Abstract
The arterial baroreflex is fundamental for evoking and maintaining appropriate cardiovascular adjustments to exercise. We sought to investigate how aging influences carotid baroreflex regulation of blood pressure (BP) during dynamic exercise. BP and heart rate (HR) were continuously recorded at rest and during leg cycling performed at 50% HR reserve in 15 young (22 ± 1 yr) and 11 older (61 ± 2 yr) healthy subjects. Five-second pulses of neck pressure and neck suction from +40 to −80 Torr were applied to determine the full carotid baroreflex stimulus response curve and examine baroreflex resetting during exercise. Although the maximal gain of the modeled stimulus response curve was similar in both groups at rest and during exercise, in older subjects the operating point (OP) was located further away from the centering point (CP) and toward the reflex threshold, both at rest (OP minus CP; −10 ± 3 older vs. 0 ± 2 young mmHg, P < 0.05) and during exercise (OP minus CP; −10 ± 2 older vs. 1 ± 3 young mmHg, P < 0.05). In agreement, older subjects demonstrated a reduced BP response to neck pressure (simulated carotid hypotension) and a greater BP response to neck suction (simulated carotid hypertension). In addition, the magnitude of the upward and rightward resetting of the carotid baroreflex-BP stimulus response curve with exercise was ∼40% greater in older individuals. These data indicate that despite a maintained maximal gain, the ability of the carotid baroreflex to defend against a hypotensive challenge is reduced, whereas responses to hypertensive stimuli are greater with advanced age, both at rest and during exercise.
Keywords: cardiovascular control, autonomic nervous system, baroreflex sensitivity, arterial baroreceptors, total vascular conductance
the arterial baroreflex (ABR) plays a critical role in the moment-to-moment regulation of arterial blood pressure (BP) via modulation of autonomic neural activity to the heart and vasculature. Although a wealth of data has indicated that aging is associated with impairments in resting cardiac baroreflex function (3, 14, 19, 29), studies examining ABR control of muscle sympathetic nerve activity (SNA) have reported equivocal results demonstrating impaired, preserved, or augmented responsiveness in older healthy adults (11, 14, 24, 29, 30, 46). Moreover, regarding overall ABR regulation of BP and aging, limited studies have been performed suggesting either reduced (24, 27) or unchanged (4, 41) control with advanced age. Furthermore, to date, the effect of aging on ABR control of BP during dynamic exercise has not been studied.
During exercise it has been shown in both animals (1, 8) and young healthy subjects (35, 38) that the ABR continues to regulate BP by resetting to operate around the exercise-induced elevation in BP with no change in sensitivity (i.e., gain). In young healthy individuals the resetting of the ABR during exercise occurs in direct relation to the intensity of exercise and is mediated by the activation and interaction of central signals arising from higher brain centers (central command) (21, 23, 32, 37) and by peripheral feedback arising from exercising skeletal muscle (exercise pressor reflex) (6, 20, 23, 32). Given this, one cannot simply assume that age-related alterations in ABR control noted at rest are similar during exercise, because the activation of central command and the exercise pressor reflex and their potential modulatory effects on ABR sensitivity and resetting may differ in young and older subjects (7, 28). Moreover, a properly functioning ABR appears essential for an appropriate neural cardiovascular response to exercise (25). In exercising dogs, acute baro-denervation leads to an exaggerated increase in BP (49, 50), while in humans who have surgically denervated carotid baroreceptors, not only is resting BP variability elevated but the BP response to exercise is greater (42, 47). Such observations raise the possibility that age-related impairments in ABR function may contribute to the augmented BP often observed in older individuals during exercise (9, 19, 34, 45). However, whether aging affects the resetting, sensitivity, and general parameters for the ABR control of BP during dynamic exercise is presently unknown.
Given the limited information regarding aging and baroreflex function during exercise, the present study was designed to provide a thorough assessment of ABR control of BP during dynamic exercise in young and older healthy subjects. The application of neck pressure (NP) and neck suction (NS) was utilized to examine responses to simulated carotid hypotension and hypertension, respectively, at rest and during moderate intensity cycling. In addition, full carotid baroreflex stimulus response curves were derived to examine exercise resetting and to determine the operating point gain and maximal gain of the carotid baroreflex BP curve. We tested the hypothesis that the carotid baroreflex control of BP would be impaired in older individuals during exercise.
