SUMMARY
The I4898T (IT) mutation in type 1 ryanodine receptor (RyR1), the Ca2+ release channel of the sarcoplasmic reticulum (SR) is linked to a form of central core disease (CCD) in humans and results in a non leaky channel and excitation-contraction uncoupling. We characterized age- and fiber type-dependent alterations in muscle ultrastructure, as well as the magnitude and spatiotemporal properties of evoked Ca2+ release in heterozygous Ryr1I4895T/WT (IT/+) knock-in mice on a mixed genetic background. The results indicate a classical but mild CCD phenotype that includes muscle weakness and the presence of mitochondrial-deficient areas in type I fibers. Electrically-evoked Ca2+ release is significantly reduced in single FDB fibers from young and old IT/+ mice. Structural changes are strongly fiber type-specific, affecting type I and IIB/IIX fibers in very distinct ways, and sparing type IIA fibers. Ultrastructural alterations in our IT/+ mice are also present in wild type, but at a lower frequency and older ages, suggesting that the disease mutation on the mixed background promotes an acceleration of normal age-dependent changes. The observed functional and structural alterations and their similarity to age-associated changes are entirely consistent with the known properties of the mutated channel, which result in reduced calcium release as is also observed in normal aging muscle. In strong contrast to these observations, a subset of patients with the analogous human heterozygous mutation and IT/+ mice on an inbred 129S2/SvPasCrl background exhibit a more severe disease phenotype, which is not directly consistent with the mutated channel properties.
Keywords: muscle, excitation-contraction coupling, central core disease, ryanodine receptor
INTRODUCTION
The I4895T (in mouse or I4898T in human) mutation of the skeletal muscle sarcoplasmic reticulum (SR) Ca2+ release channel, or type 1 ryanodine receptor (RyR1), results in a form of central core disease (CCD) in humans, marked by skeletal muscle weakness and the presence of core regions devoid of mitochondria and oxidative enzyme activity (Lynch et al. 1999). The IT mutation is located in the middle of the selectivity filter region of the channel (Gao et al. 2000) and homotetrameric IT release channels in myotubes do not support excitation-contraction coupling (termed EC uncoupling); that is, they lack depolarization-induced Ca2+ release, despite normal junctional RyR1 expression and sarcoplasmic reticulum (SR) Ca2+ content (Avila & Dirksen 2001; Avila et al. 2003; Zvaritch et al. 2007). These studies indicate that, unlike several other CCD mutations in RyR1 (Avila & Dirksen 2001; Chelu et al. 2006), the IT mutation does not enhance RyR1 Ca2+ leak and SR store depletion. Heterotypic (WT:IT) release channels exhibit a functional behavior intermediate to that of homotetrameric (WT:WT and IT:IT) channels, but also do not enhance SR Ca2+ leak or store depletion following homologous expression in myotubes (Avila & Dirksen 2001) or in adult muscle fibers isolated from heterozygous Ryr1I4895T/WT (IT/+) knock-in mice (Zvaritch et al. 2009).
In order to more rigorously address the pathogenic nature of the human I4898T RyR1 mutation in skeletal muscle, a knock-in mouse line carrying the analogous mutation in murine RyR1 (I4895T) was generated (Zvaritch et al. 2007). Whereas homozygous I4895T knock-in mice (RyR1I4895T/I4895T or IT/IT) die at birth from respiratory distress as a result of muscle paralysis, heterozygous I4895T knock-in mice (RyR1I4895T/WT or IT/+) survive, breed normally, and exhibit a normal lifespan (Zvaritch et al. 2009). By carefully selecting muscles (soleus and extensor digitorum longus or EDL) and identifying fiber types from IT/+ knock-in mice on a mixed 129S6/SvEvTac and 129S2/SvPasCrl background, we demonstrate a strong fiber type-dependence of structural alterations. These reflect acceleration of a phenotype similar to that observed during normal aging (Boncompagni et al. 2010) due, to EC uncoupling arising from a reduction in Ca2+ release (Jimenez-Moreno et al. 2008) in dihydropyridine receptor (DHPR) expression (Wang et al. 2000) and in the frequency of Ca2+ release units (Boncompagni et al. 2006).
RESULTS
1. Ultrastructural identification of primary fiber types in EDL and soleus
Since the RyR1I4895T/WT (IT/+) mutation may affect muscle ultrastructure in a fiber type-specific manner, we first established criteria for identifying the major fiber types of EDL and soleus muscles at the electron microscopic (EM) level. We selected to use the classical “fast” and “slow” muscles, extensor digitorum longus (EDL) and soleus muscles, respectively (Eccles & Sherrington 1930). In a separate publication (Boncompagni et al. 2010), we define how the three most frequent fiber types in EDL (IIA, IIB, IIX) can be differentiated on the basis of mitochondrial content and distribution. EDL contains a majority ~70–75% type IIB, ~10–15% type IIX, ~8% type IIA, and ~4% type I (Hughes et al. 1999; Danieli-Betto et al. 2005). Soleus muscle contains ~67% type I and ~33% type IIA fibers (Asmussen & Marechal 1989) and the two can be distinguished on the basis of Z line width (Eisenberg 1983). Types I, IIA and IIX fibers are also found in human leg muscles and they are essentially similar to those in mouse, although with considerably lower mitochondria content (Lovering & Russ 2008; Arbanas et al. 2009). Type IIB fibers are present in mice but not human leg muscles (Smerdu et al. 1994). Since type IIX and IIB fibers in mice respond very similarly to this mutation as well as to aging (see Boncompagni et al. 2010), we may consider that both are essentially equivalent to the human IIX.
2. Effects of the I4895T mutation are fiber type-specific in muscles from 2 and 12 month-old mice
2.1 Z line streaming in type I fibers of soleus
The most notable alteration in soleus muscle fibers from IT/+ mice and, to a much lesser extent, in fibers from wild type (WT) mice, is the presence of variable-sized regions of “Z line streaming” and associated disruption of the myofibrils (Figs. 1 and 2). “Z line streaming” is a term used to describe the presence of elongated smears of amorphous material, with electron density essentially identical to the protein assembly forming the Z line (Fig. 1A and inset). Streaming Z lines occupy the position of the natural Z line and also extend longitudinally, invading either parts of or whole sarcomeres. All stages of Z line streaming in muscle from IT/+ and WT mice are found exclusively in type I fibers, identified on the basis of a wide Z line (Fig. 2B). Z line streaming is not observed in either Type IIA fibers in soleus and EDL or type IIB and IIX fibers in EDL.
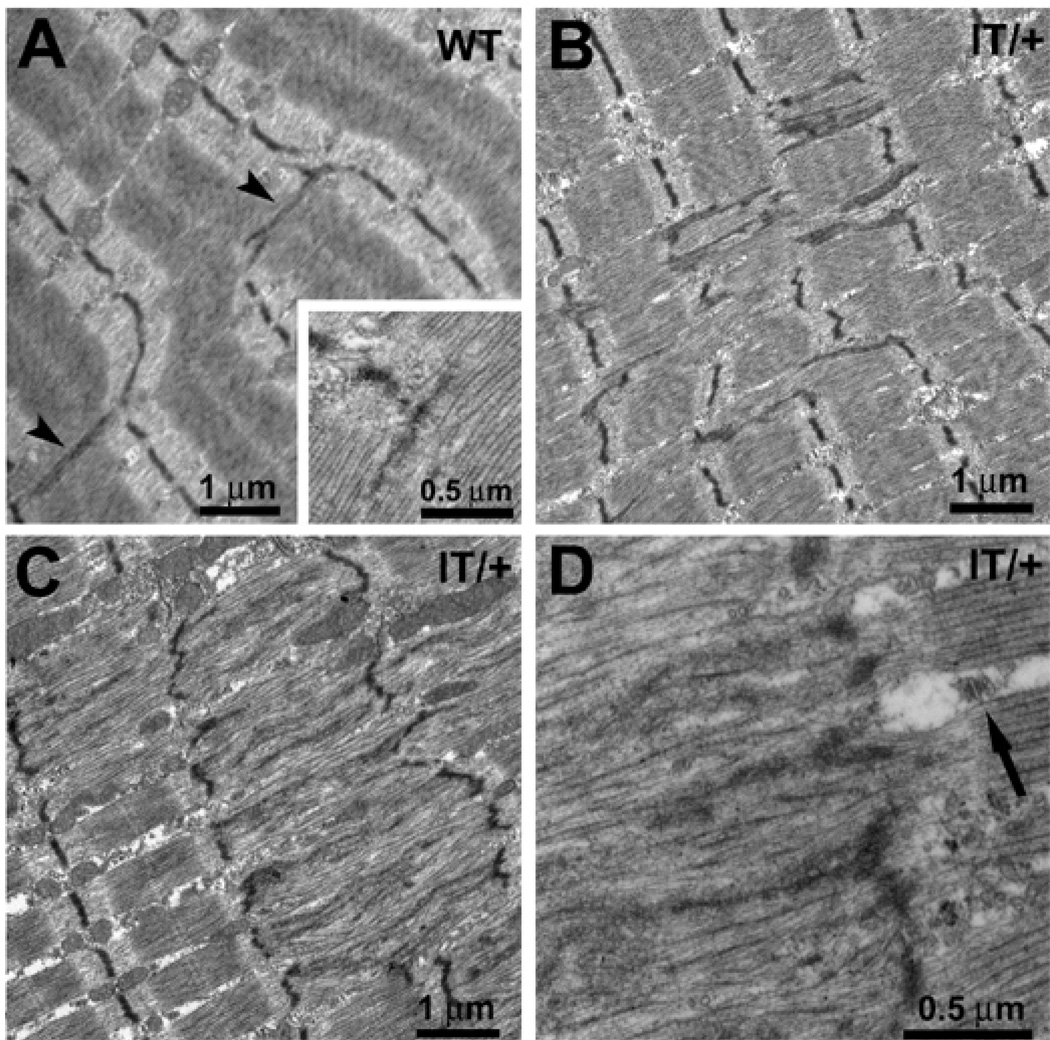
Figure 1. Z line streaming areas are more extensive in IT/+ versus WT soleus.
