Abstract
Aim
To test the hypothesis that rheumatoid arthritis influenced levels of salivary biomarkers of periodontal disease.
Methods
Medical assessments, periodontal examinations, and pain ratings were obtained from 35 rheumatoid arthritis, 35 chronic periodontitis and 35 age and gender-matched healthy controls in a cross-sectional, case-controlled study. Unstimulated whole saliva samples were analyzed for interleukin-1β (IL-1β), matrix-metalloproteinase-8 (MMP-8) and tumor necrosis factor-α (TNF)-α concentrations.
Results
The arthritis and healthy groups had significantly less oral disease than the periodontitis group (p<0.0001), with the arthritis group having significantly more sites bleeding on probing (BOP) than matched controls (p=0.012). Salivary levels of MMP-8 and IL-1β were significantly elevated in the periodontal disease group (p≤0.002), and IL-1β was the only biomarker with significantly higher levels in the arthritis group compared with controls (p=0.002). Arthritis patients receiving anti-TNF-α antibody therapy had significantly lower IL-1β and TNF-α levels compared with arthritis patients not on anti-TNF-α therapy (p=0.016, p=0.024) and healthy controls (p<0.001, p=0.011), respectively.
Conclusion
Rheumatoid arthritis patients have higher levels of periodontal inflammation than healthy controls, ie. increased BOP. Systemic inflammation appears to influence levels of select salivary biomarkers of periodontal disease, and anti-TNF-α antibody-based disease modifying therapy significantly lowers salivary IL-1β and TNF-α levels in rheumatoid arthritis.
Keywords: Interleukin 1β, matrix metalloproteinase (MMP), tumor necrosis factor (TNF)-α, salivary biomarkers, periodontal disease, rheumatoid arthritis, saliva, inflammation, biological markers
The diagnosis of periodontal disease in dentistry is historically based on the clinical detection of bleeding on probing (BOP), pocket depth (PD), clinical attachment loss (CAL), plaque index (PI), and radiographic evidence of bone loss. The diagnostic utility of these clinical measures results from their ease of use, relative non-invasiveness and reliability. However, the time, cost and professional expertise required to complete these procedures accurately, have led the profession to search for additional chair side measures to aid in early diagnosis and monitoring. Previous studies have shown that constituents present in oral fluids (i.e., gingival crevicular fluid and saliva) can provide important complimentary diagnostic information (Malamud 1992, Fox 1993, Kaufman & Lamster 2000, Kaufman & Lamster 2002, Giannobile et al. 2009), with saliva being appealing for its advantages of being a rapid, non-invasive, readily abundant diagnostic fluid potentially useful for point-of-care analysis for dental professionals and the general public (Malamud et al. 2005, Christodoulides et al. 2007).
Studies have shown that levels of certain salivary biomarkers are elevated in individuals who have periodontal disease (Pederson et al. 1995, Lamster et al. 2003, Christodoulides et al. 2005, Christodoulides et al. 2007, Ng et al. 2007, Scannapieco et al. 2007, Frodge, et al. 2008, Tobon-Arroyave et al. 2008). In particular, biomarkers associated with distinct biological phases of periodontal disease (i.e., inflammation, collagen degradation and bone remodeling) have been suggested to be useful for early recognition of patients with periodontitis (Miller et al. 2006). Within this context, several biomarkers including interleukin (IL)-1β, C-reactive protein, matrix metalloproteinase (MMP)-8 and -9, tissue inhibitor of matrix metalloproteinase (TIMP)-1, tumor necrosis factor (TNF)-α, receptor activator of nuclear factor-kappa B ligand (RANKL), and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) have been investigated (Bertolini et al. 1986, Assuma, et al. 1998, Azuma et al. 2000, Gorska & Nedzi-Gora 2006, Miller et al. 2006, Gursoy et al. 2010, Miller et al. 2010).
