched immunoglobulin was used instead of primary antibodies (62). All pictures were taken at ×200 magnification.
Leptin analysis.
Leptin was measured in serum in a single assay by a multispecies leptin RIA kit from Linco Research (St. Charles, MO), following the manufacturer's instructions and as previously validated in our laboratory (11). The intra-assay coefficient of variation was 4.8%, and assay sensitivity was 0.5 ng/ml.
Lipid analyses.
A Boehringer Mannhiem/Hitachi 912 analyzer was used to quantitate plasma cholesterol, high-density lipoprotein, low-density lipoprotein, very-low-density lipoproteins, and triglyceride (Roch Diagnostics, Indianapolis, IN) contents as previously described (25). The lipid analyses were conducted by Veterinary Diagnostic and Investigational Laboratory, College of Veterinary Medicine, University of Georgia.
Real-time RT-PCR analysis.
Total RNA was extracted using Trizol Reagent (cat. no. 15596-018; Invitrogen, Carlsbad, CA), treated with DNase I (cat. no. 79254; Qiagen), and cleaned up with RNeasy Mini kit (cat. no.74104; Qiagen). cDNA was synthesized with SuperScript III first-strand synthesis for RT-PCR kit (cat. no. 18080-051; Invitrogen). Real-time RT-PCR was conducted on a Bio-Rad (Hercules, CA) iQ5 machine. Primers used in this study were synthesized by Invitrogen. β-Tubulin was used as the housekeeping gene. Primer information for FATP1, FATP4, CD36, FABP1, FABP3, FABP4, FABP5, FABPpm, lipoprotein lipase, and β-tubulin were listed in Table 1. PPARγ forward primer, 5′-CCGCATCTTCCAGGGGTGTC-3′, and reverse primer, 5′-CAAGGAGGCCAGCATCGTGTAAAT-3′ were synthesized according to the published sequence (23).
Table 1.
Primer sets used for real-time RT-PCR
| Gene Name | Product Size, bp | Direction | Sequence, 5′-/-3′ |
|---|---|---|---|
| FABP1 | 78 | Forward: | CATGACTGGGGAGAAGATCAA |
| Reverse: | TTGATGCCCTTGAAAGTTGTC | ||
| FABP3 | 81 | Forward: | AAGTCACTCGGTGTCGGTTTT |
| Reverse: | GTCCCCATTCACTTCGATGAT | ||
| FABP4 | 75 | Forward: | GCTTTGCTACCAGGAAAGTGG |
| Reverse: | TGTTGACCACATCCCCATTTA | ||
| FABP5 | 117 | Forward: | CCTGTTTCTCAACCCAAATAGC |
| Reverse: | ATGGTACTAGCTCCCCAAGGAT | ||
| FABPpm | 100 | Forward: | ACAAACCCTGTCAGTCACACCA |
| Reverse: | GTGCTGTTTTCAGGCTGGTTCT | ||
| FATP1 | 102 | Forward: | ACTGTCTGCCCCTGTACCAC |
| Reverse: | GGCTGGCTGAAAACTTCTTG | ||
| FATP4 | 88 | Forward: | GGCACCAACGACAAGAAGAT |
| Reverse: | GCTCGTCCATCACTAGCACA | ||
| CD36 | 93 | Forward: | CAAGAAAAATGGGGTGCAAT |
| Reverse: | CTGGCATTAGAATCCCTCCA | ||
| LPL | 129 | Forward: | TGTGCCGCCTTGGGTTCAGC |
| Reverse: | GCACACGCTCAGAGCCAGCA | ||
| β-tubulin | 141 | Forward: | CGAGAGCTGTGACTGTCTGC |
| Reverse: | GGCATGACGCTAAAGGTGTT |
Statistics.
Data were analyzed as a complete randomized design using General Linear Model of Statistical Analysis System (38). Each ewe was considered as an experimental unit, and each time point was analyzed separately. Means separation was performed using LSMEANS. Means ± SE were considered different when P < 0.05, and a trend was indicated when P < 0.10.
