Abstract
We tested the hypothesis that tonic adrenergic and nonadrenergic receptor-mediated sympathetic vasoconstriction would increase at rest and during exercise with advancing age. Young (n = 6; 22 ± 1 mo; means ± SE) and old (n = 6; 118 ± 9 mo) beagles were studied. Selective antagonists for alpha-1, alpha-2, neuropeptide Y (NPY), and purinergic (P2x) receptors were infused at rest and during treadmill running at 2.5 mph and 4 mph with 2.5% grade. Prazosin produced similar increases in vascular conductance in young and old beagles at rest (Young: 158 ± 34%; Old: 98 ± 19%) and during exercise at 2.5 mph (Young: 80 ± 10%; Old: 58 ± 12%) and 4 mph and 2.5% grade (Young: 57 ± 5%; Old: 26 ± 4%). Rauwolscine caused similar (P > 0.05) increases in vascular conductance in old compared with young dogs at rest (Young: 119 ± 25%; Old: 64 ± 22%) and at 2.5 mph (Young: 86 ± 13%; Old: 60 ± 7%) and 4 mph with 2.5% grade (Young: 61 ± 5%; Old: 43 ± 7%). N2-(diphenylacetyl)-N-[4-hydroxyphenyl)methyl]-d-arginine amide (BIBP) caused a smaller increase (P < 0.05) in vascular conductance in old compared with young dogs at rest (Young: 179 ± 44%; Old: 91 ± 22%), whereas similar increases (P > 0.05) of experimental limb vascular conductance in young and old dogs occurred following BIBP during exercise at 2.5 mph (Young: 56 ± 16%; Old: 50 ± 12%) and 4 mph and 2.5% grade (Young: 45 ± 10%; Old: 25 ± 7%). Pyridoxal-phosphate-6-azophenyl-2′-4′-disulfonic acid infusion produced a larger increase in vascular conductance in old compared with young beagles at rest (Young: 88 ± 14%; Old: 191 ± 58%), whereas similar increases were observed at 2.5 mph (Young: 47 ± 18%; Old: 31 ± 11%) and 4 mph with 2.5% grade (Young: 26 ± 13%; Old: −18 ± 8%). At rest, NPY receptor-mediated restraint of skeletal muscle blood flow was reduced with advancing age, whereas P2x receptor-mediated restraint of skeletal muscle blood flow was increased. During exercise, the magnitude of adrenergic and nonadrenergic sympathetic vasoconstriction was not different between young and old dogs. Overall, these data demonstrate that adrenergic receptor-mediated vasoconstriction was not elevated at rest, but nonadrenergic sympathetic vasoconstriction was altered under basal conditions in aged beagles.
Keywords: aging, sympathetic vasoconstriction, skeletal muscle, exercise
aging is associated with a decline in aerobic capacity (12–14). An inability to augment cardiac output during exercise has been functionally linked to the reduced exercise tolerance with aging (1, 23, 36, 53). A blunted skeletal muscle blood flow response to dynamic exercise has also been reported in older humans and animals (1, 33, 37, 47, 48, 50). However, other studies have documented preserved skeletal muscle blood flow during exercise in aged humans (38, 40, 44, 49). Thus, the effect of aging on the control of skeletal muscle blood flow appears to be an unresolved issue in the scientific literature.
The observation of elevated efferent muscle sympathetic nerve activity in older adults at rest (16, 43) has led to the notion that augmented sympathetic vasoconstriction may blunt the blood flow response to exercise with aging. It should be recognized, though, that no direct measurements of sympathetic nerve activity to exercising muscle have been made in old animals or humans, and indirect evidence from norepinephrine (NE) spillover measurements is inconclusive. During moderate-intensity (50–60% of V̇o2peak) cycling exercise, whole-body (39) and leg NE spillover (49) did not differ between young and old men. In another study, leg NE spillover was similar during mild- and moderate-intensity cycling, but it was increased at near-maximal intensity in older compared with younger endurance-trained men (50). These exercise data appear to be consistent with other studies that have documented similar increases in sympathetic outflow in old relative to young adults in response to physiological stressors such as hypoxia, thermal stress (cold-pressor test), and ischemic handgrip (11, 42). Thus, the accumulated scientific evidence suggests that the sympathetic response to physiological stressors, such as exercise, is not increased in old compared with young adults.
The functional consequence of sympathetic efferent nerve activity in the skeletal muscle vascular bed is vasoconstriction. The literature on the effect of aging on tonic sympathetic vasoconstriction in the skeletal muscle vascular bed is limited and contradictory. In the human forearm, nonselective α-adrenergic receptor blockade produced a smaller increase in resting blood flow in old compared with young adults (15), suggesting that tonic α-adrenergic receptor-mediated vasoconstriction was reduced with age. In contrast, in the leg, nonselective α-adrenergic receptor blockade elicited greater increases in resting blood flow and vascular conductance in older adults, suggesting that tonic α-adrenergic receptor-mediated vasoconstriction was elevated with age (18). Not only is the effect of aging on resting α-adrenergic vasoconstriction unsettled, no previous studies have investigated the effect of aging on the magnitude of tonic α-adrenergic vasoconstriction during dynamic exercise.
Traditionally, it was believed that sympathetic vasoconstriction was mediated entirely by NE binding to postsynaptic α1-adrenergic receptors. However, it is now recognized that NE also binds to postsynaptic α2-receptors and that the nonadrenergic neurotransmitters ATP and NPY contribute to sympathetic vasoconstriction through binding with purinergic (P2x) and neuropeptide Y (NPY) receptors on vascular smooth muscle (2, 4–7, 10, 20–22, 34, 35, 46, 51, 52, 54, 55). The type, pattern, and quantity of neurotransmitter released are sensitive to the frequency of neuron firing (2, 20, 22, 34, 35, 46, 54, 55). In particular, low-discharge frequencies favor ATP release followed by NE, whereas midrange discharge frequencies produce both ATP and NE release, and high discharge frequencies favor NPY release (21, 34, 46, 51). Our laboratory has previously documented (4–7) the presence of P2x and NPY-Y1 receptor-mediated tonic vasoconstriction in the skeletal muscle vascular bed of young dogs at rest and during exercise. A greater magnitude and frequency of basal muscle sympathetic activity in the old compared with the young may increase sympathetic cotransmitter release in the old, which would provide a proportionally greater role for nonadrenergic receptor-mediated sympathetic vasoconstriction in aged skeletal muscle. Thus, a thorough analysis of the effect of aging on tonic sympathetic vasoconstriction must take into account the effects of both adrenergic and nonadrenergic receptors, and the effect of aging on nonadrenergic receptor-mediated sympathetic vasoconstriction has yet to be investigated.