A concern with the clinical use of salivary biomarkers as an adjunct to identify periodontal disease is the possibility that systemic inflammation resulting from various chronic inflammatory diseases may confound the utility of the biomarkers. This concern arises because whole saliva contains serum derived components, and mediators of inflammation, collagen breakdown or bone remodeling that are elevated in serum of persons with conditions such as rheumatoid arthritis (RA) could appear in saliva (Ben-Aryeh et al. 1978, Miller et al. 2010). RA is a chronic inflammatory disorder that exhibits soft and hard tissue destruction similar to that of periodontal disease (Snyderman & McCarty 1982). In as much as certain inflammatory mediators such as IL-1β, MMP-8, and TNF-α have been shown to be elevated in the inflamed joints and serum of patients with RA (Tchetverikov et al. 2004, Eklund et al. 2007, Marti et al. 2009), it is possible that persons with RA or other inflammatory arthritides could have increased levels of these potential biomarkers in their saliva. Of great interest also, periodontal disease has a positive association with RA (de Pablo et al. 2008, Pischon et al. 2008, de Pablo et al. 2009), and it has been proposed that chronic P. gingivalis infection of the periodontal sulcus may be a source of citrullinated peptides that could serve as antigens that trigger RA (Wegner et al. 2010). For these reasons, it may be useful to identify periodontal disease in patients with RA or at risk for RA. In the absence of published studies on this topic, this investigation sought to test the hypothesis that RA influences levels of salivary biomarkers of periodontal disease.
Material and Methods
One hundred five patients were enrolled in this cross-sectional case-controlled clinical study performed at the University of Kentucky. Thirty five patients with the diagnosis of active RA for at least 3 years, as defined by the American College of Rheumatology criteria (Arnett et al. 1988), and under the care of a board certified rheumatologist at the University, 35 patients with chronic adult periodontitis based on the criteria defined by the American Academy of Periodontology (Armitage 1999, Armitage 2004), and 35 healthy controls were enrolled. The groups were matched by age and gender.
Inclusion criteria included ≥18 years of age who were in good general health, (excluding the case definition) and had ≥18 erupted teeth. Subjects in the periodontitis group had ≥30% of sites with BOP, ≥20% of sites with PD ≥4 mm, ≥10% of sites with interproximal CAL >2 mm, and evidence of alveolar crestal bone loss ≥2 mm at ≥30% of sites visible in posterior vertical bitewing films. The healthy controls had <10% sites with BOP, <2% of sites with PD ≥5 mm, no sites with PD ≥6 mm, <1% of sites with clinical AL >2 mm, and no radiographic bone loss evident in posterior vertical bitewings films. Exclusion criteria were a history of alcoholism; liver, kidney, or salivary gland dysfunction; inflammatory bowel disease; granulomatous diseases; or were undergoing or had undergone organ transplant or cancer therapy. Pregnancy, use of antibiotics or immunosuppressant medication (non-RA groups only) within the last 6 months, need for antibiotics for infective endocarditis prophylaxis during dental procedures, symptoms of acute illness (i.e., fever, sore throat, body aches, and diarrhea), orthodontic appliances or presence of an oral mucosal inflammatory condition (e.g., aphthous, lichen planus, leukoplakia, and oral cancer) also were exclusion criteria. The use of disease modifying antirheumatic drugs (DMARDs) was permitted in the RA group. The study was performed at the University of Kentucky between August 2005 and October 2007 and was approved by the University Institutional Review Board. All subjects understood the study, provided written informed consent and received incentives (i.e., monetary compensation and a clinicalexamination) as part of the study protocol.
Clinical Evaluation
Complete medical and dental histories were obtained from the patient’s records and confirmed by interview. Clinical periodontal indices including PI, PD, BOP, and CAL were recorded for each subject by one calibrated examiner (periodontist (DRD]) after the collection of saliva. Measures for PI, PD and BOP were recorded from six locations per tooth (mesial-buccal, mid-buccal, distal-buccal, mesial-lingual, mid-lingual, and distal-lingual) using a PUNC 15 probe (Hu-Friedy, Chicago, IL, USA). CALs were obtained by measuring interproximal sites only, and gingival recession was measured on the facial and lingual surfaces only. The level of overall body pain for each RA patient at the time of saliva collection was recorded using a 10 cm line visual analog scale as previously reported (Danhauer et al. 2002).