RESULTS
Body weight of OB ewes averaged 71.6 ± 3.2 kg at the time of experimental diet initiation and had increased (P < 0.05) 30% from diet initiation to conception (92.8 ± 3.0 kg). These OB ewes then went on to exhibit 38% and 60% increases in body weight from diet initiation to necropsy on day 75 (98.5 ± 3.1 kg) or day 135 (114.7 ± 3.9 kg) of gestation, respectively. In contrast, while body weight of control ewes were similar to that of OB ewes at diet initiation (68.3 ± 2.9 kg) they only exhibited insignificant increases in body weight from diet initiation to conception (70.3 ± 3.1; 2.9% increase) or necropsy on day 75 (73.9 ± 4.2 kg; 8.2% increase) but had increased (P < 0.05) their body weight by day 135 of gestation (90.8 ± 4.3 kg; 32.9%). Consistent with increases in body weight, OB ewes increased (P < 0.05) their body condition score from 5.0 ± 0.3 at diet initiation to 7.7 ± 1.0 at day 75 and 7.7 ± 0.2 at day 135. Body condition scores of control ewes were similar to those of OB ewes at diet initiation averaging 4.7 ± 0.4 and then increased to 4.9 ± 0.4 on day 75 and 6.3 ± 0.3 on day 135 of gestation, respectively. Both body weights and body condition scores were markedly greater (P < 0.05) in OB than control ewes at conception and on days 75 and 135 of gestation. Furthermore, circulating maternal leptin concentrations were higher (P < 0.01) in OB than control ewes at both gestational ages (Table 2).
Table 2.
Triglyceride and cholesterol concentrations in maternal and fetal plasma on 75 dG and 135 dG ewes and fetuses
| 75 dG |
135 dG |
|||
|---|---|---|---|---|
| Control | OB | Control | OB | |
| Maternal plasma | n = 5 | n = 5 | n = 4 | n = 6 |
| Cholesterol, mg/dl | 64.2 ± 4.7 | 76.0 ± 4.7* | 70.8 ± 5.3 | 90.0 ± 4.3* |
| Triglyceride, mg/dl | 26.2 ± 3.3 | 36.6 ± 3.3* | 19.3 ± 3.6 | 34.3 ± 2.9* |
| HDL, mg/dl | 31.8 ± 2.3 | 27.7 ± 2.3 | 29.0 ± 2.6 | 27.5 ± 2.1 |
| LDL, mg/dl | 33.8 ± 6.4 | 36.9 ± 6.4 | 39.0 ± 7.2 | 47.0 ± 5.8 |
| VLDL, mg/dl | 5.4 ± 0.7 | 5.4 ± 0.7 | 4.3 ± 0.9 | 6.5 ± 0.7 |
| Cholesterol/HDL | 2.0 ± 0.2 | 2.8 ± 0.2* | 2.5 ± 0.2 | 3.4 ± 0.2* |
| Leptin, ng/ml | 2.7 ± 0.2 | 7.7 ± 1.0‡ | 7.9 ± 0.6 | 11.4 ± 1.0‡ |
| Fetal plasma | n = 5 | n = 5 | n = 5 | n = 5 |
| Cholesterol, mg/dl | 34.0 ± 4.2 | 42.0 ± 4.2* | 33.0 ± 3.6 | 46.0 ± 3.6* |
| Triglyceride, mg/dl | 38.8 ± 2.5 | 48.0 ± 2.5* | 18.1 ± 2.1 | 17.9 ± 2.1 |
| HDL, mg/dl | 6.8 ± 0.6 | 7.6 ± 0.6* | 9.6 ± 0.5 | 10.1 ± 0.5 |
| LDL, mg/dl | 20.0 ± 4.5 | 23.8 ± 4.5 | 20.0 ± 3.8 | 30.7 ± 3.8† |
| VLDL, mg/dl | 8.5 ± 0.8 | 8.7 ± 0.8 | 3.6 ± 0.5 | 3.6 ± 0.5 |
| Cholesterol/HDL | 5.0 ± 0.3 | 5.6 ± 0.3* | 3.5 ± 0.4 | 4.5 ± 0.4* |
Values are means ± SE.
P < 0.05 vs. control;
P < 0.10 vs. control;
P < 0.01 vs. control. dG, days of gestation; OB, obese groups. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoproteins.
