Abstract
Autonomic inputs from the sympathetic and parasympathetic nervous systems, as measured by heart rate variability (HRV), have been reported to correlate to the severity injury and responses to infectious challenge among critically-ill patients. In addition, parasympathetic/vagal activity has been shown experimentally to exert anti-inflammatory effects via attenuation of splanchnic tissue TNFα production. We sought to define the influence of gender on HRV responses to in vivo endotoxin challenge in healthy humans and to determine if baseline HRV parameters correlated with endotoxin mediated circulating cytokine responses. Young (< 30 years of age), healthy subjects (n=30) received endotoxin (2ng/kg) and HRV and blood samples were obtained serially thereafter. Plasma cytokines were measured by ELISA and HRV parameters were determined by analysis of serial 5-minute epochs of heart rate monitoring. In addition, calculation of multi-scale entropy deriving from cardiac monitoring data was performed. The influence of factors such as gender, body mass index (BMI) and resting heart rate on HRV after endotoxin exposure was assessed. We found that neither, gender, BMI nor resting heart rate significantly altered the HRV response following endotoxin exposure. Using entropy analysis, we observed that females had significantly higher entropy values at 24 hours after endotoxin exposure. Using a serially sampling protocol for cytokine determination, we found a significant correlation of several baseline HRV parameters (pNN50: r=0.42, p<0.05; HF r=0.4, p<0.05; LF/HF: r=-0.43, p<0.05) on TNFα release following endotoxin.
Keywords: endotoxin, heart rate variability, volunteer, gender, cytokine
Introduction
The acute host response to systemic inflammation includes activation of endocrine and innate immune signals (1) as well as changes in autonomic nervous activity. These overlapping signals initially promote a trajectory for the restoration of normal systemic and tissue functions. Over an extended period, however, the persistence of these signals and an associated acute phase response may also be associated with impaired recovery (2).
Physiological and biochemical measures of the acute systemic inflammatory response demonstrate some inter-individual variation of the clinical phenotype. This variability undoubtedly results from endogenous, patient-specific factors, including inherited characteristics, age, and gender(3). These factors also likely interact with pre-existing illness and therapies to elicit differing adaptive, host responses and promote uncertainty regarding the recovery capacity of patients over time. The inability to quantify the summative influences of patient-specific and treatment modified clinical response dynamics suggests that additional measures of host adaptability are needed.
Several recent studies have reported that surrogate measures of host adaptability and physiologic complexity, including heart rate variability (HRV) and entropy assessments (4-6) may provide insight into the dynamic status of host adaptability during inflammation. Measures of HRV are non-invasive assessments that may reflect real-time alterations of physiologic status. (7-9) Under normal circumstances, variability parameters measure homeostatic feedback between organ systems such as the central nervous system and heart, whereas decreased variability implies physiologic decomplexification(10) that may manifest as diminished organ responsiveness to autonomic signals.(11) In addition to HRV, other parameters have been suggested to quantify the physiologic complexity between organ systems including multiscale entropy (MSE)(12). Entropy measures the disorderliness within data and increases with greater variability between values and decreases with increased regularity between values.
Assessments of time and frequency domain analysis of HRV constitute a non-invasive method to evaluate vagal(8), parasympathetic tone(13) and sympathovagal balance(14-16). It is known that parameters of HRV are influenced by several relevant individual conditions, such as age(17, 18), body fatness(19), physical fitness(20) and, perhaps also, by genetic background(21). The above noted time and frequency assessments of HRV analysis have received increasing attention as predictors of outcome risk(6, 22-24) in patients presenting with both sterile and infectious injuries as well as potential adjuncts to the ongoing management of patients with complicated inflammatory states (4, 25). In addition, HRV assessment has been suggested to provide insights into the acute influence of both the sympathetic(26, 27) and cholinergic (28)anti-inflammatory pathways. Consistent with the hypothesis that higher indices of HRV determined parasympathetic activity are associated with attenuation of pro-inflammatory cytokines production, recent studies (29)have observed reduced TNFα appearance in an ex vivo endotoxin (LPS) challenge model.
This influence of parasympathetic activity is somewhat surprising given current evidence that the cholinergic anti-inflammatory pathway influences principally tissue-fixed immune cell populations in animal models(28, 30). This lead us to further investigate the relationship of HRV derived measures of sympatho-vagal activity and adaptability in a well described in vivo human endotoxin challenge model.(1, 27, 31) We also took the opportunity to further assess the influence of gender differences upon heart rate variability responses in this model (32). We hypothesized that individual subject factors such as gender, resting heart rate and body mass index could influence the HRV response following endotoxin. In addition we hypothesized that some basal HRV parameters, such as those measuring parasympathetic activity, would influence the subsequent cytokine response to in vivo endotoxin(29, 33). To test these hypotheses, we recruited human volunteers and grouped them based on their gender, resting heart rate and body mass index. We used a serial sampling protocol to quantify HRV and cytokine measures to assess the response following intravenous endotoxin exposure.