Saliva Collection
Unstimulated whole expectorated saliva was collected from each subject between 9 and 11 a.m. according to a modification in the method described by Navazesh.31 Subjects rinsed their mouth with tap water, then expectorated whole saliva into sterile tubes while seated in an upright position. Collected samples were placed immediately on ice and aliquoted prior to freezing at −80°C. Samples were thawed and analyzed within six months of collection.
Biomarker Analysis
Concentrations of salivary IL-1β and TNF-α were determined in duplicate using Luminex human cytokine/chemokine multiplex kits (Millipore, St. Charles, MO, USA) and salivary levels of MMP-8 were determined in duplicate for each subject using human quantikine MMP-8 enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s directions by technologists in the University of Kentucky General Clinical Research Center Core laboratory. Standards were included on all runs and all results are reported within the linearity of the assays.
Statistical Analysis
Demographic variables were compared among RA, healthy and periodontitis groups using a Chi square test or Fisher’s exact test for categorical responses and analysis of variance (ANOVA) for interval level responses. Mean periodontal indices and concentrations of the salivary analytes were compared between the groups using analysis of covariance with adjustments for race, tobacco use, and alcohol use. To determine the sensitivity of the results to large values of the biomarkers, a re-analysis using log transformed responses was conducted. Since these usually produced similar results, this re-analysis is not reported here with the exception of TNF-α where the Kolmogrov-Smirnov test for normality indicated a log transformation was warranted. The periodontal indices were regressed on the biomarkers with possible differences in intercepts and slopes due to group membership. A re-analysis of the comparison of mean periodontal indices and the regression models which account for differences among the groups on alcohol use, tobacco use, and race were conducted but since the results did not change, only the simpler analyses are reported here. All analyses were performed using the PC SAS 9.1 (SAS Institute Inc., Cary, NC, USA) with statistical significance determined at the 0.05 level.
Results
Thirty-five RA patients ranging in age from 22 to 64 years were enrolled and matched with 35 healthy controls and 35 periodontitis patients (Table 1). Subjects were predominantly female and Caucasian. There were more smokers in the RA and periodontitis groups, and alcohol use was significantly more common in the RA and healthy groups than the periodontitis group. The mean number of teeth for the three groups was similar, as was the %CAL ≥2 mm between the healthy and RA groups. A higher PI, %PD sites ≥4 mm and %PD sites ≥5 mm was observed in the RA group compared with the controls (p>0.05), additionally the %BOP was significantly higher in the RA group than the controls (p=0.012). The periodontal disease group had significantly higher values for all clinical periodontal measures compared with the RA and healthy groups (p<0.0001).
Table 1.
Comparison of demographics and clinical characteristics between study groups.
| RA (n=35) | Healthy (n=35) | Periodontitis (n=35) | P Value | |
|---|---|---|---|---|
| Age (years; mean +/− SD) | 46.8 +/− 10.5 | 43.0 +/− 10.6 | 44.2 +/− 8.2 | |
| Female (%) | 77.2 | 74.3 | 71.4 | |
| White (%) | 94.3 | 85.7 | 54.3 | <0.006* |
| Hispanic (%) | 2.9 | 5.7 | 17.1 | |
| African American | 0 | 0 | 11.4 | |
| Asian (%) | 2.9 | 8.6 | 14.3 | |
| Current tobacco use (%) | 11.4 | 0 | 17.1 | 0.065* |
| Current alcohol use (%) | 25.7 | 37.1 | 11.4 | <0.001* |
| # Teeth | 26.4 +/− 3.0 | 27.0 +/− 2.5 | 26.3 +/− 1.9 | |
| Periodontal indices (% sites; mean +/− SD) | ||||
| Plaque sites | 48.4 +/− 31.5 | 41.7 +/− 41.8 | 136.8 +/− 21.3 | <0.0001** |
| BOP Sites | 13.0 +/− 10.4 | 4.6 +/− 5.5 | 0.012*** | |
| 55.0 +/− 23.5 | <0.0001** | |||
| PD Sites ≥4 mm | 4.9 +/− 8.6 | 1.8 +/− 2.3 | 27.0 +/− 15.0 | <0.0001** |
| PD Sites ≥5 mm | 1.7 +/− 3.4 | 0.2+/− 0.5 | 16.4 +/− 11.4 | <0.0001** |
| CAL ≥2 mm | 3.4 +/− 6.7 | 2.7 +/− 5.9 | 17.6 +/−11.8 | <0.0001** |
determined by Fisher’s exact test.
determined by ANOVA and applies to comparisons of Periodontitis vs. RA or Healthy.
determined by ANCOVA and applies to comparisons of RA vs. Healthy.
