Abstract
In the developing fetus, cerebral arteries (CA) show striking differences in signal transduction mechanisms compared with the adult, and these differences are magnified in response to high-altitude long-term hypoxia (LTH). In addition, in the mature organism, cerebrovascular acclimatization to LTH may be associated with several clinical problems, the mechanisms of which are unknown. Because PKC plays a key role in regulating CA contractility, in fetal and adult cerebral arteries, we tested the hypothesis that LTH differentially regulates the PKC-mediated Ca2+ sensitization pathways and contractility. In four groups of sheep [fetal normoxic (FN), fetal hypoxic (FH), adult normoxic (AN), and adult hypoxic (AH)], we examined, simultaneously, responses of CA tension and intracellular Ca2+ concentration and measured CA levels of PKC, ERK1/2, RhoA, 20-kDa myosin light chain, and the 17-kDa PKC-potentiated myosin phosphatase inhibitor CPI-17. The PKC activator phorbol 12,13-dibutyrate (PDBu) produced robust contractions in all four groups. However, PDBu-induced contractions were significantly greater in AH CA than in the other groups. In all CA groups except AH, in the presence of MEK inhibitor (U-0126), the PDBu-induced contractions were increased a further 20–30%. Furthermore, in adult CA, PDBu led to increased phosphorylation of ERK1, but not ERK2; in fetal CA, the reverse was the case. PDBu-stimulated ERK2 phosphorylation also was significantly greater in FH than FN CA. Also, although RhoA/Rho kinase played a significant role in PDBu-mediated contractions of FN CA, this was not the case in FH or either adult group. Also, whereas CPI-17 had a significant role in adult CA contractility, this was not the case for the fetus. Overall, in ovine CA, the present study demonstrates several important maturational and LTH acclimatization changes in PKC-induced contractile responses and downstream pathways. The latter may play a key role in the pathophysiologic disorders associated with acclimatization to high altitude.
Keywords: high altitude, fetus, ERK1/2, RhoA, 20-kDa myosin light chain, CPI-17
for the developing fetus in utero, hypoxia constitutes a stress of critical importance. Severe acute hypoxia or more moderate prolonged hypoxia can result in major neurological sequelae with life-long consequences. In normoxic near-term sheep, in response to acute hypoxia [arterial Po2 (PaO2) = 12 ± 1 Torr], we previously demonstrated a decrease in fetal cortical tissue Po2 from 9 ± 1 to 2 ± 1 Torr and a 48 ± 10% increase in relative cerebral blood flow (CBF) (33). In contrast, in a related study in the fetus acclimatized to high-altitude long-term hypoxia (LTH), superimposed acute hypoxia had significantly less effect on increasing CBF (19 ± 2%) or decreasing cerebral oxygenation (cortical tissue Po2 decreased from 6 ± 1 to 2 ± 1 Torr) than in sea-level controls (3, 48, 58, 59). In adults who ascend to high altitude with associated hypoxia, initially CBF increases by vasodilation and then, as the process of acclimatization occurs, returns to near-normal values (26, 55). This CBF acclimatization response is regulated by a number of factors that alter the activity of arterial smooth muscle cell surface receptors and signal transduction cascade to modulate cerebrovascular tone via inositol trisphosphate-dependent Ca2+ release from intracellular stores and to augment intracellular Ca2+ concentration ([Ca2+]i) (18) and a PKC Ca2+-independent/sensitization pathway (19). Ca2+-dependent and -independent pathways produce a contractile response by regulating thin-filament (actin) and thick-filament (myosin) interaction. The most-studied myosin is a 20-kDa regulatory myosin light chain (MLC20) filament pathway, the activity of which depends on the balance of activities of MLC kinase (MLCK, which phosphorylates MLC20) and MLC phosphatase (MLCP, which dephosphorylates MLC20) (23, 56). MLCP and MLCK activities are regulated by a complex signal transduction cascade/network that includes modulation by PKC, as well as by other kinases and pathways.
Critical components of the PKC-mediated pathway include MAPK (2), ERK1 and ERK2 (ERK1/2) (2, 4), and Rho kinase (ROCK), the 17-kDa PKC-potentiated myosin phosphatase inhibitor CPI-17, and other enzymes (19, 25). Although the relation of PKC-ERK activation to gene expression with cell proliferation and differentiation is well established (8), less is known of the roles of this signaling pathway in vascular smooth muscle contractility. During the past decade, accumulating evidence from our and other laboratories has suggested that components of the MAPK cascade may be involved in vascular contraction, via altered sensitivity of the contractile machinery (10, 14, 22, 30, 69, 74). Recently, we demonstrated that, with maturation from fetus to adult, there is a significant alteration in these cerebral artery (CA) PKC-activated downstream signaling pathways (19). However, the extent to which these developmentally associated changes result from maturation, per se, or from oxygenation, as the fetus (PaO2 ∼25 ± 2 Torr) is in a relative hypoxemic state compared with the adult (PaO2 ∼95 ± 5 Torr) (28, 33), is unknown.
With PKC, along with ERK1/2 and RhoA, activation, myofilament Ca2+ sensitization occurs through its effector ROCK, which, in turn, modulates vascular smooth muscle sensitivity to [Ca2+]i by inhibiting the MLCP activity, thus enhancing MLC20 phosphorylation (9, 35, 56, 57, 64). Recently, we reported that the Rho/ROCK pathway undergoes significant change with maturation from the fetal to the adult stage (19). Also we demonstrated that PKC activation can lead to phosphorylation of the Thr38 residue of CPI-17, which converts it to a potent MLCP inhibitor (74), leading to arterial smooth muscle contraction (34). Recently, we also demonstrated that CPI-17 is expressed at a very low level in fetal, compared with adult, CA (19). The extent to which this low CPI-17 expression is a function of maturation or low O2 levels is unknown, however, and was examined in the present study. In addition, the effect of LTH on PKC-Rho/ROCK interaction during fetal and adult life on CA contractility is unknown. Thus, in the present study, in fetal and adult CA, we tested the hypothesis that LTH differentially regulates the PKC-mediated Ca2+ sensitization pathways and contractility.
METHODS
Experimental animals and tissues.
