Abstract
Rationale
A homozygous disruption or genetic mutation of the bag3 gene, a member of the Bcl-2-associated athanogene (BAG) family proteins, causes cardiomyopathy and myofibrillar myopathy that is characterized by myofibril and Z-disc disruption. However, the detailed disease mechanism is not yet fully understood.
Objective
bag3−/− mice exhibit differences in the extent of muscle degeneration between muscle groups with muscles experiencing the most usage degenerating at an accelerated rate. Usage-dependent muscle degeneration suggests a role for BAG3 in supporting cytoskeletal connections between the Z-disc and myofibrils under mechanical stress. The mechanism by which myofibrillar structure is maintained under mechanical stress remains unclear. The purpose of the study is to clarify the detailed molecular mechanism of BAG3-mediated muscle maintenance under mechanical stress.
Methods and Results
To address the question of whether bag3 gene knockdown induces myofibrillar disorganization caused by mechanical stress, in vitro mechanical stretch experiments using rat neonatal cardiomyocytes and an shRNA-mediated gene knockdown system of the bag3 gene were performed. As expected, mechanical stretch rapidly disrupts myofibril structures in bag3 knockdown cardiomyocytes. BAG3 regulates the structural stability of F-actin through the actin capping protein, CapZβ1, by promoting association between Hsc70 and CapZβ1. BAG3 facilitates the distribution of CapZβ1 to the proper location, and dysfunction of BAG3 induces CapZ ubiquitin-proteasome-mediated degradation. Inhibition of CapZβ1 function by overexpressing CapZβ2 increased myofibril vulnerability and fragmentation under mechanical stress. On the other hand, overexpression of CapZβ1 inhibits myofibrillar disruption in bag3 knockdown cells under mechanical stress. As a result, heart muscle isolated from bag3−/− mice exhibited myofibrillar degeneration, and lost contractile activity after caffeine contraction.
Conclusions
These results suggest novel roles for BAG3 and Hsc70 in stabilizing myofibril structure and inhibiting myofibrillar degeneration in response to mechanical stress. These proteins are possible targets for further research to identify therapies for myofibrillar myopathy or other degenerative diseases.
Keywords: BAG-family proteins, BAG3, molecular chaperone, myofibrillar myopathy, heart failure
Introduction
Molecular chaperones and co-chaperones have critical roles in protein folding, inhibition of protein aggregation and degradation of misfolded proteins. There are two major cytosolic molecular chaperones in the cellular protein folding machinery: stress inducible Hsp70 and constitutively expressed Hsc70. BAG3 is a member of the Bcl-2-associated athanogene (BAG) family of proteins that regulate Hsp70/Hsc70 chaperone activity via their conserved C-terminal domains1. The upstream region of BAG family proteins diverges, which presumably allows the different family members to act in a variety of settings. BAG3 carries both a WW domain and PXXP motif that are typical of proteins that interact with the cytoskeleton2. BAG3 expression is notably high in skeletal and cardiac muscle tissue3. Consistent with this finding, bag3-deficient mice show severe muscle dysfunction and die on average four weeks after birth3. Recently, identical mutations in BAG3 were identified in three myofibrillar myopathy patients. This mutation involved a substitution of proline for leucine at amino acid 209 (P209L), where no distinct protein motifs are located4. All three patients experienced symptoms beginning in childhood that included progressive limb and axial weakness and each developed cardiomyopathy.
Myofibrillar myopathy is a genetically heterogeneous group of diseases characterized by progressive muscle weakness, and, at a cellular level, disrupted Z-disc structure and myofibrillar degeneration5. Mutations in genes encoding Z-disc proteins can result in increased vulnerability of muscle tissue to mechanical stress, causing cardiomyopathy and myofibrillar myopathy6, 7. Myofibrillar myopathy is sometimes accompanied by cytosolic aggregated protein and ectopic accumulation of various different myofibrillar proteins, which may be due to abnormalities in protein folding and assembly of Z-disc proteins8, 9. These data suggest that BAG3 may play an important role in facilitating protein folding and maintaining the stability of myofibrillar proteins under mechanical stress.
The length of actin filaments is tightly regulated and may grow or shrink depending on the rate of globular actin (G-actin) polymerization to form filamentous-actin (F-actin) with concomitant ATP hydrolysis. Filament length is dependent in part on the activity of two types of capping proteins, tropomodulin 1 and F-actin capping protein, which act on the pointed and barbed ends, respectively, of F-actin. In addition, a giant protein, nebulin regulates actin filament lengths by stabilizing the filaments10. A specific interaction demonstrated between CapZ and the nebulin C-terminus may aid in positioning the actin filaments at the Z-disc and also facilitate linkage of adjacent sarcomeres11. F-actin capping protein is termed CapZ because of its localization at the Z-disc. CapZ binds with high affinity to the barbed (+) end of actin to prevent the dissociation of actin monomers from the filament end12. CapZ is comprised of a 1:1 heterodimer of α and β subunits. Vertebrates have three α subunits, which are encoded by three different genes, while threeβ subunits arise from one gene as a result of alternative splicing13–15. In muscle, capping protein α1 subunit and β1 subunit are localized at the Z-disc, and form CapZ13, 16. CapZ binds to α-actinin at the Z-disc, and anchors the actin thin filament to the Z-disc17. Inhibition of CapZ function using a CapZ-specific monoclonal antibody resulted in abnormal actin architecture12, while expression of CapZ mutants caused abnormal myofibrillar assembly12, suggesting that CapZ plays important roles in maintaining both actin and Z-disc structures. In yeast and dictyostelium, null mutants or underexpressors of capping proteins have aberrant actin structure and cell morphology, but these mutations are not lethal18–20. In contrast, capping protein has an essential function in higher organisms, as evidenced by Drosophila with mutations in capping protein β showing a lethal phenotype during the early larval stage21. The two isoforms of the CapZβ subunit have distinctive functions with CapZβ1 distributing to the Z disk and CapZβ2 preferentially localizing to the intercalated disk. Mice expressing the CapZβ2 isoform under the control of the α-MyHC promoter, in which the physiological function of CapZβ1 was inhibited by overexpressed CapZβ2, died with severe myofibril disruption between 7 and 26 days after birth22. These mice exhibited stunted growth, an irregular gait, and labored breathing for several days before death22.
In this study, we report that BAG3 is crucial for the maintenance of myofibrillar integrity under mechanical stress. In the absence of BAG3, myofibril structure was disrupted. We demonstrate that BAG3 regulates myofibril stability by facilitating the association of Hsc70 with CapZβ1, which resulted in the tight association of CapZ with F-actin. The absence of BAG3 in both bag3 knockdown cardiomyocytes and bag3-deficient mice rendered CapZ vulnerable to degradation. Inhibition of CapZβ1 function by overexpressing CapZβ2 increased Z-disc vulnerability and fragmentation of myofibril structure under mechanical stress. On the other hand, overexpression of CapZβ1 inhibits myofibrillar disruption in bag3 knockdown cells under mechanical stress. As a result, heart muscle isolated from bag3−/− mice exhibited myofibrillar degeneration, and lost contractile activity after caffeine contraction.
Materials and Methods
Ex-vivo Contracture and Electron Microscopy of Papillary Muscle
Small cardiac muscle strips (0.3–0.4 mm in diameter and 2 mm in length) were dissected from papillary muscle of bag3−/−or bag3+/− left ventricles as previously described in detail23. The strips were tied with a silk monofilament between a force transducer (AE801, SensoNor, Horten Norway) and an extension of a micromanipulator to adjust the muscle length, such that the bundles produced a maximum force. The strips were mounted in a well on a temperature-controlled multiple-well plate to allow for rapid solution changes. The force transducer output was digitized using PowerLab/8SP (ADInstruments, Colorado Springs, CO). The temperature was maintained at 30°C with circulating water.
To generate a large contracture in cardiac muscle, 20 mmol/L caffeine was used under Na-free, depolarizing conditions, where 150 mmol/L NaCl in the external solution was replaced with equimolar K-methanesulfonate24. The strips were repeatedly stimulated with caffeine for 5 minutes at intervals of 15 minutes in the Na-free solution for a total of six cycles. After caffeine was removed and the force was returned to resting levels, the strips were prefixed with 4% paraformaldehyde for 20 minutes at 30°C followed by fixation in 2% paraformaldehyde/2% glutaraldehyde in PBS for 2 hours, and post-fixed in osmium tetroxide for 2 hours, and embedded in araldite epoxy resin. Strips without caffeine contracture were also fixed for further electron microscopic analysis. Semi-thin sections (1μm) were stained with toluidine blue for light microscopic evaluation prior to sectioning for ultrastructural evaluation. Ultrathin sections (60–90nm) were stained with uranyl acetate and lead citrate and examined using a Siemens Elmskop 101 electron microscope at 80 kV.