Therefore, the purpose of the present study was to investigate the effect of aging on adrenergic and nonadrenergic receptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscles. We hypothesized that aging would be associated with increased adrenergic and nonadrenergic receptor-mediated vasoconstriction at rest and during exercise.
METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals. Young (n = 6; 22 ± 1) and old (n = 6; 118 ± 9 mo) beagles were selected for their willingness to run on a motorized treadmill and were chronically instrumented in a series of three sterile surgeries. For each surgery, anesthesia was induced with thiopental sodium (25 mg/kg; Gensia Pharmaceuticals, Irvine, CA). Animals were then intubated with a cuffed endotracheal tube, and a surgical level of anesthesia was maintained through mechanical ventilation with 1.5% isoflurane (Halocarbon Laboratories, River Edge, NJ), and 98.5% O2. Postoperatively, animals were given an analgesic for pain management (buprenorphrine hydrochloride, 0.3 mg; Reckitt and Coleman, Kingston-upon-Hull, UK) and received antibiotics for 10 days, (cefazolin sodium, 500 mg twice a day; Apothecon, Princeton, NJ). In the initial surgery, the carotid arteries were externalized and placed in neck skin tubes for measurement of arterial blood pressure via percutaneous cannulation. Following a 1-wk recovery, animals were instrumented with ultrasonic transit-time flow probes (4–6 mm; Transonic Systems, Ithaca, NY) around the external iliac of each hindlimb for measurement of skeletal muscle blood flow. Cables were tunneled under the skin to the back and externalized. Following a 2-wk recovery, a heparinized catheter (0.045 in. OD, 0.015 in. ID, 60-cm length; Data Science International, St. Paul, MN) was implanted in a side branch and advanced into the femoral artery of one hindlimb. The catheter was tunneled under the skin to the back, externalized, and used for infusion of experimental drugs. To maintain patency, the catheter was flushed daily with saline and filled with a heparin lock (100 IU heparin/ml in 50% dextrose solution). Dogs were given 2 days to recover from the final surgery before any experimental procedures were performed.
To minimize changes in body temperature during the exercise sessions, laboratory temperature was maintained below 20°C for all experiments. For each experiment, the dog was brought to the laboratory and rested in a sling, while the flow probes were connected to a flowmeter (Transonic Systems) and a 20-gauge intravascular catheter (Insyte, Becton-Dickinson, Sandy, UT) was inserted retrogradely into the lumen of one carotid artery and attached to a solid-state pressure transducer (Abbott, North Chicago, IL) for measurement of arterial pressure.
To investigate the effect of aging on endothelium-dependent vasodilation ACh (0.1 μg bolus) was infused into the experimental hindlimb, while the animals rested quietly.
To investigate the effect of aging on tonic adrenergic and nonadrenergic receptor-mediated sympathetic vasoconstriction, four series of investigations (series 1–4) were completed.
Adrenergic, series 1 and 2.
To investigate the effect of aging on tonic alpha-1 and alpha-2 adrenergic receptor-mediated vasoconstriction in skeletal muscle, selective alpha-1 (prazosin; 0.25 μg·ml−1·min−1; series 1) and alpha-2 (rauwolscine; 1.25 μg·ml−1·min−1; series 2) receptor antagonists were infused into the experimental hindlimb, while the animals rested quietly and while the dogs ran on a motorized treadmill at moderate (2.5 mph) and heavy (4 mph and 2.5% grade) exercise intensities. We determined the effectiveness and selectivity of prazosin and rauwolscine antagonism by infusing the selective alpha-1 receptor agonist (PE 0.1 μg·ml−1·min−1) and alpha-2 receptor agonist (clonidine; 1 μg·ml−1·min−1) prior to and ∼1 min after observation of the peak increase in limb blood flow with receptor antagonism.
Nonadrenergic, series 3 and 4.
To investigate the effect of aging on tonic NPY-Y1 and P2x receptor-mediated vasoconstriction in skeletal muscle, selective NPY-Y1 (BIBP; 2.5 mg bolus; series 3) and P2x [pyridoxal-phosphate-6-azophenyl-2′4′-disulfonic acid (PPADS); 40 mg bolus, series 4] receptor antagonists were infused into the experimental hindlimb, while the animals rested quietly and while they ran on a motorized treadmill at moderate (2.5 mph) and heavy (4 mph and 2.5% grade) exercise intensities. The effectiveness and selectivity of BIBP and PPADS antagonism were determined by infusions of the selective NPY Y1 receptor agonist [Leu31,Pro34] NPY (1 μg·ml−1·min−1) and P2x receptor agonist (α,β-methyleneadenosine 5′-triphosphate lithium salt; α,β-methylene ATP; 1 μg·ml−1·min−1) prior to and ∼1 min after observation of the peak increase in limb blood flow with receptor antagonism.
Pharmacological infusions.
Each antagonist was infused at rest and at each exercise intensity on a separate day in random order; thus, each animal was brought to the laboratory on six occasions to complete series 1 and 2 and six additional occasions to complete series 3 and 4. Infusions during exercise took place after ∼5 min of treadmill running. Small volumes of each drug (<1 ml) were infused, followed by a 3-ml saline flush. Each infusion was ∼3–5 s in duration. The effect of the infusion vehicle (prazosin: 3 parts sterile water; 1 part polyethylene glycol; rauwolscine: saline; PPADS: saline; BIBP: saline) was determined in each animal at rest and during exercise. Vehicle infusions had no effect on hindlimb blood flow or systemic hemodynamics.
