Abstract
Defects in DNA repair pathways or exposure to high levels of DNA damaging agents limit the renewal potential of adult tissues and accelerate the development of age-related degenerative pathologies.1–3 Many studies suggest these tissue homeostatic defects can result from the accumulation of DNA damage in tissue-specific stem cells.4,5 Although maintenance of genome integrity in progenitor cells is required for the renewal of adult tissues, recent studies have highlighted the importance of additional mechanisms that facilitate and direct the process of tissue regeneration. These reports indicate that the p53 tumor suppressor gene maintains adult tissue homeostasis and promotes tissue renewal by suppressing the accumulation of DNA-damaged cells.6–8 Without p53, tissue deterioration caused by the elimination of genome maintenance regulators (ATR, Hus1 or Terc) is exacerbated and, in some cases, leads to synthetic lethality at the organismal level. Importantly, the accumulation of highly damaged cells in multiple tissues appears to severely impede regeneration from undamaged progenitors, suggesting that p53-mediated removal of damaged cells is a prerequisite for effcient progenitor driven renewal. These findings argue that tissue homeostasis is governed not only by the intrinsic repopulating potential of competent progenitors, but also by mechanisms that limit the accumulation of defective cells and, thereby, promote compensatory regeneration. As discussed in this review, these findings advance our understanding of processes that counter the effects of DNA damage at the tissue level and have important implications for the development of therapeutic approaches to combating age-related pathologies and p53-deficient malignancies.
Keywords: ATR, Hus1, Chk1, telomerase, p53, DNA damage, tissue regeneration, aging, cancer
p53 and Tissue Homeostasis
It is increasingly well-established that the maintenance of many adult tissues relies on the continuous proliferation and differentiation of resident stem cells.9–11 Tissue-specific progenitors provide not only a steady supply of newly differentiated cells to support normal homeostasis, but can also be stimulated to regenerate a tissue following acute injury. The mechanisms that govern tissue renewal are becoming increasingly understood, and p53 has recently proven to be a critical regulator of this process.
A role for p53 in promoting tissue regeneration is perhaps, at first glance, at odds with many of the classical functions for p53. While the overall outcome of p53 activation on a given cell can be varied and context dependent, DNA damage- and oncogene-induced stabilization of p53 can limit the proliferative lifespan of cells.12,13 As such, broad engagement of p53 in a proliferative tissue could be expected to restrict regenerative capacity and ultimately cause tissue degeneration. Indeed, activation of p53 has been shown to negatively impact tissue homeostasis and renewal in diverse situations. For instance, developmental arrest, tissue homeostatic failures and premature cellular senescence in several mouse models harboring DNA repair gene mutations are attenuated by p53 deficiency, arguing that p53 activation promotes these degenerative phenotypes.14–25 Additionally, direct overstimulation of the p53 pathway through expression of constitutively active p53 mutants results in tissue deterioration and the appearance of several age-associated pathologies in mice.26,27 Taken together, these studies have confirmed that p53 activity can drive the attrition of proliferative organs and negatively impact tissue renewal.
However, mice expressing additional copies of p53 and p19ARF under normal regulatory control (s-Arf/p53 mice) accumulate fewer damaged cells in several tissues over time, exhibit enhanced antioxidant gene expression, and live significantly longer than mice with a normal complement of these genes.28 If increased lifespan results from improvements in the maintenance of vital tissues (as suggested elsewhere), these findings argue that p53 activation can be beneficial to tissue homeostasis.29,30 A role for p53 in preserving tissue integrity may ultimately be tied to its ability to limit the proliferation of damaged or aberrant cells and efficiently cull them from tissues by various means. In support of this model, Lowe and colleagues have recently described two circumstances in which p53 directs the elimination of aberrant cells from a tissue, ultimately preserving normal physiology.