Individuals in the RA group were under routine care of a rheumatologist. The mean number of DMARDs was 1.2 (± 0.8) per patient and the group mean VAS pain score was 3.6 cm (± 2.1 cm). Eighteen RA subjects took monoclonal anti-TNF medication (Mab) with or without methotrexate, nine took methotrexate, 3 took leflunomide with methotrexate, hydroxychloroquine, or Mab, and eight took no DMARDs. Four patients were on prednisone and eighteen of the RA patients also took analgesic/nonsteroidal anti-inflammatory drugs daily.
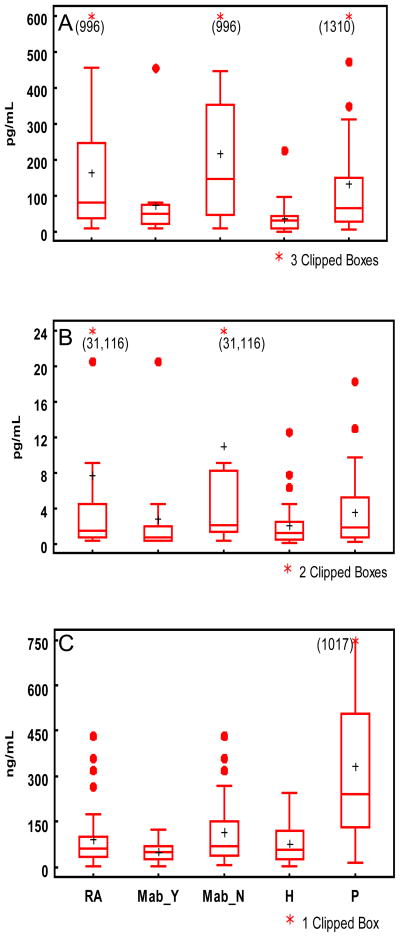
Salivary levels of MMP-8, IL-1β and TNF-α in the three groups are represented as box plots in Figure 1. When adjusted for race and use of alcohol and tobacco, mean levels of IL-1β were significantly elevated (4.18 to 5.2 times) in the RA and periodontitis groups compared to the healthy controls (p < 0.002 and P < 0.04, respectively). Subgrouping the RA patients into those on Mab-based therapy and those receiving other/no DMARDs, demonstrated that this difference was related to elevated salivary IL-1β levels from the RA patients not receiving Mab (223.24 pg/mL) vs. those on therapy (73.35 pg/mL; p=0.016) and healthy subjects (33.11 pg/mL, p<0.001).
Figure 1.
Box plots of salivary biomarker levels A) IL-1β, B) TNF-α, C) MMP-8 in the 3 study groups. Horizontal lines represent the 25th, 50th and 75th percentiles, plus sign represents the mean, and asterisks represent outliers. RA denotes rheumatoid arthritis group. Mab_Y denotes RA patients on monoclonal anti-TNF-α antibody therapy. Mab_N denotes RA patients not receiving monoclonal anti-TNF-α antibody therapy. H denotes healthy group. P denotes periodontal disease group.
Adjusted mean MMP-8 levels were significantly elevated (≥3.6 times) in the periodontitis group compared with the control and RA groups (p<0.0001), irrespective of the subgrouping of the RA patients. TNF-α levels were higher in the RA group than the healthy and periodontal disease groups although the difference was not significant (p=0.20). Subgrouping the RA patients by Mab therapy showed that raw responses of TNF-α did not differ significantly among the groups. However, the frequency of outlier TNF-α levels (>95th percentile) occurred more frequently in the RA subjects (22.9%) than the periodontal disease subjects (11.4%) and healthy controls (8.6%), thus, the results were re-analyzed based on log transformed biomarker levels. Following this analysis, TNF-α levels in the RA group not treated with Mab were significantly higher than RA treated with Mab (p=0.024) and healthy subjects (p=0.011). No periodontal clinical differences were observed between the subgroups of RA patients (data not shown).