Materials and Methods
Subjects
Male (n=16) and female (n=14) subjects were recruited by public advertisement and screened for normal health status to participate in a study approved by the Institutional Review Board of the Robert Wood Johnson Medical School. Inclusion criteria for the study were: normal general health as demonstrated by medical history and physical examination, complete blood count and basic metabolic panel within normal lab limits. In addition, we limited the age of our subjects (18-30) to eliminate the potentially confounding effects of age on HRV(17, 34) and upon cytokine responses to systemic endotoxinemia(35). Exclusion criteria included a history of any acute or chronic disease, arrhythmia, recent history of alcohol, drug or medication intake, pregnancy or prior exposure to LPS in the experimental setting. Once informed, written consent was obtained; all subjects received an initial recording of heart rate and electrocardiogram (EKG) to screen for any arrhythmic patterns or irregular heartbeats. Only subjects with a normal standard EKG were considered for admission to the protocol. This study population reported here includes a subset of subjects (n=4) from a previous report (32) as well as additional subjects accrued since then (n=26)).
Study design and procedures
Upon accrual to the study, subjects were admitted to the Center for Translational and Clinical Research at UMDNJ-Robert Wood Johnson Medical School the afternoon prior to the study. At that time, a repeat examination confirmed that no changes in health status had occurred since initial recruitment. Female subjects also underwent a urine test to exclude pregnancy.
Subjects were fasted from midnight of the admission day and given intravenous fluids (5% dextrose and 0.45% sodium chloride; 1 ml/kg-hr) via a peripheral venous catheter. As previously described, a radial arterial catheter was placed (07:00) the morning of study day. (36, 37) The arterial catheter was utilized to monitor heart rate and blood pressure as well as for periodic blood sampling at defined time points before and after endotoxin administration. A rectal thermometer was placed for continuous monitoring of core body temperature. As previously described,(31, 37) a onetime dose of endotoxin (2ng/kg, CC-RE, Lot #2) was administered over a one minute-period through a separate peripheral intravenous catheter at 09:00 (considered time point 0) on study day 1.
Clinical monitoring
Vital signs, including heart rate and mean arterial blood pressure (MAP), were recorded every 30 minutes from the arterial monitoring system for the first 6 hours (09:00-15:00 hours) and then taken manually at 9, 12, and 24 hours after LPS administration. Core temperatures were recorded every 30 minutes through +6 hours via rectal thermometer, then orally at 9, 12, and 24 hours after LPS administration. At 6 hours after LPS bolus, the arterial catheter and rectal thermometer were removed. The peripheral intravenous catheter infusing saline solution was removed once each subject tolerated a regular diet. Subjects remained in the study unit overnight and were discharged to home the following morning after the 24 hour post-LPS samples were obtained.
Assessment of heart rate variability
A base-line determination of HRV was obtained at the time of admission (Admit) as well as hourly from 0 to +6 hours following endotoxin challenge and at +24 hours after LPS. Each recording interval consisted of two consecutive 5-minute epochs. During such determinations, heart rate was monitored using a continuous electrocardiography (EKG) technique with three standard limb leads and CardioPro® 2.0 software with one Infiniti and one Procomp Plus® recorder (Thought Technology, Ltd., Montreal, P.Q., Canada). HRV parameters as well as inter-beat intervals were collected using EKG data at a rate of 256 samples/second as previously described (27, 31). In a continuous EKG record, each QRS complex was detected and the “normal-to-normal” (NN) intervals (all intervals between adjacent QRS complexes resulting from sinus node depolarization) were tabulated, thus providing a record of instantaneous heart rate.(15) For each epoch, noise artefact and irregular heartbeats were manually edited by visual inspection and interpolation prior to calculation of interbeat intervals using CardioPro software. We analyzed each epoch(31) and excluded complete measurement epochs where events such as extra systolic heartbeats, skipped beats, and other arrhythmias comprised greater than 10% of the total epoch. The power spectral density then was calculated using a Fast Fourier transformation algorithm.(15, 38) All signals were exported in standard ASCII format to Excel and EAS 9.0 for analysis and graphics(31).
Parameters of HRV were analyzed for both time domain and frequency domain measures. Time domain measures included, 1) the standard deviation of the average beat to beat intervals over a 5 minute period (SDANN), a measure of total heart rate variability and overall system adaptability and 2) the percentage of interval differences of successive interbeat intervals greater than 50 ms (pNN50), that is generally associated with respiratory sinus arrhythmia and therefore, vagus nerve activity. Frequency domain measures included, 1) high frequency variability (HF)[0.15-0.4Hz] that correlates with parasympathetic and vagal tone and 2) the low frequency/high frequency ratio (LF/HF) that is hypothesized to be associated with sympathetic:parasympathetic balance.(10, 15, 16)
Analysis of blood samples
Blood samples were collected at time points -24, 0, 0.5, 1, 1.5, 2, 3, 4, 6, and 24 hours in relation to endotoxin administration. Blood-derived plasma was then analyzed by ELISA for measurement of the soluble inflammatory markers TNFα and IL-6. The peak value of these cytokines was determined for each individual. We have previously reported the gender based cytokine response to endotoxin exposure in a larger study population that did not differ between male and female subjects (32).