Using controllable RNA interference to acutely regulate p53 expression in a mouse model of liver carcinoma, restoration of p53 in an established malignancy has been shown to significantly decrease tumor burden through the induction of cellular senescence and subsequent clearance by an innate immune response.31 The ability of p53 reactivation to cause a nascent or fully developed tumor to regress has also been demonstrated by other investigators through complementary methods.32,33 Notably, the senescence-associated immune reaction documented by the Lowe group well illustrates the ability of damaged or aberrant cells to stimulate a potent physiological response and further develops previous findings by establishing the functional importance of their clearance.34,35
The ability of a p53-dependent cell fate program to elicit an immune response, ultimately enforcing the clearance of abnormal cells, was further established using a mouse model of acute liver damage. Krizhanovsky et al. demonstrated that CCl4-induced liver injury and subsequent fibrosis was tightly associated with the accumulation of senescent hepatic stellate cells.36 Importantly, the senescence and immune-mediated clearance of these abnormal stellate cells ultimately limited the accompanying fibrosis, and this restriction of stellate cell expansion was dependent on p53. Without p53-driven clearance, hepatic stellate cells proliferated excessively in response to acute liver injury, inciting extensive fibrosis that inhibited efficient liver regeneration and ultimately perturbed organ homeostasis.36 In total, these results indicate that p53 activity can prevent the accumulation of aberrant cells in tissues through senescence and immune-mediated clearance, and this constraint can strongly influence the maintenance of tissue homeostasis and regeneration.
Beyond this work, other recent studies have helped further establish a beneficial role for p53 in the renewal of adult tissues that harbor DNA-damaged cells.6–8 These studies have collectively underscored the importance of limiting the accumulation of damaged cells, and have indicated that the persistent accrual of such cells imposes a barrier to progenitor-driven renewal. As discussed in the sections below, these findings have not only added to understanding of how organisms cope with and eliminate DNA damage at the tissue level, but may also have direct clinical relevance.
Essential Functions of ATR in DNA Replication and Tissue Homeostasis
ATR (ATM and Rad3-related) is a PIKK-family kinase that is required for the maintenance of genomic integrity during DNA replication.37 The kinase activity of ATR is potently stimulated by DNA helicase and polymerase uncoupling, and elegant work using modified replication templates in the Xenopus system has advanced the paradigm that ATR is responsive to distinct structural elements at compromised replication forks.38,39 The activation of ATR and the signaling outputs of this kinase function to ensure cell viability during DNA replication, both by inhibiting inappropriate cell cycle progression and by maintaining the stability of stalled replication forks.40,41 Together, these activities make ATR essential for the long term viability of proliferating cells.
The essential role for ATR during cell proliferation has been demonstrated in multiple systems and has been thoroughly characterized at the organismal level through targeted disruption in mice.42,43 Germline disruption of ATR in mice results in chromosomal fragmentation, cell death and early embryonic lethality.42,43 In cell culture, acute deletion of ATR using a cre/lox-conditional allele (ATRflox) has been shown to cause an exquisite sensitivity to DNA helicase and polymerase uncoupling, resulting in double strand break generation and the cessation of cell proliferation.37,44–46 This ATRflox allele, coupled with a ubiquitously expressed and inducible form of Cre-recombinase (Ubc-Cre-ERT2), has provided a unique system to examine the effects of acute ATR deletion on tissue homeostasis.47 ATR disruption in adult mice leads to the immediate degeneration of proliferative tissues, as actively replicating cells that have lost ATR are eliminated.47 Importantly, ATR loss in this system is mosaic due to a stochastic rate of failure to undergo lox recombination, allowing the minority of cells that continue to express ATR to rapidly repopulate affected tissues.47 Although residual ATR-expressing cells support this robust tissue regeneration initially, defects in renewal capacity eventually develop, leading to the accelerated onset of several aging phenotypes. This finding indicates that long-term tissue homeostasis can be compromised by the targeted removal of a fraction of contributors and suggests that the regenerative capacity of some adult tissues may be more limited than previously thought.
Germline mutations in other genome maintenance regulators have also been shown to reproduce many hallmarks of aging in mice, and several human segmental progeria syndromes are a consequence of DNA repair deficiencies.1,3 In aggregate, these examples have suggested a general model whereby unrepaired DNA damage can limit the renewal of proliferative tissues, and recent work focusing on the hematopoietic system has argued that aberrant DNA repair can specifically impact stem and progenitor cell function.5,48,49 These studies and others have advanced the concept that gradual accrual of DNA damage in stem cells activates damage checkpoints, limiting self-renewal and repopulating ability in a cell-autonomous fashion. This decline in individual stem cell performance is proposed to strongly contribute to a time-dependent degeneration of certain tissues with age.