Data analysis.
Arterial blood pressure and external iliac blood flow were recorded at 100 Hz directly to a computer with a Powerlab data acquisition system (ADInstruments, Castle Hill, Australia). Data were analyzed off-line to calculate the absolute and relative change in mean arterial pressure (MAP), experimental and contralateral (control) limb iliac blood flow, and iliac vascular conductance (iliac blood flow/mean arterial pressure) in response to intra-arterial infusions. For each infusion of antagonist, measurements were averaged over 30 s before the antagonist infusion (“Pre” values in tables). After the antagonist infusion, all variables were averaged over 5-s intervals, and the highest 5-s average was chosen as the peak response. The percent change in vascular conductance was calculated as {[(Preinfusion vascular conductance-postinfusion vascular conductance)/preinfusion vascular conductance] × 100}.
Data for each drug and receptor type were analyzed separately. Data were analyzed by two-way (age × exercise intensity) repeated-measures ANOVA. Where significant F ratios were found, Tukey's post hoc analysis was performed. All data are presented as means ± SE. A P value of <0.05 was considered statistically significant.
RESULTS
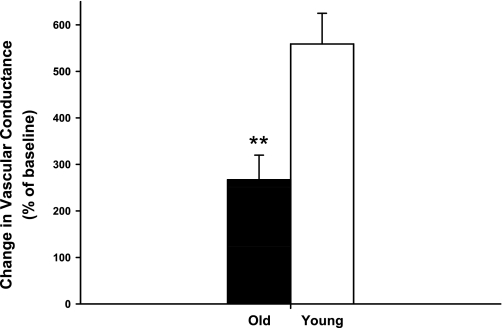
As shown in Table 1, all of the antagonists markedly inhibited the reduction in blood flow elicited by the respective agonist, and there were no age-related differences in the effectiveness of the antagonists in the canine hindlimb. Baseline hemodynamics and responses to intra-arterial infusions of antagonists are presented in Table 2 (prazosin), Table 3 (rauwolscine), Table 4 (BIBP), and Table 5 (PPADS). For all trials, control and experimental limb blood flow and vascular conductance increased from rest (P < 0.05) in an exercise intensity-dependent manner. Control and experimental limb blood flow and vascular conductance were not different between young and old dogs at rest or during exercise prior to drug infusion. The response to infusion of ACh was significantly attenuated in old compared with young dogs (Fig. 1).
Table 1.
Demonstration of effective receptor blockade
| Alpha-1, ml/min |
Alpha-2, ml/min |
P2x, ml/min |
NPY, ml/min |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 46 ± 8 | 14 ± 5 | 52 ± 21 | 32 ± 6 | 41 ± 13 | 24 ± 6 | 24 ± 7 | 3 ± 1 |
| 2.5 miles/h | 138 ± 17 | 18 ± 8 | 97 ± 13 | 25 ± 6 | 84 ± 22 | 12 ± 7 | 95 ± 14 | 6 ± 2 | |
| 4 miles/h 2.5% grade | 145 ± 22 | 21 ± 5 | 113 ± 16 | 24 ± 6 | 110 ± 11 | 21 ± 6 | 95 ± 17 | 3 ± 1 | |
| Old | Rest | 39 ± 9 | 9 ± 3 | 56 ± 15 | 22 ± 3 | 32 ± 11 | 13 ± 2 | 20 ± 5 | 4 ± 2 |
| 2.5 miles/h | 124 ± 8 | 22 ± 3 | 101 ± 14 | 33 ± 6 | 72 ± 16 | 16 ± 5 | 74 ± 24 | 9 ± 2 | |
| 4 miles/h 2.5% grade | 124 ± 16 | 17 ± 4 | 125 ± 29 | 24 ± 5 | 83 ± 19 | 37 ± 5 | 83 ± 22 | 6 ± 1 | |
Values are expressed as means ± SE. All post values are significantly different from prevalues P < 0.01. The following selective agonists and doses were utilized for each receptor type: Alpha-1 receptors: phenylephrine (0.1 μg·ml−1·min−1); Alpha-2 receptors: Clonidine (1 μg·ml−1·min−1); P2x: α,β-methylene ATP; (1 μg·ml−1·min−1); NPY: [Leu31, Pro34] NPY (1 μg·ml−1·min−1). The change in experimental limb blood flow in response to intra-arterial infusion of selective receptor agonist before (pre) and after (post) administration of the corresponding receptor antagonist.
Table 2.
Baseline hemodynamics and vascular response to intra-arterial infusion of alpha-1 receptor antagonist
| MAP, mmHg |
Control Limb Blood Flow, ml/min |
Experimental Limb Blood Flow, ml/min |
Control Limb Conductance, ml·min−1·mmHg−1 |
Experimental Limb Conductance, ml·min−1·mmHg−1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 117 ± 4 | 112 ± 1 | 56 ± 8 | 61 ± 9 | 61 ± 5 | 154 ± 27* | 0.48 ± 0.07 | 0.54 ± 0.08 | 0.53 ± 0.05 | 1.39 ± 0.24* |
| 2.5 miles/h | 129 ± 3 | 124 ± 3 | 232 ± 29 | 230 ± 45 | 209 ± 29 | 352 ± 39* | 1.77 ± 0.20 | 1.83 ± 0.36 | 1.62 ± 0.20 | 2.86 ± 0.33* | |
| 4 miles/h 2.5% grade | 136 ± 3 | 134 ± 3 | 258 ± 42 | 270 ± 45 | 248 ± 35 | 378 ± 42* | 1.93 ± 0.34 | 2.05 ± 0.36 | 1.84 ± 0.26 | 2.84 ± 0.33* | |
| Old | Rest | 123 ± 12 | 126 ± 11 | 73 ± 23 | 79 ± 33 | 57 ± 9 | 116 ± 20* | 0.61 ± 0.15 | 0.61 ± 0.20 | 0.47 ± 0.07 | 0.92 ± 0.16* |
| 2.5 miles/h | 137 ± 8 | 131 ± 11 | 230 ± 20 | 234 ± 27 | 190 ± 15 | 282 ± 24* | 1.79 ± 0.23 | 1.79 ± 0.35 | 1.42 ± 0.16 | 2.31 ± 0.40* | |
| 4 miles/h 2.5% grade | 153 ± 10 | 156 ± 10 | 279 ± 15 | 285 ± 37 | 242 ± 23 | 308 ± 26* | 1.99 ± 0.39 | 2.01 ± 0.41 | 1.59 ± 0.12 | 2.00 ± 0.18* | |
Values are means ± SE.