Equibiaxial strain system
Cardiomyocytes were plated onto a silicon sheeting (Specialty Manufacturing, Saginaw, MI) coated with 0.5μg/ml fibronectin (SIGMA) at a density of 2×105 cells/cm2. Cells were infected with adenovirus encodingβ-galactosidase or the indicated genes, and incubated for 24 hours. Cells were incubated for an additional 24 hours without serum, which halts autonomous contraction. After 24 hours of incubation in serum-free medium, mechanical stress corresponding to 20% stretch was applied to cells using an equibiaxial strain system described by Lee et al. 25.
Results
Mechanical stress induces myofibril destabilization
Mice with a homozygous disruption of the bag3 gene develop a myofibrillar myopathy characterized by non-inflammatory myofibrillar degeneration3. In bag3−/− mice, differences in the extent of muscle degeneration among muscle groups suggest that muscles experiencing the most usage degenerate at an accelerated rate3. Usage-dependent muscle degeneration suggests a role for BAG3 in supporting cytoskeletal connections between the Z-disc and myofibrils under mechanical stress. To address the question of whether bag3 gene knockdown induces myofibrillar disorganization caused by mechanical stress, in vitro experiments using rat neonatal cardiomyocytes and an shRNA-mediated gene knockdown system of the bag3 gene were performed. Cardiomyocytes were cultured on a fibronectin-coated elastic sheet (silicon sheet) and a homogenous continuous equibiaxial stretch was applied to cardiomyocytes that had been infected with adenovirus containing bag3 shRNA 48 hours prior to beginning the experiment (Figure 1A). After 2 hours of static stretch, both actin and α-actinin staining indicated disruption of myofibril structure in bag3 knockdown cardiomyocytes (Figure 1B). In the absence of static stretch, the staining pattern of both actin and α-actinin showed no clear differences between control and bag3 shRNA-treated cells (Figure 1B). Quantitative analysis of myofibril length also showed that the length of myofibril in bag3 knockdown cells is significantly shorter than that of the control after mechanical stress (Figure 1C). These results indicate that bag3 has crucial roles in maintaining myofibril structures under mechanical stress.
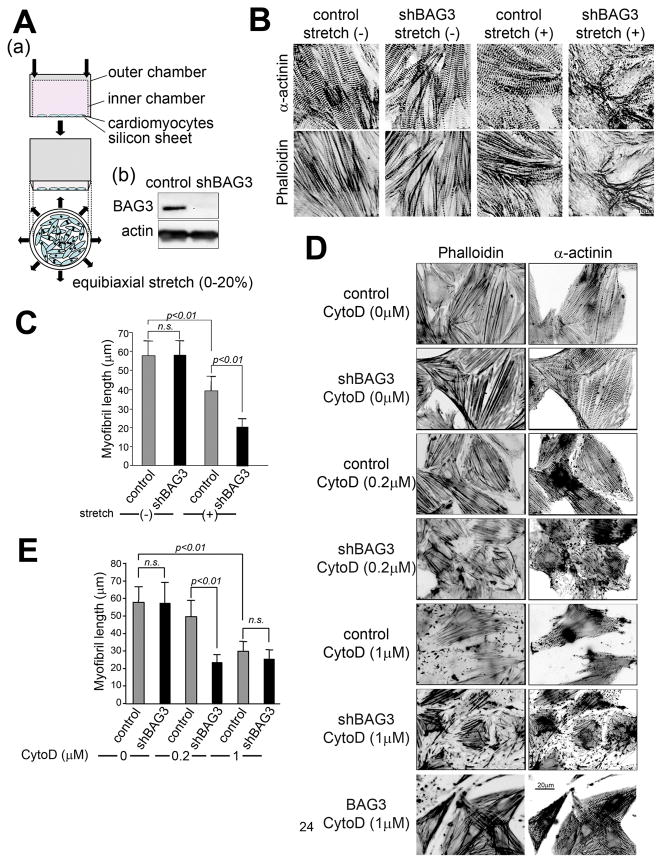
Figure 1. Mechanical stress induces myofibrillar disruption in bag3 shRNA-treated cardiomyocytes.
(A) A schematic view of the equibiaxial stretch model used in these experiments. (a) Isolated cardiomyocytes are cultured on a silicone membrane, which is attached to the bottom of the outer cylinder and a homogeneous equibiaxial strain of 0–20% can be applied by downward insertion of the inner cylinder (purchased from University of California San Diego). (b) shRNA mediated gene knockdown of BAG3. Two days after adenovirus infection, cells were harvested and an immunoblot analysis was carried out using anti-actin and anti-BAG3 antibodies to confirm that bag3 shRNA specifically reduced bag3 expression in cardiomyocytes.
(B) Mechanical stress in bag3 knockdown disrupts myofibril structure in cardiomyocytes. Cardiomyocytes infected with control or bag3 knockdown adenovirus and 20% mechanical stretch applied for 2 hours. Z-disc and F-actin structures were stained using anti-α-actinin antibody (α-actinin: upper panels) and rhodamine-phalloidin (phalloidin: lower panels).
(C) Mechanical stress affects myofibril length. The lengths of myofibril in cardiomyocytes (using at least 10 cells from various spots) were measured using NIH image J and statistically analyzed.
(D) F-actin structure is essential for proper Z-disc structure.
Cardiomyocytes infected with either control, bag3 knockdown adenovirus (shBAG3) or adenovirus carrying Flag-BAG3 (BAG3) were treated for one hour with the indicated concentration of cytochalasin D (CD) to destabilize actin (+) ends. F-actin and Z-discs were then stained with rhodamine-phalloidin (phalloidin: left panels) and anti-α-actinin antibody (α-actinin: right panels), respectively.
(E) Myofibril is vulnerable in bag3 knockdown cardiomyocytes. Myofibril length in cardiomyocytes in the presence of the indicated amount of Cytochalasin D was measured using NIH image J.
In cardiomyocytes with shRNA gene knockdown of bag3, myofibril structure was disrupted after mechanical stress (Figure 1B). Since our recent findings indicated that BAG3 can regulate cell motility by affecting actin organization2, we examined the role of BAG3 in actin organization in an in vitro model of myofibrillar degeneration using cardiomyocytes. To analyze whether BAG3 regulates actin, we used the F-actin destabilizing reagent cytochalasin D. One day following cardiomyocyte infection with bag3 knockdown adenovirus, normal actin and Z-disc structures in cardiomyocytes were maintained. Upon addition of varying amounts of cytochalasin D (0–1 μM), the myofibril structure was significantly disrupted in cardiomyocytes infected with bag3 shRNA adenovirus, but was well-maintained in control cardiomyocytes (Figure 1D). The average myofibril length was calculated and statistically analyzed; the results indicated that the length of myofibril in bag3 knockdown cardiomyocytes was statistically shorter than that of control cardiomyocytes (Figure 1E). On the other hand, bag3 overexpression rendered cells more resistant to cytochalasin D compared to control cardiomyocytes (Figure 1D). Z-disc structures were also affected in bag3 knockdown cardiomyocytes after cytochalasin D exposure, suggesting that proper thin filament structure is necessary to maintain Z-disc structure (Figure 1D). Thus, these data support a role for BAG3 in stabilizing myofibril structure via F-actin, and the absence of BAG3 results in not only myofibril disruption but also Z-disc structure destabilization under mechanical stress.
A novel function for BAG3: promoting the interaction between Hsc70/Hsp70 and CapZ proteins
The next question is how BAG3 maintains myofibril stability upon myofibril disruption caused by mechanical stress or depolymerization reagents. To address this question, an in vitro actin polymerization assay was employed to examine the effect of BAG3 on actin polymerization. Purified recombinant BAG3 protein was mixed with pyrene-conjugated G-actin, in the presence of both ATP and CaCl2 and the fluorescence intensity of polymerized pyrene-conjugated actin was monitored. Neither recombinant BAG3 nor BAG3 purified from mammalian cell lysates either with or without purified Hsp70 affected the polymerization reaction (data not shown), indicating that on their own BAG3 and/or Hsp70 do not regulate actin polymerization in vitro. This result suggests that accessory proteins are likely necessary to mediate BAG3’s effects on actin filament regulation.