On 75 dG, the body weight of OB fetuses was greater (P < 0.05) than that of control fetuses (185.7 ± 6.89 vs. 234.4 ± 6.61 g). At late gestation, however, the difference disappeared, with control and OB fetuses averaging 5.05 ± 0.28 and 5.02 ± 0.25 kg, respectively.
Maternal obesity increased (P < 0.05) cholesterol, triglyceride, and the cholesterol-to-high-density lipoprotein ratio concentration in both maternal and fetal blood on 75 dG (Table 2). On 135 dG, while the differences in cholesterol and triglycerides remained elevated (P < 0.05) in maternal blood of OB vs. control ewes, only cholesterol and low-density lipoprotein remained elevated (P < 0.05) in the blood of OB vs. control fetuses (Table 2). There is no difference in lipoprotein lipase mRNA expression between control and OB group at either gestational age (Fig. 1).
Fig. 1.
Lipoprotein lipase expression in cotyledonary (COT) tissue of control (■) and OB (□) ewes on 75 (A) and 135 (B) days of gestation (dG). Data are means ± SE; n = 8 in each group.
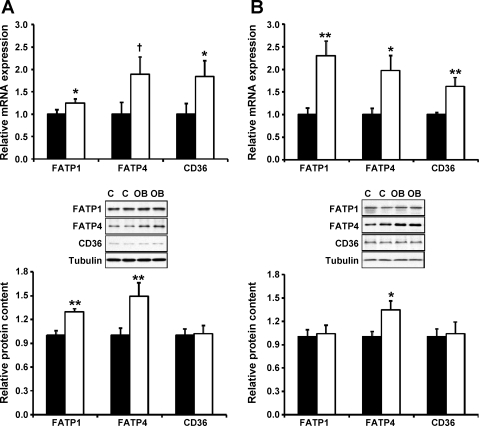
On 75 dG, Western blot analysis indicated that both FATP1 and FATP4 proteins were more abundant (P < 0.05) in cotyledonary from OB than control ewes (Fig. 2A). Consistently, FATP1 and FATP4 mRNA expression was elevated in OB ewes (Fig. 2A). In addition, FABP1, FABP3, FABP4, FABP5, and plasma membrane FABP are all expressed in sheep placentomes, but their mRNA expression did not differ between control and OB ewes (data not shown).
Fig. 2.
Fatty acid transporter (FATP)1, FATP4, and CD36 expression in COT tissue of control (■) and OB (□) ewes on 75 (A) and 135 (B) dG. Data are means ± SE; n = 8 in each group. **P < 0.01; *P < 0.05; †P < 0.10.
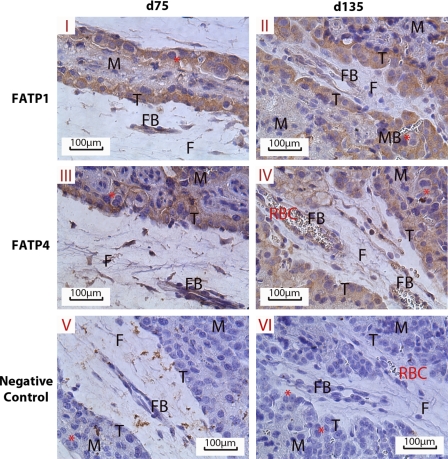
To determine whether the increased expression of FATPs in cotyledonary of OB ewes persisted into late gestation, we further analyzed FATP expression in placentomes on 135 dG. The mRNA expression of FATPs and CD36 were increased in OB placentomes, and FATP4 protein content was also found to be higher (P < 0.05) in OB placentomes at late gestation (Fig. 2B). To mediate fatty acid uptake, FATPs need to localize on the trophoblasts. Indeed, FATPs were mainly located to trophoblasts as depicted in Fig. 3.
Fig. 3.
Immunohistochemical localization of FATP1 and FATP4 in the placentome on 75 and 135 dG. I: 75 dG FATP1; II: 135dG FATP1; III: 75dG FATP4; IV: 135dG FATP4; V: negative control of 75 dG; VI: negative control of 135 dG. F, fetal COT; M, maternal caruncle; FB, COT arteriole; MB, caruncular arteriole; T, trophoblast layer; RBC, red blood cells. *Binuclear cells.