P < 0.05 indicates a significant difference from Pre value.
Table 3.
Baseline hemodynamics and vascular response to intra-arterial infusion of alpha-2 receptor antagonist
| MAP, mmHg |
Control Limb Blood Flow, ml/min |
Experimental Limb Blood Flow, ml/min |
Control Limb Conductance, ml·min−1·mmHg−1 |
Experimental Limb Conductance, ml·min−1·mmHg−1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 112 ± 3 | 113 ± 3 | 62 ± 8 | 67 ± 13 | 65 ± 10 | 145 ± 31* | 0.57 ± 0.07 | 0.59 ± 0.11 | 0.58 ± 0.09 | 1.27 ± 0.27* |
| 2.5 miles/h | 121 ± 5 | 117 ± 3 | 195 ± 31 | 198 ± 23 | 171 ± 25 | 301 ± 40* | 1.59 ± 0.20 | 1.68 ± 0.17 | 1.39 ± 0.16 | 2.58 ± 0.35* | |
| 4 miles/h 2.5% grade | 130 ± 5 | 125 ± 6 | 252 ± 36 | 266 ± 37 | 227 ± 26 | 347 ± 33* | 1.96 ± 0.32 | 2.17 ± 0.36 | 1.76 ± 0.23 | 2.83 ± 0.34* | |
| Old | Rest | 127 ± 13 | 130 ± 12 | 84 ± 20 | 88 ± 17 | 61 ± 11 | 96 ± 14* | 0.70 ± 0.19 | 0.72 ± 0.16 | 0.48 ± 0.08 | 0.75 ± 0.11* |
| 2.5 miles/h | 132 ± 7 | 132 ± 7 | 225 ± 33 | 255 ± 33 | 171 ± 14 | 277 ± 31* | 1.78 ± 0.34 | 2.01 ± 0.36 | 1.32 ± 0.16 | 2.14 ± 0.32* | |
| 4 miles/h 2.5% grade | 137 ± 6 | 135 ± 6 | 320 ± 37 | 338 ± 49 | 232 ± 32 | 324 ± 43* | 2.49 ± 0.35 | 2.68 ± 0.45 | 1.71 ± 0.24 | 2.43 ± 0.33* | |
Values are means ± SE.
P < 0.05 indicates a significant difference from Pre value.
Table 4.
Baseline hemodynamics and vascular response to intra-arterial infusion of NPY-Y1 receptor antagonist
| MAP, mmHg |
Control Limb Blood Flow, ml/min |
Experimental Limb Blood Flow, ml/min |
Control Limb Conductance, ml·min−1·mmHg−1 |
Experimental Limb Conductance, ml·min−1·mmHg−1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 120 ± 2 | 116 ± 7 | 74 ± 12 | 87 ± 17 | 61 ± 7 | 150 ± 13* | 0.62 ± 0.10 | 0.77 ± 0.17 | 0.50 ± 0.06 | 1.30 ± 0.12* |
| 2.5 miles/h | 138 ± 5 | 130 ± 12 | 166 ± 14 | 188 ± 28 | 176 ± 17 | 255 ± 36* | 1.21 ± 0.13 | 1.47 ± 0.22 | 1.26 ± 0.10 | 1.96 ± 0.25* | |
| 4 miles/h 2.5% grade | 143 ± 5 | 134 ± 9 | 212 ± 20 | 230 ± 22 | 222 ± 24 | 296 ± 31* | 1.46 ± 0.17 | 1.72 ± 0.20 | 1.57 ± 0.20 | 2.24 ± 0.26* | |
| Old | Rest | 118 ± 6 | 118 ± 19 | 65 ± 13 | 78 ± 16 | 59 ± 6 | 107 ± 8* | 0.54 ± 0.09 | 0.67 ± 0.15 | 0.50 ± 0.04 | 0.91 ± 0.06* |
| 2.5 miles/h | 155 ± 11 | 143 ± 27 | 194 ± 25 | 204 ± 29 | 155 ± 11 | 210 ± 27* | 1.34 ± 0.27 | 1.58 ± 0.34 | 1.01 ± 0.06 | 1.51 ± 0.16* | |
| 4 miles/h 2.5% grade | 155 ± 9 | 156 ± 25 | 311 ± 32 | 305 ± 37 | 222 ± 34 | 275 ± 35* | 2.16 ± 0.31 | 2.15 ± 0.35 | 1.43 ± 0.20 | 1.77 ± 0.21* | |
Values are expressed as means ± SE.
P < 0.05 indicates a significant difference from Pre value.
Table 5.