However, as described above, the accelerated decline in stem cell maintenance and tissue degeneration in the mosaic ATR-knockout mouse is not directly the consequence of genetically unstable ATR-deleted cells but, instead, is associated with the diminished ability of residual ATR-expressing progenitors to sustain tissue renewal. Thus, limited tissue renewal following genome destabilization appears to be associated with both the elimination of DNA-damaged cells and the ability of relatively intact progenitors to compensate for this loss and maintain tissue function. Although individual stem and progenitor cell potential undoubtedly plays a key role in this process, the efficiency by which damaged cells are cleared might also be expected to influence the integrity of renewal. With this model in mind, we and others have recently demonstrated that the transcription factor p53 exerts a potent, beneficial effect on the regeneration of proliferative adult tissues that contain DNA-damaged cells.
p53 is Required for Efficient Tissue Renewal Following Mosaic ATR Deletion
The genomic instability resulting from ATR deletion activates several DNA damage response pathways, including ones that promote p53 upregulation.6,37 Considering the established effects of p53 activity on tissue development and regeneration, p53 engagement following ATR deletion could drive the acute deterioration of proliferative tissues or function in a more complex manner to promote tissue regeneration. We recently tested these expectations by deleting ATR in a mosaic fashion in both p53 wild-type and p53-null mice. Remarkably, acute deletion of ATR in the absence of p53 (p53−/− ATRmKO) caused a rapid and highly penetrant lethality in mice (>94%), occurring only days after ATR elimination.6 Pathological analysis of the intestine and bone marrow, two highly proliferative tissues that are exquisitely sensitive to ATR loss, revealed that p53 deficiency failed attenuate the deterioration of these tissues after ATR deletion and, moreover, seemed to exacerbate acute intestinal degeneration.6
Consistent with previous observations, ATR-deleted cells were rapidly cleared from both the bone marrow and intestine in the presence of wild-type p53, as indicated by a loss of the recombined ATR allele from these tissues.6,47 Notably, the deleted allele was maintained at a higher level in p53−/− ATRmKO tissues, suggesting that elimination of ATR-deleted cells did not occur as efficiently in the absence of p53. Consistent with this finding, the frequency of cells exhibiting high levels of DNA damage (γ H2AX-positive) was significantly elevated in p53−/− ATRmKO tissues, an effect that was further substantiated by the level of chromosome breakage in freshly isolated p53−/− ATRmKO bone marrow. The appreciable accumulation of DNA-damaged cells relative to the modest retention of ATR-deleted cells in the absence of p53 suggests that ATR-deleted cells may accrue substantially more damage without p53. Such an effect may be caused by an increased tolerance to high levels of DNA damage or by multiple checkpoint defects leading to increased rates of genome destabilization.
The rapid death of nearly all p53−/− ATRmKO mice and the associated deficiencies in tissue homeostasis suggested that the immediate compensatory renewal driven by ATR-expressing progenitors was inhibited. Accordingly, hair follicle regeneration in p53−/− ATRmKO skin was severely delayed compared to ATRmKO controls, and this delay was accompanied by a potent inflammatory response, as characterized by redness, exfoliation and innate immune cell infiltration.6 Importantly, pharmacologic suppression of inflammation did not facilitate regeneration, indicating that immune activation was not the primary cause of short term defects in hair follicle renewal. Interestingly, not only was initial hair regeneration delayed in p53−/− ATRmKO skin, but the hair that did return in these mice showed appreciable graying, suggesting that ATR deletion in the absence of p53 leads to long-term regenerative insufficiency as well as immediate deficiencies in compensatory tissue renewal.
Similar to the bone marrow and intestine, the impairment in hair follicle regeneration strongly correlated with the persistence of DNA-damaged ATR-deleted cells in p53-null mice. Significantly increased frequencies of damaged cells (γH2AX+) in both the CD34+ progenitor populations and the total epidermis of p53−/− ATRmKO skin were observed. In total, these results strongly support a model whereby p53 limits the accumulation of damaged cells in regenerative tissues and, through this function, promotes immediate compensatory renewal by competent, undamaged progenitors.
Telomerase or Hus1 Deficiency also Leads to an Increased Dependence on p53 for Tissue Renewal
Significantly, other laboratories have also substantiated a requirement for p53 in adult tissue homeostasis following DNA damage. These studies demonstrate that co-deletion of p53 with other genome maintenance regulators, the ATR-Chk1 signaling mediator Hus1 and the Terc telomerase component, causes synergistic deterioration of tissue integrity and delays renewal in manners similar to those that follow dual deletion of ATR and p53, as described below.