Statistical analysis
Analysis of HRV parameters
Gender differences in parameters of HRV were measured by two-way analysis of variance with repeated measures on time using Statistica version 6.1 (StatSoft, Inc., Tulsa, OK).(39) P-values less than 0.05 were considered to be statistically significant. The pearson product-moment correlation coefficient was calculated to measure the association between baseline parameters of HRV and maximum recorded plasma TNFα and IL-6 levels.
Analysis of entropy
Entropy analysis was performed using the multiscale approach suggested by Costa et al. and is shown as sample entropy over increasing scale factors. (12) The pattern length of 2 was used as well as a similarity factor of 0.15 was utilized as suggested by prior reports (5, 40).
Results
Vital signs
Vital sign changes after endotoxin administration were similar to those previously described (32)and are not reported here.
Serial cytokine levels
These levels were also similar to those previously reported(32)after endotoxin administration and are not reported here.
Gender influence on HRV
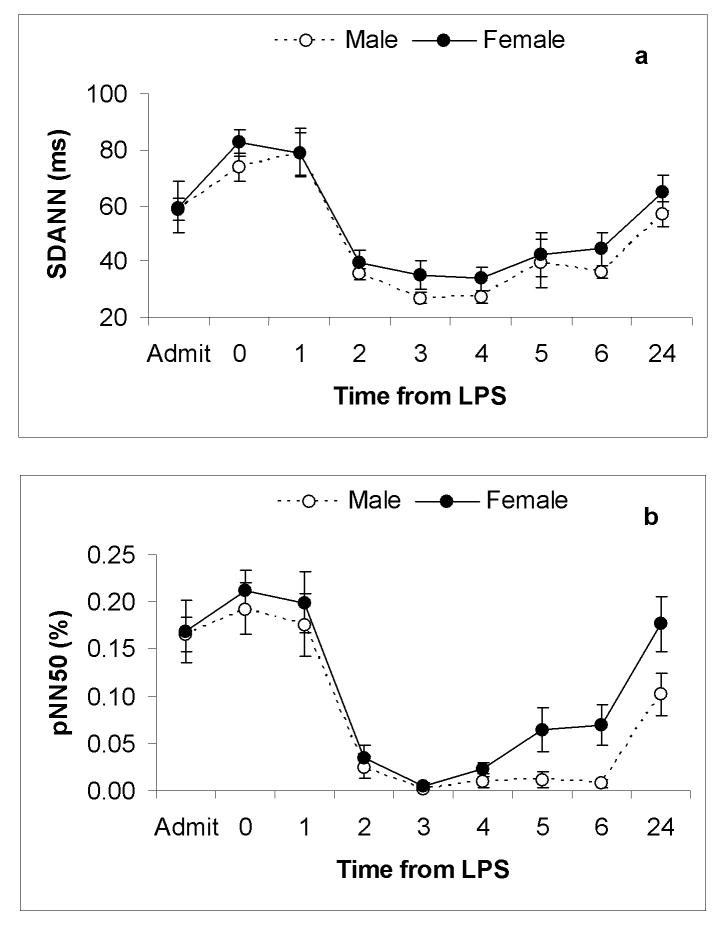
None of the HRV parameters varied at baseline between genders prior to endotoxin exposure. Consistent with previous observations, in both gender groups, the greatest change in HRV following endotoxin administration occurred between timepoint 0 and +3(27, 31). In measuring HRV from baseline to +24 hours we found that gender was not significantly associated with any measured HRV response to endotoxin (SDANN, pNN50, HF, LF/HF) (p >0.05) (Figure 1) (Figure 2). There was a trend towards an enhanced autonomic recovery in females measured by a more rapid return to baseline values of parameters of HRV including pNN50, HF, LF/HF(Figure 1) (Figure 2)
Figure 1.
Time domain measures of HRV (a)SDANN and (b)pNN50 as a function of time after intravenous endotoxin administration, given at time-point zero, in both female (n=14) and male (n=16) subjects. Results are expressed as mean ± SE. From admission to 24 hours there was no significant difference between groups as measured by (a)SDANN and (b)pNN50.
Figure 2.