Baseline hemodynamics and vascular response to intra-arterial infusion of P2x receptor antagonist
| MAP, mmHg |
Control Limb Blood Flow, ml/min |
Experimental Limb Blood Flow, ml/min |
Control Limb Conductance, ml·min−1·mmHg−1 |
Experimental Limb Conductance, ml·min−1·mmHg−1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Intensity | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Young | Rest | 117 ± 4 | 114 ± 3 | 74 ± 8 | 70 ± 18 | 112 ± 9 | 201 ± 13* | 0.64 ± 0.20 | 0.62 ± 0.15 | 0.96 ± 0.07 | 1.77 ± 0.12* |
| 2.5 miles/h | 127 ± 3 | 126 ± 2 | 184 ± 31 | 179 ± 32 | 211 ± 29 | 305 ± 45* | 1.48 ± 0.25 | 1.42 ± 0.24 | 1.68 ± 0.24 | 2.40 ± 0.32* | |
| 4 miles/h 2.5% grade | 139 ± 7 | 141 ± 6 | 267 ± 30 | 245 ± 32 | 256 ± 41 | 316 ± 40* | 1.92 ± 0.18 | 1.72 ± 0.18 | 1.81 ± 0.24 | 2.22 ± 0.25* | |
| Old | Rest | 120 ± 9 | 126 ± 9 | 80 ± 22 | 61 ± 13 | 64 ± 20 | 163 ± 31* | 0.69 ± 0.21 | 0.50 ± 0.11 | 0.54 ± 0.16 | 1.38 ± 0.31* |
| 2.5 miles/h | 141 ± 6 | 141 ± 9 | 243 ± 20 | 234 ± 23 | 186 ± 24 | 237 ± 28* | 1.77 ± 0.16 | 1.77 ± 0.23 | 1.32 ± 0.17 | 1.73 ± 0.27* | |
| 4 miles/h 2.5% grade | 156 ± 9 | 171 ± 13 | 345 ± 54 | 312 ± 35 | 232 ± 30 | 200 ± 19* | 2.40 ± 0.50 | 2.01 ± 0.33 | 1.48 ± 0.14 | 1.20 ± 0.13* | |
Values are expressed as means ± SE.
P < 0.05, indicates a significant difference from Pre value.
Fig. 1.
Percent change in experimental limb iliac vascular conductance in response to intra-arterial infusion of ACh (0.1 μg bolus) at rest in young (open bars) and old (solid bars) beagles. Values are expressed as means ± SE. **P < 0.001 indicates a significant difference between young and old.
Series 1: alpha-1-adrenergic receptor-mediated restraint of skeletal muscle blood flow in young and old dogs.
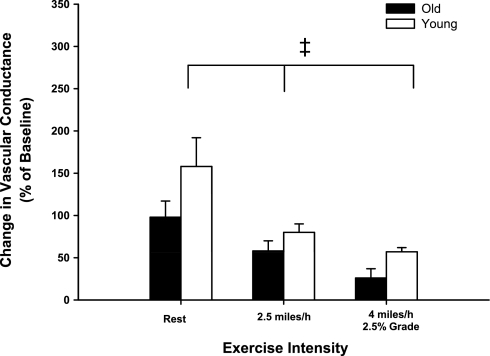
Intra-arterial infusions of prazosin caused similar increases (P > 0.05) in experimental limb vascular conductance at rest and during exercise at 2.5 mph and 4 mph and 2.5% grade in young and old dogs (Fig. 2). The amount of tonic vasoconstriction interrupted by infusion of prazosin was reduced (i.e., sympatholysis) during exercise at 2.5 mph and at 4 mph and 2.5% grade compared with rest (main effect of exercise intensity; P < 0.001). No interaction between age and exercise intensity was observed, indicating that the amount of sympatholysis was similar between old and young dogs.
Fig. 2.
Percent change in experimental limb iliac vascular conductance in response to intra-arterial infusion of the selective α1-adrenergic receptor antagonist prazosin (0.25 μg·ml−1·min−1) at rest and during treadmill running at 2.5 mph and 4 mph with 2.5% grade in young (open bars) and old (black bars) beagles. Absolute values for all hemodynamic variables prior to and following antagonist infusion are presented in Table 2. Values are expressed as means ± SE. ‡P < 0.001 indicates a main effect of exercise intensity.
Series 2: alpha-2-adrenergic receptor mediated restraint of skeletal muscle blood flow in young and old dogs.
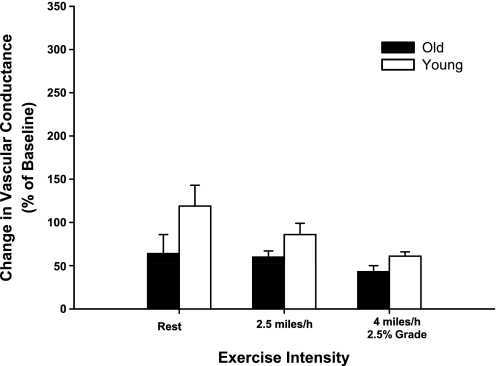
Intra-arterial infusions of rauwolscine caused similar increases (P > 0.05) in experimental limb vascular conductance (VC) at rest and during exercise at 2.5 mph and 4 mph and 2.5% grade in young and old dogs (Fig. 3). The amount of tonic vasoconstriction interrupted by infusion of rauwolscine was similar at rest and during exercise at 2.5 mph and at 4 mph and 2.5% grade.
Fig. 3.
Percent change in experimental limb iliac vascular conductance in response to intra-arterial infusion of the α2-adrenergic receptor antagonist rauwolscine (1.25 μg·ml−1·min−1) at rest and during treadmill running at 2.5 mph and 4 mph with 2.5% grade in young (open bars) and old (black bars) beagles. Absolute values for all hemodynamic variables prior to and following antagonist infusion are presented in Table 3. Values are expressed as means ± SE.
Series 3: NPY-Y1 receptor-mediated restraint of skeletal muscle blood flow in young and old dogs.
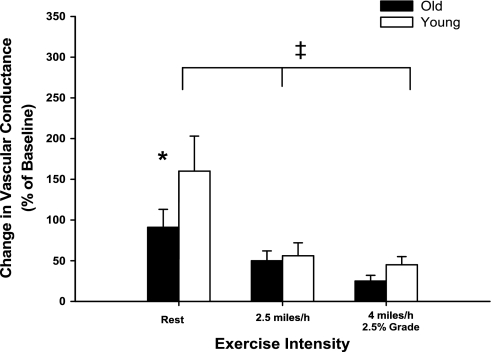
Intra-arterial infusions of BIBP caused a smaller increase (P < 0.05) in experimental limb vascular conductance in old compared with young dogs at rest, whereas similar increases (P > 0.05) of experimental limb vascular conductance in young and old dogs were observed following BIBP infusion during exercise at 2.5 mph and 4 mph and 2.5% grade (Fig. 4). The amount of tonic vasoconstriction interrupted by infusion of BIBP was reduced (i.e., sympatholysis) during exercise at 2.5 mph and at 4 mph and 2.5% grade compared with rest (main effect of exercise intensity; P < 0.001).