Cytochalasin D has been reported to destabilize F-actin by driving F-actin capping protein away from the barbed end26. This actin capping protein, CapZ, is an important molecule for regulating F-actin stability at the barbed end. Although genetic mutation of CapZ has not been reported in any human diseases, CapZ is one of the candidate proteins implicated in myofibrillar myopathy27. Like BAG3, Hsc70 is reported to bind the CapZβ1 subunit, which enhances CapZ’s actin capping activity18, 20. Here, we analyzed whether BAG3 can regulate F-actin stability by way of CapZ.
CapZ exists as a heterodimer of α and β subunits at a 1:1 molar ratio with the β1 subunit isoform preferentially localizing to the Z-disc13. To confirm the co-localization of CapZ proteins and BAG3, adenovirus expression vectors encoding either Myc-tagged CapZα or CapZβ1 were generated and used to infect cardiomyocytes along with adenovirus carrying Flag-tagged BAG3. As determined by immunofluorescence, BAG3 co-localized with CapZ proteins at the Z-disc (Figure 2A). The interaction between CapZ and Hsc70 was confirmed by an immunoprecipitation assay using endogenous or overexpressed protein. For immunoprecipitation, we used cell or tissues having sarcomere structures. CapZ proteins were solubilized from differentiated myotubes, but not from cardiomyocytes or muscle tissues. Myotubes were induced using the mouse myoblast cell line, C2C12, from which cell lysates were prepared and used for immunoprecipitation with anti-BAG3 antibody, followed by 2-dimensional gel electrophoresis. CapZβ were detected with anti-pan CapZβ antibody, which recognizes both CapZβ1 and CapZβ2. As shown in Figure 2B, at least four spots were detected by CapZβ antibody in cell lysates from differentiating myotubes. The isoform-specific antibodies (a gift from Dr. J.A. Cooper, Washington University, St. Louis) were also used for western blot, which indicated that the left three spots were CapZβ1 and the right spot was CapZβ2 (not shown). Similar 2-dimensional electrophoresis patterns were reported by Cooper’s lab previously, but the acidic shifting of CapZβ1 signals in isoelectric focusing has not been biochemically analyzed13. After immunoprecipitation with the anti-BAG3 antibody, isolated immunocomplexes revealed three different positive spots of CapZβ proteins, CapZβ1 (Figure 2B, left two spots) and CapZβ2 (Figure 2B, right spot). The intensity of each spot might indicate a different affinity of β1 and β2 isoforms for BAG3. Interestingly, the right spot of CapZβ1 had better recovery in BAG3 immunocomplexes, suggesting that post-translational modifications of CapZβ1 may potentially influence its interaction with the BAG3/Hsc70 complex. In the same immunocomplex, Hsc70 and CapZα were detected, with the absence of desmin in these complexes serving as a negative control. CapZα antibody detected two major spots in cell lysates and only one spot at the basic position recovered in immunocomplex with BAG3 (Figure 2B, right spot). According to previous reports by Dr. Cooper’s lab, the CapZα isoforms, α1 andα2, corresponded to left and right spots, respectively, at the same molecular weight on a 2-dimensional gel. Thus, we expected that the immunoprecipitated CapZα was the α2 isoform, however, since we only have antibodies to detect both CapZα1 and α2, the isoform identity of these two spots could not be confirmed in this experiment. Next, we confirmed the association among BAG3, Hsc70, and CapZ proteins using an overexpression system. Myc-tagged CapZα and CapZβ1 were expressed in HEK293 cells with or without Flag-tagged BAG3 or Flag-tagged Hsc70, and the cell lysates were incubated with anti-Flag antibody. This assay also indicated that CapZβ bound to Hsc70 as well as BAG3 (Figure 2C). Interestingly, increased expression levels of Myc-tagged BAG3 strongly promoted CapZβ1 binding to Hsc70 (Figure 2C). Although the interaction was weak compared to CapZβ1, CapZα also bound to Hsc70 in the presence of BAG3 (Figure 2C). The role of BAG3 in the association between Hsc70 and CapZ proteins was also confirmed in vitro. Both CapZα and CapZβ1 were co-expressed in bacteria, and purified as GST fusion proteins using GSH sepharose beads. Hsp70 purified from bovine brain bound to GST-CapZ proteins but not GST itself (Figure 2D). When the assay was performed in the presence of BAG3 purified from insect cells, more Hsp70 associated with CapZ proteins, confirming the immunoprecipitation assay results (Figure 2D), and suggesting that Hsp70 binds CapZ proteins directly, and that BAG3 enhances this interaction without involving other components.
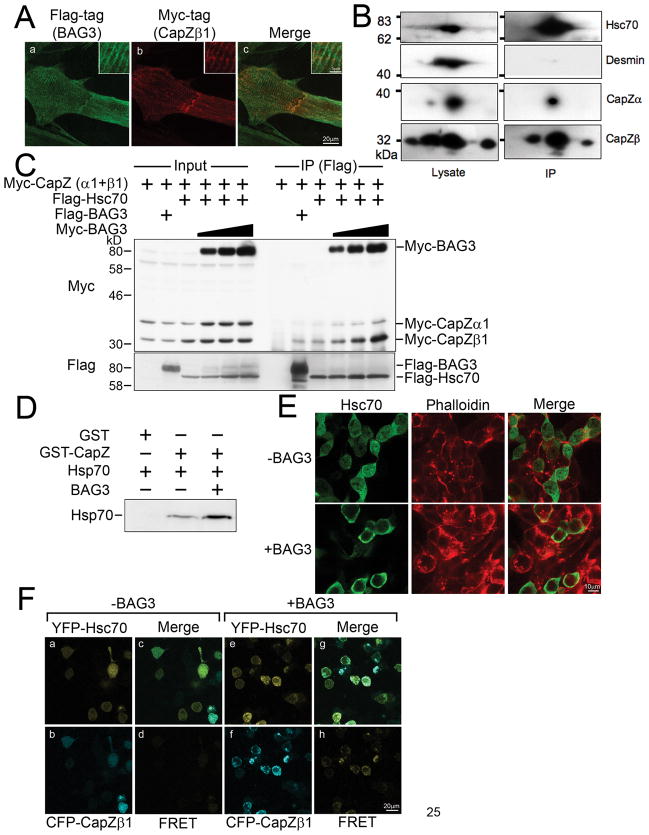
Figure 2. BAG3 increases CapZβ1-Hsc70/Hsp70 association.
(A) BAG3 localizes with CapZ protein. Cardiomyocytes were infected with adenovirus to express both Flag-tagged BAG3 and Myc-tagged CapZβ. Two days after infection, the cells were fixed, and stained with anti-Flag antibody (panel a, green) and anti-Myc antibody (panel b, red). The merged image is shown in panel c.
(B) BAG3 interacts with CapZβ in differentiating myotubes in vivo. C2C12 mouse myoblast cells were cultured in differentiation medium for 6 days and immunocomplexes were isolated by BAG3 antibody, followed by 2D electrophoresis and immunodetection. CapZβ1, β2, α, Hsc70 and desmin were detected by anti-CapZβ, anti-CapZα, anti-Hsc70 and anti-desmin antibody.
(C) BAG3 promotes the interaction between CapZ proteins and Hsc70 in mammalian cells. HEK293 cells were transfected with the indicated plasmids, and cell extracts were subjected to an immunoprecipitation assay using anti-Flag antibody. Myc-tagged CapZ proteins and Myc-tagged BAG3 were detected with anti-Myc antibody (upper panel). Precipitation of Flag-tagged BAG3 or Hsc70 was confirmed with anti-Flag antibody (bottom panel).
(D) CapZ and Hsp70 associate in vitro. Purified recombinant GST-fusion CapZα1 and CapZβ1 were mixed with purified Hsp70 with or without purified BAG3, and GST-fusion proteins were precipitated with GSH sepharose. Precipitated Hsp70 was confirmed by immunoblot assay using anti-Hsp70 antibody.
(E) BAG3 affects Hsc70 intracellular localization. Myc-tagged Hsc70 was expressed in HEK293 cells with or without Flag-tagged BAG3. The localization of Hsc70 was confirmed using anti-Myc antibody (green). F-actin was also stained using Rhodamine-Phalloidin (red).
(F) BAG3 can facilitate the interaction between Hsc70 and CapZ in vivo. For the FRET assay, CFP-CapZβ1 and YFP-Hsc70 were co-transfected into HEK293 cells with (+BAG3, panels e-h) or without (−BAG3, panels a–d) Myc-tagged BAG3. For FRET measurement, excitation at 458 nm and emission at 565–595 nm were used (d and h). Merged images are shown in panels c and g.
