Abstract
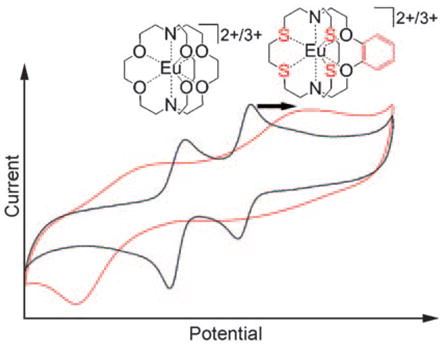
A series of cryptands have been prepared and they demonstrate the relationship between oxidative stability of aqueous EuII and ligand properties (see figure). One of these EuII complexes is more stable than FeII in hemoglobin and appears to be the most oxidatively-stable aqueous EuII species known. The high stability of EuII is expected to enable the use of the unique magnetic and optical properties of this ion in vivo.
Keywords: chelates, cryptands, europium, lanthanides, redox chemistry
The magnetic and optical properties of the divalent state of europium make this ion extremely attractive for use in materials,[1] catalysis,[2] luminescence,[3] magnetic,[4] and diagnostic-medical applications. A major hindrance to the use of EuII in many of these applications is the extreme propensity of the ion to oxidize to EuIII, especially in aqueous solution. Research efforts aimed at increasing the stability of aqueous EuII have yielded little success:[6,7] even the most stable aqueous EuII complex reported (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane europium(II), 1-Eu) is not stable enough in aqueous solution for practical use.[8,9] Our research group has generated EuII complexes in aqueous solution, and here we report the most oxidatively-stable aqueous EuII complexes known.
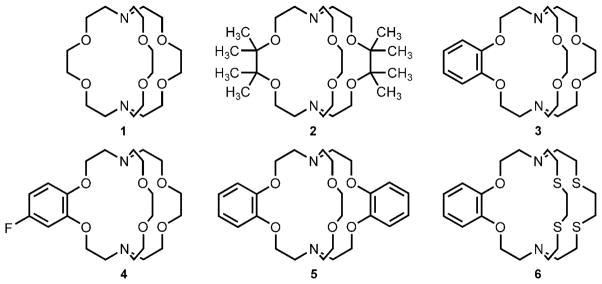
Our strategy for favoring EuII over EuIII in aqueous solution involves the synthesis and use of ligands that would preferentially coordinate to large, soft, electron-rich metals like EuII. The template for our ligand design was cryptand 1 because 1-Eu is the most oxidatively-stable, aqueous EuII complex previously reported.[8] The stability of 1-Eu is partially due to the better size match of the cavity of cryptand 1 (1.4 Å) to the EuII ion (1.25 Å) relative to the EuIII ion (1.07 Å).[10] We hypothesized that further oxidative stabilization could be achieved by modifying the structure of cryptand 1 using four principles of coordination chemistry to stabilize electron-rich metals.[6,11] Specifically, our goals were 1) to increase the steric bulk surrounding cryptand 1 to minimize interactions between EuII and its environment; 2) to reduce the Lewis basicity of cryptand 1 to favor the electron-rich EuII over EuIII; 3) to change the cavity size of the cryptand to match the size of the EuII ion preferentially; and 4) to modify the hard–soft, acid–base (HSAB) properties of cryptand 1 to coordinate EuII over EuIII. To implement these strategies, we studied cryptands 1–6 (Scheme 1).
Scheme 1.
Ligands used to observe trends in oxidative stability of aqueous EuII.
To increase the steric bulk of 1, methyl groups were added to the ethylene carbon atoms between the oxygen atoms resulting in ligand 2. This methyl substitution pattern was chosen because metal–environment interactions occur between the unmodified ethylene groups.[8] Furthermore, to examine the influence of Lewis basicity on oxidative stability, phenyl rings were introduced to decrease the electron-donating ability of the adjacent oxygen atoms of ligands 3–5 by a resonance withdrawing effect.[12] The extent of electron withdrawal was modulated by varying the electron density of the phenyl ring through the addition of a fluorine atom (4) or by increasing the number of rings (5). Phenyl-ring-containing cryptands 3–5 also have an influence on cavity size because each phenyl ring decreases the cavity size of the cryptand. We expected the seemingly minor influence of the phenyl rings on cavity size to have a noticeable effect on the oxidative stability of EuII because of selectivity studies with Group 2 cations using cryptands 1, 3, and 5.[13] Finally, relatively soft sulfur-atom donors were introduced in cryptand 6 in place of oxygen-atom donors to explore the HSAB preferences for the softer EuII ion relative to the harder EuIII ion.