Fig. 4.
Percent change in experimental limb iliac vascular conductance in response to intra-arterial infusion of the selective NPY-Y1 receptor antagonist BIBP (2.5 mg bolus) at rest and during treadmill running at 2.5 mph and 4 mph with 2.5% grade in young (open bars) and old (black bars) beagles. Absolute values for all hemodynamic variables prior to and following antagonist infusion are presented in Table 4. Values are expressed as means ± SE. *P < 0.05 indicates a significant difference between young and old at rest. ‡P < 0.001 indicates a main effect of exercise intensity.
Series 4: P2x receptor-mediated restraint of skeletal muscle blood flow in young and old dogs.
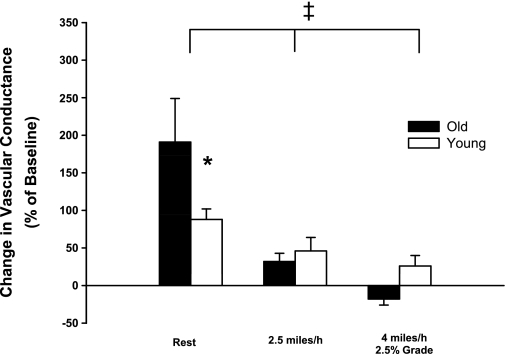
Intra-arterial infusions of PPADS caused a larger increase (P < 0.05) in experimental limb vascular conductance in old compared with young dogs at rest. During exercise at 2.5 mph, PPADS infusion resulted in a similar increase (P > 0.05) in vascular conductance in young and old dogs. Paradoxically, PPADS infusion caused a small decrease in VC at 4 mph and 2.5% grade in old dogs, whereas a small increase in vascular conductance was observed in young dogs. Despite these directionally different responses, the change in vascular conductance in response to PPADS infusion was not different (P > 0.05) between young and old beagles at 4 mph and 2.5% grade (Fig. 5). The amount of tonic vasoconstriction interrupted by infusion of PPADS was reduced (i.e., sympatholysis) during exercise at 2.5 mph and at 4 mph and 2.5% grade compared with rest (main effect of exercise intensity; P < 0.001).
Fig. 5.
Percent change in experimental limb iliac vascular conductance in response to intra-arterial infusion of the purinergic receptor antagonist PPADS (40 mg bolus) at rest and during treadmill running at 2.5 mph and 4 mph with 2.5% grade in young (open bars) and old (black bars) beagles. Absolute values for all hemodynamic variables prior to and following antagonist infusion are presented in Table 5. Values are expressed as means ± SE. *P < 0.05 indicates a significant difference between young and old. ‡P < 0.001, indicates a main effect of exercise intensity.
DISCUSSION
The purpose of this study was to investigate the effect of aging on adrenergic and nonadrenergic receptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscle. The main findings of this study were that 1) skeletal muscle blood flow and MAP were similar between young and old dogs at rest and during exercise; 2) the magnitude of alpha-1 and alpha-2 adrenergic receptor-mediated restraint of skeletal muscle blood flow was not different in old compared with young beagles at rest or during dynamic exercise; 3) the magnitude of NPY-Y1 receptor-mediated restraint of skeletal muscle blood flow was reduced in old beagles at rest but similar in young and old dogs during dynamic exercise; 4) the magnitude of P2x receptor-mediated restraint of skeletal muscle blood flow was increased in old dogs at rest but not different in old compared with young beagles during dynamic exercise.
Skeletal muscle blood flow was similar between young and old beagles at rest and during treadmill exercise at 2.5 mph and 4 mph and 2.5% grade. Previous studies of the effects of aging on skeletal muscle blood flow in humans and animals have produced conflicting results. Consistent with the present findings, several other studies have reported no decline in skeletal muscle blood flow with aging (38, 40, 44, 49), whereas others have reported reduced blood flow to skeletal muscles at rest (16–18) and during exercise (1, 33, 37, 47, 48, 50). Whether the maintained skeletal muscle blood flow with aging in the present study reflects an inherent difference between experimental models of aging or lifestyle factors that affect vascular function cannot be discerned.
The decreased skeletal muscle blood flow with aging in other studies has been attributed to increased efferent sympathetic outflow (16) and elevated postsynaptic receptor responsiveness (19). Although there is good evidence of an age-related increase of sympathetic efferent nerve activity to resting muscle (16, 43), there is little experimental evidence of elevated efferent muscle sympathetic outflow during exercise in aged animals or humans (39, 49, 50). Aging-related changes in canine muscle sympathetic nerve activity have not yet been characterized. The only published data show that renal sympathetic nerve activity at rest was elevated in old compared with young dogs at similar carotid sinus pressures (32). It seems likely that there would be a similar age-related increase in sympathetic outflow to the canine skeletal muscle vascular bed; however, this has not been established experimentally, and the effect of exercise on the magnitude and pattern of sympathetic outflow in aged animals remains unknown.