METHODS
Subjects
Fifteen young (21–29 yr) and eleven older (54–71 yr) healthy subjects were recruited for voluntary participation in the study with a mean age difference between groups of 39 yr. Subject characteristics are provided in Table 1. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board. Following a full verbal explanation of the experimental procedures and measurement techniques, subjects provided written informed consent.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Men/Women | 11/4 | 8/3 |
| Age, yr | 22 ± 1 | 61 ± 2* |
| Weight, kg | 73 ± 3 | 80 ± 5 |
| Height, cm | 173 ± 2 | 174 ± 3 |
| BMI, kg/m2 | 24 ± 1 | 26 ± 1 |
| Cholesterol, mg/dl | 198 ± 7 | |
| Triglycerides, mg/dl | 100 ± 13 | |
| LDL, mg/dl | 123 ± 6 | |
| HDL, mg/dl | 55 ± 5 | |
| Glucose, mg/dl | 100 ± 4 | |
| BUN, mg/dl | 16 ± 1 | |
| Na+, meq/l | 140 ± 1 | |
| K+, meq/l | 4.1 ± 0.1 | |
| Exercise workload, W | 104 ± 7 | 95 ± 8 |
| Peak HR, beats/min | 186 ± 3 | 159 ± 2* |
Values are means ± SE. BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BUN, blood urea nitrogen; HR, heart rate.
P < 0.05 vs. young.
Each subject completed a medical health history questionnaire, underwent a physical exam by a physician investigator, and in older subjects a 12-h fasting blood chemistry screening was performed (Table 1). Notably, no subject had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease. Subjects were nonsmokers and none were using prescribed or over-the-counter medications. Older subjects underwent Duplex ultrasound imaging within the University Radiology Department to screen for significant carotid artery plaques prior to performing NP and NS (see below). Both young and older subjects were moderately active and typically engaged in low (e.g., walking)-to-moderate (e.g., jogging, stationary bike) intensity aerobic activities (2–3 days/wk), but none competed in endurance events.
Prior to any experimental sessions, subjects were requested to abstain from eating for 2 h, caffeinated beverages for 12 h, and strenuous physical activity and alcohol for at least 24 h. All subjects were familiarized with the equipment and procedures prior to the experimental session.
Experimental Measurements
Arterial BP was measured on a beat-to-beat basis using photoplethysmography obtained from a finger of the left hand, while it rested on an adjustable bedside table at the level of the right atrium (Finometer; Finapres Medical Systems, Amsterdam, Netherlands). Beat-to-beat BP recordings were verified using an automated sphygmomanometer equipped with a microphone for the detection of Kortokoff sounds (Tango+; SunTech Medical Instruments, Raleigh, NC), which has been validated for use during dynamic exercise (5). Heart rate (HR) was monitored using a lead II ECG (model Q710; Quinton Instrument, Bothell, WA). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA). Ratings of perceived exertion were obtained using the standard 6–20 Borg scale (2). The BP waveform and ECG signal were sampled at 1,000 Hz and beat-to-beat values of BP and HR were stored for off-line analysis (Chart version 5.2; Powerlab, AD Instruments, Bella Vista, NSW, Australia).
Experimental Procedures
Incremental maximal exercise test.
All subjects performed a continuous incremental exercise test to ascertain peak HR for the determination of steady-state exercise workloads, as previously described (19). Briefly, subjects were seated in a semirecumbent position on a medical exam table equipped with an electrically braked cycle ergometer (Angio V2; Lode, Groningen, The Netherlands), while HR (12-lead ECG) and BP (automated sphygmomanometer) were measured. Following a 3-min warm-up period of cycling at 60 rpm, the workload was increased by 25 Watts every minute. Peak responses were determined at the power output where the subject could no longer maintain a pedal frequency of 60 rpm despite strong verbal encouragement. All subjects gave a maximal rating of perceived exertion (i.e., 19–20) at exhaustion.
Carotid baroreflex control of BP.