A) Z line streaming is defined as a flow of electron dense Z line material longitudinally in elongated dense patches (arrow heads and inset). In this soleus from 12 months old WT mice the “small” area of Z line streaming is at the site of a Vernier striation displacement, where n sarcomeres on one side (two in this case) are against n+1 sarcomeres on the other (one in this case) and affects only part of one sarcomere. B–D) In soleus muscle fibers from 12 months old IT/+ mice, Z line streaming is significantly more frequent and extensive, affecting “medium” and “large” sized areas (see also Table 1). Large areas of streaming in fibers from IT/+ mice involve the entire structure of several sarcomeres and myofibrils. Separation between affected and normal areas is abrupt (e.g. see intact triad next to disrupted Z line in D).
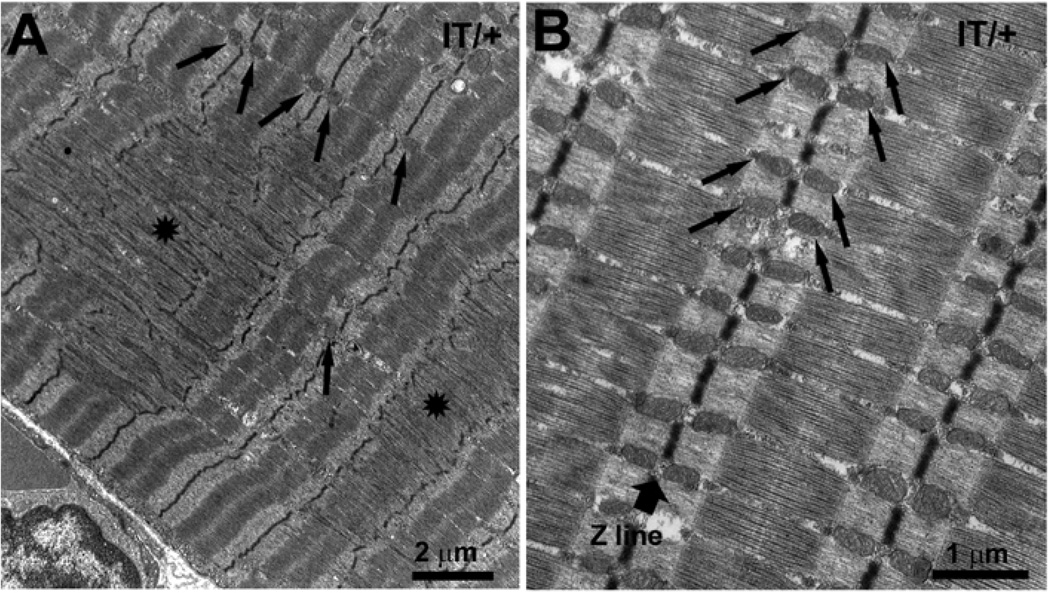
Figure 2. Streaming is present only in slow-twitch (type I) fibers and is a very localized phenomenon that affects local mitochondrial distribution.
A) Two extensive, well-delimited areas of Z line streaming and sarcomeric degeneration (asterisks) in a soleus muscle fiber from 12 months old IT/+ mouse. Mitochondria are completely missing in the disrupted regions, but are present and normal immediately outside regions of Z line streaming (arrows). B) An unaffected region from the same fiber shows well aligned striation and a perfect distribution and disposition of mitochondria (arrows) on either side of the Z line. Note wide width of the Z line in a type I fiber (wide arrow).
Early stages of streaming involve a limited portion of the Z line (Fig. 1A, arrow heads) as the dense material extends partially into the A band and the streaming affects only one myofibril. These “small” regions of streaming are most frequently found at sites of “Vernier” striation displacements (Fig. 1A). In “medium-sized” streaming areas, Z lines of several adjacent myofibrils are affected (Fig. 1B). In “large” areas of streaming, sarcomeric structure is severely disrupted across several myofibrils and over the length of multiple sarcomeres (2–5 sarcomeres per 10–15 myofibrils) (Figs. 1C and D and 2A, stars). Z lines typically maintain their individuality (Fig. 1B, from IT/+ muscle), but in some areas several Z line regions fuse together to form a continuous transverse structure (Fig. 1C and D). This is particularly true of medium and large streaming areas, where all elements of the sarcomere are markedly altered, with loss of thick filament alignment at the A band and a very disordered striation.
Note that in the areas of streaming mitochondria, triads and longitudinal SR are essentially absent (Figs. 1C and D and 2A), whereas mitochondria and triads exhibit normal spacing and orientation immediately adjacent to the disrupted areas (Fig. 1D and 2A arrows) and elsewhere in the fiber (Fig. 2B, arrows). Except in close proximity of the affected sarcomeres, the cross striation maintains a perfect alignment (Fig. 2B).
Streaming areas are randomly disposed along the length and across the diameter of affected fibers and are present in both wild type and mutant muscles, even if their frequency and extent varies with age and genotype (Table 1). In wild type soleus fibers at 3 and 12 months, streaming is rare (0.03–0.3 sites/100 mm length) and restricted to extremely small alterations, though a mild increase is seen with age during this period. In soleus fibers from age-matched IT/+ mice, streaming is significantly more frequent, affects larger areas, and the frequency increases with age between 3 and 12 months in a marked fashion (Figs. 1 and 2, Table 1).
Table 1.
Frequency and size of Z line streaming areas in type I fibers of soleus muscle
| Muscle type (age) |
Sample Size | Streaming sites/100 µm fiber length |
Small& Streaming % of total |
Medium& Streaming % of total |
Large& Streaming % of total |
|---|---|---|---|---|---|
| WT (3 months) |
2 mice, 40 fiber segments |
0.03# | 100% | ||
| IT/+ (3 months) |
2 mice, 46 fiber segments |
0.14# | 94% | 6% | |
| WT (12 months) |
3 mice, 40 fiber segments |
0.30 ± 0.57* | 92% | 7% | 1% |
| IT/+ (12 months) |
3 mice, 46 fiber segments |
1.01 ± 1.11* | 70% | 6% | 24% |
See text for criteria used to classify approximate size of streaming areas.
The frequency of streaming areas at 3 months, was very low and is thus referred to the sum of all fiber segments rather than to individual fibers. This did not allow a stringent statistical analysis.
Data are shown as mean±SD. P<0.0004 (Student’s t-test; standard deviation calculated from data of individual fibers).
2.2 T-tubule swelling in type I fibers of soleus
Apparently empty, membrane-limited vesicles of variable size are observed in type I fibers from soleus muscles of IT/+ mice (Fig. 3). These vesicles are not evenly distributed, but are located within domains involving several sarcomeres and occupy only a portion of the fiber width (Fig. 3A, arcs). The vesicles are identified as sectioned profiles of focally dilated T-tubules, based on their location adjacent to the Z line (Fig. 3B) and their position adjacent to jSR cisternae associated with RyRs (Fig. 3C and D). Enlarged T-tubules are present in type I fibers from soleus muscles of both 12 month-old WT and IT/+ mice, but they are more frequent and of larger size in the latter. Specifically, wider T-tubules are present in 14 ±36% (from 35 fibers each in 3 mice) of longitudinally-sectioned fiber segments in WT mice and 27 ±45% of identically sectioned fiber segments in IT/+ mice (33 fibers each in 3 mice. Student’s t-test: p <0.0001).
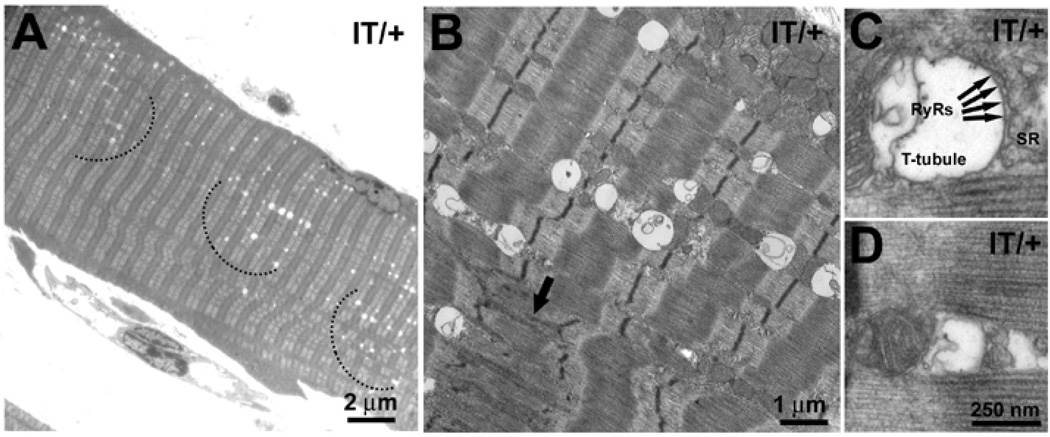
Figure 3. T tubule swelling in soleus muscle fibers from 12 months IT/+ mice.