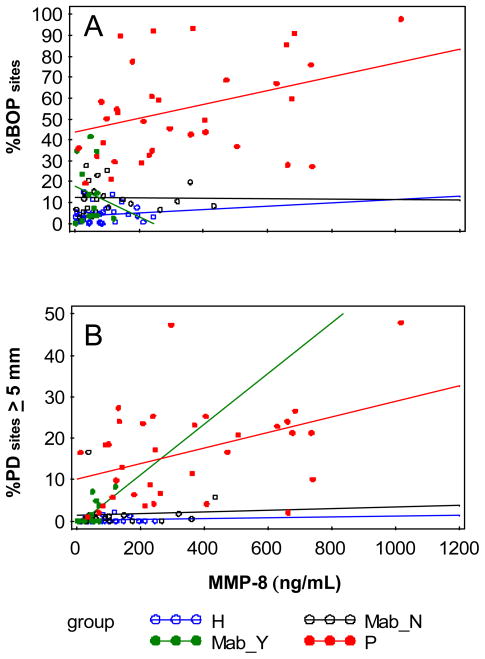
We next investigated whether RA group displayed unique relationships between salivary biomarker levels and any of the clinical parameters of periodontal disease compared with the periodontal disease and healthy groups. As expected, levels of MMP-8 in the periodontitis group significantly correlated with %PD sites ≥4 mm, %PD sites ≥5 mm and %BOP (p=0.04, 0.01, 0.04, respectively, Fig. 2). However, the relationship between MMP-8 levels and periodontal measures was only observed with %PD sites ≥5 mm in the Mab arthritis group (Pearson’s r = 0.63, p = 0.02), not in the healthy group, RA group or non-Mab arthritis group, and MMP-8 levels in the Mab arthritis group were much lower than in the periodontal disease group. IL-1β levels showed a positive correlation with %PD sites ≥4 and ≥5 mm in the periodontitis group (r=0.29 and r=0.27, respectively), however this relationship was not significant and did not occur in the healthy, RA, or RA subgroups. TNF-α levels significantly correlated with %CAL≥2 mm (r=0.38, p=0.026) in the healthy group only. Other factors, (i.e., the number of teeth, level of pain, DMARD use, and demographics) did not correlate with the levels of salivary biomarkers or significantly influence the level of salivary biomarker per group (data not shown).
Figure 2.
Scattergram and least squares line between A) MMP-8 level and %BOP, and B) MMP-8 level and %PD sites ≥5 mm by group. Group H denotes healthy, Mab_Y denotes RAon anti-TNF-α therapy, Mab_N denotes RA not on anti-TNF-α therapy, and P denotes periodontitis. In A) the slopes for the Mab_Y and P group were significant (r=0.63, P=0.02; r=0.42, P=0.01, respectively), and in B) only the slope for the P group was significant (r=0.36, P=0.04).
Discussion
This study investigated the influence of RA on salivary levels of three biomarkers (IL-1β, MMP-8 and TNF-α) associated with periodontitis. The effects were examined using three study groups (healthy, RA and chronic adult periodontitis) with the premise that if RA was influential, then the salivary biomarker levels would be altered with respect to levels observed in the periodontitis or healthy groups. We found that salivary levels of IL-1β were significantly elevated in the overall RA group compared to a control group with similar levels of periodontal disease, whereas MMP-8 and TNF-α were not. As expected, we demonstrated elevations in the concentrations of these salivary biomarkers in patients with periodontal disease compared with healthy controls. However, salivary levels of IL-1β and TNF-α did not reliably differ between the periodontal disease group and RA group who had less severe periodontal disease. When the RA subjects were stratified into subgroups based upon the presence or absence of anti-TNF-α Mab therapy we showed that salivary IL-1β and TNF-α biomarker levels were strongly influenced by this treatment, whereas MMP-8 levels were not.