Full carotid baroreflex stimulus-response curves for the control of BP were derived at rest and during moderate intensity cycling (16, 35) at least 48 h following the incremental maximal exercise test. Following instrumentation, subjects were fitted with a malleable lead collar that encircled the anterior two-thirds of the neck for the application of NP and NS. Appropriate neck chamber placement was ensured by identifying the location of the carotid sinus bifurcation with ultrasound imaging and then fitting the subjects based on observed neck size. In addition, resting trials of NP and NS were always performed during a screening session to determine directionally appropriate and consistent mean BP responses. Carotid baroreflex function was determined by applying random-ordered, single 5-s pulses of NP and NS ranging from +40 to −80 Torr (i.e., +40, +20, −20, −40, −60, −80 Torr).
To minimize respiratory-related modulation of HR and BP the 5-s pulses of pressure and suction were delivered to the carotid sinus during a 10- to 15-s breath hold at end expiration under resting conditions. During exercise the breath hold was eliminated to avoid any potential for straining maneuvers and inadvertent activation of the ABR and/or chemoreflex (38). In addition, no differences have been identified between the responses to neck collar stimuli during inspiration and expiration at a breathing frequency of > 24 breaths/min as occurs with dynamic exercise (15). Four to five trials of NP and NS at each chamber pressure were performed at rest. However, during exercise, to allow subjects to be at steady state before carotid baroreflex testing began and to minimize any potential confounding effects of cardiovascular drift on carotid baroreflex function, only two to three perturbations at each chamber pressure were performed (33). A minimum of 45 and 30 s of recovery was allotted between NP and NS trials at rest and during exercise, respectively, to allow all physiological variables to return to prestimulus values. The exercise bout began with a low workload (∼30 Watts), which was then adjusted to elicit a target HR corresponding to 50% HR reserve, while pedal frequency was maintained at 60 rpm. Once the target HR was achieved, subjects exercised for ∼5 min to assure steady-state conditions, after which carotid baroreflex function was assessed.
Derivation of carotid baroreflex stimulus response curves.
At rest and during steady-state exercise, carotid-BP responses were evaluated by plotting the peak and nadir changes in mean BP evoked by NP and NS, respectively, against the estimated carotid sinus pressure (ECSP), which was calculated as mean BP minus neck chamber pressure. Beat-to-beat changes in mean BP measured by photoplethysmography were uniformly corrected to the absolute BP recorded via automated sphygmomanometry to provide accurate estimates of ECSP and mean BP. The carotid baroreflex stimulus response data were fitted to the logistic model described by Kent et al. (26) using the following equation: mean BP = A1{1 + exp[A2·(ECSP−A3)]}−1 + A4, where mean BP is the dependent variable, ECSP is the ECSP, A1 is the mean BP range of response (maximum-minimum), A2 is the gain coefficient, A3 is the carotid sinus pressure required to elicit an equal pressor and depressor response (centering point), and A4 is the minimum mean BP response. The data were fit to this model by nonlinear least-squares regression (using a Marquardt-Levenberg algorithm), which minimized the sum of squares error term to predict a curve of best fit for each set of raw data. The overall fit of the curves was similar in the young and older subjects with r2 values of 0.989 ± 0.001 vs. 0.980 ± 0.006, respectively, at rest and 0.983 ± 0.004 vs. 0.978 ± 0.005, respectively, during exercise. The coefficient of variation for the overall fit of this model to the individual responses was 18% for the younger subjects and 19% for the older subjects. The carotid-BP maximal gain and operating point gain were calculated using the following equations: GMAX = − A1A2/4 and GOP = A1A2 exp[A2(ECSPOP − A3)]/{1 + exp[ECSPOP − A3]}2, where GMAX is the maximal gain of carotid-BP stimulus response curve, GOP is the gain of carotid baroreflex stimulus response curve at the operating point, and ECSPOP is the ECSP at the operating point (i.e., prestimulus mean BP). The GMAX was calculated as the gain at the centering point and used as an index of overall carotid baroreflex responsiveness, whereas the GOP was calculated as the gain at the operating point and used to provide a measure of responsiveness at the operating point of the carotid-BP stimulus response curve. The latter measure becomes important during exercise as the operating point may move away from the centering point to a locus of reduced responsiveness (39). Thus, a reduction in the operating point gain can occur independently of a change in maximal gain (i.e., the gain at the centering point).