A, B) Apparently empty, membrane-limited vesicles of variable size are located in wide patches (arcs) within the fiber interior. Note an area of streaming in a fiber together with swollen vesicles (B, arrow). C, D) Vesicles are identified as enlarged T-tubules based on higher magnification images where they clearly are associated with the SR via electron dense “feet” (arrows in C). Swollen T-tubules are present in fibers with both wide- and narrow-Z line width and at higher frequency in IT/+ than WT fibers, but are absent in EDL.
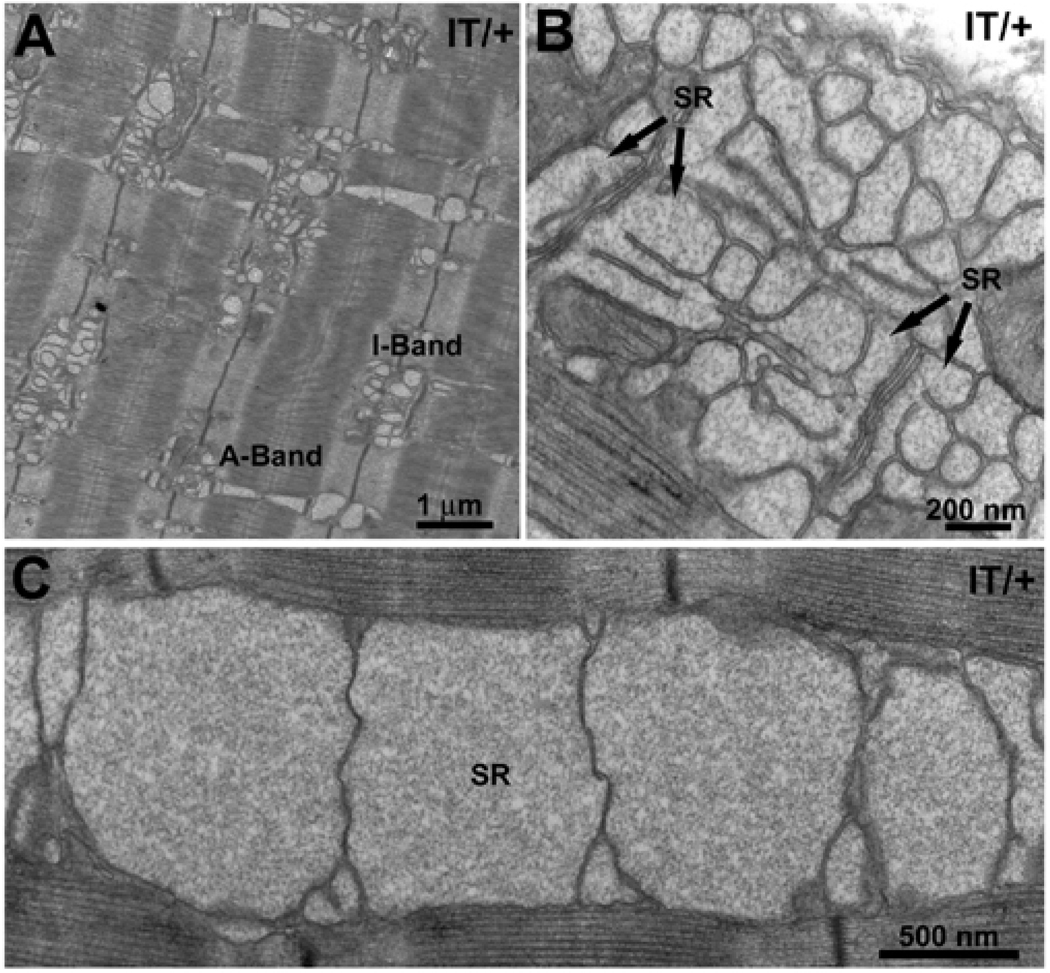
2.3 Free-SR alterations in type IIB and IIX fibers of EDL
Type IIB and IIX fibers in EDL, but none of the others in EDL and none in soleus, show an enlargement (or swelling) of longitudinal SR tubules filled with an electron dense material, presumably calsequestrin (CASQ) (Figs. 4 and 5). This alteration is age dependent in WT muscle fibers (Boncompagni et al. 2010), but the frequency and severity are significantly increased in age-matched IT/+ muscle fibers (Table 2).
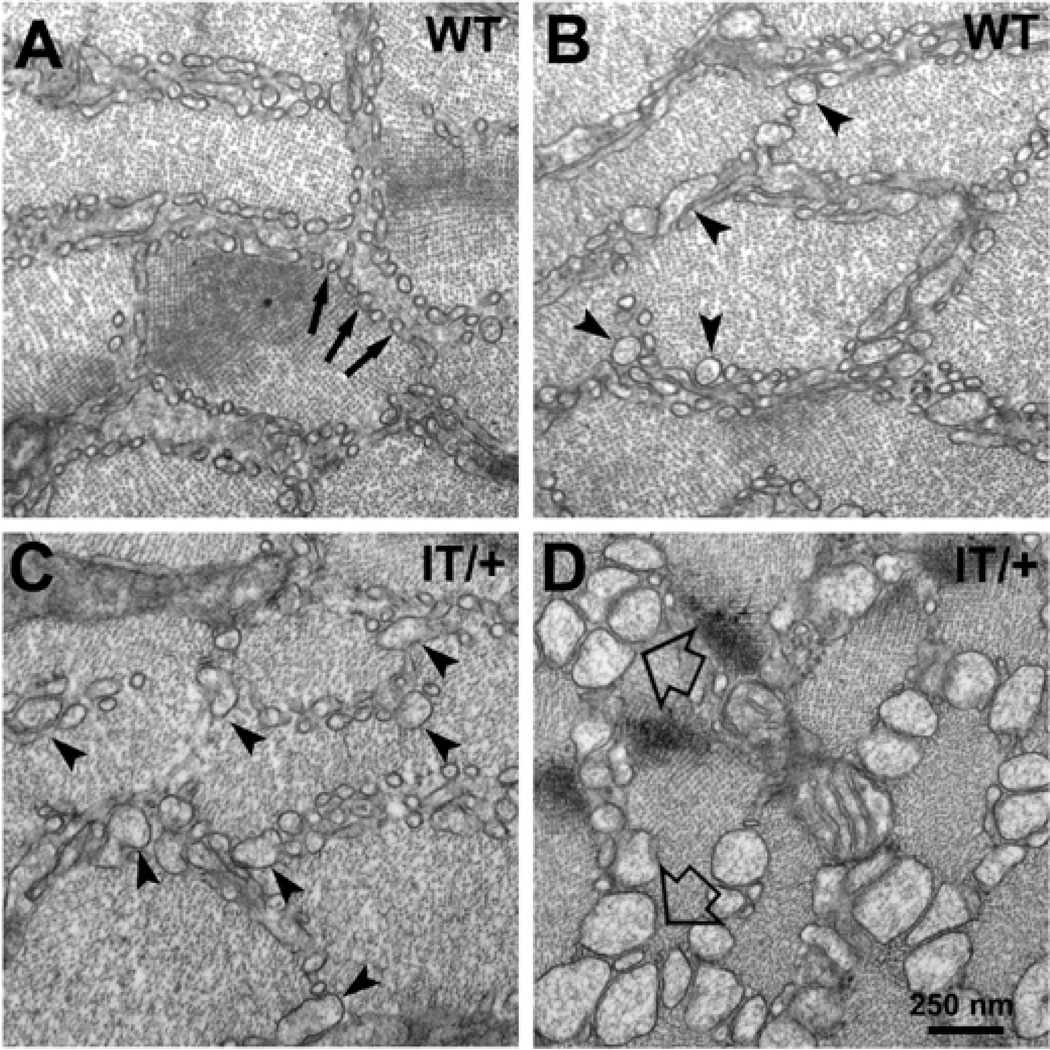
Figure 4. Enlargement of SR tubules is more extensive in type IIB/IIX fibers of EDL muscles of IT/+ versus WT mice.
Cross sectional areas at the I-Z-I level of type IIB fibers from EDL muscles in WT and IT/+ mice at 12 months of age. A,B) In WT, cross sections thorough elongate free SR tubules show two configurations: small circular profiles with an apparently empty lumen (A, arrows) and wider profiles with a visible content, presumably CASQ (B, arrow heads). C,D) Fibers from IT/+ mice show a higher frequency of wide profiles with CASQ (C, arrow heads) and also greatly enlarged profiles (D, empty arrows). (See also Table 2).
Figure 5. CASQ accumulation in free SR is extensive in IIB/IIX fibers from IT/+ mice.
All images are from 12 months EDL. A) In contrast to WT, CASQ accumulation in IT/+ fibers affects both I band and A band level SR. B, C) Greatly dilated SR and exceedingly large CASQ filled SR sacs are seen only in fibers from IT/+ mice. Note that the lateral sacs of the triads (arrows) as well as the rest of the SR are affected.
Table 2.