To explore possible mechanisms leading to enhanced FATPs expression, we analyzed the expression of PPARγ. Both the mRNA expression and protein content of PPARγ were increased in OB vs. control placentomes at midgestation (Fig. 4A). However, at late gestation, this difference in PPARγ disappeared (Fig. 4B). p38 MAP kinase activation is necessary for PPARγ to regulate the expression of FATPs, and the activation of p38 MAP kinase is through phosphorylation. Consistent with increased expression of both PPARγ and FATPs, the phosphorylation of p38 MAP kinase was also higher in OB placentomes at midgestation (Fig. 5A), and this difference in p38 MAP kinase disappeared at late gestation (Fig. 5B).
Fig. 4.
Protein and mRNA content of peroxisome proliferator-activated receptor-γ (PPARγ) in COT tissue of control (■) and OB (□) on 75 and 135 dG. Data are means ± SE; n = 8 in each group. *P < 0.05; †P < 0.10; n = 8 in each group. A: 75 dG; mRNA expression (top); protein content (bottom). B: 135 dG; mRNA expression (top); protein content (bottom).
Fig. 5.
Protein content of p38 and its phosphorylation in COT tissue of control (■) and OB (□) ewes on 75 (A) and 135 (B) dG. Data are means ± SE; n = 8 in each group. †P < 0.10.
DISCUSSION
The sheep is one of the most commonly used models for studying human pregnancy (3). Sheep have a cotyledonary placentation where nutrient and waste product exchange occurs at discrete sites called placentomes (10, 55). Placentomes are individual units of fetal/maternal exchange, and are composed of fetal cotyledonary and maternal caruncular components (10). The cotyledonary component of both the human and sheep placenta are of the same villous type and exhibit similarities in vascular development and morphology (3).
We observed that placental weight was increased due to MO, which is consistent with a previous survey study in women, where placental weights correlated with increasing MO (47). In addition, fetuses in OB ewes exhibited increased weights at midgestation, consistent with the observed macrosomia of obese human pregnancy (20). The uniqueness of our obese sheep model is that the difference in placental and fetal weight largely disappears at the late gestation; the exact mechanisms associated with the reduction of placental development during the late portion of pregnancy is unclear but could be due to feedback inhibition. Such an increase and then a decrease in placental development provide a unique model for studying the mechanisms regulating placental development and function, with high relevance to human pregnancy.
Obesity leads to low-grade inflammation (14, 46, 54). NF-κB and JNK signaling pathways are the primary pathways of inflammatory responses (1), which further enhances the expression of proinflammatory cytokines, forming a vicious cycle (19). Toll-like receptor (TLR)-4 drives the inflammatory NF-κB pathway (60) and activates the JNK pathway (34). A crucial recent finding demonstrated that free fatty acids are ligands for the TLR4 receptor (37, 39). Therefore, excessive fatty acids in the fetal circulation due to MO are expected to activate TLR4 signaling, resulting in inflammation.
Placental uptake of free fatty acids from the maternal circulation provides fatty acids both for placental metabolism and delivery to the fetus (41, 57). Placental fatty acid uptake is mediated by FATPs, including FATP1 and FATP4. In this study, we found that MO enhanced the expression of FATPs at midgestation, which is consistent with observations in ob/ob mice where the mRNA expression of FATPs is increased in adipose tissue (29). Consistent with the increased expression of FATPs observed in this study, we previously reported that the free fatty acid concentration in the fetal circulation of OB ewes at midgestation was higher than that of control ewes (63). The difference in the level of free fatty acids between control and OB ewes should not be due to the difference in triglyceride hydrolysis because there is no difference in lipoprotein lipase mRNA expression between control and OB groups at either gestational age, which indicates that increased FATP in OB placenta may be the key factor for enhanced fatty acid fluxes into the fetal circulation of OB ewes. Changes in fetal fatty acids could also be partially due to alteration in fetal metabolism, which warrants further studies.