The movement of the operating point away from the centering point was directly assessed using the equation: operating point − centering point. The threshold (THR; point where no further increase in mean BP occurred, despite reductions in ECSP) and the saturation (SAT; point where no further decrease in mean BP occurred, despite increases in ECSP) were calculated by applying equations described by McDowall and Dampney (31): THR = −2.944/A2 + A3 and SAT = 2.944/A2 + A3. These calculations of THR and SAT are the carotid sinus pressures at which the reflexly controlled variable (i.e., mean BP) is within 5% of the upper or lower plateau of the sigmoid function. In addition to the derivation of carotid-BP stimulus response curves and associated parameters, individual responses to +40 Torr NP and −80 Torr NS were assessed independently to better understand potential age-related differences in responses to hypotension and hypertension, respectively. Also, to compare the magnitude of exercise-induced baroreflex resetting in the young and older groups, we calculated the sum of the changes in A3, A4, THR, and SAT from rest to exercise for each subject as an estimate of the upward and rightward movement of the carotid-BP curve that occurs with exercise.
Statistical Analysis
Statistical comparisons of physiological variables were made using a two-way repeated-measures ANOVA test, and a Student-Newman-Keuls test was employed post hoc to investigate significant main effects and interactions. Statistical significance was set at P < 0.05. Results are presented as means ± SE. Analyses were conducted using SigmaStat (Jandel Scientific Software; SPSS, Chicago, IL) for Windows.
RESULTS
Cardiovascular Responses at Rest and During Moderate Intensity Cycling
Table 2 presents cardiovascular parameters in the young and older subjects at rest and during exercise. Resting systolic BP, pulse pressure, and HR (P > 0.05), were similar between the younger and older subjects, while mean BP and diastolic BP were lower in the young group (P < 0.05). During leg cycling, systolic and mean BP were significantly increased from rest in both groups, but remained lower in the younger subjects (P < 0.05). Diastolic BP was unchanged from rest to exercise in both groups (P > 0.05). Ratings of perceived exertion were similar in the young and older subjects during exercise (12 ± 1 vs. 13 ± 0.5 units, young vs. older, respectively; P > 0.05).
Table 2.
Selected cardiovascular parameters at rest and during moderate-intensity cycling in young and older subjects
| Rest |
Exercise |
|||
|---|---|---|---|---|
| Young | Older | Young | Older | |
| Systolic BP, mmHg*† | 116 ± 3 | 116 ± 4 | 167 ± 4 | 179 ± 6 |
| Diastolic BP, mmHg† | 65 ± 2 | 75 ± 2 | 63 ± 2 | 77 ± 2 |
| Mean BP, mmHg*† | 81 ± 2 | 89 ± 3 | 98 ± 2 | 111 ± 2 |
| Pulse pressure, mmHg*† | 51 ± 3 | 40 ± 2 | 103 ± 5 | 102 ± 6 |
| HR, beats/min*†‡ | 59 ± 2 | 61 ± 3 | 125 ± 2 | 109 ± 3 |
Values are means ± SE. BP, blood pressure;
P < 0.05, main effect of condition;
P < 0.05, main effect of group;
P < 0.05, interaction between condition and group.
Carotid Baroreflex Control of BP at Rest and During Moderate Intensity Cycling
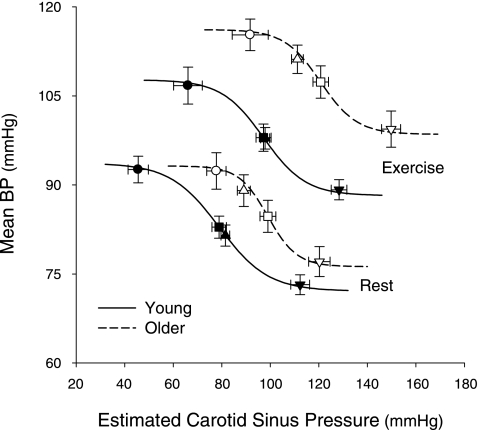
The stimulus response curves relating mean BP to ECSP at rest and during exercise in young and older subjects are shown in Fig. 1. Table 3 and Fig. 2 present the logistic model parameters and derived variables describing carotid baroreflex control of BP in the young and older individuals at rest and during exercise. The A1 was similar between young and older groups at rest (P > 0.05), and this persisted during exercise. However, the A3, A4, THR, and SAT were all significantly greater in older individuals both at rest and during exercise (P < 0.05 vs. young). An upward and rightward resetting of the carotid-BP stimulus response curve was observed in both young and older subjects as demonstrated by the significant increase in A3, A4, THR, and SAT from rest to exercise (all P < 0.05 vs. rest in young and older). Notably, the magnitude of carotid baroreflex resetting (i.e., the sum of the changes in A3, A4, THR, and SAT from rest to exercise for each subject) was ∼40% greater in the older group compared with the younger group (65 ± 9 vs. 90 ± 11 mmHg, young vs. older; P < 0.05).