Disruption of telomerase function leads to progressive telomere shortening and chromosomal instability.50 Late-generation telomerase-deficient mice with critically short telomeres exhibit defects in multiple proliferative organs, including the intestine.51,52 Initial studies indicated that concurrent deficiency in p53 helps attenuate the degenerative pathologies seen in many adult tissues, suggesting that p53 activation detrimentally affects tissue homeostasis following telomere erosion.16 Additionally, deletion of a cell cycle inhibitor induced by p53, Cdkn1a (p21Waf1/Cip1), almost universally improves the integrity of proliferative tissues in late-generation Terc-deficient mice.53 Although the exact mechanism of this outcome remains unclear, these results have led to the hypothesis that p53 activity constrains tissue renewal in response to dysfunctional telomeres. Formally, however, the influence of p53 on the development of age-associated pathologies in telomerase-deficient tissues has been incompletely defined, largely due to the diverse array of malignancies that limit the lifespan of p53/telomerase-deficient mice.54
To further examine the role of p53 activation in response to telomere shortening, Rudolph and colleagues utilized a conditional allele of p53 in concert with an inducible form of Cre-recombinase expressed solely in the intestinal epithelium, thus allowing them to directly test the influence of acute p53 loss on the intestines of adult telomerase-deficient mice. Similar to the effects of p53 deficiency when combined with ATR elimination, deletion of p53 significantly worsened the degenerative effects of telomerase deficiency, resulting in intestinal atrophy, loss of body weight, and a shortened lifespan that notably was not associated with overt tumor formation.7 Although p53 deletion did prevent p21 upregulation in the intestines of telomerase-deficient mice, the accelerated intestinal deterioration in p53Δ/ΔTerc−/− iG4 mice suggests the detrimental effects of p53 deficiency negate any benefit to tissue function conferred by p21 attenuation.7,53 A careful analysis of the intestinal crypts revealed an accumulation of highly DNA-damaged (γH2AX+) and genetically unstable cells, arguing that p53 is required for the depletion of dysfunctional intestinal progenitors. Ultimately, this build-up of damaged progenitors in the absence of a p53-mediated selection mechanism correlates with defects in tissue maintenance and overall organ function.
An essential role for p53 in proliferative adult tissues has additionally been demonstrated in recent work from Weiss and colleagues.8 The Rad9-Rad1-Hus1 complex localizes to sites of DNA damage and is required for full activation of ATR signaling that leads to Chk1 phosphorylation.37 As expected given its critical role in the ATR pathway, disruption of this complex through deletion of Hus1 results in accumulation of DNA damage during replication, eventual loss of proliferative capacity, and cell death.55,56 A conditional allele of Hus1 coupled with a form of Cre recombinase expressed from the β-lactoglobulin promoter was used to selectively delete Hus1 in adult mammary epithelium.8 Interestingly, Hus1 deletion during pregnancy and subsequent lactation did not affect mammary gland architecture or function. In fact, similar to the compensatory response that follows ATR deletion, Hus1-deleted cells were rapidly lost from the proliferating epithelium and replaced by undeleted, Hus1-expressing cells. Importantly, similar to the outcome of co-deleting p53 with ATR or Terc, highly-damaged Hus1-deleted cells accumulated to higher frequencies in the mammary epithelium in p53-deficient animals than p53 wild-type controls. Once again, the persistence of highly-damaged Hus1-deleted cells correlated with disrupted mammary gland development and function, arguing that p53 is required for compensatory mammary gland growth following mosaic Hus1 deletion. Consistent with these findings, it has been reported that tissue-restricted conditional deletion of p53 and Chk1, a critical downstream kinase of ATR and Hus1, also results in a regenerative delay in the intestinal epithelium.57
Thus, in several relatively disparate systems and multiple tissues, adult tissue homeostasis and renewal in the context of elevated levels of DNA damage have proven to be dependent on p53. This reliance on p53 seems to be tightly associated with this protein’s ability to limit the accumulation of highly damaged cells in proliferative tissues and does not appear to be the consequence of altered stem and progenitor cell potential. Although the mechanism by which the accumulation of damged cells may inhibit renewal from intact progenitors is unclear, studies of other model organisms and recent cell-based genomic analyses have suggested several attractive models, as described below.
Potential Mechanisms of Regenerative Defects After Loss of ATR and p53
In the context of high levels of genomic instability, as caused by engineered defects in the ATR-Chk1 pathway or telomere maintenance, p53 deficiency leads to disrupted tissue homeostasis and defects in regeneration. These effects appear to result from deficiencies in compensatory renewal by competent progenitors. Although the precise mechanisms by which p53 functions to safeguard tissue integrity and promote renewal in the context of genomic instability are not fully understood, several recent studies have provided valuable insight into the underlying processes that may be at work.