This study is the first to examine the influence of a systemic inflammatory condition and its treatment on salivary biomarkers of periodontal disease. IL-1β, MMP-8 and TNF-α were investigated because these salivary biomarkers have associations with biological aspects of periodontitis, and have been shown to be significantly elevated in periodontitis subjects compared to healthy controls (Miller et al. 2006, Christodoulides et al. 2007, Ng et al. 2007; Scannapieco et al. 2007, Frodge et al. 2008, Tobon-Arroyave et al. 2008, Gursoy et al. 2010). Specifically related to the findings in this report is the current emphasis and emerging potential for targeted salivary biomarkers to serve in point-of-care diagnostic monitoring of periodontal health and as an adjunct in management of periodontal disease (Ramseier et al. 2009, Miller et al. 2010). These markers were studied in the context of RA, since this chronic inflammatory disorder has a positive association with periodontitis (Kasser et al. 1997, Mercado et al. 2000, Mercado et al. 2001, Havemose-Poulsen et al. 2006, Pischon et al. 2008, Biyikoglu et al. 2009) and elevated levels of these inflammatory mediators occur in the serum of RA patients (Tchetverikov et al. 2004, Eklund et al. 2007, Marti et al. 2009). Moreover, the potential for inflammatory mediators to appear in saliva during autoimmune conditions has been demonstrated (Ben-Aryeh et al. 1978, Tishler et al. 1999).
MMP-8, a highly sensitive measure of periodontal disease, (Sorsa et al. 1994) proved to be a significant predictor of %PD sites ≥5 mm, %PD sites ≥4 mm and %BOP in the 35 periodontitis patients studied here as well as in other studies (Miller et al. 2006, Herr et al. 2007, Miller et al. 2010). Salivary levels of MMP-8 were unaffected in the RA group, irrespective of DMARD and anti-TNF-α Mab therapy. This finding is consistent with MMP levels in the gingival crevicular fluid of patients with RA and gingivitis, or periodontitis being similar to their systemically healthy counterparts (Biyikoglu et al. 2009).
Salivary IL-1β levels were significantly elevated in the periodontitis and RA group compared with the controls. Although these elevated levels were consistent with the RA group having a significantly higher %BOP score, and higher PI, %PD ≥ 4 and 5 mm, and %CAL scores than the healthy controls, this does not fully explain the elevated levels. The RA group had clinical measures less severe than the periodontitis group despite the salivary IL-1β levels being higher in the RA group suggesting that salivary IL-1β was elevated due to systemic inflammation. By stratifying the RA patients into subgroups based upon treatment with anti-TNF-α Mab therapy it was revealed that the RA group treated with Mab had significantly lower salivary IL-1β levels. Similarly, in the log transformed data analysis, salivary levels of TNF-α in the RA group treated with Mab were significantly lower than RA treated with Mab and healthy subjects. In as much as there were no periodontal clinical differences between the RA subgroups (data not shown), these findings suggest that anti-TNF Mab therapy can influence salivary biomarkers of periodontal disease. Also, the fact that treatment with a Mab reduced salivary IL-1β and TNF-α could be a reflection of reduced RA disease activity and/or a specific effect on the inflammatory components of periodontal disease.
The findings presented here are to be viewed within the context of the limitations of its cross-sectional study design and the fact that the majority of the RA subjects were female, Caucasian and medically well controlled. Seventy seven percent of the RA subjects took at least one DMARD, more than half took analgesics/nonsteroidal anti-inflammatory drugs daily and the majority had low VASs. Because these therapies could have attenuated circulating serum and salivary biomarker levels, as has been shown in a study of hydroxychloroquine, an anti-inflammatory drug for primary Sjögren's syndrome (Tishler et al. 1999), we subgrouped the RA patients by VAS, Mab and DMARD use. In these analyses only anti-TNF-α Mab use proved to demonstrate subgroup differences.