All experimental procedures were approved within guidelines of the American Physiological Society and the Animal Care and Use Committee of Loma Linda University. We used CA from nonpregnant ewes (22–24 mo) and near-term fetal sheep (∼140 days gestation) that had been maintained near sea level (300 m; PaO2 = 25 ± 2 and 95 ± 10 Torr for fetus and adult, respectively). We also obtained CA from nonpregnant and near-term fetal sheep that had been acclimatized to high altitude (3,801 m, 12,470 ft, PaO2 = 19 ± 3 and 60 ± 2 Torr for fetus and adult, respectively; Barcroft Laboratory, White Mountain Research Station, Bishop, CA) for ≥110 days (n ≥ 5 for each group).
Ewes were obtained from Nebeker Ranch (Lancaster, CA), as previously described (37). At the appropriate age, animals maintained at high altitude were transported to the Center for Perinatal Biology at Loma Linda University. Shortly after arrival, a tracheal catheter was placed in the ewe; through this catheter, N2 flowed at a rate adjusted to maintain the PaO2 of the ewe at ∼60 Torr, i.e., PaO2 at high altitude (28). At the time of the study, the ewes were anesthetized with thiopental sodium (10 mg/kg iv), and anesthesia was maintained with inhalation of 1% isoflurane in O2 throughout surgery. After delivery of the fetus by hysterotomy, the fetuses and ewes were euthanized with an overdose of Euthasol [pentobarbital sodium (100 mg/kg) and phenytoin sodium (10 mg/kg); Virbac, Ft. Worth, TX]. Brains were removed from the nonpregnant adult and fetal sheep, and CA were obtained for further analysis. Studies were performed in isolated vessels cleaned of adipose and connective tissue. To avoid the complications of endothelium-mediated effects, we removed the endothelium by carefully inserting a small wire three times, as described previously (38, 41). We used the vessels immediately for the experiments.
Simultaneous measurement of [Ca2+]i and tension.
The technique used for simultaneous measurement of [Ca2+]i and tension has been described elsewhere (19, 20). Briefly, we cut the fetal or adult middle CA into 2-mm-long rings, which were incubated at 25°C for 40 min with fura 2-AM (Molecular Probes, Eugene, OR), a fluorescent Ca2+ indicator (19, 20, 37). After loading the dye, we mounted the arterial segments in a 5-ml tissue bath mounted on an intracellular Ca2+ analyzer (model CAF-110, Jasco, Easton, MD). After stabilization of CA rings at 38°C (core temperature of sheep) for 40 min in oxygenated (bath chambers bubbled continuously with 95% O2-5% CO2) standard Krebs solution, we stimulated the isolated arterial rings with 125 mM KCl and, after the contractile force reached a plateau, applied 100 μM acetylcholine to evaluate relative quantity of contractile smooth muscle (43) and determine the status of endothelium disruption (21). The contractile force due to 125 mM KCl was measured as grams of tension, prior to stimulation of the test compound for each arterial segment, and was used for normalization of the arterial contraction with other agonists and antagonists for variation in the smooth muscle mass (38). Arteries that relaxed in the presence of acetylcholine were discarded from the study. Before treatment with the PKC agonist PDBu, with or without the ERK antagonist U-0126 or the ROCK antagonist Y-27632 as a positive control, we measured the KCl-induced contraction (Kmax) and increase in [Ca2+]i. During all contractility experiments, we continuously digitized, normalized, and recorded contractile tensions and the fluorescence ratio (F340/380) using an online computer. In CA from normoxic and hypoxic animals used to study PKC-ERK1/2-mediated contraction, we administered 3 × 10−6 M PDBu (a dose chosen on the basis of our previous dose-response studies) to achieve near-maximal response (19, 42). We incubated a second set of arteries in U-0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], a selective inhibitor of MEK1 and MEK2 (13), at 2 × 10−5 M (74) (Promega, Madison, WI) for 30 min and then treated them with PDBu (3 × 10−6 M). In a similar manner, to study involvement of PKC-ROCK1/2-mediated contraction in vessels of both age groups, we measured PDBu-induced contraction and [Ca2+]i change in the presence or absence of the ROCK inhibitor Y-27632 (3 × 10−7 M) (24, 44). After stabilization, on the basis of our previous length-tension studies, optimum resting tension was 0.6 and 0.7 g for fetal and adult CA, respectively, as at these tensions the contractility response to 125 mM KCl was maximal (41, 47).
PKC measurement.
We measured PKC as described previously (42). Briefly, arteries (∼30 mg wet wt) were homogenized in 2 ml of Tris·HCl buffer, and the homogenate was centrifuged at 100,000 g for 30 min. Assay of the supernatant was defined as cytoplasmic PKC activity. Membrane-bound PKC activity was extracted from the pellet and assayed in the supernatant after recentrifugation. We measured proteins in the membrane and cytosolic fractions by a modification of the Bradford method (6). We determined PKC activity by an assay (Amersham Biosciences, Piscataway, NJ) based on PKC-catalyzed transfer of the gamma-phosphate group of ATP to a PKC-specific peptide. Samples were incubated with a PKC assay mixture and 32P-labeled ATP for 20 min at 37°C. Aliquots of the mixture were spotted on paper disks and washed twice with 75 mM orthophosphoric acid, and the disks were counted for 2 min in 10 ml of scintillation fluid. Data were expressed as picomoles of PO4 incorporated per minute per milligram of protein. The intra- and interassay coefficients of variation for the assay were 4% and 3%, respectively.
Western immunoblot analysis.
CA from normoxic and hypoxic fetal and adult sheep were isolated, cleaned in Na-HEPES buffer (pH 7.5), and frozen rapidly in liquid nitrogen. Frozen samples were homogenized in the 1× cell lysing buffer (Cell Signaling Technology, Beverly, MA) containing 1× phosphatase and protease inhibitor cocktail (Sigma, St. Louis, MO) and 1 mM phenylmethanesulfonyl fluoride. Nuclei and debris were pelleted by centrifugation at 1,000 g for 10 min. The supernatant was collected and stored at −80°C. As we have reported, SDS gel and Western blot analyses were performed using phosphorylated and total ERK1/2 antibody (Cell Signaling Technology) (72) and α-actin as an internal control for uniform protein loading (73). For phosphorylated (Thr38) CPI-17, the methods for immunoblots were similar to those for ERK1/2 with appropriate antibodies. For MLC20 immunoblots, tissues were frozen in a freezing buffer (5% trichloroacetic acid, 10 mM DTT, 5 mM NaF, and 95% acetone) on dry ice. The tissues then were brought to room temperature in washing buffer (10 mM DTT, 100% acetone, and 5 mM NaF) and washed three times. Proteins were extracted (0.04 g wet wet/ml) in extraction buffer (8.0 M urea, 20 mM Tris base, 23 mM glycine, 10 mM DTT, 10 mM EGTA, 10% glycerol, 0.05% bromphenol blue, and 5 mM NaF, pH 8.6) at room temperature for 2 h. Then 6 μg of protein from each sample were loaded onto an SDS gel and electrophoresed at 100 V for 3 h. Proteins were transferred to a nitrocellulose membrane and subjected to immunoblotting with phospho-specific (Ser19) MLC20 antibody (1:1,000 dilution; Cell Signaling Technology). The same blots were stripped and blotted for total MLC20 (1:300 dilution; Sigma). Bands were detected with enhanced chemiluminescence using a ChemiImager (Alpha-Innotech, San Leandro, CA). For calculation of MLC20 phosphorylation, we divided the integrated density value of the phosphorylated band by that of the total MLC20 band and then normalized to control. The results are expressed as fraction of control.