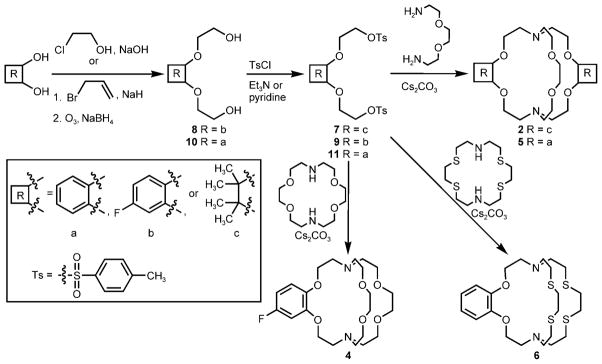
To synthesize the diverse set of cryptands 1–6, a three-step procedure was devised that involved common intermediates 7, 9, and 11 (Scheme 2).[14] Briefly, the synthesis involved the conversion of the appropriate ethylenediols or catechols into the corresponding ditosylates (7, 9, and 11) and subsequent ring closure with 1,4,10,13-tetraoxa-7,16-diazacyclooctadecane, 2,2′-(ethylenedioxy)bis(ethylamine), or 1,4,10,13-tetrathia-7,16-diazacyclooctadecane. Metal complexation was achieved in situ by mixing Eu(NO3)3·5H2O and the desired cryptand (1–6) in aqueous solution under an Ar atmosphere. The resulting solution was placed in a standard three-electrode cell (glassy-carbon working electrode, platinum-wire auxiliary electrode, and Ag/AgCl (1.0M KCl) reference electrode). The potential at the carbon electrode was held at −0.8 V (vs. Ag/AgCl) while stirring to produce EuII in situ for metalation.[7] After metalation, cyclic voltammograms were obtained for each complex in solution with ferrocene as an internal standard:[9,15] a new anodic peak was observed for each complex at a more positive potential than the peak corresponding to oxidation of the aqueous EuII (Table 1). These data indicate that each cryptand imparted additional stability to EuII as hypothesized.
Scheme 2.
Synthetic route to cryptands 2 and 4–6 using common intermediates 7, 9, and 11.
Table 1.
Anodic peak potentials (Epa) with respect to ferrocene/ferrocenium (Fc/Fc+).
| Sample | Epa (V vs. Fc/Fc+)[a] | Sample | Epa (V vs. Fc/Fc+)[a] | ||
|---|---|---|---|---|---|
| 1 | Eu(NO3)3 | −0.701 ± 0.030 | 5 | 2-Eu | −0.169 ± 0.006 |
| 2 | 1-Eu | −0.336 ± 0.016 | 6 | 4-Eu | −0.079 ± 0.007 |
| 3 | 5-Eu | −0.211 ± 0.004 | 7 | hemoglobin | −0.070 ± 0.003 |
| 4 | 3-Eu | −0.208 ± 0.009 | 8 | 6-Eu | −0.035 ± 0.010 |
Potentials are listed as mean ± standard error.
The cyclic voltammetric data of europium-containing solutions of 1 and 2 demonstrate that the increased steric bulk in cryptand 2 leads to increased oxidative stability over unmodified cryptand 1. Furthermore, a more targeted examination of the influence of Lewis basicity on oxidative stability was achieved by examining the impact of ligands 3–5. We observed that one phenyl ring on cryptand 3 was sufficient to stabilize EuII oxidatively by 128 mV with respect to the unmodified cryptand 1-Eu. This stabilization is likely due to a combination of the decrease in Lewis basicity of the adjacent oxygen atoms (better for electron-rich metals) and the reduction in cavity size caused by the phenyl ring (better match for the size of the EuII ion). However, the addition of a second phenyl ring (5) caused no difference in the anodic peak potential compared to the monophenyl cryptand 3 (p = 0.76). This effect is likely due to reduction of the cavity size counteracting the decreased basicity of the ligand, thus suggesting that a minimum cavity size for EuII stabilization was achieved with cryptand 3. Further decrease in Lewis basicity through the addition of a fluorine substituent to the phenyl ring (4) led to 129 mV greater stability than what was observed with unsubstituted monophenyl cryptand 3. In addition, the oxidative stability of 4-Eu is not different from that of FeII in hemoglobin (p = 0.45).
Finally, replacement of the harder oxygen atoms with softer sulfur atoms (6) produced the most dramatic stabilization effect of our cryptand series. This modification increased the oxidative stability of EuII by 173 mV compared to the structurally similar cryptand 3. The cavity size of cryptand 6 increases slightly because of the increased bond length of C–S compared to C–O, thus suggesting that a decrease in stability should be observed based on the difference between cryptands 1 and 3. However, the effect of cavity size is small relative to the influence of HSAB matching between EuII and sulfur. Cryptand 6 with EuII produces an oxidative potential that is 666 mV more positive than the aqueous EuII and 35 mV more positive than FeII in hemoglobin. To the best of our knowledge, this oxidative stability of EuII is the highest reported in aqueous solution and indicates the potential for the use of EuII in vivo.
We observed dramatic oxidative stabilization of EuII using modified cryptands. These trends in stability suggest that further stabilization of aqueous EuII and other lanthanide ions is possible. We are currently pursuing these avenues of research in addition to measuring the thermodynamic stability of the EuII complexes reported here. Finally, our most stable complex, with an oxidation potential indicative of biological oxidative stability, opens the door for the use of the magnetic and spectroscopic properties of EuII in vivo.
Supplementary Material
Footnotes
This research was supported by start-up funds from Wayne State University and a Pathway to Independence Career Transition Award (R00EB007129) from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201002789.