Since BAG3 interacts with Hsc70 and promotes the interaction between Hsc70 and CapZ proteins, we were prompted to determine the intracellular localization of Hsc70 when BAG3 is overexpressed. Flag-tagged Hsc70 was expressed in HEK293 cells, and an immunostaining assay was performed with an anti-Flag antibody. Hsc70 expressed alone had a diffuse cytoplasmic localization, but BAG3 overexpression directed Hsc70 to the juxtamembrane region where CapZ proteins and F-actin are enriched (Figure 2E).
We also verified the function of BAG3 using a FRET assay. CFP-conjugated CapZβ was expressed with YFP-conjugated Hsc70 in HEK293 cells. After 24 hours of culture, the cells were visualized by confocal microscopy using emission and excitation wavelengths as described in the Methods. Only very weak signals from these two molecules were detected by FRET, but when BAG3 was also expressed, the fluorescence signal was strongly enhanced (Figure 2F), indicating that BAG3 promotes a tight interaction between CapZ and Hsc70 in vivo.
BAG3 enhances the proper localization and stabilization of CapZ proteins
To understand the significance of the association between CapZ and Hsc70 in the presence of BAG3, Myc-tagged CapZβ1 was expressed in cardiomyocytes with or without Flag-tagged BAG3, and an immunostaining assay was performed to detect differences in CapZβ1 localization. Overexpression of CapZβ1 alone produced a diffuse cytosolic localization, but upon BAG3 co-overexpression, CapZβ1 translocated to the Z-disc (Figure 3A). To investigate the mechanism of CapZβ1 localization, we used cellular fractionation to separate lysates of rat neonatal cardiomyocytes infected with CapZβ1 adenovirus with or without adenovirus encoding flag-tagged bag3 into two fractions, the G-actin and F-actin fraction, followed by analysis of CapZ distribution. CapZα was also expressed together with CapZβ1 to determine the localization and stability of this subunit, since both isoforms are reported to be necessary for physiological dimerization at the Z-disc and overexpression of CapZα1 or β1 protein subunits singly could destabilize both isoforms28.
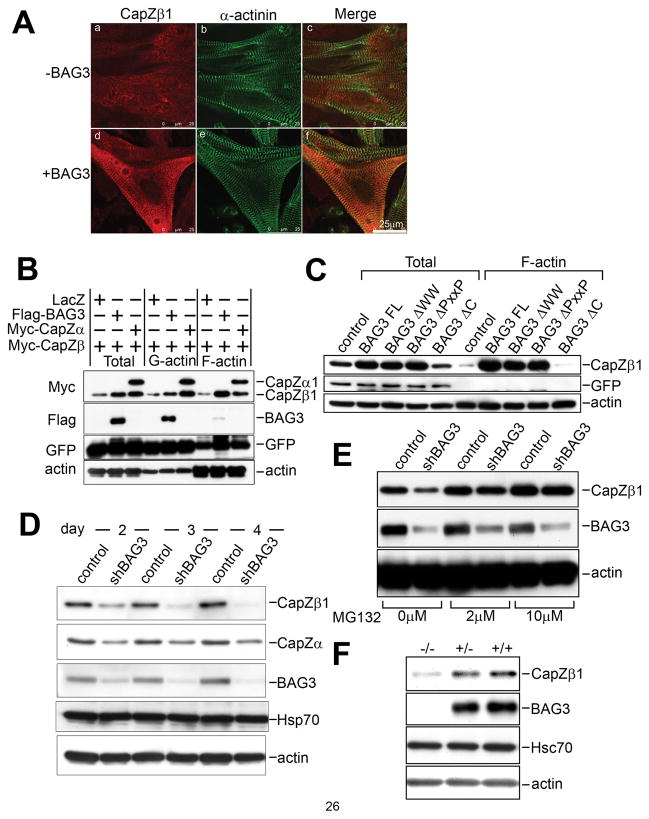
Figure 3. BAG3 is crucial for proper localization and expression of CapZ proteins.
(A) BAG3 promotes CapZ localization to Z-discs. Myc-tagged CapZβ1 adenovirus was infected to cardiomyocytes with LacZ (−BAG3, a, b and c) or Flag- tagged BAG3 (+BAG3, d, e and f) adenovirus. After 2 days of infection, the cells were fixed, and stained. CapZβ1 was detected using anti-Myc antibody (panels a and d, red). Z-disc was stained using anti-sarcomeric alpha actinin antibody (panels b and e, green). The merged images are shown in panels c and f.
(B) BAG3 promotes both CapZβ1 expression and translocation to the F-actin fraction. Cardiomyocytes were co-infected with Myc-tagged CapZβ1 and either LacZ (control), Flag-tagged BAG3 (BAG3) or Myc-tagged CapZα (CapZα) with GFP adenovirus. G-actin and F-actin fractions were applied to an immunoblot assay using anti-Myc antibody (top panel), anti-Flag antibody (middle panel) or anti-actin antibody (bottom panel) and anti-GFP antibody (third panel) for confirming same infection efficiency.
(C) The BAG domain is required for BAG3 action towards CapZ. HEK293 cells were transfected with empty vector (mock), BAG3 full length (FL), WW domain deletion mutant (ΔWW), PxxP motif deletion mutant (ΔPxxP) or BAG domain deletion mutant (ΔC), and fractionated into F-actin or G-actin fractions. Myc-tagged CapZβ1 was detected with anti-Myc antibody (CapZβ1). Anti-actin and anti-GFP antibodies were used for a loading and infection efficiency control.
(D) CapZβ1 is destabilized in bag3 knockdown cells. After 2, 3 and 4 days of infection of bag3 shRNA adenovirus or control LacZ virus, cardiomyocytes were harvested for immunoanalysis of endogenous CapZβ1 and CapZα1. A BAG3-specific antibody was used to confirm the efficient knockdown of BAG3 protein expression. Immunoblots of Hsp70 and actin are shown as loading controls.
(E) CapZβ1 destabilization is inhibited by addition of proteasome inhibitor MG132. shRNA bag3 adenovirus infected cells were incubated with 2μM or 10μM MG132 at 24 hours after infection. After an additional 24 hours, the cells were harvested for immunoblot assay using anti CapZβ1 antibody (top panel), anti-BAG3 antibody (second panel) and anti-actin antibody (bottom panel).
(F) CapZβ1 is destabilized in bag3−/− mice. Hearts were extracted from 14 day old wild type (+/+), heterogenous (+/−) or bag3−/− (−/−) mice, homogenized, and applied to immunoblot assay using anti-CapZβ1, Hsc70/Hsp70, BAG3, or actin antibodies.
As shown in the western blot of the total lysate, co-expression of CapZα increased the intensity of CapZβ1 signals in the lysate (Figure 3B). Similarly, when BAG3 was overexpressed with CapZβ1 in cardiomyocytes, immunodetection of CapZβ1 indicated high intensity of signals on a western blot of the protein compared to lysates from cells expressing only CapZβ1, suggesting that BAG3 as well as CapZα increased CapZβ1 expression (Figure 3B).
When CapZα1 is co-expressed with CapZβ1, both proteins are detected in the G-actin and F-actin fractions, however with overexpression of BAG3, the majority of stabilized CapZβ1 localized to the F-actin filament (Figure 3B).
To understand the importance of the interaction of BAG3 with Hsc70 for the stability and translocalization of CapZ proteins, we expressed various BAG3 mutants in HEK293 cells with CapZβ1, and performed a fractionation assay. The BAG3 protein possesses three known protein interaction motifs: the WW domain, which interacts with the PPXY sequence found in various subcellular compartments; the PxxP motif, a candidate interaction site for SH3 domain proteins; and the BAG domain, which interacts with Hsp70/Hsc70. While wild-type BAG3, ΔWW, and ΔPxxP each promoted CapZβ1 translocation to the juxtamembrane region, the BAG mutant, which does not bind to Hsc70, failed to do so (Figure 3C). BAG also did not stabilize CapZβ1 in this experiment, suggesting that the interaction between BAG3 and Hsc70 is crucial for both CapZ stability and localization. Hsc70 itself has a weak effect on the localization and stability of CapZβ1 but this effect is enhanced synergistically in the presence of BAG3 (Online Figure I). To understand the importance of BAG3 in this role, bag3 knockdown 293 cells were transfected with a plasmid expressing Hsc70. As expected, increased destabilization of CapZβ1 was observed in bag3 knockdown cells, and importantly, the presence of Hsc70 had little or no effect on CapZβ1 stability in these cells (Online Figure II). Taken together, BAG3 enhances the interaction between Hsc70 and CapZ proteins (Figure 2B and 2B), and modulates the interaction between Hsc70 and CapZ proteins to ensure the proper expression and localization of CapZ proteins.