Approximate sizes of cross sectioned free SR tubules at the I band level in type IIB/IIX fibers of EDL
| Muscle type (age) |
Sample size | Small (≤ 70 nm)# profiles % |
Medium (~80–150 nm)# profiles % |
Large (~150–200 nm)# profiles % |
X-Large (>200 nm)# profiles % |
|---|---|---|---|---|---|
| WT (2–3 months)* | 3 mice, 31 fibers | 98 ± 2 | 2 ± 2 | 0 | 0 |
| WT (12 months)* | 3 mice, 31 fibers | 89 ± 10 | 9 ± 8 | 2 ± 5 | 0 |
| IT/+ (12 months) | 3 mice, 31 fibers | 63 ± 17 | 23 ± 11 | 12 ± 10 | 3 ± 4 |
approximate diameter of cross sectioned free SR tubules.
These data are copied from the manuscript Boncompagni et al. 2010
Data are shown as mean ± SD.
Student’s t-test: WT vs IT/+ in the same size category. P<0.005 for small profile and P<0.0001 in all other categories.
A quantitative estimate of SR swelling magnitude and frequency in WT and IT/+ muscles was obtained by roughly classifying the profiles of SR tubules seen in cross sections at the level of the I band into four categories. The first category (small) includes small circular profiles up to ~70 nm in diameter that are apparently empty (see the majority of profiles in Fig. 4A, arrows). Profiles in the other categories have a size range of ~ 80–150 nm (medium profiles, Fig. 4B and C, arrows heads), ~150–200 (large profiles, Fig. 4D, empty arrows) and, >200 (extra large profiles, Fig. 5, C) nm in diameter and exhibit greater variability in shape and content of electron dense material. The data for WT are presented in Boncompagni et al. 2010 and are included again here for reference. In WT muscles from 2–3 and 12 month-old mice, the majority of profiles (89–98%) are small, contain little or no CASQ and fall within the first category (Table 2). At 12 months, there is a small but definite increase in frequency of medium sized free SR tubules. By contrast, muscles from 12 month-old IT/+ mice show a much higher frequency of medium profiles (23%), some large profiles (12%) and occasionally exceedingly large profiles with diameters of 0.5–1 µm (Fig. 5C). In WT muscles (Boncompagni et al. 2010), large profiles are present only rarely and at much later ages.
In addition to being more pronounced, SR swelling in IT/+ muscles unusually extends into the SR at the A band level (Fig. 5A and B), as well as into the triad (Fig. 5B, arrows) while in WT muscle involvement of A band level SR is rare and mostly seen only at advanced ages. Thus, free SR swelling is age-dependent in type IIB/IIX fibers in muscle from both WT and IT/+ mice, but the latter show a premature appearance and much stronger effect.
Two additional structural alterations are premature and more prominent in type IIB/IIX fibers of IT/+ mice versus WT muscles: an accumulation of SR-derived tubular aggregates (data not shown) and a shift of triads from the normal transverse to a longitudinal orientation (Supplemental Fig. S1). The typical orientation of triads in WT adult fibers is with the long axis of the central T tubule in transverse planes. Thus, in muscle cross sections triads appear as dark elongated profiles in which the two rows of RyRs (junctional feet) are often resolved (Supplemental Fig. S1, arrows). Triads with a longitudinally oriented axis appear as two SR flanking a T-tubule profile in muscle cross sections (Supplemental Fig. S1, arrow heads). The ratio of longitudinal to transverse triads, counted in cross sections, is very low in type IIB/IIX fibers from wild type muscles at one year of age (0.09 ±0.10, from 3 mice, 45 fibers), but significantly (P<1.910−6) higher in IT/+ muscles (0.46 ±0.47, from 3 mice, 45 fibers). In 5 out of 45 IT/+ fibers closely examined, the ratio of longitudinal to transverse triads was even higher than 1. The orientation of all triads remains transverse in type I and IIA fibers of both WT and IT/+ muscles.
3. Aged Muscles
3.1 Z line streaming
The frequency of Z line streaming areas, particularly those involving larger segments, increases at late ages in WT muscles. On average, soleus muscles from four 24–25 month-old WT mice have 0.94 ±0.56 Z line streaming areas/100 µm fiber length and, of these, 20% are “large” and 45% “medium” sized (as defined above). The standard deviation is large because the frequency of streaming areas varies substantially between individual animals. Interestingly, the frequency and size of Z line streaming areas in aged IT/+ mice is also variable, but is, surprisingly, lower than that observed in either aged WT mice or 12 month-old IT/+ mice. Specifically, the average frequency for combined data from one 16.5 month-old and two 24 month-old IT/+ mice is 0.33±0.31/100 µm and the majority of the streaming areas (50–71%) are small.
3.2 CASQ accumulation
Enlarged SR profiles containing CASQ are observed in type IIB/IIX fibers of EDL in aged muscles from both WT and IT/+, but the enhanced accumulation of CASQ in the IT/+ SR is even more prominent at older ages, leading at very extended SR sacs over the whole sarcomere (Fig. 5). Additionally, both WT and IT/+ muscles show extensive accumulation of tubular aggregates. These details were not subjected to a specific quantitative analysis.
3.3 Nemaline rods
In the process of examining thin sections of soleus muscles from WT aged mice, we observed occasional regions containing nemaline rods (Conen et al. 1963; Shy et al. 1963), or electron dense material accumulation showing a fine periodicity similar to that seen in Z lines (Supplemental Fig. S2). The frequency and abundance of nemaline rods varied considerably. Of four soleus muscles from 24–25 month-old WT mice, one had none, one had only a few in a single fiber, and the other two had frequent rods in 30–50% of fibers In this work, no rods were observed in two 24–25 month-old IT/+ mice in this work, but Zvaritch et al. (2009) observed rods in 15 to 20% of soleus muscles from 2 out of 5 IT/+ mice at 18–20 months on the alternate 129S2/SvPas background.
4. Contractures are not characteristic of muscle fibers from IT/+ mice
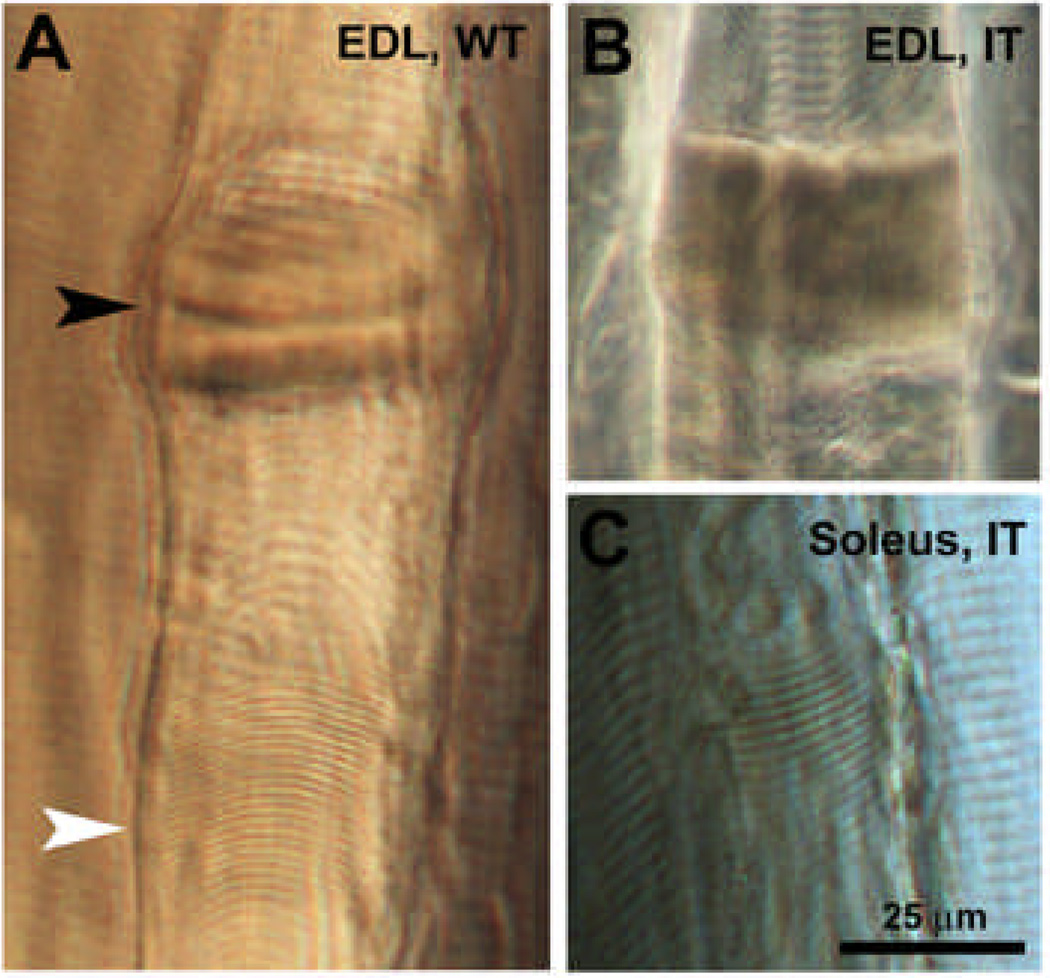
Contractures, localized regions of severe sarcomere shortening maintained during chemical fixation, have been described in muscle biopsies of individuals that carry RyR1 mutations linked to malignant hyperthermia and/or core disease. Widespread regions of contracture lacking SR and mitochondria are a prominent feature in heterozygot knock-in mice expressing the MH/central core Y522S RyR1 mutation (Boncompagni et al. 2009) and were reported in soleus muscle of IT/+ mice (Zvaritch et al. 2009). In fixed tissue, however, localized contractures with misplaced mitochondria can also result artifactually from microdamage during dissection that induces local entry of extracellular Ca2+. The best way of determining whether a variable (e.g. knock-in mutation) is specifically responsible for the presence of contractures is to determine if the frequency and appearance of contractures in muscles from mutants is significantly higher than that observed in identically prepared WT muscle and whether or not the frequency and/or appearance of contractures correlates with age and genotype. We used light microscopy to image teased preparations of osmium postfixed fibers (see Experimental procedures) for an extensive quantitative analysis of a large sample of fibers. In teased fibers, phase contrast optics offer excellent identification of contractures as segments with stretched and/or distorted sarcomeres adjacent to regions of contractures (Fig. 6). Fiber bundles damaged during dissection are also readily detected because clusters of severely contracted fibers are found in close proximity to each other (Supplemental Fig. S3A and B). These are referred to as “clustered” contractures in Supplemental Table S1. Single isolated contractures of the type shown in Figure 6 were extremely rare in our samples. Except for regions of contractures and their immediate proximity, cross striations were otherwise perfectly well aligned across all fibers regardless of origin (Suppl. Fig. S3C and D).