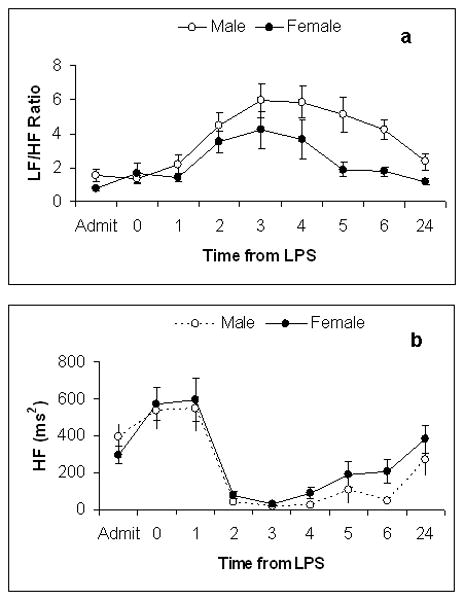
Frequency domain measures of HRV (a)LF/HF and (b)HF as a function of time after intravenous endotoxin administration, given at time-point zero, in both female (n=14) and male (n=16) subjects. Results are expressed as mean ± SE. From admission to 24 hours there was no significant difference between groups as measured by (a)LF/HF and (b)HF.
Entropy
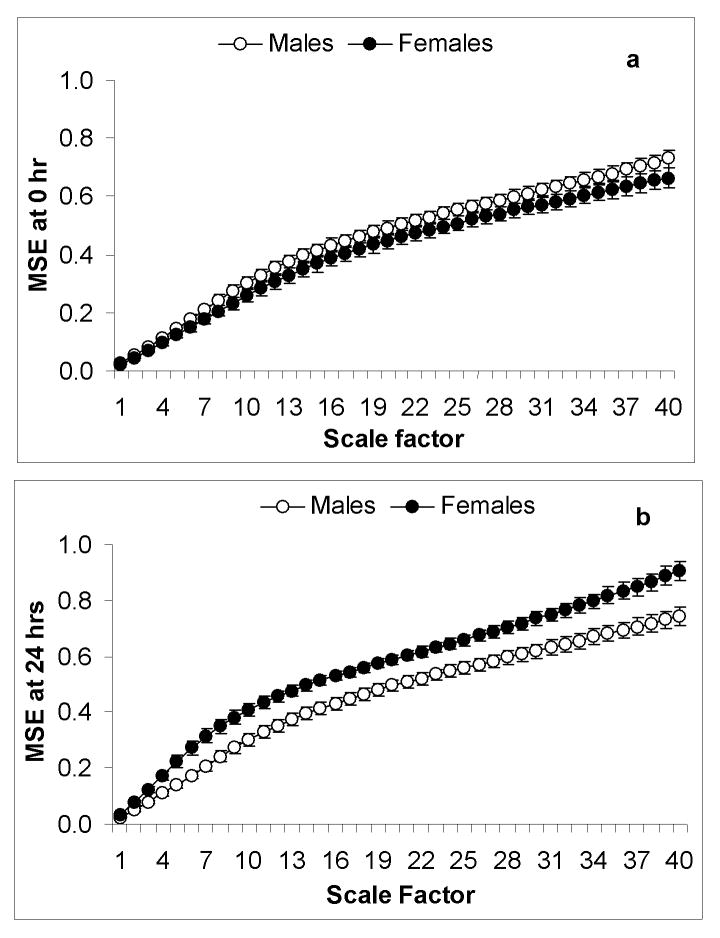
Baseline entropy was not significantly different between males and females at timepoint zero (p>0.05). At 24 hours, MSE analysis revealed a significant difference in the gender related response to endotoxin where females had significantly higher entropy over increasing scale factors (p<0.05). At 24 hours post endotoxin males return to baseline entropy values. In females, higher entropy than at baseline was observed (Figure 3).
Figure 3.
Multiscale entropy measuring sample entropy at increasing scale factors comparing male (n=16) and female (n=14) volunteers. At timepoint zero (a) prior to endotoxin administration there was no significant difference in entropy between male and female volunteers. At 24 hours post endotoxin (b), female volunteers demonstrated a significantly higher entropy over increasing scale factors (p<0.05) and also had higher entropy at 24 hours vs baseline where male volunteers returned to baseline entropy.
Body Mass Index
Body mass index (BMI) was calculated using height and weight data measured on admission. Groups were separated by those with a BMI less than 25 (n=21) and those with a BMI greater than or equal to 25 (n=9) according to World Health Organization guidelines(41). Across the modest range of BMI exhibited by our healthy, young subjects, BMI was not correlated with any basal or endotoxin-induced change in parameters of heart rate variability (data not shown).
Heart Rate
Resting heart rate (HR) was measured at timepoint 0 and groups were separated by those with a resting HR less than 70 (n=21) and those with a HR greater than or equal to 70 (n=9). Resting HR, whether assessed as a continuous or dichotomous variable, as above, did not correlate with any basal or endotoxin-induced change in parameters of HRV (data not shown).