Using anti-CapZα1 and β1 specific antibodies, we performed additional experiments to investigate how BAG3 might stabilize CapZ protein. After 2, 3, and 4 days of shRNA bag3 adenovirus infection, cardiomyocytes were harvested and cell lysates were subjected to SDS-PAGE, followed by western blot analysis. Immunoblot analysis showed that endogenous CapZβ1 levels were decreased when bag3 gene expression was depleted (Figure 3D, upper panel). Interestingly, CapZα1 protein expression was also detected at lower levels compared to control infected cells. The decreased expression of CapZβ1 observed in bag3 knockdown cardiomyocytes likely does not occur at the RNA level since RT-PCR showed no difference in RNA levels between control and bag3 knockdown cardiomyocytes (Online Figure III). From the above results, we may conclude that BAG3 is important for the proper localization and protein stability of CapZβ1.
BAG family proteins are co-chaperones with Hsp70/Hsc70 family molecular chaperones and their activity has been reported to be critical for protein folding or degradation pathways29, 30. To investigate the regulation of CapZβ1 protein stability by ubiquitin proteasome degradation, we used the proteasome inhibitor MG132. With increasing MG132 doses, CapZβ1 signals recovered even at low levels of BAG3 (Figure 3E). Thus, CapZ proteins appear to be destabilized and degraded via the proteasome in the absence of BAG3 assistance. Finally, the reduction of CapZβ1 was also verified in protein lysates from hearts isolated from bag3−/− mice at the age of 14 days (Figure 3F and Online Figure IV). Taken together, these results indicate that BAG3 promotes the interaction between Hsc70 and CapZ proteins, which is crucial for the proper localization and expression of CapZ proteins, and the absence of BAG3 leads to inappropriate localization and proteasomal degradation of CapZ proteins.
BAG3 prevents mechanical stress-induced myofibrillar disorganization through CapZβ1 function
The previous experiments determined that BAG3 has a critical function in maintaining CapZβ1 localization and stability. Under mechanical stress, myofibril structure is disorganized in bag3 knockdown cardiomyocytes, which is followed by disruption of Z-disc structure. To address whether CapZ proteins play a pivotal role in these abnormalities in bag3 knockdown cardiomyocytes, two strategies were taken: inhibition of CapZβ1 function and overexpression of CapZβ1 in cardiomyocytes.
First, we studied whether inhibition of CapZβ1 function increased the vulnerability of Z-discs in cardiomyocytes. Using adenoviral short hairpin shRNA expression vectors to reduce CapZβ1 expression in rat neonatal cardiomyocytes, we observed moderate myofibril and Z-disc disruption after 20% static stretch for 2 hours. This result occurred despite a reduction in CapZβ1 expression of 40% compared to control vector-infected cardiomyocytes (Online Figure V). The moderate effect of CapZβ1 shRNA expression was likely due to the presence of multiple CapZ isoforms. Although CapZβ1 and β2 are translated from alternatively spliced forms of RNA they differ only in their C-terminal sequence and have similar affinity for F-actin. The two isoforms have distinct distributions and physiological functions, and cannot be replaced by each other22. Indeed, CapZβ2 overexpressing transgenic mice reportedly have severely disrupted Z-discs in vivo, suggesting inhibition of CapZβ1 function by CapZβ2 overexpression22. Thus, we constructed Myc-tagged CapZβ2 adenovirus expression vectors to overexpress this protein in cardiomyocytes to determine the effect of inhibiting CapZβ1 function in cardiomyocytes. As shown in Figure 4A, protein expression was confirmed by western blot analysis. Interestingly, the CapZβ1 signal was reduced when CapZβ2 was overexpressed in cardiomyocytes, possibly because CapZα was occupied by overexpressed CapZβ2 and CapZβ1 was destabilized28. Such reductions in CapZβ1 were also observed in transgenic mice overexpressing CapZβ222. Next, cardiomyocytes were stained with anti-Myc antibody, which revealed a diffuse or granular distribution of Myc-CapZβ2 (Figure 4B). Cardiomyocytes were cultured on a stretchable silicone sheet, infected with Myc-CapZβ2 or LacZ adenovirus, followed by mechanical stretch for 2 hours and the cytoskeletal structure examined. CapZβ2 overexpressing cardiomyocytes showed myofibril structure fragmentation after mechanical stretch, and a similar pattern was seen in bag3 knockdown cells (Figure 4C). The expression of CapZβ1 and β2 in CapZβ1 knockdown or CapZβ2 overexpressing cardiomyocytes is shown in Online Figure VI. Interestingly, induction of BAG3 expression was observed in CapZβ1 knockdown and CapZβ2 overexpressing cardiomyocytes, probably due to a compensatory mechanism against low CapZβ1 expression.
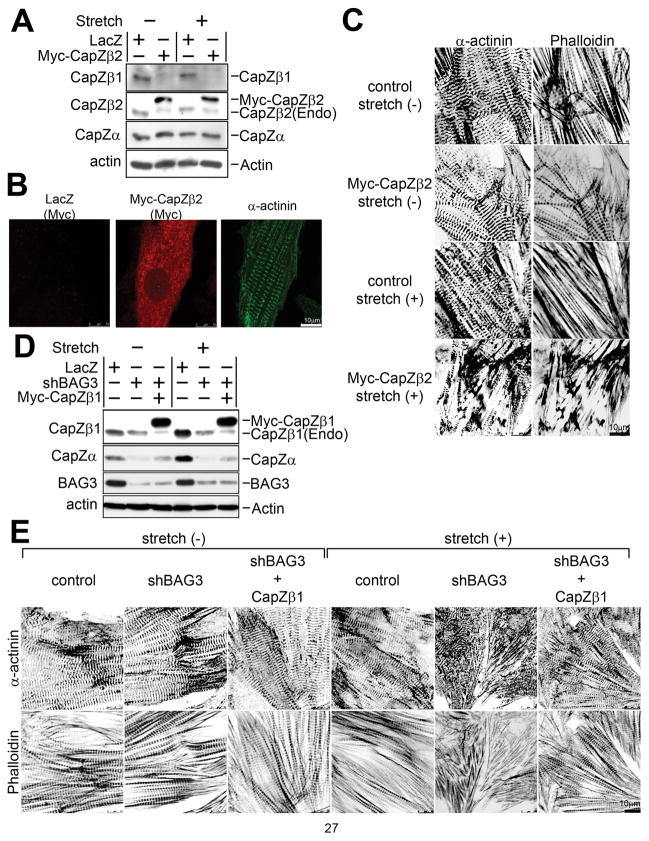
Figure 4. CapZβ1 plays pivotal roles in myofibrillar and Z-disc degeneration caused by mechanical stress.
(A) Expression of CapZ proteins in cardiomyocytes with overexpression of Myc-CapZβ2. Myc-tagged CapZβ2 and LacZ control adenovirus was infected into cardiomyocytes. Expression of CapZβ1, β2 and α was confirmed by antibody against each protein. Lysates were prepared from cardiomyocytes with or without stretch.
(B) Distribution of overexpressed CapZβ2 in cardiomyocytes. After infection of Myc-CapZβ2 and LacZ control virus, fixed cells were incubated with anti-Myc antibody to detect localization of myc-CapZβ2 (Myc: left and middle panels), indicating granular staining of overexpressed CapZβ2. For visualization of Z-discs, sarcomeric α-actinin antibody was used for double staining on the same sample (α-actinin: right panel).
(C) Overexpression of CapZβ2 results in myofibrillar and Z-disc degeneration under mechanical stress. Cardiomyocytes were infected with adenovirus LacZ or Myc-tagged CapZβ (Myc-CapZβ2), followed by 20% mechanical stress for 2 hours and stained with anti-α-actinin (α-actinin: left panels) and Rhodamine-Phalloidin (Phalloidin: right panels).
(D) Western blot analysis of cardiomyocytes with CapZβ1 overexpression. Cardiomyocytes were infected with adenovirus for control (LacZ) or bag3 knockdown (shBAG3) with or without Myc-tagged CapZβ1. The expression of CapZ and BAG3 were confirmed with anti-CapZβ1 antibody, anti-CapZα1 antibody or anti-BAG3 antibody, respectively. The same membrane was also blotted with anti-actin antibody. (Endogenous CapZβ1 was decreased after the expression of Myc-CapZβ1 probably due to compensation to maintain total expression levels.)
(E) Overexpression of CapZβ1 protein attenuates disruption of myofibrillar structure in bag3 knockdown cardiomyocytes exposed to mechanical stress. Myc-tagged CapZβ1 adenovirus was infected to cardiomyocytes with bag3 knockdown adenovirus, and mechanical stress (20%) was applied for 2 hours. Cells were fixed and stained with anti-α-actinin antibody (α-actinin: upper panels) and rhodamine-phalloidin (Phalloidin: lower panels).