Statistics.
We analyzed the data using unpaired, two-tailed Student's t-test and one-way ANOVA with Newman-Keuls post hoc test to determine significant differences between groups by the use of GraphPad Prism (GraphPad Software, San Diego, CA). The hypothesis was accepted at P < 0.05. For each study, n (i.e., 4–6) is the number of animals from which we obtained CA.
RESULTS
PKC interacts differentially with the MEK/ERK pathway in fetal and adult sheep CA.
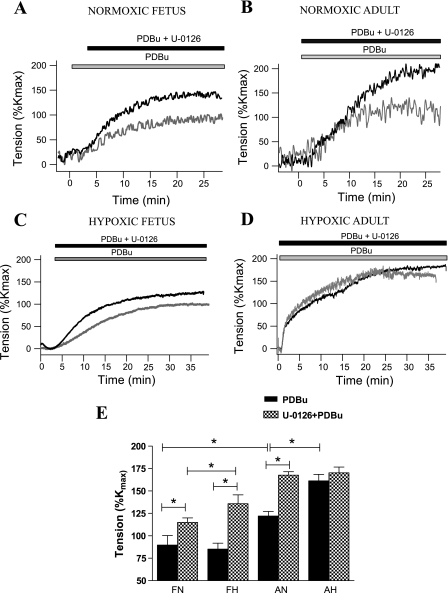
In each of the four experimental groups, PDBu induced a robust contractile response (Fig. 1). PDBu-induced tension was significantly higher in adult than fetal CA. Also the PDBu-induced contraction was significantly greater in hypoxic than normoxic adult CA (Fig. 1, B and D). In the presence of MEK inhibition by U-0126, PDBu-induced CA tension was 20–30% higher in all except the adult hypoxic group (Fig. 1; P < 0.05, n = 5 for each age group). For each of the four groups, Fig. 1E shows PDBu-mediated tension responses as %Kmax in the absence and presence of U-0126. In response to PDBu with or without U-0126, there was no change in [Ca2+]i in any group (data not shown).
Fig. 1.
Phorbol 12,13-dibutyrate (PDBu)-induced contractile response (gray trace) and effect of MEK inhibition by 10−5 M U-0126 (black trace) in normoxic fetal (A), normoxic adult (B), hypoxic fetal (C), and hypoxic adult (D) CA. E: PDBu (3 × 10−6 M)-induced contraction in the absence (filled bars) and presence (cross-hatched bars) of 10−5 M U-0126 in the 4 study groups [normoxic and hypoxic fetuses (FN and FH) and normoxic and hypoxic adults (AN and AH)]. Values (means ± SE) are expressed as percentage of KCl-induced contraction (%Kmax); n = 5 for each group. *Statistically significant difference (P < 0.05).
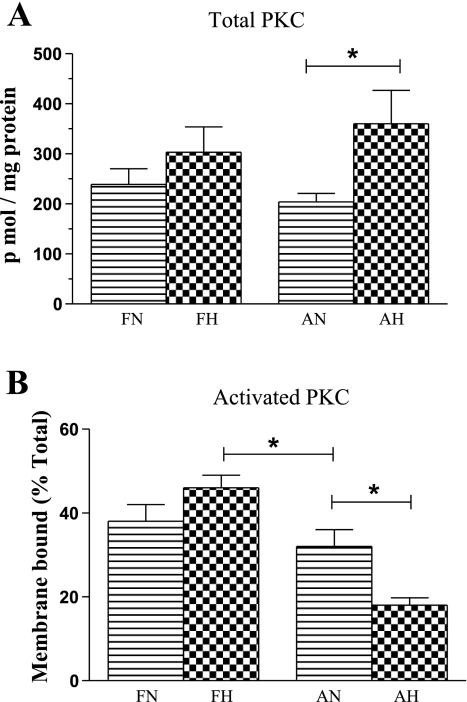
To determine the extent to which the variation in PDBu-induced contractile responses was a consequence of total or phosphorylated (activated, membrane-bound) PKC protein, we measured these levels. Neither total basal PKC protein (Fig. 2A) nor activated PKC (Fig. 2B) levels were altered with maturation. Nonetheless, in adult, but not fetal, CA, as a consequence of LTH acclimatization, the amount of total PKC increased, while the amount of activated PKC decreased (P < 0.05, n = 5 for each age group).
Fig. 2.
Total (A) and activated (B) PKC levels in cerebral arteries from normoxic fetus, hypoxic fetus, normoxic adult, and hypoxic adult. Values are means ± SE; n = 5 for each group. *Statistically significant difference (P < 0.05).