Fig. 1.
Summary data demonstrating the upward and rightward resetting of the modeled carotid baroreflex blood pressure (BP) stimulus response curve from rest to moderate intensity cycling in young and older subjects. The lines and symbols denote actual group data for all subjects. Black symbols represent young subjects and white symbols denote older subjects. Squares indicate the centering points, triangles indicate the operating points, circles indicate the carotid sinus pressure thresholds, and inverted triangles represent the carotid sinus pressure saturations. Values are means ± SE.
Table 3.
Logistic model parameters and derived variables describing carotid baroreflex control of BP at rest and during moderate-intensity cycling in young and older subjects
| Rest |
Exercise |
|||
|---|---|---|---|---|
| Young | Older | Young | Older | |
| A1, mmHg | 22 ± 2 | 17 ± 2 | 20 ± 2 | 18 ± 2 |
| A2, au† | 0.10 ± 0.01 | 0.17 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 |
| A3, mmHg*† | 79 ± 3 | 99 ± 3 | 97 ± 3 | 121 ± 3 |
| A4, mmHg*† | 72 ± 2 | 76 ± 3 | 88 ± 2 | 99 ± 3 |
| Threshold, mmHg*† | 46 ± 4 | 78 ± 4 | 60 ± 5 | 92 ± 7 |
| Saturation, mmHg*† | 112 ± 4 | 120 ± 4 | 128 ± 3 | 150 ± 4 |
Values are means ± SE. A1, response range; A2, gain coefficient; A3, centering point; A4, minimum response; au, arbitrary units.
P < 0.05, main effect of condition (rest vs. exercise);
P < 0.05, main effect of group (young vs. older).
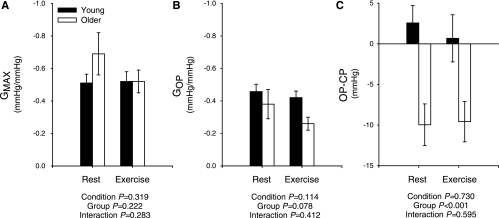
Fig. 2.
Summary data comparing maximal (A) and operating point (B) gains (GMAX, GOP, respectively) for carotid baroreflex control of BP in young and older subjects at rest and during moderate intensity cycling. C: operating point-to-centering point (OP-CP) relationship of the modeled carotid baroreflex BP stimulus response curve at rest and during cycling in young and older subjects. Values are means ± SE. P values indicate ANOVA results.
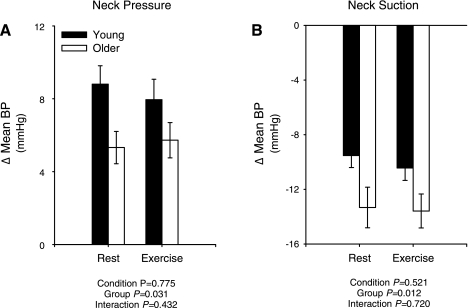
The GMAX for the carotid baroreflex BP stimulus response curve was similar in young and older subjects both at rest and during exercise, whereas the gain at GOP tended to be lower in the older group (Fig. 2, A and B). Of note, this tendency for a difference in GOP became significant (P = 0.004) with the omission of one older individual with an exceptionally high gain (−1.11 mmHg/mmHg). Compared with the young group, the operating point of the modeled stimulus response curve was located further away from the centering point and toward the reflex THR both at rest and during exercise in the older subjects (Fig. 2C), indicating a reduced ability to respond to hypotension with an augmented capacity to defend against hypertension. In line with this, compared with the younger group, the change in mean BP with +40 Torr NP was significantly attenuated, while the change in mean BP with −80 Torr NS was significantly greater in the older subjects, both at rest and during exercise (Fig. 3). Notably, in a subset analysis of seven young and seven older subjects with a similar resting mean BP (84 ± 1 vs. 86 ± 1 mmHg, young vs. older, P > 0.05), the changes in mean BP with +40 Torr NP (Δ9 ± 1 vs. 4 ± 1 mmHg, young vs. older, P < 0.05) and −80 Torr NS (Δ−9 ± 1 vs. −15 ± 1 mmHg, young vs. older, P < 0.05) were similar to the larger cohort.