Next, CapZβ1 adenovirus was infected with or without bag3 knockdown and we examined whether cytoskeletal disruption was reversed. Cardiomyocytes were cultured on a stretchable silicone sheet, infected with bag3 shRNA adenovirus with or without CapZβ1 adenovirus and mechanical stress was applied, followed by observation of changes in myofibril and Z-disc structures. The expression level of CapZβ1 was confirmed by western blot (Figure 4D). After 2 hours of mechanical stress, myofibril structure was severely disrupted in bag3 knockdown cardiomyocytes, but overexpression of CapZβ1 in the bag3 knockdown cardiomyocytes attenuated the disorganization of myofibril structure (Figure 4E). The statistical analysis of myofibril length also supported the attenuation of cytoskeletal disorganization in bag3 knockdown cardiomyocytes provided that CapZβ1 was expressed (Online Figure VII).
These results show that BAG3 and CapZβ1 cooperatively stabilize the Z-disc following mechanical stress. These experimental results suggest that myofibrillar myopathy in bag3−/− mice is likely caused by altered Z-disc structure due to dysfunction of BAG3 in myofibril stabilization through CapZβ1.
Mechanical stress causes degeneration of myofibrillar components leading to muscle contraction failure
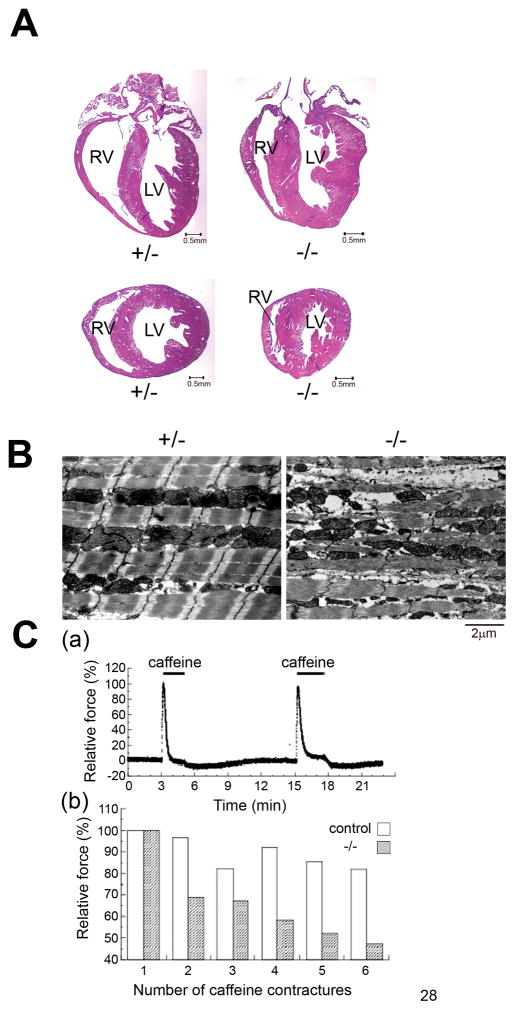
Myofibrillar myopathy is characterized by myofibrillar and Z-disc disruption in striated muscle. Cardiomyopathy is one of the symptoms observed in this disease, although the pathological features of this cardiomyopathy have not been well-characterized. We showed that mechanical stress causes degeneration of myofibrillar structures in bag3 knockdown cardiomyocytes (Figure 1B). Using heart tissue from bag3−/− mice, the ultrastructure of papillary muscles from hearts of bag3−/− and control mice at 12 day after birth was analyzed. Ultrastructural changes were observed with Z-disc widening or smearing as well as irregularities in the I-band in bag3−/− papillary muscle (Online Figure VIII). Gross pathological examination indicated that hypertrophic cardiomyopathy was present in hearts from bag3−/− mice (Figure 5A). Interestingly, patients with the P209L bag3 mutation also showed hypertrophic cardiomyopathy4. To test whether mechanical stress directly influences the degeneration of myofibrillar structure and muscle contractile activity, ex vivo contracture experiments were performed using caffeine, with active muscle force monitored by a force transducer. Small strips of papillary muscle were connected to the force transducer and the relevant solution applied. After six 5 minute exposures to caffeine (20mM) conducted at 15 minute intervals, Z-disc destruction and myofibrillar degeneration were observed similarly to the ultrastructural changes present in bag3−/− mouse skeletal muscle3 or in bag3 knockdown cardiomyocytes (Figure 1B, Figure 5B). As expected, a rapid reduction of both active and passive tension was observed in bag3−/−specimens (~50%), while the control tissue displayed only a 10–20% reduction (Figure 5C).
Figure 5. Mechanical stress causes myofibrillar degeneration and contraction failure.
(A) Hematoxylin and eosin staining detects asymmetric hypertrophy in bag3−/− heart. The left two panels show a low magnification section of control (bag3+/−) heart and the right two panels depict bag3−/− heart. The sagittal and transverse sections are displayed in the upper and lower panels, respectively (bar indicates 0.5mm).
(B) Myofibrillar degeneration is induced by ex vivo contracture experiment. Papillary muscle fibers (~0.4 mm in width and 2 mm in length) were dissected from left ventricles of wild-type, heterozygous or bag3−/−mice. One end of the fibers was tied with monofilament silk to a force transducer (AE801, SensoNor, Horton Norway) and the other to a micromanipulator to adjust the muscle length. The fibers were soaked in the high (124mM) K+ and low (30mM) Na+ solution to facilitate a large caffeine-induced contracture in cardiac muscle. After the sixth contracture, the papillary muscle fibers were removed, and the myofibrillar ultrastructure was examined. Myofibrillar structure and Z-disc was disrupted in bag3−/− papillary muscle after contracture (right), but not in control papillary muscle (Left).
(C) Time course of repeated caffeine contractures in control cardiac muscle fibers. (a) 20mM caffeine was repeatedly applied for 3 min in the high K+ solution at each 12 min interval. Panel (b) shows a rapid deterioration of the repeated caffeine-induced contractures in bag3−/− cardiac muscle compared to control (bag3−/− n=4, control n=4). At the sixth contracture, the amplitude was less than 50% of the original in the bag3−/−muscle while control muscle maintained more than 80% amplitude.
Discussion
In this study we propose a novel function for the BAG family protein BAG3 in stabilizing CapZ and preventing myofibrillar degeneration under mechanical stress. Myofibrillar myopathy is characterized by myofibrillar disruption and Z-disc destruction, sometimes accompanied by protein aggregation and ectopic accumulation of myofibrillar proteins. The pathogenesis of myofibrillar myopathy is believed to be induced by abnormal function of proteins on or connecting to the Z-disc, but the precise mechanism by which this occurs or whether protein aggregation is involved remains unclear. In this report, we showed that mechanical stress induces myofibrillar and Z-disc disruption in bag3 knockdown cardiomyocytes or papillary muscle from bag3−/− mice. We also demonstrated that the Z-disc protein CapZβ1 directly interacts with Hsc70 and this interaction was enhanced by the presence of BAG3. Without BAG3, CapZβ1 fails to localize to the Z-disc and is degraded by the proteasome pathway.
BAG3-mediated CapZ stabilization and localization
The BAG family consists of six members, each possessing a BAG domain, which is thought to be a catalytic domain for ATP exchange in the cytosolic 70kDa molecular chaperone (Hsc70/Hsp70)29. As such, BAG family proteins may act as functional homologues of the bacterial chaperone GrpE31, 32, despite the absence of structural homology between the two proteins. What the exact molecular function of BAG domain proteins might be and why mammals need six different BAG proteins remains an interesting question. BAG family proteins have a conserved Hsc70-interacting domain at their C-terminus, but upstream their sequences diverge and a distinct function for each member has been reported from gene knockout studies3, 33, 34. In this report, we found that endogenous BAG3 and Hsc70 formed a complex with CapZ proteins in vivo (Figure 2B) and BAG3 strongly enhances the binding of Hsc70 to CapZ proteins without requiring additional components (Figure 2C and 2D), suggesting that at minimum BAG3 functions by promoting the binding of Hsc70/Hsp70 to a specific substrate, which is in contrast to the previously proposed BAG mechanism of facilitating Hsc70/Hsp70 substrate release. BAG3 also affects the intracellular localization of Hsc70, probably due its enhancement of the Hsc70/Hsp70-CapZ interaction (Figure 2E and 2F). The thinking that each BAG protein may have a specific substrate is strengthened by the finding that BAG3 is the only BAG family member that promotes the Hsc70 and CapZ interaction (Online Figure IX) and regulates CapZβ1 localization to F-actin (Online Figure X). Importantly, BAG3 itself does not bind to CapZβ1 directly (Online Figure XI) but enhances the interaction between CapZβ1 and Hsc70 (Figure 2). To our knowledge, this is the first demonstration of a BAG protein acting as a co-chaperone protein to link Hsc70/Hsp70 to its substrate specifically.