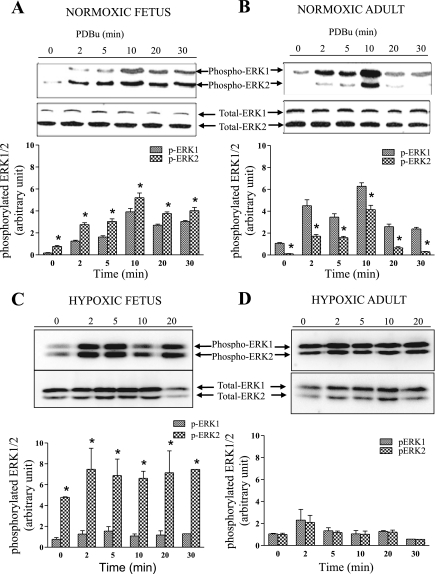
To elaborate further the interaction of PKC with ERK, we examined the time course of phosphorylated ERK1/2 levels in response to PDBu. In control experiments, we determined the optimum dose of PDBu to activate ERK (data not shown; see Ref. 74). In fetal and adult vessels, increasing the dose of PDBu from 10−8 to 10−5 M resulted in a significant increase in phosphorylated ERK1/2. In both age groups, at 3 × 10−6 M PDBu, the increase in phosphorylated ERK1/2 was maximal. In another set of control experiments, to confirm that PDBu-mediated ERK1/2 phosphorylation occurred through activation of upstream MEK, we examined these responses in the absence or presence of the MEK inhibitor U-0126. In CA of both age groups, U-0126 eliminated the PDBu-induced increases of phosphorylated ERK1 and ERK2, whereas total protein levels were unaffected (data not shown; see Ref. 19). As seen in Fig. 3A in normoxic fetal vessels, PDBu resulted in a significant increase in phosphorylated ERK1 (p44) and ERK2 (p42) by 2 and 5 min, with a further four- to fivefold increase above control that peaked at ∼10 min (ERK phosphorylation correlated well with PDBu-induced contractile response). Moreover, in the fetus, in the basal state and in response to PDBu, the level of phosphorylated ERK2 was significantly greater than the level of phosphorylated ERK1 (total ERK1/2 remained constant). In normoxic adult CA, Fig. 3B illustrates an increase in phosphorylated ERK1 and ERK2 levels at 2 min, which remained elevated at 10 min, and total ERK1/2, which remained constant during this time. In contrast to the fetus, in normoxic adult CA, the amount of phosphorylated ERK1 greatly exceeded the amount of phosphorylated ERK2 (P < 0.05). In association with LTH, fetal arteries showed a much more pronounced increase in phosphorylated ERK2 than phosphorylated ERK1 (Fig. 3C). In contrast, in adult CA, there was a significant increase in levels of phosphorylated ERK1 and ERK2 at 2 min that lasted <5 min (Fig. 3D; P < 0.05, n = 5 for each age group); however, ERK1 did not exceed ERK2.
Fig. 3.
Western immunoblots and histograms (densitometric analysis) of phosphorylated ERK1 (p-ERK1, p44) and ERK2 (p-ERK2, p42) showing time course of PDBu (3 × 10−6 M)-induced activation of ERK1/2 in cerebral arteries from normoxic fetus (A), normoxic adult (B), and hypoxic fetus (C), and hypoxic adult (D) cerebral arteries at 2, 5, 10, 15, 20, and 30 min. Levels of total ERK1/2 are also shown. Histograms demonstrate relative integrated density of phosphorylated ERK1 and ERK2. Values are means ± SE; n = 5 for each group. *P < 0.05 compared with control.
PKC interacts differentially with ROCK pathway in fetal and adult CA.
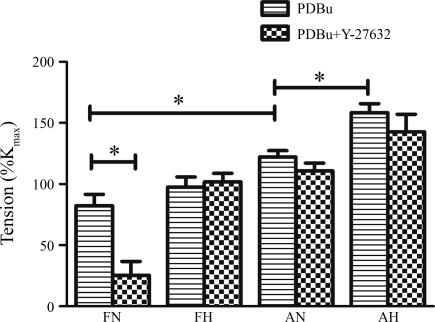
To examine the effect of PKC stimulation on the ROCK pathway, we measured PDBu-induced contractions in the presence and absence of the ROCK inhibitor Y-27632 (3 × 10−7 M). As seen in Fig. 4, in normoxic fetal CA, Y-27632 significantly reduced the PDBu-induced contractile response; however, this was not observed in any other group. In high-altitude-acclimatized fetal CA, the Y-27632-mediated decrease in PKC-induced contraction was lost. In neither normoxic nor hypoxic adult CA, did ROCK inhibition by Y-27632 show an effect on PDBu-induced tension (P < 0.05, n = 5 for each age group). Also, in neither fetal nor adult CA, did [Ca2+]i change significantly in response to Y-27632 (3 × 10−7 M), alone or with PDBu stimulation (data not shown).
Fig. 4.
PDBu (3 × 10−6 M)-induced contraction in the absence and presence of the ROCK inhibitor Y-27632 (3 × 10−7 M) in normoxic fetal, hypoxic fetal, normoxic adult, and hypoxic adult cerebral arteries. Values are means ± SE; n = 5 for each group. *Statistically significant difference (P < 0.05).
PKC interacts differentially with MLC20 in fetal and adult CA.
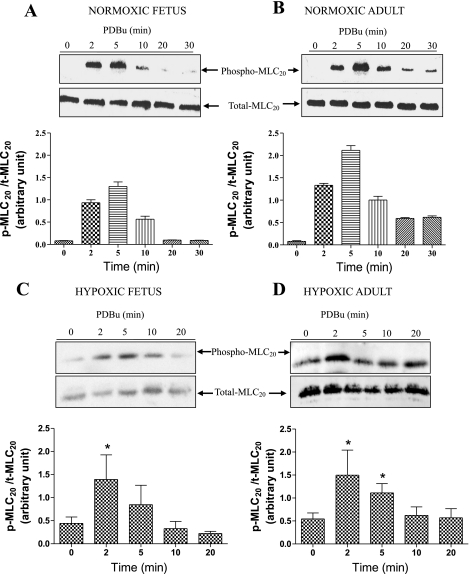
To explore further the mechanisms by which PKC induces Ca2+-independent CA contraction via thick-filament (myosin) regulation, we measured the extent to which PDBu (3 × 10−6 M) increased MLC20 phosphorylation. In normoxic fetal CA (Fig. 5A), Western immunoblot density of phosphorylated MLC20 levels increased severalfold at 2 min, peaked by 5 min, and then declined to near-control values. Similarly, in adult CA (Fig. 5B) treated with PDBu, phosphorylation of MLC20 increased at 2 min, peaked by 5 min, and declined thereafter but remained significantly higher than control. In comparison, in response to LTH acclimatization, MLC20 phosphorylation peaked at 2 min in fetal and adult vessels and started to decline at ∼5 min (Fig. 5, C and D; P < 0.05, n = 5 for each age group).
Fig. 5.
Western immunoblots of total and phosphorylated 20-kDa myosin light chain (t-MLC20 and p-MLC20) at 2, 5, 10, 20, and 30 min after stimulation with PDBu in normoxic fetal (A), normoxic adult (B), hypoxic fetal (C), and hypoxic adult (D) cerebral arteries. Densitometric analysis of phosphorylated MLC20 is shown below each immunoblot. Values are means ± SE; n = 5 for each group. *P < 0.05 compared with control.