PPARs belong to a group of the ligand-activated nuclear receptor superfamily and function as transcription factors regulating the expression of genes related to lipid metabolism and adipogenesis (9). PPARγ is known to be essential for placental development (57) and placental uptake of fatty acids (40). Activation of PPARγ enhances the uptake of fatty acids by increasing the expression of FATP1 and FATP4 (41). In this study, both mRNA and protein of PPARγ were higher in the placenta of obese ewes. p38 MAP kinase is also an important regulator of lipid metabolism, and its activation is necessary for PPARγ-induced FATP expression (40). Consistent with increased PPARγ and FATPs expression, the phosphorylation of p38 was also increased in OB placentomes at midgestation. These data show that PPARγ and p38 MAP kinase may regulate the enhanced expression of FATPs in OB ewes.
The increase in PPARγ expression in OB placentomes could be due to the elevated inflammatory response associated with MO. It was reported previously that inflammation upregulates PPARγ expression (48). Obesity in pregnant women induces exaggerated inflammatory responses in the placenta (6), consistent with our observations in this study in the ewes (58). The increase in PPARγ expression might also be due to enhanced adipogenesis in the placenta, because PPARγ is a marker of adipogenesis. Interestingly, adipose tissue has a very similar transcriptome to the human placenta (6). The activation of p38 MAP kinase might be also associated with the inflammation and stress in OB placentomes. p38 MAP kinase is known to be activated in OB conditions in mice (7, 27).
A very interesting observation of this study is that the difference in FATPs and associated regulatory signaling pathways between control and OB placentomes attenuates at late gestation. The exact reason for the downregulation of FATPs in the placenta in late gestation is unclear, but might be due to feedback inhibition. MO induces placental inflammation at midgestation (63), and inflammation itself is able to induce anti-inflammatory TGF-β signaling in mice and humans (4, 44), which should downregulate inflammation and, correspondingly, the expression of FATPs. Indeed, in our studies in fetal gut at late gestation, enhanced TGF-β signaling was observed in OB fetuses compared with control fetuses (Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ, unpublished observations). Consistent with the downregulation of FATPs in late gestation, the concentration of lipids in fetal circulation in OB was also reduced during late gestation, and the difference between OB and control disappeared on 135 dG. This reduction of lipids in fetal circulation might also contribute to the downregulation of PPARγ and transcription of FATPs in OB sheep placenta in late gestation. Another factor for the lack of difference in fetal lipid profile between control and OB could be due to the change in overall placentome weight. Using the same cohort of animals, we previously reported that the total placentome weight at 75 dG did not differ between control and OB ewes (709.5 ± 38.9 g vs. 654.6 ± 37.6), but at 135 dG, the placentome weight was lower in OB compared with control ewes (control vs. OB = 623.3 ± 15.8 vs. 486 ± 14.2, P < 0.05). As a result, at midgestation OB fetal weight was much higher than control weight (control vs. OB = 185.7 ± 6.9 vs. 234.4 ± 6.6 g), but at late gestation such difference disappeared (control vs. OB = 5024.5 ± 163.4 vs. 4,827.4 ± 169.1 g). When expressed as the ratio of fetal to placenta weight, i.e., placenta efficiency, they are still higher in OB ewes at both gestations (midgestation: control vs. OB 0.27 ± 0.02 vs. 0.37 ± 0.02; late gestation: control vs. OB 8.1 ± 0.3 vs. 10.0 ± 0.4) (26). The downregulation of nutrient transporters and reduction in placentome weight during the later portion of gestation should be mainly responsible for the lack of difference in body weight at birth (26). The reason for the reduction in OB placental weight may be associated with the downregulation of growth signaling pathways in OB placenta (64).