Fig. 3.
Summary data showing changes in mean BP to neck pressure (+40 Torr; A) and neck suction (−80 Torr; B) at rest and during moderate intensity cycling in young and older subjects. Values are means ± SE. P values indicate ANOVA results.
DISCUSSION
In the present study, we have for the first time assessed the carotid baroreflex control of BP in older healthy subjects during dynamic exercise. The major novel findings are that the upward and rightward resetting of carotid baroreflex-BP stimulus response curve with exercise is augmented in older individuals and that compared with the younger group the reflex operating point is located further away from the centering point and toward the THR. This age-related relocation of the operating point was also present at rest and indicates that despite a maintained maximal gain, the ability of the carotid baroreflex to defend against a hypotensive challenge is reduced, whereas responses to hypertensive stimuli are greater with advanced age. Given the latter, these data indicate that impairments in the maximal gain of the carotid baroreflex control of BP do not appear to underlie the augmented pressor response to exercise in older adults.
There is convincing evidence that a properly functioning ABR is requisite for an appropriate neural cardiovascular response to exercise. Indeed, previous studies in both dogs (49, 50) and humans (42, 47) have demonstrated that baroreceptor denervation can lead to exaggerated BP responses to exercise. It has been suggested that the ABR acts to partially restrain the BP response to exercise by buffering increases in vasomotor tone (25). Thus, reductions in ABR gain (i.e., sensitivity) during exercise could lead to inappropriate increases in vascular tone and elevations in BP (25). In the present study, we hypothesized that older individuals would have reductions in ABR control of BP during exercise. Our rationale was that impairments in ABR function with increased age may contribute to the augmented BP often observed in older individuals during exercise (9, 19, 34, 45). However, the results suggest that although the carotid baroreflex BP function curve was reset further upward and rightward during exercise in the older group, no age-related differences in the maximal gain were observed. As such, reports of exaggerated BP responses to exercise in older individuals do not appear to be related to impaired ABR gain and may be attributable to dysregulation in other mechanisms implicated in the control of the vasculature and BP during exercise [e.g., impaired metabolic vasodilatation (40) and exaggerated sympathetic vasoconstriction (13, 17)].
Interestingly, our results show that although the maximal gain of the carotid baroreflex BP stimulus response curve was similar in young and older subjects, the operating point was located further away from the centering point and toward the reflex THR of the baroreflex curve in older individuals. Shi et al. (41) also reported that the resting sensitivity of the carotid-BP stimulus response curves were similar in young and older subjects; however, the location of the operating point on the reflex function curve was not examined. Moreover, Studinger et al. (46) demonstrated that age-related differences in ABR-mediated responses of SNA to rises and falls in BP were not evident when the responses were combined; that is, the gain of the integrated ABR control of SNA was not different between the young and older groups. However, sympathetic responses to reductions in BP were clearly attenuated in older subjects, while greater sympatho-inhibition to pharmacologically induced hypertension was also present. Taken together, these findings highlight the importance of examining the ability of the ABR to respond to rises and falls in BP separately. Indeed, although overall carotid baroreflex gain was similar between groups in the present study, older subjects had a reduced response to simulated carotid hypotension and a greater response to hypertensive stimuli both at rest and during exercise.