One attractive explanation for the observed defects in tissue homeostasis without p53 has been suggested by recent studies in Drosophila indicating an essential role for p53 in driving compensatory proliferation.58,59 Co-expression of a proapoptotic gene along with a caspase inhibitor generates “undead cells” in a developing region of Drosophila, and this manipulation has previously been shown to induce compensatory growth of the tissue occupied by these cells.60 Importantly, p53 is required to stimulate this excessive growth response to undead cells.58 The high amount of cleaved caspase-3 within these undead cells implies that the engagement of initiator caspases might act as an initiating signal that ultimately stimulates compensatory proliferation. Indeed, the initiator caspase Dronc is required for compensatory expansion,60 and appears to mediate this effect through a non-apoptotic function that regulates p53.58
In the murine models described above, high levels of apoptosis in the absence of p53 were observed in at least some tissues, suggesting that engagement of caspases might be able to stimulate compensatory renewal in the presence of p53, but not in its absence.6,7,8 However, although cleaved caspase-3 staining was observed at a relatively high frequency in the intestines of p53−/− ATRmKO mice, it was not observed to any significant degree in the bone marrow or skin.6 These inconsistencies suggest that the generation of a positive cue for compensatory renewal by a caspase-p53 interaction cannot be the only means by which p53 facilitates tissue regeneration.
Alternatively, rather than directly stimulating tissue regeneration, p53 may facilitate tissue renewal indirectly by limiting the accumulation of damaged cells that could impede the ability of competent progenitors to drive renewal. Given the intricate signaling cues required for stem and progenitor cells to divide and maintain tissue integrity,9–11 it is conceivable that the persistence of DNA-damaged cells might disrupt key morphogen gradients or other environmental cues, thus precluding efficient regeneration. As of yet, however, there is little direct evidence in support of this theory and the relatively incomplete understanding of the essential factors and cues that guide tissue renewal make testing such a hypothesis challenging.
However, beyond simply occupying key physical or functional space in a regenerative tissue, highly damaged cells may actively inhibit renewal by secreting trans-dominant inhibitory factors that prevent the division of intact progenitors. This model is supported by several recent studies showing that a defined cohort of cytokines and other factors is released from highly damaged or senescent cells, and this release is significantly augmented by p53 deficiency.61–63 Although the outcome of this cytokine signaling is context-dependent, these factors can induce cell cycle arrest or senescence in certain situations.63–65 If such a mechanism were utilized, it may function as a tissue regeneration checkpoint, delaying progenitor-driven renewal until damaged cells have been effectively cleared.
Notably, the secretion of senescence-inducing cytokines by highly damaged, p53-deficient cells could account for many features of the aforementioned mouse models, including the non-cell autonomous inhibition of tissue renewal and associated inflammation.6–8 Importantly, although blocking immune cell infiltration in p53−/− ATRmKO skin with dexamethasone treatment did not ameliorate the regenerative delay in hair follicles,6 it is possible be that this treatment was sufficient only to inhibit inflammatory cell recruitment but not enough to suppress local signaling by key inhibitory factors. Further studies will be required to investigate any potential roles for secreted factor production in the regenerative defects observed following co-deletion of p53 with ATR, Hus1 or Terc. Together, the recent studies described in this review have argued that the efficient elimination of DNA-damaged and senescent cells is a prerequisite for robust progenitor driven renewal. This process is notably distinct from the influence that preservation of individual stem and progenitor cell potential has on tissue renewal. Cooperation and coordinate regulation of these components may assure the overall efficiency and fidelity of regeneration and the prevention of age-related disease. It is likely that premature or inappropriate renewal prior to the removal damaged or senescent cells may lead to long-lasting effects on tissue architecture and function, effects that extend beyond the eventual clearance of such functionally compromised cells. In this light, it is currently unclear on a tissue-by-tissue basis whether individual stem cell potential or the prevention of premature renewal imposes a greater limitation to tissue integrity over time. Therefore, elucidating the mechanisms responsible for the non-cell autonomous inhibition of regeneration in these and other model systems will lend great insight into the causes of age-related disease.