MO enhances the expression of FATPs, which should increase fatty acid uptake, further increasing fetal adiposity at midgestation. In addition, excessive fatty acids are expected to induce inflammation, because fatty acids are ligands for TLR4, which drives the inflammatory response (34, 37, 39, 60). Indeed, in our previous study, we detected elevated inflammation in OB placenta compared with control placenta (63). Inflammation in fetuses induces developmental changes (8). Furthermore, previous studies showed that the activity of amino acid transporter system A is enhanced by inflammatory cytokines in cultured primary trophoblast cells (17). Maternal high-fat diet and obesity upregulates placental glucose transporter-1 and amino acid transporter (18). These data combined with our observations show that MO enhances the expression of critical nutrient transporters for fatty acids, glucose, and amino acids, which should account for the fetal obesity and overgrowth in the midgestation. Because major organ development occurs before midgestation and adipogenesis initiates around midgestation, such an excessive supply of fatty acids may alter fetal development and promote adipogenesis, increasing preadipocytes and adipocytes in fetuses (8). In the late gestation, the expression of FATPs was downregulated and fetal weight was normal, which might be too late to correct altered fetal development, predisposing offspring to obesity in later life. This was recently confirmed by Ford et al. (12) who reported a significant increase in adiposity at birth in lambs born to OB compared with control ewes. Furthermore, when these lambs reached maturity (2 yr of age), they exhibited an increased appetite, altered glucose and insulin regulation, and a greater accumulation of body fat in response to a bout of ad libitum feeding compared with offspring born to ewes fed to requirements (24).
Perspectives and Significance
Our observation that MO increases FATPs expression has important clinical applications. MO is known to increase the incidence of fetal overgrowth and adiposity and is associated with the development of preeclampsia and associated fetal death (32, 43). Our data demonstrate that MO enhances the expression and content of FATPs in cotyledonary in midgestation, which should at least partially account for the fetal overgrowth and adiposity. The increase in FATPs is associated with higher PPARγ content and enhanced p38 signaling. Because most of the developmental events of fetuses occur before or around midgestation, the excessive delivery of fatty acids into fetal circulation in midgestation is expected to alter fetal development, exerting long-term effects on offspring health.
GRANTS
This project was supported by National Research Initiative Competitive Grant 2008-35203-19084 from the USDA Cooperative State Research, Education and Extension Service, and Agriculture and Food Research Initiative Competitive Grant 2009-65203-05716 from the USDA National Institute of Food and Agriculture and by University of Wyoming INBRE Grant P20- RR-016474.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Adam Uthlaut for assistance with animal care and tissue collection.
REFERENCES
- 1.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab 13: 364–368, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology 69: 55–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleux C, Bobe P, Kanellopoulos-Langevin C. A mouse placental immunoregulatory factor different from transforming growth factor beta. J Interferon Cytokine Res 15: 351–357, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32: 1076–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29: 274–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptorγ coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem 282: 15439–15450, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 82: 4–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duttaroy AK. Fetal growth and development: roles of fatty acid transport proteins and nuclear transcription factors in human placenta. Indian J Exp Biol 42: 747–757, 2004 [PubMed] [Google Scholar]
- 10.Ford SP. Cotyledonary placenta. In: Encyclopedia of Reproduction, edited by Knobil E., Neil J. D. San Diego, CA: Academic, 2000, p. 730–738 [Google Scholar]
- 11.Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol 297: R835–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol 572: 5–15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83: 461S–465S, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hanebutt FL, Demmelmair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr 27: 685–693, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Yan X, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Du M. Enhanced transforming growth factor-β signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. Am J Physiol Endocrinol Metab 298: E1254–E1260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol 297: C1228–C1235, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434–439, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol 24: 697–705, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 15: 986–993, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Larque E, Demmelmair H, Klingler M, De Jonge S, Bondy B, Koletzko B. Expression pattern of fatty acid transport protein-1 (FATP-1), FATP-4 and heart-fatty acid binding protein (H-FABP) genes in human term placenta. Early Hum Dev 82: 697–701, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lomax MA, Sadiq F, Karamanlidis G, Karamitri A, Trayhurn P, Hazlerigg DG. Ontogenic loss of brown adipose tissue sensitivity to β-adrenergic stimulation in the ovine. Endocrinology 148: 461–468, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and high nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci In Press [DOI] [PubMed] [Google Scholar]