The finding that the BP responses to NS (i.e., simulated carotid hypertension) were greater in the older group during exercise suggests that the ability to inhibit sympathetic outflow to the peripheral vasculature was greater in the older group than in the younger individuals during exercise. This possibility is in accordance with recent work demonstrating greater ABR-mediated sympatho-inhibition in older subjects in response to pharmacological increases in BP at rest (46). However, whether an enhanced ability to inhibit sympathetic outflow persists during dynamic exercise in older subjects remains to be determined. In addition, alterations in vascular responsiveness cannot be excluded. In contrast to the augmented responses to carotid hypertension, the BP responses to simulated carotid hypotension (i.e., NP) were attenuated in older individuals. This observation concurs with the findings of Studinger et al., (46) who reported that the sympatho-excitatory responses to a pharmacologically induced reduction in systemic BP was attenuated in older compared with younger individuals. This blunted response in older individuals was attributed to a decrement in the mechanical transduction of the fall in BP to barosensory vessels due to increased arterial stiffening. While this may explain our observations, decreased α-adrenergic receptor sensitivity has also been reported in healthy older subjects (12, 22, 43), suggesting that NP-induced increases in sympathetic outflow may be less effective in increasing BP due to impaired neural vascular transduction. However, previous studies examining the influence of age on vascular responses to sympathetic activation have reported both blunted (10) and preserved (14) responses in older individuals. In addition, the reduced BP response to carotid baroreceptor unloading may also be related to an attenuated tachycardic response (18, 19). Further work is required to examine the influence of aging on the ABR-regulation of vascular conductance and the relative contribution of central hemodynamics to overall ABR control of BP.
Age-related impairments in ABR-mediated sympatho-excitation have previously been suggested to be linked with impairments in neural vascular transduction (13, 43) and/or increases in barosensory vessel stiffness (46). It may be reasonable to expect that these factors are modulated by dynamic exercise. Indeed, while resting α-adrenergic sensitivity appears to be reduced in older individuals (12, 22), the normal metabolic modulation of sympathetic vascular regulation (i.e., functional sympatholysis) is blunted (13, 17), and as such, sympathetic vasoconstrictor tone may be enhanced during exercise. Furthermore, exercise-induced changes in carotid artery distensibility may alter SNA responses to NP in older individuals. However, despite the potential for exercise to modulate the BP response to NP, we observed similarly attenuated BP responses to carotid baroreceptor unloading in the older subjects both at rest and during exercise. An alternative explanation for the impaired ability of the older subjects to increase BP in response to NP during exercise may be related to increased central blood volume and the consequent influence of cardiopulmonary baroreceptor loading (39). Indeed, cardiopulmonary baroreceptor loading may exert a central inhibitory effect during sympatho-excitatory maneuvers, such as carotid baroreceptor unloading or exercise. However, as cardiopulmonary baroreceptor loading during exercise is strongly associated with an attenuated carotid baroreflex resetting (36, 48), it is unlikely that this would explain the age-related relocation of the operating point observed in the present study, as we observed an augmented carotid baroreflex resetting in older individuals. Further studies are needed to determine the mechanisms underlying the age-related attenuation in the BP responses to carotid baroreflex unloading during exercise.
In the present study, the variable-pressure neck chamber was utilized, as it has the advantages of being noninvasive and nonpharmacological and permits the derivation of full carotid baroreflex stimulus response curves and its associated parameters during dynamic exercise, unlike alternative methodologies (e.g., modified Oxford technique, sequence technique). Importantly, this allows statistical comparisons of baroreflex function to be made between young and older subjects as rest and during exercise. However, there are limitations that should be considered. As we did not perform the required invasive procedure of placing a pressure transducer at the carotid artery during NP and NS, we cannot definitively know whether the transmission of pressure to the carotid sinus differs between young and older groups. Nevertheless, if one assumes that the older adults have less mechanical deformation for a given level of NS or NP, this would further support our main finding that the gain of the carotid baroreflex is not impaired during exercise in older individuals. Furthermore, as the variable-pressure neck chamber technique is selective for the carotid baroreflex, no measure of aortic baroreflex control is presented. However, because of the parallel activity of the carotid and aortic baroreceptors (44), the assumption is made that a selective modeling of the carotid baroreflex would be characteristic of the overall ABR. Finally, we recognize that the results of the present study are specific to steady-state dynamic exercise at moderate, relative intensities and thus, the potential for age-related differences at higher exercise intensities or when comparing at absolute workloads cannot be dismissed.