Since infusion of selective adrenergic antagonists produced similar increases in vascular conductance at rest, we conclude that the magnitude of tonic alpha-1 and alpha-2 adrenergic receptor-mediated restraint of skeletal muscle blood flow was similar in old compared with young beagles at rest. Previous studies in humans have produced conflicting results. Nonselective α-adrenergic receptor blockade produced a smaller increase in forearm blood flow in old compared with young adults, (15), suggesting that tonic α-adrenergic-receptor-mediated vasoconstriction was reduced in older adults. In contrast, however, basal leg blood flow and vascular conductance were lower in old compared with young adults and nonspecific α-adrenergic blockade abolished the group differences between old and young (18). The present study demonstrated, for the first time, that there is tonic P2x and NPY-Y1 receptor-mediated vasoconstriction in skeletal muscle vasculature of old dogs. The magnitude of tonic NPY-Y1 receptor mediated vasoconstriction was lower in old compared with young beagles at rest, suggesting that in older dogs, NPY makes a smaller contribution to the overall control of skeletal muscle vascular tone. P2x receptor-mediated vasoconstriction was increased in old compared with young dogs at rest. Overall, these data demonstrate that adrenergic receptor-mediated vasoconstriction was not elevated at rest, but nonadrenergic sympathetic vasoconstriction was altered under basal conditions in aged beagles. NPY-Y1 receptor-mediated vasoconstriction was reduced in old dogs, whereas P2x receptor-mediated vasoconstriction was increased in old dogs. Potentially, age-related changes in the frequency of sympathetic nerve discharge may be responsible for the altered contribution of the nonadrenergic neurotransmitters ATP and NPY to the overall regulation of skeletal muscle vascular tone at rest in aged animals. Changes in the responsiveness of postsynaptic NPY and P2x receptors with aging may also be involved in the altered contribution of these neurotransmitters to resting vascular tone. Collectively, the similar magnitude of adrenergic receptor-mediated restraint of skeletal muscle blood flow in old and young dogs and the directionally opposite age-related changes in the contribution of NPY and P2x to tonic vasoconstriction are consistent with the maintenance of basal skeletal muscle vascular conductance with aging in the present study.
To our knowledge, the present study is the first to investigate the effect of aging on the magnitude of adrenergic and nonadrenergic receptor-mediated vasoconstriction in the skeletal muscle vascular bed during exercise. The results demonstrate a similar magnitude of alpha-1 and alpha-2 adrenergic receptor-mediated restraint of skeletal muscle blood flow in old and young dogs during dynamic exercise. Moreover, P2x and NPY-Y1 receptor-mediated vasoconstriction was not different between young and old dogs during exercise. These findings suggest that there is not an obligatory increase in adrenergic and/or nonadrenergic receptor-mediated sympathetic vasoconstriction during exercise as a function of aging. Interestingly, the age-related differences in nonadrenergic receptor-mediated vascular control under basal conditions were eliminated during exercise. This finding implies that the neural regulation of the circulation may differ at rest compared with exercise with aging. Advancing age may alter the pattern of sympathetic outflow and the relative contributions of different neurotransmitters to overall sympathetic vasoconstriction under basal conditions; however, in response to physiological stress, such as exercise, where sympathetic outflow is increased several-fold above resting levels, the pattern and magnitude of sympathetic outflow appear to be similar in young and older dogs.
Experimental considerations.
A major strength of the present experimental approach is the ability to study basic physiological mechanisms of vascular control in conscious dynamically exercising animals by delivering selective agonists and antagonists to a discrete vascular bed without altering blood pressure or blood flow in other vascular beds. The findings of the present study in young and old dogs were similar in several respects to previous studies from our laboratory in young mongrel dogs (5, 6, 8, 9). Consistent with those studies, local and systemic hemodynamic variables increased in a manner dependent on exercise intensity and evidence of tonic adrenergic and nonadrenergic sympathetic vasoconstriction was observed at rest and during exercise. The magnitude of sympathetic vasoconstriction has been shown to vary as a function of exercise intensity (3). A limited number of exercise intensities were used in the present study, and it is conceivable that the effect of aging on sympathetic vasoconstriction may be different at other intensities. It should also be recognized that infusion of pharmacological agents into the entire hindlimb does not enable determination of the relative contribution of different segments of the vascular tree to the vascular response. The findings of Musch et al. (40), who reported similar hindlimb blood flows in old and young rats running at the same absolute intensity, but an altered distribution of hindlimb limb flow toward type II muscles in aged animals, highlight the potential for differential vascular control in different vascular beds and segments of the vascular tree that could not be investigated with the present experimental approach.
The canine model has been used previously to investigate the effect of aging on the cardiovascular system (24–32). The mean lifespan of beagles is 12.6 yr (41). The aged dogs in the present study had a mean age of 9.8 yr and were, therefore, on average in the last quarter of their expected lifespan. Studies of the comparative longevity of dogs and humans indicate that a chronological age of ∼10 years in beagles corresponds to a physiological age of ∼66 years in humans (45). The vascular response to the infusion of ACh was also blunted in the aged dogs in the present study. Thus, the dogs used in the present study appear to be “old” in both chronological and physiological terms.
Because of the long lifespan of this experimental model, the experimental design was necessarily cross-sectional. The animals used in this study were raised in a temperature-controlled, enriched environment in which they were provided balanced nutrition and regular veterinary supervision. Presumably, this lifestyle was associated with minimal behavioral stress and a decreased likelihood of disease related to negative lifestyle choices available to humans, and this may have had a protective effect of vascular function in the present study. Parker et al. (44) recently reported that leg vascular conductance was significantly higher during incremental exercise in older men with a V̇o2max equal to or greater than 60% of age predicted compared with older men with a V̇o2max below the 30th percentile for age group norms. These findings illustrate the potential positive impact of lifestyle choices that maintain functional capacity and vascular function.
In conclusion, this study demonstrated that basal and contracting skeletal muscle blood flow does not inexorably decline as a function of age in beagles. Alpha-1 and alpha-2 adrenergic receptor-mediated restraint of resting skeletal muscle blood flow did not differ between young and old beagles. NPY-Y1 receptor-mediated vasoconstriction at rest was reduced with aging, whereas P2x receptor-mediated vasoconstriction in resting muscle was increased with aging, suggesting a decreased contribution of NPY and an increased contribution of ATP to the control of vascular tone in resting skeletal muscle of old dogs. During exercise, the magnitude of adrenergic and nonadrenergic receptor-mediated restraint of hindlimb skeletal muscle blood flow was not different in young and old beagles.
GRANTS
Support for this project was provided by the National Heart, Lung, and Blood Institute and the Medical Research Service of the Department of Veterans Affairs. D. S. DeLorey was supported by a postdoctoral research fellowship from the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors acknowledge the expert technical assistance of Mr. Paul Kovac.