These studies are of particular relevance as a relationship between periodontal disease and other chronic inflammatory diseases, including RA, are being suggested as phenotypic manifestations of a hyperinflammatory genotype in a subset of the population. Accumulating evidence suggests that periodontitis and RA are inter-related and possibly interdependent with respect to the elevated levels of serum pro-inflammatory molecules that likely contribute to the milieu of the gingival crevicular fluid and thus the saliva. Thus, the potential altered abilities of RA patients to perform effective oral hygiene could result in increased BOP that exacerbates the risk for enhanced tissue destruction in periodontitis. Moreover, interesting observations regarding the complexity of the oral and systemic challenge provide unique mechanisms by which dysregulation of host responses could occur. For example, P. gingivalis has the ability to generate citrullinated proteins that can serve as antigens that can drive an autoimmune response (Wegner et al. 2010). Finally, the observation that anti-TNF-α Mab therapy was associated with lower levels of periodontal disease biomarkers in patients with RA further supports some common hyperinflammatory mechanisms in these diseases. This type of finding emphasizes opportunities to evaluate biological therapies for periodontitis that would specifically target selected pro-inflammatory mediators for control in the local oral environment (Assuma et al. 1998).
Conclusions
This study provides evidence that salivary IL-1β, MMP-8 and TNF-α levels are clearly influenced by the local periodontal environment, and selectively influenced by a systemic inflammatory condition such as RA. The findings provide further support for the clinical utility of salivary biomarkers in assessing periodontal disease in otherwise healthy adults, although additional studies are required to more clearly delineate the impact of systemic inflammatory disease(s) and co-morbidities on profiles of salivary biomarkers that could be used in monitoring health and/or defining oral disease.
Clinical Relevance.
Scientific rationale for study: Systemic inflammation may influence concentrations of salivary constituents. In this study, we measured levels of three biomarkers known to be associated with periodontal disease in whole saliva to test the hypothesis that rheumatoid arthritis influenced levels of salivary biomarkers of periodontal disease. Principal findings: Salivary levels of IL-1β and TNF-α were significantly elevated in arthritis patients not receiving anti-TNF-α antibody therapy compared with arthritis patients receiving anti-TNF-α therapy and healthy controls. Practical implications: Rheumatoid arthritis in the absence of disease modifying antirheumatic drugs appears to influence levels of select salivary biomarkers of periodontal disease.
Acknowledgments
Sources of Support: This study was supported by grants from the National Institute of Health P20 RR020145 and M01-RR02602 and the University of Kentucky General Clinical Research Core.
The authors thank Jenny O’Nan, Donna Mischel, Vanessa Hodges-Reed, Dawn Dawson study coordinators, Jason Stevens, research analyst, and Malini Bharadwaj, data management specialist, of the Center for Oral Health Research of the University of Kentucky for clinical, laboratory and data management support. This study was supported by grants P20 RR020145 and M01-RR02602 from the National Institutes of Health, Bethesda, Maryland, and the University of Kentucky General Clinical Research Core.
Footnotes
Conflict of interest: The authors report no conflicts of interest related to this study.