Figure 6. Examples of contractures in fixed EDL and soleus muscle fibers from 12 months WT and IT/+ mice.
Contractures are found randomly, but at low frequency in all fixed muscles. Although variable in extent of shortening and frequency (see text for details), contractures do not show distinctive differences in either parameter between fibers from WT and IT/+ mice. A) Extreme, raised contracture segment (black arrowhead) and an extensive region of highly shortened sarcomeres with still visible striations (white arrowhead) in an EDL fiber from a WT mouse. B and C) Similar contractures are also observed at a similar frequency (Supplemental Table S1) in fibers from IT/+ mice.
Overall, the frequency of contractures varied greatly between preparations, irrespective of age or genotype. As a result, average values shown in Supplemental Table S1 exhibit relatively large standard deviations. Despite these variations, the large sample size (725–1858 fibers for each group) allows for a well-powered statistical evaluation. A comparison of contracture frequencies shows that no statistically significant difference exists for: 1. combined data from all WT mice at all ages versus all IT/+ mice at all ages; 2. separate data from EDL and soleus at all ages in WT mice versus IT/+ mice; 3. data from all young adult WT mice versus all old WT mice; and 4. data from all young adult IT/+ mice versus all old IT/+ mice. Thus no muscle-, age- or genotype-dependent trends in contracture frequency were observed, indicating that the contractures are random events most likely due to microdamage during isolation. In order to enable a direct comparison with previous results reported from Y522S knock-in mice (Boncompagni et al. 2009), we calculated the frequency of contracture per 10 mm fiber length. Assuming a fiber length of 7 mm, the minimum and maximum values shown in Supplemental Table S1 extrapolate to 0.5 and 0.01 contractures/10 mm fiber length.
The limited number of contractures, and specifically the absence of contractures in central regions of the muscle that are unlikely to be caused by dissection damage, was also confirmed via electron microscopic analysis (Supplemental Fig. S4). Additionally the few contractures observed in thin sections of IT/+ muscle do not differ from those found in wild type both in severity and in the fact that mitochondria are present in all contractures (Supplemental Fig. S4A and B), although they tend to be less visible and pushed into columns where the contractures are severe.
5. Mitochondrial ultrastructure and disposition are largely unaltered in fibers from IT/+ mice
Mitochondria were absent in regions of Z line streaming, which were only observed in type I fibers of soleus muscles from IT/+ mice. Thus, mitochondria-free Z line streaming regions could fully account for the focal “core-like” regions that stain negatively for oxidative enzyme activity in soleus of IT/+ mice reported by Zvaritch et al. (2009). Apart from these areas, mitochondria did not show any specific ultrastructural alterations in muscle from IT/+ mice, nor was there a significant change in their overall positioning or frequency compared to WT mice. This finding was confirmed by morphometric measurements of mitochondrial volume in soleus. Mitochondrial volume as a percentage of fiber volume (exclusive of the fiber periphery) was 12 ±5% in WT (mean ± SD, from 60 fibers, 3 mice) and 14 ±6% (same sample size) in IT/+, which was not statistically significant (student’s T test, P>0.01).
6. Electrically-evoked Ca2+ release is reduced, but spatially uniform, in fibers from IT/+ mice
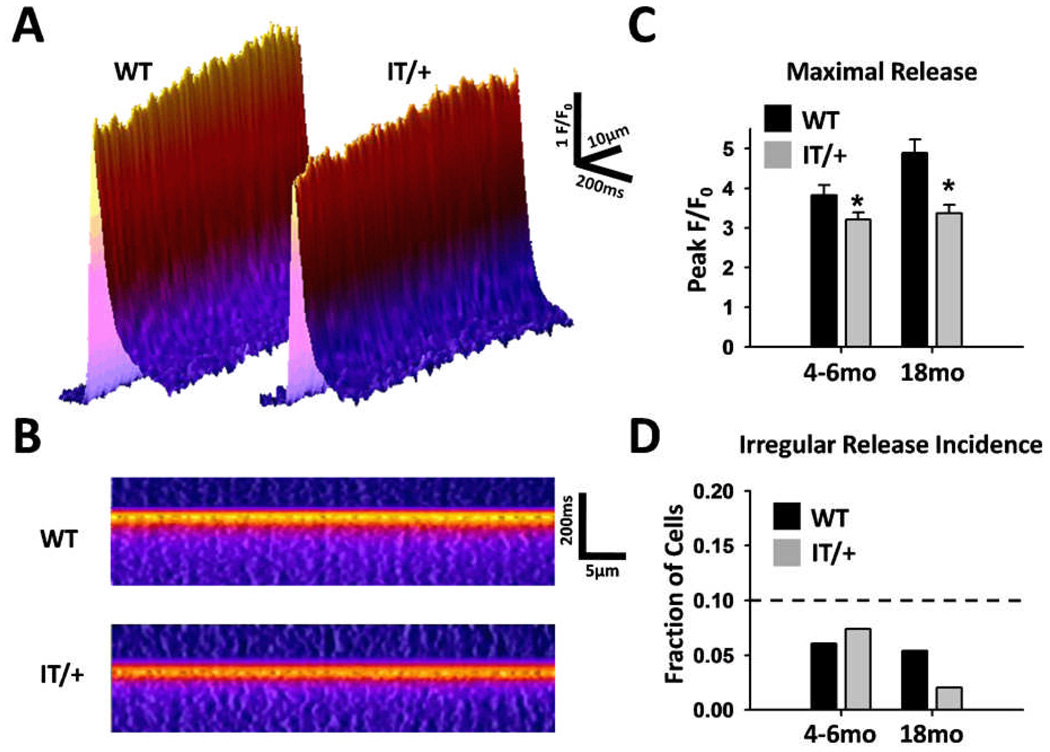
In two recent papers documenting the progression of core formation in knockin mice expressing the Y522S leaky channel mutation (Boncompagni et al. 2009) or the I4895T EC-uncoupled channel mutation (Zvaritch et al. 2009), spatial inhomegeneity in local Ca2+ releases in muscles was suggested as a possible mechanism for the formation of localized alterations in muscle structure. This concept was extended further by (Zvaritch et al. 2009), who suggested that functional heterogeneity in WT:IT RyR1 tetramers may lead to non-uniform Ca2+ release that results in shear forces that, over time, cause structural alterations and a marked progression of muscle dysfunction with age in IT/+ mice. Thus, we evaluated the magnitude, kinetics, and spatial uniformity of electrically-evoked Ca2+ release during EC coupling using confocal line scan microscopy in fluo-4-loaded FDB fibers obtained from young (4–6 month-old) and aged (18 month-old) WT and IT/+ mice. Fluo-4 fluorescence line scan profiles in Figs. 7A and B show representative Ca2+ release events in response to electrical stimulation in single FDB fibers from WT and IT/+ mice. Consistent with prior study (Zvaritch et al. 2009), the magnitude of the peak Ca2+ transient (averaged across the longitudinal 47.7µm line of interest) was significantly (p < 0.05) reduced in fibers from both young (WT: 3.82 ± .25 vs IT/+: 3.21 ± 0.18) and old (WT: 4.89 ± 0.34 vs IT/+: 3.37 ± 0.21) IT/+ mice (Fig. 7A–C). Relative spatiotemporal uniformity of Ca2+ release across the line of interest was evaluated empirically by setting a detection threshold for irregular release (Fig. 7D) by simulating data with variable amplitude “cold spots” of Ca2+ release (see Methods and Supplemental Figure S5). Using this approach, the fraction of cells showing detectable non-uniform release was not significantly different between FDB fibers from either young or old WT and IT/+ mice. These results confirm a significant reduction in global electrically-evoked Ca2+ release during EC coupling in FDB fibers from young and old IT/+ mice that occurs in the absence of a detectable change in release uniformity.
Figure 7. Magnitude and spatiotemporal properties of Ca2+ release in FDB fibers from young and old WT and IT/+ mice.
A) Representative 3D surface plots from longitudinal confocal line scan images along the fiber center of electrically-evoked Ca2+ transients in fluo-4-loaded FDB fibers from WT (left) and IT/+ (right) mice. B) Linearized rendering of the representative plots shown in A. C) Mean peak F/F0 (± SEM) results for FDB fibers from young (4–6 months) and old (18 months) WT (black bars) and IT/+ (gray bars) mice. D) Fraction of fibers showing non-uniform Ca2+ release in FDB fibers from young (left) and old (right) WT (black bars) and IT/+ (gray bars) mice.