Implications for Preventive Treatments and Therapies
A role for p53 in enforcing tissue quality and long-term maintenance is consistent with the extended lifespan of mice expressing additional copies of p53 under normal regulatory control. According to this model, bolstering the innate ability of p53 to mediate damaged and senescent cell clearance might enhance the maintenance of tissues that are not limited by stem and progenitor cell potential. Similarly, the complete elimination of pathologically degenerated tissues could likely be expected to improve the ultimate quality of regenerated tissue derived from intact progenitors. Finally, augmenting the trans-dominant inhibition of renewal until damaged-cell clearance can be completed may also have a constructive, therapeutically useful effect following acute insult. Although these concepts are logical extensions of the conclusions from the studies herein, their broad practical application to regenerative medicine awaits both technical advances and the development of a more complete understanding of the cell-intrinsic and cell-extrinsic factors that govern tissue renewal.
However, a more immediately tangible and clinically relevant application of these findings and others may be in combating p53-deficient malignancies. A cell-autonomous synthetic lethal interaction between dual abrogation of the ATR-Chk1 and p53 pathway has been well-documented and may be the consequence of additive genome destabilization resulting from either redundant or aberrant S-phase entry.66–69 The accumulation of cells with high levels of phosphorylated H2AX in many proliferative tissues of both p53−/− ATRmKO and p53−/− Hus1Δ2,3/Δ1 mice supports the idea of a cellular interaction between p53 deficiency and ATR signaling defects in addition to any non-cell autonomous mechanism inhibiting efficient tissue regeneration.6,8 Additionally, Oscar Fernandez-Capetillo’s group has recently developed a mouse model of Seckel Syndrome, a rare human disorder which can be caused by mutations in ATR that result in abnormal splicing and decreased ATR expression.70,71 ATRseckel/seckel mice showed evidence of DNA damage during embryogenesis and were born at less than normal numbers, indicating embryonic lethality conferred by hypomorphic levels of ATR.71 Importantly, p53−/− ATRseckel/seckel mice were born at an even lower frequency and exhibited an accentuated accumulation of damaged cells during embryogensis.71 Thus, even ATR suppression to below heterozygous levels can interact with p53 deficiency, leading to the accrual of highly damaged cells and impairment of tissue growth during embryonic development. In aggregate, these findings suggest that inhibition of the ATR-Chk1 pathway may be uniquely useful for the treatment of p53-deficient cancers by causing synergistic increases in genomic instability and the degenerative propagation of terminally damaged cells.
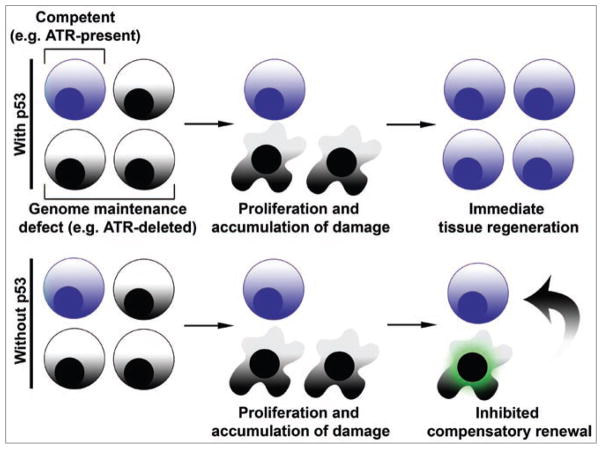
Figure 1.
An important role for limiting the accumulation of damaged cells in facilitating tissue renewal. (Top) Defects in genome maintenance (e.g. ATR, Hus1 or Terc loss) lead to the accumulation of DNA damage during replication. In the presence of p53, extensively damaged cells are rapidly eliminated from proliferative tissues, and these tissues are subsequently reconstituted by less damaged or fully competent cells. (Bottom) In the absence of p53, damaged cells accumulate in proliferative tissues and may accrue higher levels of genomic instability. The persistent accumulation of these highly damaged cells ultimately impedes the immediate compensatory regeneration of the tissue by intact progenitors.
Acknowledgments
We thank members of the Brown laboratory for helpful discussions. This work was supported by the National Institute on Aging, R01AG027376 (E.J.B.) and F30AG034027 (D.W.S.), and the Abramson Family Cancer Research Institute.