Relationship of basal HRV and peak in vivo TNFα and IL-6 response
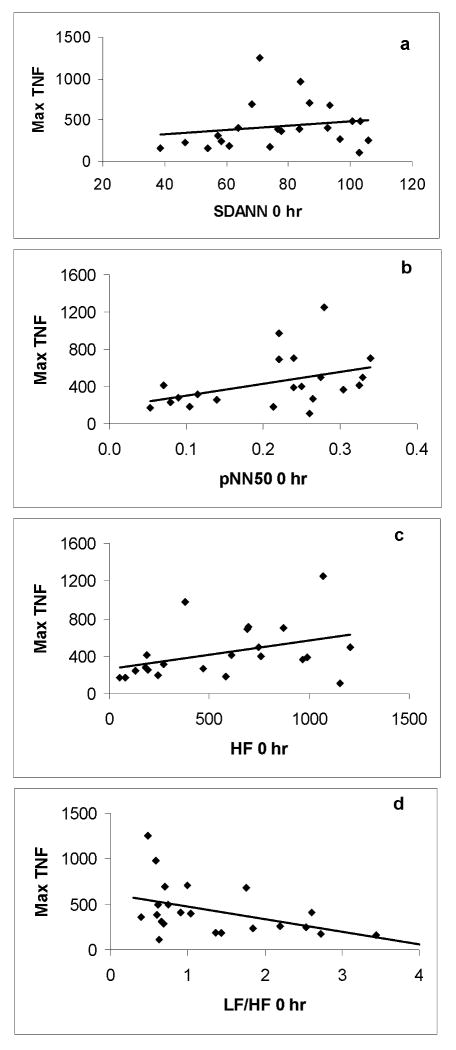
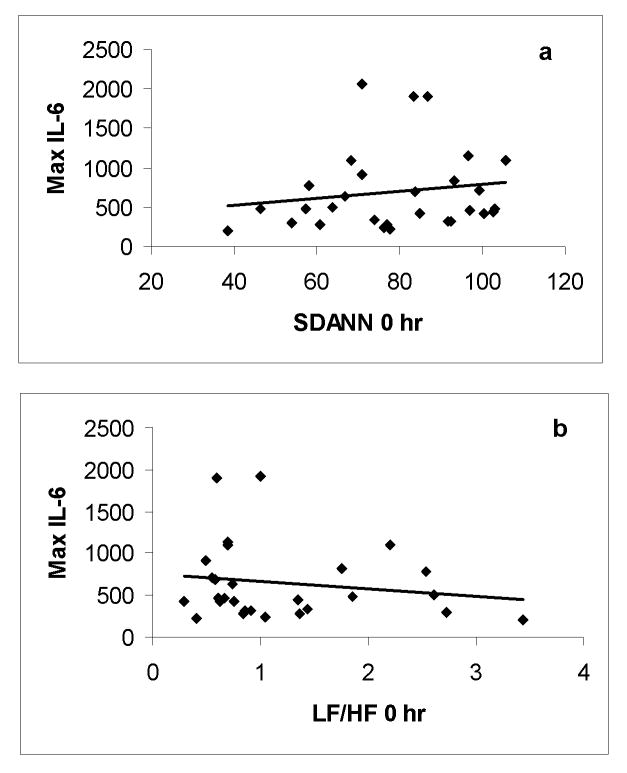
Basal HRV was determined immediately prior to endotoxin administration and compared to the maximum subsequent plasma TNFα and IL-6 level. Baseline LF/HF (r=-0.43; p<0.05), pNN50 (r=0.42; p<0.05) and HF (r=0.4; p<0.05) demonstrated a significant association with plasma TNFα level where baseline SDANN (r=0.19) did not correlate to TNFα production after endotoxin administration. Given that initial HRV levels did not vary by gender before LPS, the depiction of this relationship combined both genders (Figure 4). No baseline parameter of HRV (SDANN: r=0.15, pNN50: r=0.17, HF: r=0.17, LF/HF: r= -0.16) was significantly associated with the production of IL-6 after in vivo endotoxin (Figure 5).
Figure 4.
A scatterplot of associations between zero hour (a) SDANN, (b) pNN50, (c) HF, (d) LF/HF and maximal TNFα level following endotoxin exposure in all study subjects (n=30). There was a significant correlation between maximal TNFα level and (b) pNN50 (r=0.42; p <0.05), (c) HF (r=0.4; p<0.05) and (d) LF/HF (r=-0.43; p<0.05). There was no significant correlation between maximal TNFα level and (a) SDANN.
Figure 5.
A scatterplot of associations between zero hour (a)SDANN, (b) LF/HF and maximal IL-6 level following endotoxin exposure in all study subjects (n=30). There was no significant correlation between maximal IL-6 level and (a) SDANN (r=0.15, p>0.05) and (b) LF/HF (r=-0.16, p>0.05).