DISCUSSION
Muscle fibers from IT/+ mice in a mixed 129S6/SvEvTac and 129S2/SvPasCrl background exhibit structural alterations of the type seen in CCD patients (Hayashi et al. 1989; Lamont et al. 1998) as well as in WT mice at older ages (Boncompagni et al. 2010). The two major structural alterations observed routinely in muscles from IT/+ mice are Z line streaming in type I fibers (see also Zvaritch et al. 2009) and widening of free SR, apparently due to excess CASQ loading, followed by accumulation of tubular aggregates in type IIB/IIX fibers of EDL (Boncompagni et al. 2010). Type IIA fibers in EDL and soleus of IT/+ mice were unaffected. The reason for this fiber type specific response is not known, but it reflects the different embryonal origins of these fibers (Rubinstein & Kelly 1978) and it is consistent with their differential responses to a variety of conditions (Pellegrino & Franzini 1963; Takekura et al. 2003; Banker & Engel 2004)
Nemaline rods were found in soleus of aging WT (this paper) and IT/+ mice (Zvaritch et al. 2009). Thus, rod formation most likely reflects the stochastic presentation of a low frequency event associated with aging and not a direct result of the mutation. High-resolution spatiotemporal recordings of electrically-evoked Ca2+ transients demonstrate significantly reduced, but spatially uniform Ca2+ release in fibers from young and old IT/+ mice, consistent with an independent assortment of WT and Ca2+ permeation-deficient IT subunits, as suggested previously (Xu et al. 2008; Zvaritch et al. 2009). We also find that contractures of any size are not a significant phenotypic aspect in muscles from our IT/+ mice, consistent with the reduced and uniformly distributed Ca2+ transients.
The alterations we document in soleus type I fibers of IT/+ mice are entirely consistent with those observed in human central core disease (Engel et al. 1961; Telerman-Toppet et al. 1973; Isaacs et al. 1975). Indeed, the Z line streaming areas we observed in restricted regions in type I fibers correspond to core regions of reduced oxidative enzyme staining characteristic of CCD since mitochondria and striations in these regions are either missing or strongly disarranged (Zvaritch et al. 2009). As argued below, we propose that cumulative effects of long term mechanical stress imposed on fatigue-resistant type I fibers involved in the continual control and maintenance of posture result in structural damage to the sarcomere that leads to the observed areas of Z line streaming. This defect in mechanical stress may be compounded in type I fibers of IT/+ muscle in which reduced contractility results in greater stretch and structural damage during eccentric contractions. Indeed, our findings that greater structural defects and preferential Z-line streaming is observed in type I fibers of IT/+ mice are consistent with the well-documented preferential involvement and type I fiber predominance that is characteristic of human CCD (Engel et al. 1961; Telerman-Toppet et al. 1973; Isaacs et al. 1975).
Z line streaming is a structural alteration linked to mechanical stress, since it is frequently observed following eccentric contractions (Newham et al. 1983). In WT muscles of young adults, this effect is limited and is found primarily at sites of Vernier mismatches of the striation. It is logical to assume that myofibrils at the boundary line undergo a certain degree of mechanical stress during contraction. The continuous activity and high frequency of Vernier displacements in type I fibers could explain the higher incidence of Z line streaming in normal type I fibers and its absence in other fiber types. Following this line of reasoning, enlarged “Z line streaming” areas observed in fibers from aging and even in younger IT/+ mice would indicate an accumulation of enhanced mechanical stress in these muscles. The correspondence of Z line streaming (e.g. in aged WT and adult IT/+ mice) and weakness due to reduced levels of activating calcium may reflect muscle fibers being exposed to focal regions of eccentric contraction and stress even during normal ambulation. The potential effect of this stress over time on muscle structure is discussed below in relation to Ca2+ release events.
Our finding that global electrically-evoked Ca2+ transients are significantly reduced in single FDB fibers from both young and aged IT/+ mice (Fig. 7) would certainly contribute to the muscle weakness and reduced twitch contraction demonstrated previously (Zvaritch et al. 2009). The reduced global Ca2+ transient could be due to either a uniform reduction in Ca2+ release or summation of local regions of normal and lower Ca2+ release, as proposed by Zvaritch et al. (2009). In contrast to the later suggestion, our results indicate that peak Ca2+ transient magnitude is significantly reduced in FDB fibers from both young and old IT/+ mice in the absence of a detectable change in spatial release homogeneity. However, since our Ca2+ transient measurements were conducted in fibers isolated from FDB muscle, which consists of primarily fast twitch type II fibers (Gonzalez et al. 2003), it is possible that inhomogeneous Ca2+ release may occur preferentially in type I fibers, particularly in areas of prominent Z line streaming. The two structural alterations of type IIB/IIX fibers (accumulation of luminal CASQ and the shift of triads from transverse to longitudinal orientation) are also present during normal aging, but are increased in extent and frequency in fibers from IT/+ mice. Such changes are occasionally noticeable in published micrographs from CCD patients (Lamont et al., 1998), but are often overlooked. An explanation for these effects is difficult to find. However, since type IIB and IIX fibers are less continuously active than either type I or IIA fibers, reduced Ca2+ cycling may promote CASQ accumulation and thus enhance type IIB/X SR Ca2+ storage capacity, compared to type I and IIA fibers. In support of this idea, Ca2+ release is reduced (Jimenez-Moreno et al. 2008) and CASQ accumulation is increased (Figs. 4 and 5) in fibers from aged mice. Under this scenario, the reduction in global Ca2+ release during EC coupling in fibers from young IT/+ mice (Fig. 7) could eventually lead to the observed enhanced SR accumulation of CASQ and accelerated age-dependent changes in SR structure.
The muscle phenotype of IT/+ mice is significantly different from that recently reported for muscle from Ryr1Y522S/WT (YS/+) knock-in mice (Boncompagni et al. 2009), a mutation that results in enhanced RyR1 Ca2+ leak and sensitivity to activation (Tong et al. 1999; Avila & Dirksen 2001; Dirksen & Avila 2002; Dirksen & Avila 2004; Chelu et al. 2006) The major points of divergence involve both mitochondrial involvement and the incidence of contractures. In muscles from IT/+ mice, mitochondria are apparently normal and do not decrease in frequency, although they are clearly missing in the relatively small areas of Z line streaming observed in type I fibers. In muscles from YS/+ mice, on the other hand, mitochondria are the first organelle to be affected (as early as 2 months), exhibiting a translucent matrix, marked swelling, cristae remodeling, and alterations in the outer membrane that result in increased variability of organelle shape. Importantly, altered mitochondria in YS/+ mice are affected within discrete subcellular domains that evolve over time into cores (Boncompagni et al. 2009).
Contraction cores that completely lack mitochondria and SR and exhibit greatly shortened sarcomeres are observed at high frequency in soleus fibers from YS/+ mice (Boncompagni et al. 2009). This is to be expected from a mutation that enhances RyR1 Ca2+ leak, promotes SR store depletion, and hypersensitizes the release channel to activation (Chelu et al. 2006; Durham et al. 2008; Andronache et al. 2009). In contrast, contractures in muscles from our subline of IT/+ mice are rarely observed, are not associated with a lack of mitochondria, and occur with a frequency that is not affected by age, muscle type and/or genotype, indicating that they reflect infrequent microdamage during dissection. Specifically, contracture frequencies extrapolated from the data in Supplemental Table S1 for muscles from WT and IT/+ mice are between 0.01 and 0.5/10 mm fiber length, while the frequencies in YS/+ mice are ~100 fold higher (23–42/10 mm fiber length).
The mild functional and structural phenotypes of our subline of IT/+ mice are entirely consistent with the known properties of the mutated channel. Mutation of the conserved I4895 residue in the middle of the selectivity filter reduces release channel Ca2+ conductance (Gao et al. 2000) and RyR1 Ca2+ flux during physiological channel activation of mice in our colony (Fig. 7). Thus, the mild functional and structural phenotypes in our subline of IT/+ mice are consistent with the reduced Ca2+ release during EC coupling and absence of Ca2+ leak at rest. The same mutation bred onto a C57Bl6 background also exhibits a similar mild structural phenotype (unpublished observations). Since the mutation auses a loss of Ca2+ release function in the absence of any spatial inhomegenities, we do not expect that the heterozygote phenotype should be strongly affected by variations in the level of allele gene expression, unless under extreme conditions. On the other hand, this mutation on a congenic 129S2/SvPasCrl background manifests a much stronger disease phenotype with more severe structural lesions that appear at even younger ages (Zvaritch et al. 2009). In view of these observations and the fact that heterozygous individuals in human families carrying the I4898T CCD mutation also exhibit a highly variable disease presentation (Lynch et al. 1999; Monnier et al. 2001; Davis et al. 2003), it is apparent that genetic background has a strong effect on the phenotypic presentation of the I4898T mutation.
A recent study of patients with multiminicore diseases found a correlation between variations in clinical phenotype and RyR1 expression levels, indicating that allele silencing can play an important role in disease presentation (Zhou et al. 2006; Zhou et al. 2007). However, direct gene silencing is probably not a major factor in IT/+ mice since both WT and mutant alleles are expressed at the same level in even the most affected colony (Zvaritch et al. 2009). But other factors including differences in epigenetic modulators or stability of the mutant protein may be at play. In any event, phenotypic differences between the two partially overlapping genetic lines of IT/+ mice (Zvarich et al. 2009 and this work) offer a unique opportunity for unraveling factors underlying the well-established variable penetrance of CCD in humans (Lynch et al. 1999; Monnier et al. 2001; Davis et al. 2003).
