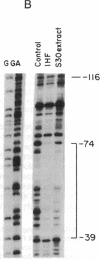
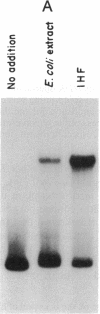
Abstract
We have detected activity of the nitrogen fixation regulatory protein NIFA of Klebsiella pneumoniae in vitro. To do so we directed synthesis of NIFA in a coupled transcription-translation system and detected its ability to activate expression of a translational fusion between the nifH and lacZ genes. We infer that NIFA stimulates initiation of transcription by sigma 54 holoenzyme from the nifHDK promoter. The activity of NIFA was lost rapidly under both aerobic and anaerobic conditions at 30 degrees C and was lost somewhat less rapidly at 0 degrees C. Loss of activity was not accompanied by degradation of NIFA polypeptide. Loss of activity was approximately exponential and was not affected by NIFA concentration over a 5-fold range. Therefore, NIFA inactivation does not appear to be due to self-association. We found that the factor in crude extracts previously demonstrated to bind to the nifHDK promoter-regulatory region [Beynon, J., Cannon, M., Buchanan-Wollaston, V., and Cannon, F. (1983) Cell 34, 665-671] is the integration host factor, which is known to bend DNA. Since the binding site for integration host factor lies between the upstream binding site for NIFA and the nifHDK promoter, integration host factor may bend the DNA between these two sites to facilitate productive interactions between NIFA and sigma 54 holoenzyme.
Full text
PDF
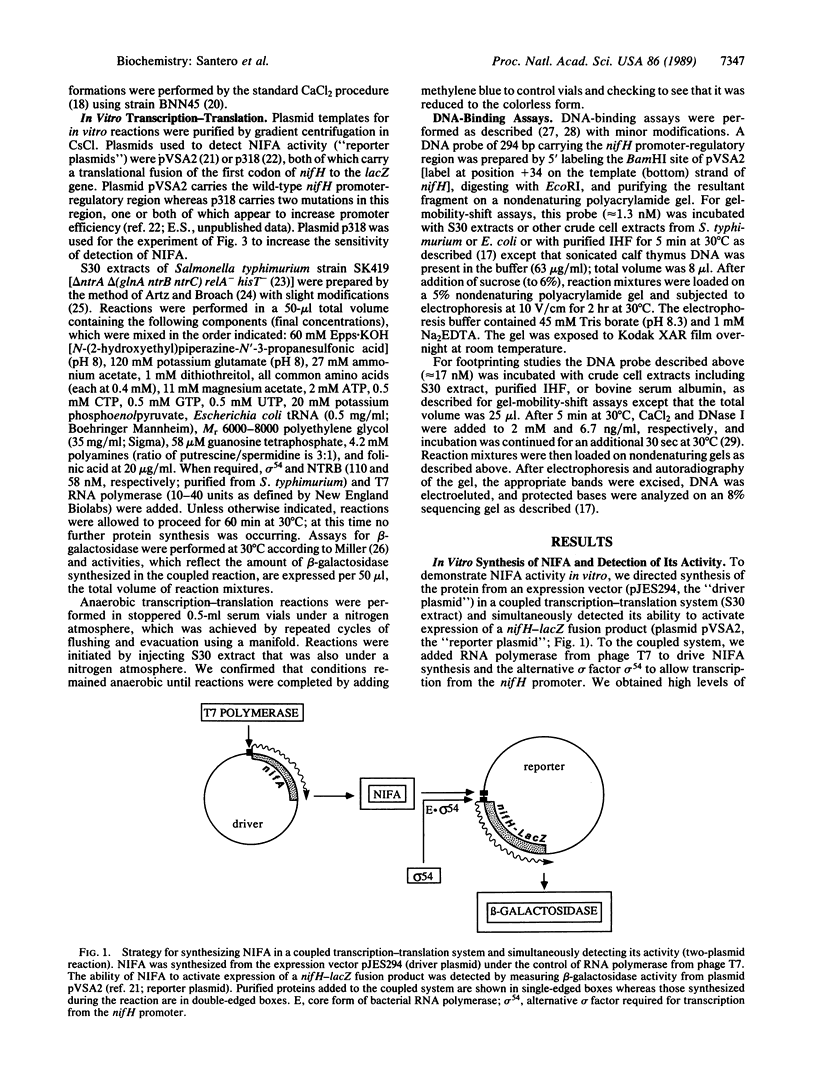
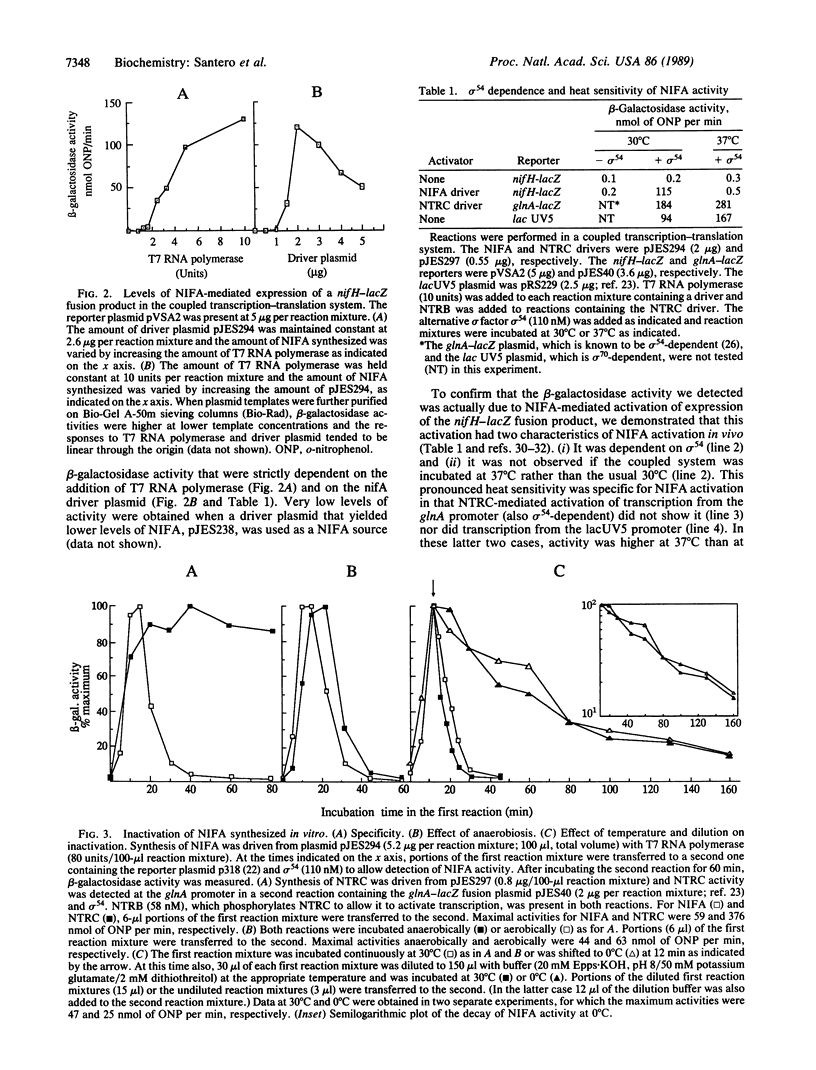

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. T., Cannon F., Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol. 1988 May;2(3):315–321. doi: 10.1111/j.1365-2958.1988.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Beynon J. L., Williams M. K., Cannon F. C. Expression and functional analysis of the Rhizobium meliloti nifA gene. EMBO J. 1988 Jan;7(1):7–14. doi: 10.1002/j.1460-2075.1988.tb02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Buck M., Cannon W., Woodcock J. Transcriptional activation of the Klebsiella pneumoniae nitrogenase promoter may involve DNA loop formation. Mol Microbiol. 