In summary, our findings suggest that the magnitude of resetting of the carotid baroreflex-BP stimulus response curve during exercise is augmented in older individuals, and although maximal gain was similar in young and older subjects, the operating point of the modeled curve was located further away from the centering point and toward the reflex THR in older individuals. This age-related relocation of the operating point was also present at rest and indicates that despite a maintained maximal gain, the ability of the carotid baroreflex to defend against a hypotensive challenge is reduced, whereas responses to hypertensive stimuli are greater with advanced age. Given the latter, these data suggest that impairments in the maximal gain of the carotid baroreflex control of BP do not appear to underlie the augmented pressor response to exercise in older adults.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-093167 (to P. J. Fadel). J. P. Fisher was supported by the American Heart Association and the Royal Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors appreciate the time and effort expended by all the volunteer subjects.
REFERENCES
- 1.Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest 45: 132–142, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 3.Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol 202: 45P–46P, 1969 [PubMed] [Google Scholar]
- 4.Brown CM, Hecht MJ, Weih A, Neundorfer B, Hilz MJ. Effects of age on the cardiac and vascular limbs of the arterial baroreflex. Eur J Clin Invest 33: 10–16, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cameron JD, Stevenson I, Reed E, McGrath BP, Dart AM, Kingwell BA. Accuracy of automated auscultatory blood pressure measurement during supine exercise and treadmill stress electrocardiogram-testing. Blood Press Monit 9: 269–275, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Carrington CA, White MJ. Exercise-induced muscle chemoreflex modulation of spontaneous baroreflex sensitivity in man. J Physiol 536: 957–962, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrington CA, White MJ. Spontaneous baroreflex sensitivity in young and older people during voluntary and electrically evoked isometric exercise. Age Ageing 31: 359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Coote JH, Dodds WN. The baroreceptor reflex and the cardiovascular changes associated with sustained muscular contraction in the cat. Pflügers Arch 363: 167–173, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clin Proc 71: 445–452, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol Heart Circ Physiol 275: H1768–H1772, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 263: H798–H803, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol 304: 489–502, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol 88: 671–680, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561: 893–901, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JP, Kim A, Young CN, Ogoh S, Raven PB, Secher NH, Fadel PJ. Influence of ageing on carotid baroreflex peak response latency in humans. J Physiol 587: 5427–5439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H777–H783, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol 533: 871–880, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol 533: 861–870, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogikyan RV, Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab 266: E717–E724, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol Heart Circ Physiol 272: H1157–H1164, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation 104: 2424–2429, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology 57: 295–310, 1972 [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 53: 96–104, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Markel TA, Daley JC, 3rd, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation 107: 675–678, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst 60: 209–212, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J Auton Nerv Syst 73: 182–185, 1998 [DOI] [PubMed] [Google Scholar]
- 31.McDowall LM, Dampney RA. Calculation of threshold and saturation points of sigmoidal baroreflex function curves. Am J Physiol Heart Circ Physiol 291: H2003–H2007, 2006 [DOI] [PubMed] [Google Scholar]
- 32.McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280: H1454–H1463, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Norton KH, Gallagher KM, Smith SA, Querry RG, Welch-O'Connor RM, Raven PB. Carotid baroreflex function during prolonged exercise. J Appl Physiol 87: 339–347, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol 566: 599–611, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogoh S, Fisher JP, Fadel PJ, Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol 581: 405–418, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol 543: 349–364, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol 91: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi X, Gallagher KM, Welch-O'Connor RM, Foresman BH. Arterial and cardiopulmonary baroreflexes in 60- to 69- vs. 18- to 36-yr-old humans. J Appl Physiol 80: 1903–1910, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Smit AA, Timmers HJ, Wieling W, Wagenaar M, Marres HA, Lenders JW, van Montfrans GA, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation 105: 1329–1335, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional α-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA, Querry RG, Fadel PJ, Weiss MW, Olivencia-Yurvati A, Shi X, Raven PB. Comparison of aortic and carotid baroreflex stimulus-response characteristics in humans. Auton Neurosci 88: 74–85, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation 89: 1648–1655, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Cardiovascular responses to stress after carotid baroreceptor denervation in humans. Ann NY Acad Sci 1018: 515–519, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand 181: 289–295, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res 52: 253–262, 1983 [DOI] [PubMed] [Google Scholar]
- 50.Walgenbach SC, Shepherd JT. Role of arterial and cardiopulmonary mechanoreceptors in the regulation of arterial pressure during rest and exercise in conscious dogs. Mayo Clin Proc 59: 467–475, 1984 [DOI] [PubMed] [Google Scholar]