REFERENCES
- 1.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol 284: H2007–H2014, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JB, Hamann JJ, Clifford PS. Neuropeptide Y1 receptor vasoconstriction in exercising canine skeletal muscles. J Appl Physiol 99: 2115–2120, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2x purinergic receptors? J Appl Physiol 95: 953–959, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JB, Hamann JJ, Kluess HA, Clifford PS. Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol 287: H144–H149, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Do P2x purinergic receptors regulate skeletal muscle blood flow during exercise? Am J Physiol Heart Circ Physiol 286: H633–H639, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol Heart Circ Physiol 277: H33–H39, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol 83: 1575–1580, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Coney AM, Marshall JM. Contribution of α2-adrenoceptors and Y1 neuropeptide Y receptors to the blunting of sympathetic vasoconstriction induced by systemic hypoxia in the rat. J Physiol 582: 1349–1359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davy KP, Jones PP, Seals DR. Influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Am J Physiol Regul Integr Comp Physiol 273: R690–R695, 1997 [DOI] [PubMed] [Google Scholar]
- 12.DeLorey DS, Kowalchuk JM, Paterson DH. Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy-intensity exercise in young and older adults. J Appl Physiol 98: 1697–1704, 2005 [DOI] [PubMed] [Google Scholar]
- 13.DeLorey DS, Kowalchuk JM, Paterson DH. The effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97: 165–172, 2004 [DOI] [PubMed] [Google Scholar]
- 14.DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metab 32: 1251–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Dinenno FA, Seals DR, Desouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531: 573–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elam M, Sverrisdottir YB, Rundqvist B, McKenzie D, Wallin BG, Macefield VG. Pathological sympathoexcitation: how is it achieved? Acta Physiol Scand 177: 405–411, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Evans RJ, Cunnane TC. Relative contributions of ATP and noradrenaline to the nerve evoked contraction of the rabbit jejunal artery. Dependence on stimulation parameters. Naunyn Schmiedebergs Arch Pharmacol 345: 424–430, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Flavahan NA, Vanhoutte PM. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J Pharmacol Exp Ther 239: 784–789, 1986 [PubMed] [Google Scholar]
- 23.Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78: 890–900, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Haidet GC. Alpha-adrenergic-mediated reflex responses to induced muscular contraction are changed with age in dogs. Am J Physiol Heart Circ Physiol 265: H1899–H1908, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Haidet GC. Effects of age on beta-adrenergic-mediated reflex responses to induced muscular contraction in beagles. Mech Ageing Dev 68: 89–104, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Haidet GC. Effect of age on cardiovascular responses to static muscular contraction in beagles. J Appl Physiol 73: 2320–2327, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Haidet GC. Dynamic exercise in senescent beagles: oxygen consumption and hemodynamic responses. Am J Physiol Heart Circ Physiol 257: H1428–H1437, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Haidet GC, Parsons D. Reduced exercise capacity in senescent beagles: an evaluation of the periphery. Am J Physiol Heart Circ Physiol 260: H173–H182, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Haidet GC, Wennberg PW, Finkelstein SM, Morgan DJ. Effects of aging per se on arterial stiffness: systemic and regional compliance in beagles. Am Heart J 132: 319–327, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Haidet GC, Wennberg PW, Rector TS. Aging and vasoreactivity: in vivo responses in the beagle hindlimb. Am J Physiol Heart Circ Physiol 268: H92–H99, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Hajduczok G, Chapleau MW, Abboud FM. Increase in sympathetic activity with age. II. Role of impairment of cardiopulmonary baroreflexes. Am J Physiol Heart Circ Physiol 260: H1121–H1127, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Hajduczok G, Chapleau MW, Johnson SL, Abboud FM. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol Heart Circ Physiol 260: H1113–H1120, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Hammer LW, Boegehold MA. Functional hyperemia is reduced in skeletal muscle of aged rats. Microcirculation 12: 517–526, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Haniuda K, Nakane T, Chiba S. Different contributions of ATP and noradrenaline to neurotransmission in the isolated canine intermediate auricular artery. Eur J Pharmacol 333: 163–168, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Johnson CD, Coney AM, Marshall JM. Roles of norepinephrine and ATP in sympathetically evoked vasoconstriction in rat tail and hindlimb in vivo. Am J Physiol Heart Circ Physiol 281: H2432–H2440, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Magnusson G, Kaijser L, Isberg B, Saltin B. Cardiovascular responses during one- and two-legged exercise in middle-aged men. Acta Physiol Scand 150: 353–362, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Mazzeo RS, Rajkumar C, Jennings G, Esler M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J Appl Physiol 82: 1869–1874, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council Mammalian Models for Research on Aging. Washington D.C: National Academy Press, 1981, p. 587 [Google Scholar]
- 42.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol 267: H344–H353, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci 52: B171–B178, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Pernow J, Schwieler J, Kahan T, Hjemdahl P, Oberle J, Wallin BG, Lundberg JM. Influence of sympathetic discharge pattern on norepinephrine and neuropeptide Y release. Am J Physiol Heart Circ Physiol 257: H866–H872, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol 94: 1859–1869, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Ren LM, Burnstock G. Prominent sympathetic purinergic vasoconstriction in the rabbit splenic artery: potentiation by 2,2′-pyridylisatogen tosylate. Br J Pharmacol 120: 3: 530–536, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revington M, McCloskey DI. Neuropeptide Y and control of vascular resistance in skeletal muscle. Regul Pept 23: 331–342, 1988 [DOI] [PubMed] [Google Scholar]
- 53.Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol 97: 781–789, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Tanaka K, Yang XP, Chiba S. Purinergic and adrenergic cotransmission in canine isolated and perfused gastroepiploic arteries. Clin Exp Pharmacol Physiol 30: 678–683, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Tarasova O, Sjoblom-Widfeldt N, Nilsson H. Transmitter characteristics of cutaneous, renal and skeletal muscle small arteries in the rat. Acta Physiol Scand 177: 157–166, 2003 [DOI] [PubMed] [Google Scholar]