1987 Sep;1(2):243–249. doi: 10.1111/j.1365-2958.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Szeto W. W., Lemley P. V., Orme-Johnson W. H., Ausubel F. M. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 1985 Jun 25;13(12):4539–4555. doi: 10.1093/nar/13.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J., Roberts G. P., Brill W. J. Posttranscriptional control of Klebsiella pneumoniae nif mRNA stability by the nifL product. J Bacteriol. 1986 Oct;168(1):173–178. doi: 10.1128/jb.168.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H M, Hennecke H. Direct response of Bradyrhizobium japonicum nifA-mediated nif gene regulation to cellular oxygen status. Mol Gen Genet. 1987 Oct;209(3):621–626. doi: 10.1007/BF00331174. [DOI] [PubMed] [Google Scholar]
- Fischer H. M., Alvarez-Morales A., Hennecke H. The pleiotropic nature of symbiotic regulatory mutants: Bradyrhizobium japonicum nifA gene is involved in control of nif gene expression and formation of determinate symbiosis. EMBO J. 1986 Jun;5(6):1165–1173. doi: 10.1002/j.1460-2075.1986.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Shanmugam K. T. Temperature control of nitrogen fixation in Klebsiella pneumoniae. Arch Microbiol. 1979;123(3):259–265. doi: 10.1007/BF00406659. [DOI] [PubMed] [Google Scholar]
- Hill S., Kennedy C., Kavanagh E., Goldberg R. B., Hanau R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature. 1981 Apr 2;290(5805):424–426. doi: 10.1038/290424a0. [DOI] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo J. M., Goldberg R. B. Regulation of nitrogen metabolism in glutamine auxotrophs of Klebsiella pneumoniae. J Bacteriol. 1980 Apr;142(1):99–110. doi: 10.1128/jb.142.1.99-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masepohl B., Klipp W., Pühler A. Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet. 1988 Apr;212(1):27–37. doi: 10.1007/BF00322441. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E., Cannon W., Buck M. The DNA-binding domain of the transcriptional activator protein NifA resides in its carboxy terminus, recognises the upstream activator sequences of nif promoters and can be separated from the positive control function of NifA. Nucleic Acids Res. 1988 Dec 23;16(24):11469–11488. doi: 10.1093/nar/16.24.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Regulation of nitrogen metabolism genes by nifA gene product in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):307–313. doi: 10.1038/301307a0. [DOI] [PubMed] [Google Scholar]
- Ow D. W., Xiong Y., Gu Q., Shen S. C. Mutational analysis of the Klebsiella pneumoniae nitrogenase promoter: sequences essential for positive control by nifA and ntrC (glnG) products. J Bacteriol. 1985 Mar;161(3):868–874. doi: 10.1128/jb.161.3.868-874.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Santero E., Toukdarian A., Humphrey R., Kennedy C. Identification and characterization of two nitrogen fixation regulatory regions, nifA and nfrX, in Azotobacter vinelandii and Azotobacter chroococcum. Mol Microbiol. 1988 May;2(3):303–314. doi: 10.1111/j.1365-2958.1988.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Jones J. D., Ow D. W., Ausubel F. M. Klebsiella pneumoniae nifA product activates the Rhizobium meliloti nitrogenase promoter. Nature. 1983 Feb 24;301(5902):728–732. doi: 10.1038/301728a0. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli R., Merrick M. J. Over-production and characterization of the nifA gene product of Klebsiella pneumoniae--the transcriptional activator of nif gene expression. J Gen Microbiol. 1988 Feb;134(2):425–432. doi: 10.1099/00221287-134-2-425. [DOI] [PubMed] [Google Scholar]
- Zhu J., Brill W. J. Temperature sensitivity of the regulation of nitrogenase synthesis by Klebsiella pneumoniae. J Bacteriol. 1981 Feb;145(2):1116–1118. doi: 10.1128/jb.145.2.1116-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]