Discussion
Recent data supports the role of efferent, vagus nerve outflow as a regulator of systemic pro-inflammatory mediator activity during inflammation and infection.(42) As an effector arm of the parasympathetic nervous system, vagal activity may regulate inflammation through several mechanisms. Vagus nerve efferent signals have been shown to reduce production of the pro inflammatory cytokine TNFα via acetylcholine (Ach) binding to the α-7 subunits of nicotinic receptors on mononuclear phagocytes of the reticulo-endothelial system(28, 42, 43). Vagotomy has been demonstrated experimentally to enhance pro-inflammatory cytokine release in a murine model of intraperitoneal sepsis.(44) Pharmacologic activation of nicotinic receptors has also been shown to reduce TNFα release from alveolar macrophages exposed to LPS.(45) We have also recently confirmed that antecedent transcutaneous administration of the known α-7 agonist, nicotine, reduced systemic phenotype and pro-inflammatory mediator responses to endotoxin in humans.(37)
Both parasympathetic and sympathetic nervous system activity contribute to protective host responses during systemic inflammation and sepsis. While parasympathetic activity may exert anti-inflammatory effects through via vagally mediated anti-inflammatory pathways(28), sympathetic activity is also of importance in regulating vascular tone during sepsis(46). The influence of both of these systems can be estimated by parameters of HRV(15). Observations in the literature are divided as to whether relative parasympathetic(28) or sympathetic predominance(22) is beneficial during stress. Interestingly in critically ill patients, increased parasympathetic tone and decreased sympathetic tone has been associated with mortality (22) and specific parameters of HRV measuring parasympathetic tone (HF, RMSSD) have been identified as independent predictors of mortality(22) and development of septic shock(23). Other measures of the complex interaction between sympatho-vagal balance and other regulatory systems, such as those reflected in very low frequency variability, have also been suggested to associate with outcome.(47)
Previous studies have suggested that relative enhancement of parasympathetic activity assessed by high-frequency power spectral analysis and time domain components of HRV are inversely correlated with ex-vivo blood production of the pro-inflammatory mediators, TNFα and IL-6(29). In order to study this correlation in-vivo, we measured the parameters of HRV at timepoint zero (immediately prior to endotoxin exposure) and sought correlates to maximal in vivo TNFα and IL-6 production. We detected a correlation between parasympathetic/vagal parameters of HRV (HF and pNN50) and maximal TNFα level but not for IL-6 response following endotoxin exposure. However, our finding is in contrast to what might be anticipated from the results of prior studies that suggest that greater basal parasympathetic/vagal activity would lead to a lesser pro-inflammatory mediator response via vagally mediated cholinergic anti-inflammatory pathways(28). Several factors may account for this difference, including the younger age of our subjects compared with the generally older subjects evaluated in prior studies. In addition, prior studies utilized in vitro incubation to assess peak cytokine production whereas the current study determined peak in vivo responses with a serial sampling protocol. Interestingly enough, gender differences have been observed in ex-vivo studies wherein higher TNFα and IL-6 levels were detected in male subjects(29). This is consistent with prior ex-vivo stimulated blood observations based on gender(48). By contrast, in a previous report, higher IL-6 monocyte expression was observed among females throughout the circadian cycle(49). These results underscore the blurring of lines that separate pro and anti-inflammatory signaling mechanisms based upon discrete sympathetic and parasympathetic components(33).
It remains to be determined what influence the autonomic nervous system exerts over the dynamic processes that result from stress. A previous study measuring the influence of antecedent epinephrine on endotoxin induced systemic inflammation in healthy subjects suggests that this catecholamine is associated with a modest reduction in vagally mediated heart rate variability (27). This is consistent with previous studies of this α and β agonist in humans (50, 51). However, epinephrine excess was associated with decreased TNFα levels after endotoxin challenge and this result appears to contrast to an enhanced production that might result from decreased activity of cholinergic anti-inflammatory pathways. This suggests that autonomic signals regulating cytokine release have a more complex interface with the hormonal and nervous systems than previously appreciated(27).
Gender did not significantly influence parameters of HRV following endotoxin exposure. Because the recruitment process for these studies sought to exclude females that might be in a peri-ovulatory phase of the menstrual cycle, only one female subject demonstrated an estradiol level greater than 100pg/ml. This precluded a detailed analysis of the influence of increased estrogen on the systemic response following endotoxin administration. Interestingly, during recovery at 6 hours after endotoxin, female subjects exhibited a relative decrease in sympathetic activity, as measured by LF/HF ratio, as well as a trend towards increased vagal tone reflected by increased HF and pNN50. These findings may suggest that females may undergo more rapid autonomic recovery after a limited acute systemic stressor. In order to further analyze gender-related differences in the time to recovery of autonomic balance, we undertook entropy analysis(12) in these subjects. By this analysis, male subjects returned to base-line physiologic complexity at 24 hours, whereas female subjects appeared to exhibit greater entropy values than at baseline. The enhanced autonomic recovery in females at 24 hours may be influential in modulating the response to subsequent stressors.