BAG3 increases the Z-disc distribution of CapZ, and failure to achieve this localization leads to CapZ degradation through the 26S proteasome (Figure 3). Little or no functional CapZβ1 rapidly leads to destabilization of myofibrillar structures in muscle (Figure 4) 12, 22, which in turn can promote a degenerative disease state35. Finally, overexpression of CapZ protein in bag3 knockdown cardiomyocytes rendered cells resistant to mechanical stress-induced disruption of myofibril structure (Figure 4E). This evidence indicates that low level expression and mislocalization of CapZ is responsible for the myopathy seen in bag3−/− mice.
Since interaction with proteins having ubiquitin ligase activity appears to be a general feature of BAG family proteins36–40, a future direction of study will be to investigate whether BAG3 can also modulate ubiquitin ligase function, especially given our finding that CapZβ1 can be a substrate of the proteasomal degradation pathway and this degradation can be inhibited by BAG3.
In cultured myotubes, BAG3 and Hsc70 form complexes with CapZ proteins, CapZβ1, β2 and CapZα (Figure 2B). In this report, we focused on the regulation of CapZβ1 function by BAG3 and Hsc70 at the Z-disc, owing to the abnormal Z-disc structure found in bag3 gene knockout mice and knockdown cardiomyocytes. But since CapZβ2 may also participate in this complex, it is possible that BAG3/Hsc70 may have a general function to regulate the CapZβ complex at the barbed end of F-actin. It will be of interest to investigate how BAG3 and Hsc70 regulate CapZβ function in non-muscle cells. Indeed, we reported that BAG3 regulates cell motility and metastasis in cancer cells through actin organization2. Further investigation will be necessary to elucidate the possibility of the regulation of CapZβ function by BAG3/Hsc70 in non-muscle cells.
Guardian for mechanical stress
The muscle sarcomere has important roles in mechanotransduction and mutations in genes expressing costamere proteins can induce various muscular dystrophies and myopathies, as well as heart failure41. LIM domain proteins, MLP and Cypher (ZASP), associate with α-actinin and have an important role in signaling of mechanical stress. Interestingly, the titin cap protein (t-cap/telethonin) interacts with MLP. Mutations in T-cap result in protein instability, which can cause dilated cardiomyopathy42. In addition, mechanical stress induces degradation of titin capping protein, which also induces Z-disc disruption. Genetic mutations of Cypher have been found in human myofibrillar myopathy patients, so it would be of interest to determine whether signal transduction of mechanical stress and Z-disc disruption require common pathways that involve BAG3/CapZ and MLP/Cypher/actinin.
Mechanical stress on the Z-disc induces expression of proteins that prevent Z-disc disruption under physiological conditions. Online Figure XII shows the analysis of endogenous CapZβ1 protein expression levels after mechanical stress and demonstrates that CapZβ1 as well as BAG3 is rapidly upregulated after 12 hours of 20% static strain. Recent microarray or proteomics experiments revealed changes in CapZ expression levels under different stress conditions, including skeletal muscle injury model43, smooth muscle under hemodynamic stress 44 and smooth muscle in pulmonary hypertension45. These changes in CapZ levels suggest that BAG3 may have important roles in mechanical stress-induced pathophysiological conditions of striated muscle, such as muscle injury, atrophy, heart failure and cardiomyopathy. In addition, BAG3 expression was reported to be upregulated when eccentric contraction is applied to skeletal muscle, suggesting that bag3 may have a role in repairing skeletal muscle injuries caused by mechanical stress46. A mouse model of congenital non-dystrophic myopathy, nemalin myopathy, also displays high BAG3 expression levels, again suggesting that BAG3 may function in the regenerating process of myopathy47. Thus, it would be important to determine whether CapZβ1 as well as BAG3 is a downstream target of the mechanical stress signaling.
Mechanical Stress on myofibrillar myopathy
Although various gene mutations have been reported to cause myofibrillar myopathy, overall myofibrillar myopathy has many similar clinical and histological features. One of the typical clinical manifestations of myofibrillar myopathy is progressive muscle weakness48. Muscle weakness is observed not only in patients with bag3 mutation but also in bag3-deficient mice3, 4. In most cases, muscle weakness is late-onset, and then progresses but why the disease proceeds this way is unknown. In this article, we raise the possibility that mechanical stress causes myofibrillar degeneration with a disease background, which is followed by muscle weakness (Figure 5B and C). Myofibrillar degeneration progresses after birth upon repeated and/or strong muscle contractions, which accompanies muscle weakness. On the other hand, the cardiac muscle maintains its myofibrillar structure relatively well as compared to other skeletal muscles in bag3−/− mice. We only observed subtle changes in myofibrillar structures between bag3−/− and control hearts (Online Figure VIII). This result may be due to differences of the stretch in terms of the strength and direction between cardiac and skeletal muscle. According to the Frank-Starling law, the heart is working in the ascending limb of sarcomere length-tension curve. Even at 2.0 μm of sarcomere length where the thin and thick filaments are maximally overlapping each other, physiological contraction is markedly enhanced in response to positive inotropic agents such as isoprenaline, suggesting that cardiac muscle fibers are working below 50% of maximum force that they can produce. In contrast, skeletal muscle fibers stimulated by motor nerve impulses always produce equal to or, if not, near 100% of maximum force. In addition, skeletal muscle fibers are often stretched during contraction in keeping the balance of movement while cardiac muscle fibers are stretched at the relaxed state under normal conditions. Furthermore, cardiac muscle fibers are more protected against stretch because of more collagen, microtubules and intermediate filaments. Together, these suggest that the stress generated at the Z-disks during contraction is much higher in skeletal than cardiac muscle fibers. In fact, myofibrillar disruption occurred after the caffeine contraction in cardiac muscle (Figure 5B), followed by contraction failure (Figure 5C). It is noteworthy that myofibrillar myopathy is not always accompanied by cardiomyopathy, and about 1/6 patients with myofibrillar myopathy also suffer from cardiomyopathy27.
BAG3 modulates the substrate of Hsc70/Hsp70 specifically, and the absence of BAG3 causes the mislocalization and destabilization of the Hsc70 substrate CapZβ1, which, with mechanical stress, eventually leads to myofibrillar degeneration, muscle weakness and early death. Further analysis may help us understand the detailed molecular mechanism of myofibrillar myopathy as well as other diseases caused by abnormal Z-disc function. We hope to find translational implications of BAG3 and CapZβ1 association for muscle protection not only in pathological conditions but also in physiological settings, such as myofibrillar remodeling under mechanical stress.
Novelty and Significance.
What is known?
BAG3 regulates protein folding and degradation by directly interacting with cellular chaperones Hsc70/Hsp70.
In BAG3 knockout mice, myofibrillar degeneration occurs in a use-dependent fashion, suggesting that BAG3 can protect myofibril structures from mechanical stress.
The F-actin capping protein CapZ maintains both actin and Z-disc structures and aberrant distribution of CapZ isoforms induces abnormal myofibrillar assembly.
What new information does this article contribute?
BAG3 is essential for maintaining myofibril structure under mechanical stress.
BAG3 and Hsc70 regulate proper localization of CapZ to F-actin at Z-disc.
Without the support of BAG3 and Hsc70, the CapZ protein failed to interact with actin filaments, and was degraded via the ubiquitin-proteasome system.
Summary
BAG3 mutations in human and mouse causes progressive myofibrillar myopathy with severe loss of skeletal and cardiac muscle function, but the molecular mechanism of this degeneration is unclear. Using bag3 gene knockdown, rapid myofibrillar degeneration and Z-disc disruption was observed in cardiomyocytes exposed to mechanical stretch. We found that BAG3 interacts with Hsc70 and increases localization and stabilization of the actin capping protein CapZ at Z-disc F-actin. Without BAG3, CapZ protein is degraded via the ubiquitin proteasome pathway, which results in myofibrillar structure disruption upon exposure to mechanical stress. Our report indicates a novel function for BAG3 and Hsc70 in supporting myofibril structure and that CapZ is a client protein for the Hsc70/BAG3 molecular chaperone complex. This study promotes our understanding of how myofibrillar structure is maintained under excessive muscle contraction, and suggests that CapZ is an important player in myofibrillar myopathy pathogenesis. Further analysis of this system may provide potential therapeutic targets for myofibrillar myopathy as well as other diseases caused by mechanical stress on the Z-disc.