EXPERIMENTAL PROCEDURES
Generation and handling of Ryr1I4895T/+ (IT/+) knock-in mice
The generation and genotyping of Ryr1I4895T/wt mice (IT/+) is described in (Zvaritch et al. 2007). A subline of IT/+ mice on a mixed 129S6/SvEvTac and 129S2/SvPasCrl background was maintained at the University of Rochester by brother-sister matings, beginning in 2006.and used for all experiments conducted in this study. The mice used in the present study differ from the congenic subline described by (Zvaritch et al. 2009), in which the mice had been backcrossed for ~15 generations onto 129S2/SvPasCrl. All mice were housed in a pathogen-free area at the University of Rochester and experiments were carried out in accordance with procedures reviewed and approved by the local University Committee on Animal Resources. Mice were euthanized by regulated delivery of compressed CO2 followed by cervical dislocation.
Muscle isolation and fixation
Soleus and EDL muscles were carefully dissected while irrigated with Ringer’s solution consisting of (mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4. Isolated muscles were fastened to sylguard-coated plates, rinsed once with Ringer’s, immersed in fixative (3.5% glutaraldehyde in 0.1 M NaCaCo buffer, pH 7.4) at room temperature for >20 minutes and then transferred and stored at 4 °C for variable periods of time.
Electron microscopy
Muscle segments were post-fixed in 2% OsO4 in the same buffer for 1–2 hr at 4°C, en block-stained in saturated uranyl acetate and embedded in Epon 812. Ultrathin sections (40–60 nm) were cut with an ultra-microtome Leica Ultracut R (Leica Microsystem, Austria) using a Diatome diamond knife (DiatomeLtd. CH-2501 Biel, Switzerland) and stained in 4% uranyl acetate and lead citrate solutions.
Light microscopy of fiber bundles
Following fixation and postfixation in OsO4 as for EM, portions of muscles were teased into very thin bundles/single fibers and whole-mounted in glycerol for light microscopy. The bundles were examined under direct illumination and/or phase contrast optics. The number of contractures was counted and referred to the approximate length of the fiber bundle (1/3, 1/2 or whole fiber length) and finally normalized to whole fiber length. Note was taken whether contractures were clustered within adjacent regions of a group of fibers or were present singly within an individual fiber. In all cases, we directly compared age-matched WT and IT/+ muscles that were dissected by the same operator within a single session and the counts at the microscope were performed by an operator blinded to genotype.
Random sample collection
In order to insure unbiased collection of data, we followed two approaches. Some data (e.g., frequency of Z line streaming areas, T-tubule swelling and contractures) were obtained from all fibers in the field of view in either thin sections for EM or teased fiber bundles for light microscopy. Counts were done directly at the light/electron microscopes, in order to extract data rapidly from large sample areas. For data requiring micrographs, images were taken at random, usually one for each fiber as it first appeared in the field of view as the grid was scanned. In all cases sample sizes were equivalent, though not identical, in mutated and WT muscles (see data) and examiners were also blinded to genotype prior to completing the structural analyses.
The relative frequency of free SR tubule profiles of different sizes was performed in random photographs from cross sections, taken at magnification of 84.400 × (see Table 2). Classification of profile was based on an approximate size estimate. A circle of ≤70 nm of diameter covered the smallest profiles; ~ 80–150 nm; ~150–200 nm and >200 nm identified medium, large and extra large profiles respectively.
Isolation of flexor digitorum brevis (FDB) muscle fibers
Flexor Digitorum Brevis (FDB) muscle fibers were isolated as previously described (Beam & Knudson 1988). Briefly, FDB muscles were dissected while bathed in Ringer’s solution (see above) Muscles were then enzymatically dissociated in Ringer’s solution supplemented with 1 mg/mL collagenase A (Roche) for 60 min while rocking gently at 37°C. Individual fibers were dispersed by gentle trituration and then plated onto glass coverslips. Only fibers with a clean morphology, clear striations, and no signs of swelling or damage were used for recordings. All experiments were conducted within 8 hours of fiber isolation.
Confocal line scan analysis of electrically-evoked Ca2+ release
Single FDB fibers were loaded with 10 µM Fluo-4 acetomethoxy ester (AM) in Ringer’s solution for 30 min at room temperature and then bathed in dye-free Ringer’s solution supplemented with 25 µM 4-methyl-N-(phenylmethyl)benzenesulfonamide (BTS, Tocris Biosciences), a skeletal muscle myosin II ATPase inhibitor, for >20 min to allow for both de-esterification of dye and inhibition of contraction. Fluo-4 was excited using a 488 nm argon laser (8× attenuation, 530-nm emission). Data were collected in linescan mode (1024 lines at a rate of 485 lines per second) along a longitudinally oriented 47.7 µm line (covering >20 sarcomeres) within a central region of the fiber using a Nikon Eclipse C1 Plus Confocal microscope equipped with a SuperFluor 40× 1.3 NA oil immersion objective (Nikon Instruments, Melville, NY). During linescan acquisition, electrically-evoked Ca2+ transients were elicited using individual electrical stimuli (8V, 20 ms) delivered using an extracellular electrode filled with 1% agarose in 200 mM NaCl placed adjacent to the cell of interest. Data were analyzed offline using NIH ImageJ (http://rsb.info.nih.gov/ij/). Image noise was reduced by removing bright and dark outliers with a radius set to 3 and threshold of 50. Images were then lowpass Gaussian filtered using a threshold of 200 and a second pass of outlier removal to complete image processing. This filtering sequence was empirically determined to not significantly alter signal rise time or spatial resolution. For each pixel in the X (temporal) direction, all values 25 ms immediately prior to electrical stimulation were averaged and used to divide fluorescence values obtained after stimulation to generate F/F0 values for each row of pixels. The mean of all peak F/F0 values (one peak value for each pixel) was determined for each fiber and peak responses across all fibers were then averaged (± SEM) within a group (i.e. young WT, old WT, young IT/+, old IT/+). Interactive 3D Surface Plot (bundled with NIH ImageJ was used to generate and export surface plots to visually inspect for potential regions of inhomogeneous release. To determine the threshold of detectable inhomogeneous release, simulation, “burns” were used on the raw linescan images (See Supplemental Figure S1) with varying intensity and spatial dimension to produce a series of progressively altered surface plots exhibiting non-uniform release. The fraction of cells exceeding a 15% burn across a 1.0 µm distance (Supplemental Fig. S1B) was used as an objective index of fibers displaying a detectable level of inhomogeneous release (Figure 7D).
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. B. Silverstein for writing the ImageJ line scan analysis plug-in used in this study and Nosta Glaser for expert EM support. We also thank Drs. D. H. MacLennan and E. Zvaritch for providing access to the Ryr1I4895T / WT mice used in this study. Supported by research grants from the National Institutes of Health (AR052354 to CFA and RTD as well as AR044657 and AR053349 to RTD) and a National Institutes of Health Dental and Craniofacial Training Grant (to REL).
Footnotes
AUTHORS’ CONTRIBUTIONS
Simona Boncompagni, Address: Interuniversitary Institute of Myology, Department of Neuroscience and Imaging, Ce.S.I.-Centro Scienze dell’Invecchiamento, Università degli Studi G. d’Annunzio, 66013 Chieti, Italy. Tel: +39 0871-541218, Fax: +39 0871-541422, s.boncompagni@unich.it, Planned experiments, collected and analysed data, wrote paper.
Ryan E. Loy, Address: University of Rochester Medical Center. Department of Pharmacology and Physiology. 601 Elmwood Avenue. Rochester, NY 14642. Tel: 585-273-2170, Fax: 585-273-2652, Ryan_Loy@URMC.Rochester.edu, Planned experiments, collected and analysed data, wrote paper.
Robert T. Dirksen, Address: University of Rochester Medical Center. Department of Pharmacology and Physiology. 601 Elmwood Avenue. Rochester, NY 14642. Tel: 585-275-4824, Fax: 585-273-2652, Robert_Dirksen@URMC.Rochester.edu, Designed research, planned experiments, analysed data, wrote paper.
Clara Franzini-Armstrong, Address: Dept. of Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104. Tel: 215 898 3345, armstroc@mail.med.upenn.edu, Designed research, planned experiments, collected and analysed data, wrote paper.
SUPPORTING INFORMATION
Supplemental Figures
Fig. S1 Longitudinal triads are frequently observed in 12 months EDL fibers from IT/+ mice.
Fig. S2 Nemaline Rods are present in aged soleus muscles from WT mice.
Fig. S3 Dissection damage is well detectable in whole mounts for the light microscope.
Fig. S4 Contractures in fibers from WT and IT/+ fibers have similar characteristics.
Fig. S5 Simulation and detection threshold assessment of non-homogeneous Ca2+ release.
Supplemental Table
Table S1 Frequency of contractures in fibers from fixed WT and IT/+ muscles.
Contributor Information
Simona Boncompagni, Email: s.boncompagni@unich.it.
Ryan E. Loy, Email: Ryan_Loy@URMC.Rochester.edu.
Robert T. Dirksen, Email: Robert_Dirksen@URMC.Rochester.edu.
Clara Franzini-Armstrong, Email: armstroc@mail.med.upenn.edu.