PKC interacts differentially with CPI-17 and caldesmon in fetal and adult sheep CA.
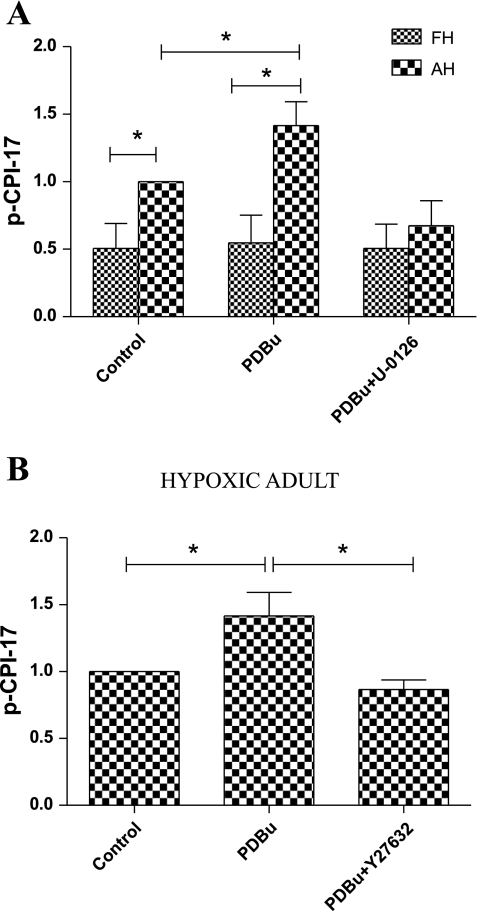
PKC also can sensitize the contractile machinery to Ca2+ by activating CPI-17, which in turn inhibits MLCP, thereby increasing contractile response. To explore this, we examined the effect of PDBu on CPI-17 phosphorylation in the presence of MEK and ROCK inhibitors. As shown in Fig. 6A and as we reported previously (19), levels of phosphorylated CPI-17 are significantly lower in fetal than adult CA. In adult CA, PDBu increased the levels of phosphorylated (Thr38) CPI-17, which were inhibited by U-0126 (Fig. 6A) and Y-27632 (Fig. 6B; P < 0.05, n = 5 for each age group).
Fig. 6.
A: densitometric analysis of phosphorylated CPI-17 (p-CPI-17) in response to 3 × 10−6 M PDBu alone or with U-0126 in hypoxic fetal and adult cerebral arteries, as detected by Western immunoblot analysis. B: densitometric analysis of phosphorylated CPI-17 in response to 3 × 10−6 M PDBu alone or with Y-27632 in hypoxic adult cerebral arteries. Values are means ± SE; n = 5 for each group. *P < 0.05.
DISCUSSION
The present study extends our previous work to understand signal transduction mechanisms in ovine CA and to elucidate further issues of relevance to the developing fetus compared with the adult. In addition, we have attempted to define further the fundamental mechanisms of great importance of LTH-induced acclimatization responses. Several important and novel findings are demonstrated in the present study. 1) PKC activation produced a significantly greater contractile response in adult than fetal CA, regardless of oxygenation status, indicating that this is a maturational phenomenon (Fig. 1). 2) In adult, but not fetal, CA, the PDBu-induced contractile response was increased in response to acclimatization to LTH; also in the adult, high-altitude LTH acclimatization was associated with a loss of the ERK1/2-mediated negative regulation of PKC-induced contractile response. This may have been a consequence of the significantly reduced ERK1/2 phosphorylation (Fig. 3). In fetal CA, in contrast, the ERK1/2-mediated augmentation of PKC-induced contraction remained intact (Fig. 1) and phosphorylation of ERK2 increased (Fig. 3). 3) With maturation from fetus to adult, total and activated PKC remained unaltered (Fig. 2). However, in adult CA acclimatized to high-altitude LTH, basal total PKC increased while activated PKC decreased. In contrast, in fetal CA, these levels did not change significantly (Fig. 2). 4) In each of the four groups of arteries, PKC stimulation increased phosphorylated ERK1/2 levels (Fig. 3): in adult CA, basal and phosphorylated ERK1 were greater than ERK2; in fetal CA, the reverse was the case. Also, in the fetus at high altitude, PDBu-induced ERK2 phosphorylation was significantly greater than in the normoxic control (Fig. 3). 5) In normoxic fetal, but not adult, CA, the RhoA/ROCK pathway plays a key role in PKC-mediated contractility (Fig. 4). However, as a consequence of acclimatization to LTH in fetal CA, ROCK inhibition lost its role in the PKC-mediated contractile response. 6) In normoxic adult and fetal CA, PKC activation resulted in robust and sustained MLC20 phosphorylation. In the LTH-acclimatized animals, particularly the adult, this response was less robust (Fig. 5). 7) In adult, but not fetal, CA, CPI-17 phosphorylation is an important regulatory pathway, and PDBu-induced CPI-17 phosphorylation was blocked by inhibitors of ERK and ROCK (Fig. 6). Table 1 summarizes some of these LTH-associated responses in fetal and adult CA.
Table 1.