This study was specifically limited to subjects less than 30 years of age and hence it is unlikely that age influenced the parameters of HRV. Adiposity did not influence either time domain or frequency domain parameters of HRV following endotoxin exposure. Physical fitness, based on non-exercise, oximetric testing(52), has been associated with increased baseline vagal/parasympathetic profile as determined by increased pNN50 and HF following mental stress versus a non-fit cohort (53). In this study, physical fitness, estimated by a resting heart rate less than seventy, did not appear to influence parameters of HRV following endotoxin challenge. Other reports suggest that improved fitness increases both time and frequency domain parameters of parasympathetic activity (54). A recent study has confirmed that an extended period of submaximal exercise training is associated with reduced ex vivo TNFα production in response to endotoxin(55). Further studies are needed to determine how mechanisms of physical fitness induced autonomic activity might interact with other humoral modulators of innate immunity to derive these potential benefits.
Limitations
There may be age and chronic illness associated influences(3, 56,) that preclude direct extension of our observations to either much older or younger subjects. Age does influence parameters of HRV(17) and endocrine responsiveness,(34) although many elderly subjects appear to maintain innate immune activity.(35) Female subjects were almost exclusively in a lower estradiol background and so the influence of increased estrogen cannot be assessed. Nevertheless, this is the first such assessment of this purported relationship using the human endotoxin model and has sought to control for the important confounding effect of age on autonomic activity (17). Our study population included healthy volunteers and it is unclear whether our observations would differ in subjects with ongoing sterile or infectious stress. HRV also exhibits circadian variation (57) and it is conceivable that diurnal variations in autonomic function might differentially influence endotoxin responses when assessed at other points in the circadian cycle(58).
Acknowledgments
This work was supported by the National Institutes of Health (R01 GM34695).
References
- 1.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock (Augusta, Ga. 2005;24 1:94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 2.Renckens R, van Westerloo DJ, Roelofs JJ, Pater JM, Schultz MJ, Florquin S, van der Poll T. Acute phase response impairs host defense against Pseudomonas aeruginosa pneumonia in mice. Critical care medicine. 2008;36:580–587. doi: 10.1097/01.CCM.0B013E3181620652. [DOI] [PubMed] [Google Scholar]
- 3.Lowry SF. The stressed host response to infection: the disruptive signals and rhythms of systemic inflammation. Surg Clin North Am. 2009;89:311–326. doi: 10.1016/j.suc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris JA, Jr, Norris PR, Ozdas A, Waitman LR, Harrell FE, Jr, Williams AE, Cao H, Jenkins JM. Reduced heart rate variability: an indicator of cardiac uncoupling and diminished physiologic reserve in 1,425 trauma patients. J Trauma. 2006;60:1165–1173. doi: 10.1097/01.ta.0000220384.04978.3b. discussion 1173-1164. [DOI] [PubMed] [Google Scholar]
- 5.Norris PR, Stein PK, Morris JA., Jr Reduced heart rate multiscale entropy predicts death in critical illness: a study of physiologic complexity in 285 trauma patients. J Crit Care. 2008;23:399–405. doi: 10.1016/j.jcrc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock (Augusta, Ga. 2007;28:549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- 7.Norris PR, Ozdas A, Cao H, Williams AE, Harrell FE, Jenkins JM, Morris JA., Jr Cardiac uncoupling and heart rate variability stratify ICU patients by mortality: a study of 2088 trauma patients. Ann Surg. 2006;243:804–812. doi: 10.1097/01.sla.0000219642.92637.fd. discussion 812-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 9.Winchell RJ, Hoyt DB. Analysis of heart-rate variability: a noninvasive predictor of death and poor outcome in patients with severe head injury. The Journal of trauma. 1997;43:927–933. doi: 10.1097/00005373-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Current opinion in critical care. 2002;8:311–315. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–1116. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 13.Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285:R927–931. doi: 10.1152/ajpregu.00452.2003. [DOI] [PubMed] [Google Scholar]
- 14.Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Critical care medicine. 1996;24:1117–1124. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 16.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, Katus HA. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. Journal of cardiovascular electrophysiology. 2003;14:791–799. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- 18.De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol. 2007;74:165–173. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 20.Pichot V, Roche F, Denis C, Garet M, Duverney D, Costes F, Barthelemy JC. Interval training in elderly men increases both heart rate variability and baroreflex activity. Clin Auton Res. 2005;15:107–115. doi: 10.1007/s10286-005-0251-1. [DOI] [PubMed] [Google Scholar]