REFERENCES
- Andronache Z, Hamilton SL, Dirksen RT, Melzer W. A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice. Proc Natl Acad Sci U S A. 2009;106:4531–4536. doi: 10.1073/pnas.0812661106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbanas J, Klasan GS, Nikolic M, Jerkovic R, Miljanovic I, Malnar D. Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat. 2009;215:636–641. doi: 10.1111/j.1469-7580.2009.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen G, Marechal G. Maximal shortening velocities, isomyosins and fibre types in soleus muscle of mice, rats and guinea-pigs. J Physiol. 1989;416:245–254. doi: 10.1113/jphysiol.1989.sp017758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol. 2001;118:277–290. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila G, O'Connell KM, Dirksen RT. The pore region of the skeletal muscle ryanodine receptor is a primary locus for excitation-contraction uncoupling in central core disease. J Gen Physiol. 2003;121:277–286. doi: 10.1085/jgp.200308791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker BQ, Engel AG. Basic Reactions of Muscle. In: Engel AG, Franzini-Armstrong C, editors. Myology (third edition) 2004. pp. 691–747. McGraw- Hill Med. Publish. Div., vol.I, chap. 30. [Google Scholar]
- Beam KG, Knudson CM. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988;91:781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, d'Amelio L, Fulle S, Fano G, Protasi F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance. J Gerontol A Biol Sci Med Sci. 2006;61:995–1008. doi: 10.1093/gerona/61.10.995. [DOI] [PubMed] [Google Scholar]
- Boncompagni S, Protasi F, Franzini-Armstrong C. Sequential Stages in the Formation of Tubular Aggregates in Aging Fast Twitch Muscle Fibers. SERCA and Calsequestrin Involvement. Submitted. 2010 doi: 10.1007/s11357-011-9211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, Rossi AE, Micaroni M, Hamilton SL, Dirksen RT, Franzini-Armstrong C, Protasi F. Characterization and temporal development of cores in a mouse model of malignant hyperthermia. Proc Natl Acad Sci U S A. 2009;106:21996–22001. doi: 10.1073/pnas.0911496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelu MG, Goonasekera SA, Durham WJ, Tang W, Lueck JD, Riehl J, Pessah IN, Zhang P, Bhattacharjee MB, Dirksen RT, Hamilton SL. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. Faseb J. 2006;20:329–330. doi: 10.1096/fj.05-4497fje. [DOI] [PubMed] [Google Scholar]
- Conen PE, Murphy EG, Donohue WL. Light and Electron Microscopic Studies of "Myogranules" in a Child with Hypotonia and Muscle Weakness. Can Med Assoc J. 1963;89:983–986. [PMC free article] [PubMed] [Google Scholar]
- Danieli-Betto D, Esposito A, Germinario E, Sandona D, Martinello T, Jakubiec-Puka A, Biral D, Betto R. Deficiency of alpha-sarcoglycan differently affects fast- and slow-twitch skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1328–R1337. doi: 10.1152/ajpregu.00673.2004. [DOI] [PubMed] [Google Scholar]
- Davis MR, Haan E, Jungbluth H, Sewry C, North K, Muntoni F, Kuntzer T, Lamont P, Bankier A, Tomlinson P, Sanchez A, Walsh P, Nagarajan L, Oley C, Colley A, Gedeon A, Quinlivan R, Dixon J, James D, Muller CR, Laing NG. Principal mutation hotspot for central core disease and related myopathies in the C-terminal transmembrane region of the RYR1 gene. Neuromuscul Disord. 2003;13:151–157. doi: 10.1016/s0960-8966(02)00218-3. [DOI] [PubMed] [Google Scholar]
- Dirksen RT, Avila G. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca(2+) release channels? Trends Cardiovasc Med. 2002;12:189–197. doi: 10.1016/s1050-1738(02)00163-9. [DOI] [PubMed] [Google Scholar]
- Dirksen RT, Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys J. 2004;87:3193–3204. doi: 10.1529/biophysj.104.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Sherrington CS. Reflex summation in the ipsilateral spinal flexion reflex. J Physiol. 1930;69:1–28. doi: 10.1113/jphysiol.1930.sp002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, Adrian RH, editors. Handbook of Physiology, section 10, skeletal muscle. Baltimore Md.: Am Physiol Soc.; 1983. pp. 191–213. [Google Scholar]
- Engel WK, Foster JB, Hughes BP, Huxley HE, Mahler R. Central core disease-an investigation of a rare muscle cell abnormality. Brain. 1961;84:167–185. doi: 10.1093/brain/84.2.167. [DOI] [PubMed] [Google Scholar]
- Gao L, Balshaw D, Xu L, Tripathy A, Xin C, Meissner G. Evidence for a role of the lumenal M3–M4 loop in skeletal muscle Ca(2+) release channel (ryanodine receptor) activity and conductance. Biophys J. 2000;79:828–840. doi: 10.1016/S0006-3495(00)76339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Miller RG, Brownell AK. Central core disease: ultrastructure of the sarcoplasmic reticulum and T-tubules. Muscle Nerve. 1989;12:95–102. doi: 10.1002/mus.880120203. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Chi MM, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J Cell Biol. 1999;145:633–642. doi: 10.1083/jcb.145.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs H, Heffron JJ, Badenhorst M. Central core disease. A correlated genetic, histochemical, ultramicroscopic, and biochemical study. J Neurol Neurosurg Psychiatry. 1975;38:1177–1186. doi: 10.1136/jnnp.38.12.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J. 2008;94:3178–3188. doi: 10.1529/biophysj.107.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont PJ, Dubowitz V, Landon DN, Davis M, Morgan-Hughes JA. Fifty year follow-up of a patient with central core disease shows slow but definite progression. Neuromuscul Disord. 1998;8:385–391. doi: 10.1016/s0960-8966(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Lovering RM, Russ DW. Fiber type composition of cadaveric human rotator cuff muscles. J Orthop Sports Phys Ther. 2008;38:674–680. doi: 10.2519/jospt.2008.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch PJ, Tong J, Lehane M, Mallet A, Giblin L, Heffron JJ, Vaughan P, Zafra G, MacLennan DH, McCarthy TV. A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease. Proc Natl Acad Sci U S A. 1999;96:4164–4169. doi: 10.1073/pnas.96.7.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N, Romero NB, Lerale J, Landrieu P, Nivoche Y, Fardeau M, Lunardi J. Familial and sporadic forms of central core disease are associated with mutations in the C-terminal domain of the skeletal muscle ryanodine receptor. Hum Mol Genet. 2001;10:2581–2592. doi: 10.1093/hmg/10.22.2581. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Pellegrino C, Franzini C. An Electron Microscope Study of Denervation Atrophy in Red and White Skeletal Muscle Fibers. J Cell Biol. 1963;17:327–349. doi: 10.1083/jcb.17.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein NA, Kelly AM. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978;62:473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Shy GM, Engel WK, Somers JE, Wanko T. Nemaline Myopathy. a New Congenital Myopathy. Brain. 1963;86:793–810. doi: 10.1093/brain/86.4.793. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Takekura H, Tamaki H, Nishizawa T, Kasuga N. Plasticity of the transverse tubules following denervation and subsequent reinnervation in rat slow and fast muscle fibres. J Muscle Res Cell Motil. 2003;24:439–451. doi: 10.1023/a:1027356912404. [DOI] [PubMed] [Google Scholar]
- Telerman-Toppet N, Gerard JM, Coers C. Central core disease. A study of clinically unaffected muscle. J Neurol Sci. 1973;19:207–223. doi: 10.1016/0022-510x(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Tong J, McCarthy TV, MacLennan DH. Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J Biol Chem. 1999;274:693–702. doi: 10.1074/jbc.274.2.693. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78:1947–1954. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang Y, Yamaguchi N, Pasek DA, Meissner G. Single channel properties of heterotetrameric mutant RyR1 ion channels linked to core myopathies. J Biol Chem. 2008;283:6321–6329. doi: 10.1074/jbc.M707353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Brockington M, Jungbluth H, Monk D, Stanier P, Sewry CA, Moore GE, Muntoni F. Epigenetic allele silencing unveils recessive RYR1 mutations in core myopathies. Am J Hum Genet. 2006;79:859–868. doi: 10.1086/508500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Jungbluth H, Sewry CA, Feng L, Bertini E, Bushby K, Straub V, Roper H, Rose MR, Brockington M, Kinali M, Manzur A, Robb S, Appleton R, Messina S, D'Amico A, Quinlivan R, Swash M, Muller CR, Brown S, Treves S, Muntoni F. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain. 2007;130:2024–2036. doi: 10.1093/brain/awm096. [DOI] [PubMed] [Google Scholar]
- Zvaritch E, Depreux F, Kraeva N, Loy RE, Goonasekera SA, Boncompagni S, Kraev A, Gramolini AO, Dirksen RT, Franzini-Armstrong C, Seidman CE, Seidman JG, Maclennan DH. An Ryr1I4895T mutation abolishes Ca2+ release channel function and delays development in homozygous offspring of a mutant mouse line. Proc Natl Acad Sci U S A. 2007;104:18537–18542. doi: 10.1073/pnas.0709312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaritch E, Kraeva N, Bombardier E, McCloy RA, Depreux F, Holmyard D, Kraev A, Seidman CE, Seidman JG, Tupling AR, MacLennan DH. Ca2+ dysregulation in Ryr1(I4895T/wt) mice causes congenital myopathy with progressive formation of minicores, cores, and nemaline rods. Proc Natl Acad Sci U S A. 2009;106:21813–21818. doi: 10.1073/pnas.0912126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.