References
- 1.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Ruzankina Y, Asare A, Brown EJ. Replicative stress, stem cells and aging. Mech Ageing Dev. 2008;129:460–6. doi: 10.1016/j.mad.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeijmakers JH. DNA damage, aging and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 4.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–13. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 5.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–96. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet. 2009;41:1144–9. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begus-Nahrmann Y, Lechel A, Obenauf AC, Nalapareddy K, Peit E, Hoffmann E, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet. 2009;41:1138–43. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 8.Yazinski SA, Westcott PM, Ong K, Pinkas J, Peters RM, Weiss RS. Dual inactivation of Hus1 and p53 in the mouse mammary gland results in accumulation of damaged cells and impaired tissue regeneration. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0904965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–64. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 13.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Wlodarski P, Wasik M, Ratajczak MZ, Sevignani C, Hoser G, Kawiak J, et al. Role of p53 in hematopoietic recovery after cytotoxic treatment. Blood. 1998;91:2998–3006. [PubMed] [Google Scholar]
- 15.Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, et al. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–51. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–38. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 17.Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, et al. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature. 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 19.Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW. Partial rescue of Brca1 (5–6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16:298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- 20.Lim DS, Hasty P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–43. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim DS, Vogel H, Willerford DM, Sands AT, Platt KA, Hasty P. Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol. 2000;20:3772–80. doi: 10.1128/mcb.20.11.3772-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet. 2001;28:266–71. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol Cell Biol. 1998;18:4385–90. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Komarov PG, Maurer M, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 2000;60:5002–6. [PubMed] [Google Scholar]
- 25.Orii KE, Lee Y, Kondo N, McKinnon PJ. Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc Natl Acad Sci USA. 2006;103:10017–22. doi: 10.1073/pnas.0602436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 27.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–19. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–9. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Cao I, Garcia-Cao M, Tomas-Loba A, Martin-Caballero J, Flores JM, Klatt P, et al. Increased p53 activity does not accelerate telomere-driven ageing. EMBO Rep. 2006;7:546–52. doi: 10.1038/sj.embor.7400667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–22. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 31.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–7. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 33.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 34.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDougall CA, Byun TS, Van C, Yee MC, Cimprich KA. The structural determinants of checkpoint activation. Genes Dev. 2007;21:898–903. doi: 10.1101/gad.1522607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–52. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–36. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedel AM, Pike BL, Gasser SM. ATR/Mec1: coordinating fork stability and repair. Curr Opin Cell Biol. 2009;21:237–44. doi: 10.1016/j.ceb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 43.de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, et al. Targeted disruption of the cell cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol. 2000;10:479–82. doi: 10.1016/s0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 44.Smith KD, Fu MA, Brown EJ. Tim-Tipin dysfunction creates an indispensible reliance on the ATR-Chk1 pathway for continued DNA synthesis. J Cell Biol. 2009;187:15–23. doi: 10.1083/jcb.200905006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–28. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–6. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 47.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–26. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 49.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 50.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 51.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 52.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, et al. Longevity, stress response and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 53.Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 54.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–5. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 55.Bao S, Lu T, Wang X, Zheng H, Wang LE, Wei Q, et al. Disruption of the Rad9/Rad1/Hus1 (9-1-1) complex leads to checkpoint signaling and replication defects. Oncogene. 2004;23:5586–93. doi: 10.1038/sj.onc.1207753. [DOI] [PubMed] [Google Scholar]
- 56.Zhu M, Weiss RS. Increased common fragile site expression, cell proliferation defects and apoptosis following conditional inactivation of mouse Hus1 in primary cultured cells. Mol Biol Cell. 2007;18:1044–55. doi: 10.1091/mbc.E06-10-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenow KR, Clarke AR, Jones RH. Chk1 deficiency in the mouse small intestine results in p53-independent crypt death and subsequent intestinal compensation. Oncogene. 2009;28:1443–53. doi: 10.1038/onc.2008.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–15. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baehrecke EH. Growth control: p53, the guardian angel of compensatory proliferation. Curr Biol. 2006;16:840–2. doi: 10.1016/j.cub.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 60.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–6. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 64.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 65.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 66.Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Natl Acad Sci USA. 2001;98:9092–7. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–65. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 68.Koniaras K, Cuddihy AR, Christopoulos H, Hogg A, O’Connell MJ. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene. 2001;20:7453–63. doi: 10.1038/sj.onc.1204942. [DOI] [PubMed] [Google Scholar]
- 69.Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–25. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 70.O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 71.Murga M, Bunting S, Montana MF, Soria R, Mulero F, Canamero M, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41:891–8. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]