References
- 1.a) Stadler F, Oeckler O, Höppe HA, Möller MH, Pöttgen R, Mosel BD, Schmidt P, Duppel V, Simon A, Schnick W. Chem Eur J. 2006;12:6984–6990. doi: 10.1002/chem.200600409. [DOI] [PubMed] [Google Scholar]; b) Denis G, Deniard P, Gautron E, Clabau F, Garcia A, Jobic S. Inorg Chem. 2008;47:4226–4235. doi: 10.1021/ic702240q. [DOI] [PubMed] [Google Scholar]; c) Huxter VM, Mirkovic T, Nair PS, Scholes GD. Adv Mater. 2008;20:2439–2443. [Google Scholar]
- 2.a) Evans WJ, Perotti JM, Brady JC, Ziller JW. J Am Chem Soc. 2003;125:5204–5212. doi: 10.1021/ja020957x. [DOI] [PubMed] [Google Scholar]; b) Datta S, Gamer MT, Roesky PW. Organometallics. 2008;27:1207–1213. [Google Scholar]; c) Edelmann FT. Chem Soc Rev. 2009;38:2253–2268. doi: 10.1039/b800100f. [DOI] [PubMed] [Google Scholar]
- 3.a) Richter MM, Bard AJ. Anal Chem. 1996;68:2641–2650. doi: 10.1021/ac960211f. [DOI] [PubMed] [Google Scholar]; b) Su FH, Chen W, Ding K, Li GH. J Phys Chem A. 2008;112:4772–4777. doi: 10.1021/jp8008332. [DOI] [PubMed] [Google Scholar]; c) Petrykin V, Kakihana M. Chem Mater. 2008;20:5128–5130. [Google Scholar]
- 4.a) Ahn K, Pecharsky AO, Gschneidner KA, Pecharsky VK. J Appl Phys. 2004;97:1–5. [Google Scholar]; b) Hasegawa Y, Adachi T, Tanaka A, Afzaal M, O’Brien P, Doi T, Hinatsu Y, Fujita K, Tanaka K, Kawai T. J Am Chem Soc. 2008;130:5710–5715. doi: 10.1021/ja710165m. [DOI] [PubMed] [Google Scholar]; c) Regulacio MD, Kar S, Zuniga E, Wang G, Dollahon NR, Yee GT, Stoll SL. Chem Mater. 2008;20:3368–3376. [Google Scholar]
- 5.a) Tóth É, Burai L, Merbach AE. Coord Chem Rev. 2001;216–217:363–382. [Google Scholar]; b) Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chem Rev. 2010;110:2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Yee EL, Gansow OA, Weaver MJ. J Am Chem Soc. 1980;102:2278–2285. [Google Scholar]; b) Burai L, Tóth É, Moreau G, Sour A, Scopelliti R. Chem Eur J. 2003;9:1394–1404. doi: 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]
- 7.Gansow OA, Kausar AR, Triplett KM, Weaver MJ, Yee EL. J Am Chem Soc. 1977;99:7087–7089. [Google Scholar]
- 8.Burai L, Scopelliti R, Tóth É. Chem Commun. 2002:2366–2367. doi: 10.1039/b206709a. [DOI] [PubMed] [Google Scholar]
- 9.See the Supporting Information.
- 10.a) Sabbatini N, Ciano M, Dellonte S, Bonazzi A, Bolletta F, Balzani V. J Phys Chem. 1984;88:1534–1537. [Google Scholar]; b) Yuan JL, Zeng XY, Zhao JT, Zhang ZJ, Chen HH, Zhang GB. J Solid State Chem. 2007;180:3310–3316. [Google Scholar]
- 11.a) Evans WJ, Johnston MA, Greci MA, Ziller JW. Organometallics. 1999;18:1460–1464. [Google Scholar]; b) Hauber S, Niemeyer M. Inorg Chem. 2005;44:8644–8646. doi: 10.1021/ic0514602. [DOI] [PubMed] [Google Scholar]; c) Guo H, Zhou H, Yao Y, Zhang Y, Shen Q. Dalton Trans. 2007:3555–3561. doi: 10.1039/b705353c. [DOI] [PubMed] [Google Scholar]; d) Puchta R, Meier R, Eldik RV. Aust J Chem. 2007;60:889–897. [Google Scholar]; e) Dugah DT, Skelton BW, Delbridge EE. Dalton Trans. 2009:1436–1445. doi: 10.1039/b816916k. [DOI] [PubMed] [Google Scholar]
- 12.a) Cox BG, Truong NV, Garcia-Rosas J, Schneider H. J Phys Chem. 1984;88:996–1001. [Google Scholar]; b) Dantz DA, Buschmann HJ, Schollmeyer E. Polyhedron. 1998;17:1891–1895. [Google Scholar]
- 13.Bemtgen JM, Springer ME, Loyola VM, Wilkins RG, Taylor RW. Inorg Chem. 1984;23:3348–3353. [Google Scholar]
- 14.Cryptands 1 and 3 were obtained from commercial sources.
- 15.Gagne R, Koval C, Licenski G. Inorg Chem. 1980;19:2854–2855. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.