- 21.Andrich J, Schmitz T, Saft C, Postert T, Kraus P, Epplen JT, Przuntek H, Agelink MW. Autonomic nervous system function in Huntington's disease. J Neurol Neurosurg Psychiatry. 2002;72:726–731. doi: 10.1136/jnnp.72.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WL, Chen JH, Huang CC, Kuo CD, Huang CI, Lee LS. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med. 2008;26:395–401. doi: 10.1016/j.ajem.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med. 2007;14:392–397. doi: 10.1197/j.aem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Cooke WH, Salinas J, Convertino VA, Ludwig DA, Hinds D, Duke JH, Moore FA, Holcomb JB. Heart rate variability and its association with mortality in prehospital trauma patients. J Trauma. 2006;60:363–370. doi: 10.1097/01.ta.0000196623.48952.0e. discussion 370. [DOI] [PubMed] [Google Scholar]
- 25.Annane D. Glucocorticoids in the treatment of severe sepsis and septic shock. Current opinion in critical care. 2005;11:449–453. doi: 10.1097/01.ccx.0000176691.95562.43. [DOI] [PubMed] [Google Scholar]
- 26.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jan BU, Coyle SM, Oikawa LO, Lu SE, Calvano SE, Lehrer PM, Lowry SF. Influence of Acute Epinephrine Infusion on Endotoxin-Induced Parameters of Heart Rate Variability: A Randomized Controlled Trial. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181a40193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 29.Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosomatic medicine. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- 30.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. Journal of endotoxin research. 2007;13:358–368. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 32.Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock (Augusta, Ga. 2006;26:538–543. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- 33.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofek K, Krabbe KS, Evron T, Debecco M, Nielsen AR, Brunnsgaad H, Yirmiya R, Soreq H, Pedersen BK. Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med. 2007;85:1239–1251. doi: 10.1007/s00109-007-0226-x. [DOI] [PubMed] [Google Scholar]
- 36.Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 37.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clinical and experimental immunology. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen H, Cooley JW. The Numerical Solution of the Time-Dependent Nernst-Planck Equations. Biophys J. 1965;5:145–162. doi: 10.1016/s0006-3495(65)86707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Statsoft. I. STATISTICA data analysis software system. 2003. [Google Scholar]
- 40.Norris PR, Anderson SM, Jenkins JM, Williams AE, Morris JA., Jr Heart rate multiscale entropy at three hours predicts hospital mortality in 3,154 trauma patients. Shock (Augusta, Ga. 2008;30:17–22. doi: 10.1097/SHK.0b013e318164e4d0. [DOI] [PubMed] [Google Scholar]
- 41.Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ. 1995;73:673–680. [PMC free article] [PubMed] [Google Scholar]
- 42.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257:156–166. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 44.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 45.Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock (Augusta, Ga. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 46.Korach M, Sharshar T, Jarrin I, Fouillot JP, Raphael JC, Gajdos P, Annane D. Cardiac variability in critically ill adults: influence of sepsis. Critical care medicine. 2001;29:1380–1385. doi: 10.1097/00003246-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Stein PK. Measures of parasympathetic function and risk stratification in critical care. Critical care medicine. 2008;36:1025–1027. doi: 10.1097/CCM.0B013E318164EC6D. [DOI] [PubMed] [Google Scholar]
- 48.Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–258. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 49.O'Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 50.Cogliati C, Colombo S, Ruscone TG, Gruosso D, Porta A, Montano N, Malliani A, Furlan R. Acute beta-blockade increases muscle sympathetic activity and modifies its frequency distribution. Circulation. 2004;110:2786–2791. doi: 10.1161/01.CIR.0000146335.69413.F9. [DOI] [PubMed] [Google Scholar]
- 51.Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosomatic medicine. 2001;63:788–796. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Kelley GA, Lowing L, Kelley K. Gender differences in the aerobic fitness levels of young African-American adults. J Natl Med Assoc. 1999;91:384–388. [PMC free article] [PubMed] [Google Scholar]
- 53.Rossy LA, Thayer JF. Fitness and gender-related differences in heart period variability. Psychosomatic medicine. 1998;60:773–781. doi: 10.1097/00006842-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Madden KM, Levy WC, Stratton JK. Exercise training and heart rate variability in older adult female subjects. Clin Invest Med. 2006;29:20–28. [PubMed] [Google Scholar]
- 55.Sloan RP, Shapiro PA, Demeersman RE, McKinley PS, Tracey KJ, Slavov I, Fang Y, Flood PD. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 56.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clinical and experimental immunology. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sztajzel J, Jung M, Bayes de Luna A. Reproducibility and gender-related differences of heart rate variability during all-day activity in young men and women. Ann Noninvasive Electrocardiol. 2008;13:270–277. doi: 10.1111/j.1542-474X.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollmacher T, Mullington J, Korth C, Schreiber W, Hermann D, Orth A, Galanos C, Holsboer F. Diurnal variations in the human host response to endotoxin. J Infect Dis. 1996;174:1040–1045. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]