- 25.Long NM, Hill GM, Baker JF, Graves WM, Froetschel MA, Keisler DH, Mullinix BG., Jr Reproductive performance of beef heifers supplemented with corn gluten feed and rumen-protected fat before breeding. Prof Anim Sci 23: 316–324, 2007 [Google Scholar]
- 26.Ma Y, Zhu MJ, Zhang L, Hein SM, Nathanielsz PW, Ford SP. Maternal obesity and overnutrition alter fetal growth rate and cotyledonary vascularity and angiogenic factor expression in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R249–R258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maekawa T, Jin W, Ishii S. The role of ATF-2 family transcription factors in adipocyte differentiation: anti-obesity effects of p38 inhibitors. Mol Cell Biol 30: 613–625, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malee MP, Verma A, Messerlian G, Tucker R, Vohr BR. Association between maternal and child leptin levels 9 years after pregnancy complicated by gestational diabetes. Horm Metab Res 34: 212–216, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Memon RA, Fuller J, Moser AH, Smith PJ, Grunfeld C, Feingold KR. Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes 48: 121–127, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. J Biol Chem 273: 16710–16714, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Murai JT, Muzykanskiy E, Taylor RN. Maternal and fetal modulators of lipid metabolism correlate with the development of preeclampsia. Metabolism 46: 963–967, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. Ilar J 47: 73–82, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Nickerson JG, Alkhateeb H, Benton CR, Lally J, Nickerson J, Han XX, Wilson MH, Jain SS, Snook LA, Glatz JF, Chabowski A, Luiken JJ, Bonen A. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem 284: 16522–16530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council Nutrient Requirements of Sheep. Washington, DC: National Academy Press, 1985 [Google Scholar]
- 37.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SAS SAS User's Guide. Version 8. Cary, NC: SAS Institute, 2000 [Google Scholar]
- 39.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-κB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 126: 233–245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-γ and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 90: 4267–4275, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Schaiff WT, Knapp FF, Jr, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor γ alters placental morphology and placental fatty acid uptake in mice. Endocrinology 148: 3625–3634, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25: 1175–1182, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Silver RM. Fetal death. Obstet Gynecol 109: 153–167, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Solomon A, Wajngarten M, Alviano F, Anteby I, Elchalal U, Pe'er J, Levi-Schaffer F. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy 35: 941–948, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab 12: 266–273, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Steinberg GR. Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle 6: 888–894, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Swanson LD, Bewtra C. Increase in normal placental weights related to increase in maternal body mass index. J Matern Fetal Neonatal Med 21: 111–113, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-δ and peroxisome proliferator-activated receptor-γ. Circ Res 91: 427–433, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Tong J, Zhu MJ, Underwood KR, Hess BW, Ford SP, Du M. AMP-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3T3–L1 cells. J Anim Sci 86: 1296–1305, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 296: E917–E924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vatnick I, Ignotz G, McBride BW, Bell AW. Effect of heat stress on ovine placental growth in early pregnancy. J Dev Physiol 16: 163–166, 1991 [PubMed] [Google Scholar]
- 52.Wang J, Ma H, Tong C, Zhang H, Lawlis GB, Li Y, Zang M, Ren J, Nijland MJ, Ford SP, Nathanielsz PW, Li J. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J 24: 2066–2076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Fujii H, Knipp GT. Expression of PPAR and RXR isoforms in the developing rat and human term placentas. Placenta 23: 661–671, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 294: R673–R680, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wooding FB, Morgan G, Forsyth IA, Butcher G, Hutchings A, Billingsley SA, Gluckman PD. Light and electron microscopic studies of cellular localization of oPL with monoclonal and polyclonal antibodies. J Histochem Cytochem 40: 1001–1009, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol 26: 3455–3467, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Y, Wang Q, Cook TJ, Knipp GT. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J Pharm Sci 96: 2582–2606, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-κB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-κB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Zhu MJ, Du M, Hess BW, Means WJ, Nathanielsz PW, Ford SP. Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes. Placenta 28: 361–368, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zhu MJ, Du M, Hess BW, Nathanielsz PW, Ford SP. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome. Placenta 28: 1192–1199, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Zhu MJ, Du M, Nathanielsz PW, Ford SP. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 31: 387–391, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Zhu MJ, Du M, Nijland MJ, Nathanielsz PW, Hess BW, Moss GE, Ford SP. Down-regulation of growth signaling pathways linked to a reduced cotyledonary vascularity in placentomes of over-nourished, obese pregnant ewes. Placenta 30: 405–410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]