Supplementary Material
Acknowledgments
We thank Drs. Emerson, Homma and Miller for critical discussions.
Sources of Funding
We thank the NIH for their generous support (AR052925).
Non-standard Abbreviations and Acronyms
- BAG
Bcl-2-associated athanogene
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki M, Homma S, Hishiya A, Dolezal SJ, Reed JC, Takayama S. BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res. 2007;67:10252–10259. doi: 10.1158/0008-5472.CAN-07-0618. [DOI] [PubMed] [Google Scholar]
- 3.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169:761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65:83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selcen D. Myofibrillar myopathies. Curr Opin Neurol. 2008;21:585–589. doi: 10.1097/WCO.0b013e32830a752b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 7.Engel AG. Myofibrillar myopathy. Ann Neurol. 1999;46:681–683. doi: 10.1002/1531-8249(199911)46:5<681::aid-ana1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Vicart P, Caron A, Guicheney P, Li Z, Prévost M-C, Faure A, Chateau D, Chapon F, Tomé F, Dupret J-M, Paulin D, Fardeau M. A missense mutation in the aB-crystallin chaperone gene causes a desmin-related myopathy. Nature Gene. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 9.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54:804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 10.Pappas CT, Krieg PA, Gregorio CC. Nebulin regulates actin filament lengths by a stabilization mechanism. J Cell Biol. 2010;189:859–870. doi: 10.1083/jcb.201001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas CT, Bhattacharya N, Cooper JA, Gregorio CC. Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol Biol Cell. 2008;19:1837–1847. doi: 10.1091/mbc.E07-07-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer DA, Hug C, Cooper JA. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol. 1995;128:61–70. doi: 10.1083/jcb.128.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer DA, Korshunova YO, Schroer TA, Cooper JA. Differential localization and sequence analysis of capping protein beta-subunit isoforms of vertebrates. J Cell Biol. 1994;127:453–465. doi: 10.1083/jcb.127.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart MC, Korshunova YO, Cooper JA. Vertebrates have conserved capping protein alpha isoforms with specific expression patterns. Cell Motil Cytoskel. 1997;38:120–132. doi: 10.1002/(SICI)1097-0169(1997)38:2<120::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.von Bulow M, Rackwitz HR, Zimbelmann R, Franke WW. CP beta3, a novel isoform of an actin-binding protein, is a component of the cytoskeletal calyx of the mammalian sperm head. Exp Cell Res. 1997;233:216–224. doi: 10.1006/excr.1997.3564. [DOI] [PubMed] [Google Scholar]
- 16.Casella JF, Craig SW, Maack DJ, Brown AE. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987;105:371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papa I, Astier C, Kwiatek O, Raynaud F, Bonnal C, Lebart MC, Roustan C, Benyamin Y. Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. J Musc Res Cell Motil. 1999;20:187–197. doi: 10.1023/a:1005489319058. [DOI] [PubMed] [Google Scholar]
- 18.Haus U, Trommler P, Fisher PR, Hartmann H, Lottspeich F, Noegel AA, Schleicher M. The heat shock cognate protein from Dictyostelium affects actin polymerization through interaction with the actin-binding protein cap32/34. Embo J. 1993;12:3763–3771. doi: 10.1002/j.1460-2075.1993.tb06054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hug C, Jay PY, Reddy I, McNally JG, Bridgman PC, Elson EL, Cooper JA. Capping protein levels influence actin assembly and cell motility in dictyostelium. Cell. 1995;81:591–600. doi: 10.1016/0092-8674(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 20.Tardieux I, Baines I, Mossakowska M, Ward GE. Actin-binding proteins of invasive malaria parasites and the regulation of actin polymerization by a complex of 32/34-kDa proteins associated with heat shock protein 70kDa. Mol Biochem Parasitol. 1998;93:295–308. doi: 10.1016/s0166-6851(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 21.Hopmann R, Cooper JA, Miller KG. Actin organization, bristle morphology, and viability are affected by actin capping protein mutations in Drosophila. J Cell Biol. 1996;133:1293–1305. doi: 10.1083/jcb.133.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart MC, Cooper JA. Vertebrate isoforms of actin capping protein beta have distinct functions In vivo. J Cell Biol. 1999;147:1287–1298. doi: 10.1083/jcb.147.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitazawa T. Effect of extracellular calcium on contractile activation in guinea-pig ventricular muscle. J Physiol. 1984;355:635–659. doi: 10.1113/jphysiol.1984.sp015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitazawa T. Caffeine contracture in guinea-pig ventricular muscle and the effect of extracellular sodium ions. J Physiol. 1988;402:703–729. doi: 10.1113/jphysiol.1988.sp017230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD. An equibiaxial strain system for cultured cells. Am J Physiol. 1996;271:C1400–1408. doi: 10.1152/ajpcell.1996.271.4.C1400. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selcen D, Ohno K, Engel AG. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain. 2004;127:439–451. doi: 10.1093/brain/awh052. [DOI] [PubMed] [Google Scholar]
- 28.Amatruda JF, Gattermeir DJ, Karpova TS, Cooper JA. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol. 1992;119:1151–1162. doi: 10.1083/jcb.119.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 30.Carra S, Seguin SJ, Landry J. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4:237–239. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- 31.Hohfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuart JK, Myszka DG, Joss L, Mitchell RS, McDonald SM, Xie Z, Takayama S, Reed JC, Ely KR. Characterization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J Biol Chem. 1998;273:22506–22514. doi: 10.1074/jbc.273.35.22506. [DOI] [PubMed] [Google Scholar]
- 33.Gotz R, Wiese S, Takayama S, Camarero GC, Rossoll W, Schweizer U, Troppmair J, Jablonka S, Holtmann B, Reed JC, Rapp UR, Sendtner M. Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci. 2005;8:1169–1178. doi: 10.1038/nn1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada H, Chen NJ, Mirtsos C, Suzuki S, Suzuki N, Wakeham A, Mak TW, Yeh WC. Role of SODD in regulation of tumor necrosis factor responses. Mol Cell Biol. 2003;23:4026–4033. doi: 10.1128/MCB.23.11.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delalle I, Pfleger CM, Buff E, Lueras P, Hariharan IK. Mutations in the Drosophila orthologs of the F-actin capping protein alpha- and beta-subunits cause actin accumulation and subsequent retinal degeneration. Genetics. 2005;171:1757–1765. doi: 10.1534/genetics.105.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzawa S, Takayama S, Froesch BA, Zapata JM, Reed JC. p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demand J, Alberti S, Patterson C, Hohfeld J. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol. 2001;11:1569–1577. doi: 10.1016/s0960-9822(01)00487-0. [DOI] [PubMed] [Google Scholar]
- 38.Kalia SK, Lee S, Smith PD, Liu L, Crocker SJ, Thorarinsdottir TE, Glover JR, Fon EA, Park DS, Lozano AM. BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron. 2004;44:931–945. doi: 10.1016/j.neuron.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Arndt V, Daniel C, Nastainczyk W, Alberti S, Hohfeld J. BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol Biol Cell. 2005;16:5891–5900. doi: 10.1091/mbc.E05-07-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C. Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J Biol Chem. 2005;280:38673–38681. doi: 10.1074/jbc.M507986200. [DOI] [PubMed] [Google Scholar]
- 41.Chien KR. Genotype, phenotype: upstairs, downstairs in the family of cardiomyopathies. J Clin Invest. 2003;111:175–178. doi: 10.1172/JCI17612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 43.Mahoney DJ, Safdar A, Parise G, Melov S, Fu M, MacNeil L, Kaczor J, Payne ET, Tarnopolsky MA. Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1901–1910. doi: 10.1152/ajpregu.00847.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGregor E, Kempster L, Wait R, Gosling M, Dunn MJ, Powell JT. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol Cell Proteomics. 2004;3:115–124. doi: 10.1074/mcp.M300046-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Laudi S, Steudel W, Jonscher K, Schoning W, Schniedewind B, Kaisers U, Christians U, Trump S. Comparison of lung proteome profiles in two rodent models of pulmonary arterial hypertension. Proteomics. 2007;7:2469–2478. doi: 10.1002/pmic.200600848. [DOI] [PubMed] [Google Scholar]
- 46.Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582:825–841. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanoudou D, Corbett MA, Han M, Ghoddusi M, Nguyen MA, Vlahovich N, Hardeman EC, Beggs AH. Skeletal muscle repair in a mouse model of nemaline myopathy. Hum Mol Genet. 2006;15:2603–2612. doi: 10.1093/hmg/ddl186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–1371. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.