Summarized findings of the present study
| Fetal |
Adult |
|||
|---|---|---|---|---|
| Finding | Normoxic* | Hypoxic | Normoxic* | Hypoxic |
| PKC-induced contractility | + | + | ++ | +++ |
| ERK1/2 inhibition-mediated increase in PKC-induced contractility | + | + | + | − |
| Basal PKC | ++ | ++ | ++ | +++ |
| Activated PKC | ++ | ++ | ++ | + |
| PKC-induced total ERK1/2 phosphorylation | +++ | +++ | +++ | +++ |
| PKC-induced ERK1 phosphorylation | + | + | +++ | + |
| PKC-induced ERK2 phosphorylation | ++ | +++ | ++ | + |
| ROCK inhibition-mediated decrease in PKC-induced contractility | + | − | − | − |
| PKC-induced MLC20 phosphorylation sustainability | 5 min | 2 min | 5 min | 2 min |
| PKC-induced CPI-17 phosphorylation* | − | − | + | + |
In the brain and other organs, blood flow is regulated chiefly by alterations of vascular smooth muscle tone, which in turn is regulated by pathways acting on endothelium and/or smooth muscle cells, as well as by cross talk among several pathways. In the present study, in an effort to dissect out the role of those signal transduction pathways activating the smooth muscle cells, we carefully removed endothelium. Previously, we showed significantly less reduction in lumen diameter as a function of pressure (greater myogenic tone) in endothelium-intact arteries than in those without endothelium (13). In the present studies, we attempted to understand the mechanistic basis of vascular smooth muscle cell signaling pathways and to achieve a deeper understanding of the PKC, ERK1/2, ROCK, CPI-17, and effector networks in the regulation of cerebrovascular reactivity, an area of vital importance. Recently, we reported important developmental changes in the PKC-mediated downstream signaling in normoxic control fetal CA compared adult CA (19). The fetus is relatively hypoxemic (PaO2 ∼25 Torr) compared with the adult (PaO2 ∼90 Torr), and mechanistic changes may result from changes in oxygenation status as well as maturation. To provide a systematic analysis of changes in PKC-induced contractility and downstream pathways, as a function of maturational development and acclimatization to high-altitude LTH, the present study compared four groups (2 ages and 2 oxygenation states). Previously, we reported on the role of significant LTH-mediated contractility and blood flow changes in CA (33, 48, 61), as well as uterine (7, 65), pulmonary (12, 36, 70), and other (71), arteries. These hypoxia-mediated responses are associated with changes in many elements of the signal transduction cascade and are reflected in decreased contractile response to α-adrenergic (39, 40) and serotonergic (39) receptor stimulation. In vascular smooth muscle, we also demonstrated several aspects of the important role of PKC and its downstream effectors in modulating contractile response (19, 42, 66, 67, 74). In a continuation of this line of investigation, the present study demonstrates some aspects of important mechanistic CA responses to maturation and acclimatization to high-altitude LTH.
During the past two decades, we and others have demonstrated that kinases such as PKC (27, 42, 49, 74), ERKs (68, 72, 74), and ROCK (19, 25) each play important roles in modulating CA Ca2+-independent contraction. Moreover, in addition to its effect on other components, evidence suggests that PKC mediates myogenic tone and its contractile response through ERK. In previous studies (19, 74), we demonstrated that inhibition of ERK1/2 activity by U-0126 (10−5 M) significantly increased PDBu-induced CA tension in fetus and adult. Similar results have been observed in the sheep uterine artery (69). Together, these findings affirm that while PKC activation leads to phosphorylation of ERK1/2, by apparent negative feedback ERK1/2 regulates PKC-mediated contractility, and this may limit the phosphorylation of MLC20 by sensitization of MLCP or MLCK. Furthermore, in the present study, we show in the high-altitude-acclimatized adult CA that the ERK1/2-mediated inhibition of PKC-induced contractility is eliminated (Fig. 1). Although PDBu phosphorylated ERK1/2 in these arteries, the phosphorylated ERK1/2 levels were not sustained and, particularly in adult, were much lower than in normoxic vessels (Fig. 3). The significance of this more transient ERK phosphorylation in high-altitude LTH-acclimatized adult CA is not known and requires further investigation. We speculate that this may be an important adaptive mechanism regulating cerebral contractility and optimizing CBF at high altitude. Potentially, this may be of great physiological importance, as at high altitude the decrease in PaO2 (hypoxemia) is associated with numerous cardiac, hemodynamic, cerebrovascular, pulmonary, endocrinological, and other adjustments, the mechanisms of which are poorly understood. In other studies, MAPK/ERK phosphorylation has been demonstrated to play a role in contraction of rabbit femoral artery (50), sheep uterine artery (69), pig carotid artery (1, 29), ferret aorta (10, 30), rat aorta, mesenteric artery, and tail artery (62), rat mesenteric artery smooth muscle cells (60), and bovine carotid artery (11). Nonetheless, in several vessels [pig carotid artery (17) and rabbit portal vein and femoral artery (46)], such a role has been denied. An additional report has demonstrated that pharmacological inhibition of ERK1/2 phosphorylation abolished CA tone developed in response to increased intraluminal pressure (32).
In the present study, we also observed in fetal CA that PDBu-mediated PKC stimulation was associated with significantly greater activation of ERK2 than ERK1 (Fig. 3). This ERK2 phosphorylation in fetal arteries was increased greatly in response to LTH acclimatization. In adult CA, the reverse was the case. With limited understanding of the role of specific ERK isoforms, a straightforward rationale for such a major change in signaling pathway with maturation and LTH is unclear. Further study with specific inhibition of ERK1 or ERK2 by small interfering RNA or specific inhibitory peptides is required.
In the developing fetus and the adult, hypoxia is associated with a significant increase in CBF, a response acquired early in life (90–103 days gestation in fetal lamb) (31). Nonetheless, in previous studies in vivo, we demonstrated that, in association with acclimatization to high altitude-induced LTH, fetal CBF compensations in response to superimposed acute hypoxia are inadequate to sustain O2 delivery for the cerebral metabolic rate (5, 33, 48). Consequently, the developing brain may be more vulnerable (than the adult brain) to cerebral hypoxia/ischemia. Thus, adaptive differences between fetus and adult are to be anticipated. The differential regulation of ERK1 and ERK2 may account, in part, for inadequate acclimatization response during fetal life. However, other differences in the Ca2+ sensitization arm, such as that of ROCK or CPI-17, may be responsible for increased vulnerability of the cerebral circulation in the developing organism.
Downstream effector proteins such as ROCK, CPI-17, MLCK, MLC20, and others act to regulate Ca2+ sensitivity of the myofilament contractile apparatus, without significant changes in [Ca2+]i per se (42). Among downstream effector proteins, the basal level of RhoA decreases with maturation (19). The inhibitory signal for “Ca2+ sensitization” is communicated by RhoA to a ROCK that phosphorylates the myosin 110–130 regulatory subunit and inhibits the catalytic activity of MLCP. This results in increased MLC20 phosphorylation, contraction, and cell motility (57). The ROCK antagonist Y-27632 inhibited the PDBu-mediated contractile response significantly in fetal, but not adult, CA, irrespective of O2 status. Importantly, this significant decrease in PDBu-mediated contractile response following ROCK inhibition was lost in the LTH-acclimatized fetus. We speculate that this may be an important adaptive mechanism to help optimize fetal CBF in the face of LTH. In a manner similar to ERK, ROCK has been shown to play an important role in pathological conditions such as cerebral vasospasm and brain ischemic injury (52, 53). The ROCK pathway also has been suggested to regulate a neuroprotective effect on ischemic brain damage (51). The present findings reinforce the idea that whereas RhoA/ROCK plays a key role in the regulation of cerebrovascular tone in the fetus, such is not the case in the adult (Fig. 4). We speculate that PDBu-stimulated RhoA/ROCK in fetal, compared with adult, CA could be an important therapeutic target in ischemic injury in premature newborns.