References
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- Ben-Aryeh H, Nahir M, Scharf Y, Gutman D, Laufer D, Szargel R. Sialochemistry of patients with rheumatoid arthritis. Electrolytes, protein, and salivary IgA. Oral Surg Oral Med Oral Pathol. 1978;45:63–70. doi: 10.1016/0030-4220(78)90224-4. [DOI] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Biyikoglu B, Buduneli N, Kardesler L, Aksu K, Pitkala M, Sorsa T. Gingival crevicular fluid MMP-8 and -13 and TIMP-1 levels in patients with rheumatoid arthritis and inflammatory periodontal disease. J Periodontol. 2009;80:1307–1314. doi: 10.1902/jop.2009.090130. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Floriano PN, Miller CS, Ebersole JL, Mohanty S, Dharshan P, Griffin M, Lennart A, Ballard KL, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV, McDevitt JT. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Fox PC, McDevitt JT. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- Danhauer SC, Miller CS, Rhodus NL, Carlson CR. Impact of criteria-based diagnosis of burning mouth syndrome on treatment outcome. J Orofac Pain. 2002;16:305–311. [PubMed] [Google Scholar]
- de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- de Pablo P, Chapple ILC, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- Eklund KK, Leirisalo-Repo M, Ranta P, Maki T, Kautiainen H, Hannonen P, Korpela M, Hakala M, Jarvinen P, Mottonen T. Serum IL-1beta levels are associated with the presence of erosions in recent onset rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:684–689. [PubMed] [Google Scholar]
- Fox PC. Salivary monitoring in oral diseases. Ann N Y Acad Sci. 1993;694:234–237. doi: 10.1111/j.1749-6632.1993.tb18356.x. [DOI] [PubMed] [Google Scholar]
- Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J Periodontol. 2008;79:1913–1919. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska R, Nedzi-Gora M. The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and MMP-8, MMP-9, and TIMP-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Arch Immunol Ther Exp (Warsz) 2006;54:419–426. doi: 10.1007/s00005-006-0047-6. [DOI] [PubMed] [Google Scholar]
- Gursoy UK, Könönen E, Pradhan-Palikhe P, Tervahartiala T, Pussinen PJ, Suominen-Taipale L, Sorsa T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37:487–493. doi: 10.1111/j.1600-051X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Havemose-Poulsen A, Westergaard J, Stoltze K, Skjodt H, Danneskiold-Samsoe B, Locht H, Bendtzen K, Holmstrup P. Periodontal and hematological characteristics associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2006;77:280–288. doi: 10.1902/jop.2006.050051. [DOI] [PubMed] [Google Scholar]
- Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci U S A. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser UR, Gleissner C, Dehne F, Michel A, Willershausen-Zonnchen B, Bolten WW. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40:2248–2251. doi: 10.1002/art.1780401221. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis--a review. J Clin Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva--a review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol. 2003;74:353–359. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- Malamud D. Saliva as a diagnostic fluid. BMJ. 1992;305:207–208. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D, Bau H, Niedbala S, Corstjens P. Point detection of pathogens in oral samples. Adv Dent Res. 2005;18:12–16. doi: 10.1177/154407370501800104. [DOI] [PubMed] [Google Scholar]
- Marti L, Golmia R, Golmia AP, Paes AT, Guilhen DD, Moreira-Filho CA, Scheinberg M. Alterations in cytokine profile and dendritic cells subsets in peripheral blood of rheumatoid arthritis patients before and after biologic therapy. Ann N Y Acad Sci. 2009;1173:334–342. doi: 10.1111/j.1749-6632.2009.04740.x. [DOI] [PubMed] [Google Scholar]
- Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267–272. doi: 10.1034/j.1600-051x.2000.027004267.x. [DOI] [PubMed] [Google Scholar]
- Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–787. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campbell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, Ebersole JL, McDevitt JT. Current developments in salivary diagnostics. Biomarkers in Medicine. 2010;4:1–18. doi: 10.2217/bmm.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA. Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol Med Microbiol. 2007;49:252–260. doi: 10.1111/j.1574-695X.2006.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch Oral Biol. 1995;40:1151–1155. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, Landau H, Brinkmann PG, Schlattmann P, Zernicke J, Buttgereit F, Detert J. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–986. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–46. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R, McCarty GA. Analogous mechanisms of tissue destruction in rheumatoid arthritis and periodontal disease. In: Genco RJ, Mergenhagen SE, editors. Host-Parasite Interactions in Periodontal Disease. Washington, DC: American Society of Microbiology; 1982. pp. 254–362. [Google Scholar]
- Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, Ohtani H, Andoh N, Takeha S, Konttinen YT. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann N Y Acad Sci. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- Tchetverikov I, Ronday HK, Van El B, Kiers GH, Verzijl N, TeKoppele JM, Huizinga TW, DeGroot J, Hanemaaijer R. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–883. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler M, Yaron I, Shirazi I, Yaron M. Hydroxychloroquine treatment for primary Sjogren's syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 1999;58:253–256. doi: 10.1136/ard.58.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobon-Arroyave SI, Jaramillo-Gonzalez PE, Isaza-Guzman DM. Correlation between salivary IL-1beta levels and periodontal clinical status. Arch Oral Biol. 2008;53:346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010 May 6; doi: 10.1002/art.27552. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]