Another mechanism by which PKC regulates Ca2+-independent contractility is phosphorylation of CPI-17, which inhibits MLCP and, thus, results in increased MLC20 phosphorylation, contraction, and cell motility (56). In a previous report, we demonstrated that whereas CPI-17 plays a significant role in CA contraction in the adult, this is not the case in the fetus. In the present report, we show this to be a maturational phenomenon, as in the acclimatized animal, PDBu-induced CPI-17 phosphorylation occurred only in adult vessels. Moreover, in the present study, we have demonstrated that inhibition of the MEK/ERK or ROCK pathway abolished PKC-mediated CPI-17 phosphorylation. This indicates that PKC phosphorylates CPI-17 directly or through a signaling molecule that is regulated by ERK and ROCK. Our results agree with studies demonstrating that increased Ca2+ sensitivity may result from an increase in MLC20-to-G protein-coupled receptor activation through ERK or RhoA/ROCK (15, 16). In turn, ERK or RhoA/ROCK may act by phosphorylating CPI-17 (54) to promote contraction (9, 64). However, further investigation is required.
Perspectives and Significance
In the United States and other countries, severe neurological impairment is far too common. In the United States, ∼4.5 million live infants are born per year; of this number, >10,000 (2–3 per 1,000) can be expected to have severe neurological disability. Among very preterm births (<32 wk gestation), the prevalence of brain damage is particularly high (45, 63). Thus, this nation faces a major crisis, in that every year, a large number of newborn infants face a life of severe disability and neurological impairment. Needless to say, the care for such infants and children requires a huge expenditure in health care dollars and utilization of state and federal resources. In addition, these neurological handicaps represent personal tragedies in the lives of these infants, as well as their families. Dysregulation of CBF is a major pathogenetic factor in such disorders; thus, maintenance of well-regulated cerebral vascular tone and CBF is essential to fetus and adult. The fetus is not a “mini-adult.” Moreover, therapeutically targeting signal transduction pathways has many more side effects during fetal/premature newborn life than in adulthood. In the present study, in fetal CA, an important observation was the greater PKC-mediated activation of ERK2 and involvement of the RhoA/ROCK pathway, with lack of participation by CPI-17. For the fetus, vulnerability of the immature cerebral vessels, with attendant dysregulation of CBF, may, in part, be a function of relatively poor development of the Ca2+-dependent and -independent arms of the agonist-induced signal transduction cascade. From a therapeutic point of view, these specific pathways may be targeted to correct dysregulation of CBF in the fetus and premature infant and, hopefully, prevent or ameliorate their profound neurological sequelae. Overall, we accept the hypothesis that, in fetal and adult CA, the signal transduction pathways acclimatize to high-altitude LTH. Also, from a clinical perspective for the adult, the present studies provide insights into cerebrovascular mechanisms altered in association with acclimatization to high-altitude LTH, i.e., reduced ERK1/2 phosphorylation with loss of ERK1/2-mediated negative regulation of PKC-induced contractile response. A challenge for the future will be to clarify the roles and importance of specific PKC, ERK1/2, and ROCK1/2 isoforms, other kinases, and their interactions in developmental maturation and in response to acclimatization to high altitude.
GRANTS
This work was supported by National Institutes of Health Grants HD/HL-03807 and HD-31226 to L. D. Longo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Brenda Kreutzer for preparing the manuscript.
REFERENCES
- 1.Adam LP, Franklin MT, Raff GJ, Hathaway DR. Activation of mitogen-activated protein kinase in porcine carotid arteries. Circ Res 76: 183–190, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini A, Crews CM, Erikson RL. Phorbol ester stimulates a protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product. Proc Natl Acad Sci USA 89: 8200–8204, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen WW, Power GG, Longo LD. Fetal O2 changes in response to hypoxic stress: a mathematical model. J Appl Physiol 42: 179–190, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343: 651–653, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol 546: 869–878, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol 296: H1840–H1849, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs TJ, Watson MH, Sanghera JS, Campbell DL, Pelech SL, Mak AS. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem 267: 22853–22859, 1992 [PubMed] [Google Scholar]
- 9.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J 367: 517–524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dessy C, Kim I, Sougnez CL, Laporte R, Morgan KG. A role for MAP kinase in differentiated smooth muscle contraction evoked by α-adrenoceptor stimulation. Am J Physiol Cell Physiol 275: C1081–C1086, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Epstein AM, Throckmorton D, Brophy CM. Mitogen-activated protein kinase activation: an alternate signaling pathway for sustained vascular smooth muscle contraction. J Vasc Surg 26: 327–332, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Raj UJ. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high-altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Geary GG, Osol GJ, Longo LD. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J Physiol 558: 883–896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerthoffer WT, Yamboliev IA, Shearer M, Pohl J, Haynes R, Dang S, Sato K, Sellers JR. Activation of MAP kinases and phosphorylation of caldesmon in canine colonic smooth muscle. J Physiol 495: 597–609, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong MC, Gorenne I, Read P, Jia T, Nakamoto RK, Somlyo AV, Somlyo AP. Regulation by GDI of RhoA/Rho-kinase-induced Ca2+ sensitization of smooth muscle myosin II. Am J Physiol Cell Physiol 281: C257–C269, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins—ras-family or trimeric proteins or both—in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci USA 93: 1340–1345, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinase does not alter smooth muscle contraction in swine carotid artery. Am J Physiol Heart Circ Physiol 275: H131–H138, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L905–L914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 297: H2242–H2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal R, Mittal A, Chu N, Zhang L, Longo LD. α1-Adrenergic receptor subtype function in fetal and adult cerebral arteries. Am J Physiol Heart Circ Physiol 298: H1797–H1806, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg B, Kishiyama S. Endothelium-dependent and -independent responses to severe hypoxia in rat pulmonary artery. Am J Physiol Heart Circ Physiol 265: H1712–H1720, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev 76: 967–1003, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Ihara E, MacDonald JA. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can J Physiol Pharmacol 85: 79–87, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57: 976–983, 2000 [PubMed] [Google Scholar]
- 25.Janssen LJ, Tazzeo T, Zuo J, Pertens E, Keshavjee S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L852–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Jensen JB, Sperling B, Severinghaus JW, Lassen NA. Augmented hypoxic cerebral vasodilation in men during 5 days at 3,810 m altitude. J Appl Physiol 80: 1214–1218, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Jiang MJ, Morgan KG. Intracellular calcium levels in phorbol ester-induced contractions of vascular muscle. Am J Physiol Heart Circ Physiol 253: H1365–H1371, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol Regul Integr Comp Physiol 266: R1778–R1785, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Katoch SS, Moreland RS. Agonist and membrane depolarization induced activation of MAP kinase in the swine carotid artery. Am J Physiol Heart Circ Physiol 269: H222–H229, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Khalil RA, Morgan KG. PKC-mediated redistribution of mitogen-activated protein kinase during smooth muscle cell activation. Am J Physiol Cell Physiol 265: C406–C411, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Kurth CD, Wagerle LC. Cerebrovascular reactivity to adenosine analogues in 0.6–0.7 gestation and near-term fetal sheep. Am J Physiol Heart Circ Physiol 262: H1338–H1342, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Lagaud GJL, Lam E, Lui A, van Breemen C, Laher I. Nonspecific inhibition of myogenic tone by PD98059, a MEK1 inhibitor, in rat middle cerebral arteries. Biochem Biophys Res Commun 257: 523–527, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Hatran DP, Tomimatsu T, Pereyra-Peña J, McAuley G, Longo LD. Fetal cerebral blood flow, electrocorticographic activity, and oxygenation: responses to acute hypoxia. J Physiol 587: 2033–2047, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol 508: 871–881, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol 50: 17–24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Gao Y, Negash S, Longo LD, Raj JU. Long-term effects of prenatal hypoxia on endothelium-dependent relaxation responses in pulmonary arteries of adult sheep. Am J Physiol Lung Cell Mol Physiol 296: L547–L554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP and KCa channel responses to long-term hypoxia. J Appl Physiol 92: 1692–1701, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Long W, Zhao Y, Zhang L, Longo LD. Role of Ca2+ channels in NE-induced increase in [Ca2+]i and tension in fetal and adult cerebral arteries. Am J Physiol Regul Integr Comp Physiol 277: R286–R294, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264: R65–R72, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol A Mol Integr Physiol 119: 683–694, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, α1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Heart Circ Physiol 270: H915–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Longo LD, Zhao Y, Long W, Miguel C, Windemuth RS, Cantwell AM, Nanyonga AT, Saito T, Zhang L. Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am J Physiol Regul Integr Comp Physiol 279: R1419–R1429, 2000 [DOI] [PubMed] [Google Scholar]
- 43.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74: 365–507, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol 325: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Nelson KB. Can we prevent cerebral palsy? N Engl J Med 349: 1765–1769, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Nixon GF, Iizuka K, Haystead CM, Haystead TA, Somlyo AP, Somlyo AV. Phosphorylation of caldesmon by mitogen-activated protein kinase with no effect on Ca2+ sensitivity in rabbit smooth muscle. J Physiol 487: 283–289, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Pereyra-Peña J, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol 578: 359–370, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen H, Forder J, Kojima I, Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun 122: 776–784, 1984 [DOI] [PubMed] [Google Scholar]
- 50.Ratz PH. Regulation of ERK phosphorylation in differentiated arterial muscle of rabbits. Am J Physiol Heart Circ Physiol 281: H114–H123, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36: 2251–2257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res 87: 195–200, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Satoh S, Utsunomiya T, Tsurui K, Kobayashi T, Ikegaki I, Sasaki Y, Asano T. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci 69: 1441–1453, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci 25: 388–391, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Severinghaus JW, Chiodi H, Eger EI, 2nd, Brandstater B, Hornbein TF. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res 19: 274–282, 1966 [DOI] [PubMed] [Google Scholar]
- 56.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomimatsu T, Pereyra-Peña J, Longo LD. Fetal hypercapnia and cerebral tissue oxygenation: studies in near-term sheep. Pediatr Res 60: 711–716, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Tomimatsu T, Pereyra-Peña J, Longo LD. Fetal hypercapnia in high-altitude acclimatized sheep: cerebral blood flow and cerebral oxygenation. Reprod Sci 14: 51–58, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res 84: 505–515, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Ueno N, Zhao Y, Zhang L, Longo LD. High altitude-induced changes in α1-adrenergic receptors and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Regul Integr Comp Physiol 272: R669–R674, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Watts S. Serotonin activates the mitogen-activated protein kinase pathway in vascular smooth muscle: use of the mitogen-activated protein kinase kinase inhibitor PD098059. J Pharmacol Exp Ther 279: 1541–1550, 1996 [PubMed] [Google Scholar]
- 63.Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics 110: 1220–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao D, Huang X, Bae S, Ducsay CA, Longo LD, Zhang L. Cortisol-mediated regulation of uterine artery contractility: effect of chronic hypoxia. Am J Physiol Heart Circ Physiol 286: H716–H722, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Xiao D, Huang X, Longo LD, Pearce WJ, Zhang L. Regulation of baseline Ca2+ sensitivity in permeabilized uterine arteries: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H413–H420, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Xiao D, Longo LD, Zhang L. α1-Adrenoceptor-mediated phosphorylation of MYPT-1 and CPI-17 in the uterine artery: role of ERK/PKC. Am J Physiol Heart Circ Physiol 288: H2828–H2835, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Xiao D, Pearce WJ, Longo LD, Zhang L. ERK-mediated uterine artery contraction: role of thick and thin filament regulatory pathways. Am J Physiol Heart Circ Physiol 286: H1615–H1622, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol 282: H292–H300, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol 104: 1786–1792, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Hu X, Longo LD. Effect of chronic hypoxia on adrenoceptor responses of ovine foetal umbilical vessels. Br J Pharmacol 125: 136–142, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y, Long W, Zhang L, Longo LD. Extracellular signal-regulated kinases and contractile responses in ovine adult and fetal cerebral arteries. J Physiol 551: 691–703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y, Xiao H, Long W, Pearce WJ, Longo LD. Expression of several cytoskeletal proteins in ovine cerebral arteries: developmental and functional considerations. J Physiol 558: 623–632, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Zhang L, Longo LD. PKC-induced ERK1/2 interactions and downstream effectors in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 289: R164–R171, 2005 [DOI] [PubMed] [Google Scholar]