SUMMARY
Neural precursor cell (NPC) transplantation may have a role in restoring brain function after stroke, but how aging might affect the brain’s receptivity to such transplants is unknown. We reported previously that transplantation of human embryonic stem cell (hESC)-derived NPCs together with biomaterial (Matrigel) scaffolding into the brains of young adult Sprague-Dawley rats 3 wks after distal middle cerebral artery occlusion (MCAO) reduced infarct volume, and improved neurobehavioral performance. In this study we compared the effect of NPC and Matrigel transplants in young adult (3-mo-old) and aged (24-mo-old) Fisher 344 rats from the National Institute on Aging’s aged rodent colony. Distal MCAO was induced by electrocoagulation and hESC-derived NPCs were transplanted into the infarct cavity 3 wks later. Aged rats developed larger infarcts, but infarct volume and performance on the cylinder and elevated body swing tests, measured 6–8 wks post-transplant, were improved by transplantation. We conclude that advanced age does not preclude a beneficial response to NPC and Matrigel transplantation following experimental stroke.
Keywords: transplant, neural precursor, ischemia, stroke, brain
INTRODUCTION
Transplantation of exogenous neural precursor cells (NPCs) is a potential strategy for restoring brain function after stroke (Bliss et al. 2010). In previous studies, NPCs of rodent (Wei et al. 2005; Zhu et al. 2005; Daadi et al. 2009a), nonhuman primate (Hayashi et al. 2006) and human (Chu et al. 2004; Ishibashi et al. 2004; Kelly et al. 2004; Chu et al. 2005; Daadi et al. 2009b; Daadi et al. 2010) origin have all been transplanted into ischemic rodent brains, where they have been found to survive and improve histological or functional outcome. Although most potential recipients of human NPC transplants for stroke are elderly and aging may adversely affect stroke outcome (Nakayama et al. 1994; Ay et al. 2005), little attention has been given to how aging might affect the brain’s receptivity to such transplants.
We and others have found that transplantation of NPCs together with biomaterial scaffolding improves transplant survival and function in the ischemic young rodent brain (Park et al. 2002; Bible et al. 2009; Jin et al. 2010; Zhong et al. 2010). In our study (Jin et al. 2010), human NPCs derived from human embryonic stem cell (hESC) line BG01 were transplanted with Matrigel scaffolding into young adult (280–310 g) Sprague-Dawley rat brain 3 wks after middle cerebral artery occlusion (MCAO). Five to nine weeks later, rats given NPC-Matrigel transplants showed increased survival of transplanted cells, reduced infarct volume, and improved performance on neurobehavioral tests, compared to rats given vehicle, NPCs, or Matrigel alone.
Here we report the results of studies to determine if the salutary effects of human NPC and Matrigel transplantation on outcome from MCAO extend to aged rodents.
RESULTS
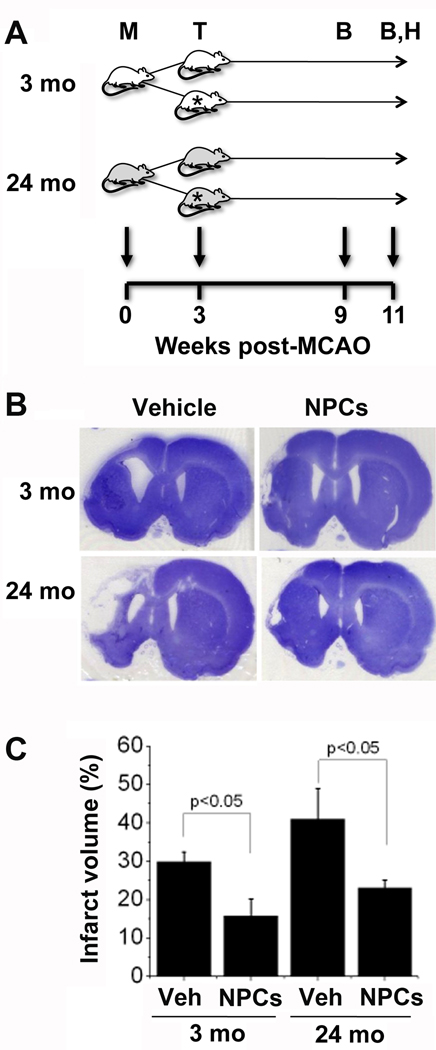
To determine if the benefits of delayed transplantation extend to older rats, who model more closely the age group most susceptible to stroke in humans, the same nestin+/SOX1+/calbindin−/GFAP−/OX4− NPCs used in our previous study (Jin et al. 2010), mixed with Matrigel scaffolding, were transplanted 3 wks post-MCAO into the infarct cavity of 3- and 24-mo-old Fisher rats. A different rat strain was used because the NIA aged rodent colony from which we obtained our animals employs Fisher rather than Sprague-Dawley rats. Figure 1 shows that infarct volume 11 wks post-MCAO (8 wks post-transplant) was ~30% greater in 24- than in 3-mo control rats given aCSF injections into the infarct cavity. An age-related increase in infarct size or evolution rate in rodents has been reported in some but not other prior studies (Popa-Wagner et al. 2007). In rats who received NPC transplants, infarct volume was reduced—by ~50% (p<0.05) in 3-and ~40% (p<0.05) in 24-mo-old animals.
Figure 1. Infarction volume in young adult and aged rats after MCAO and NPC transplantation.
(A) Experimental design: 3- and 24-mo-old rats underwent MCAO (M), followed 3 wks later by transplantation (T) of vehicle or NPCs; behavioral testing (B) was conducted 6 and 8 wks after transplantation, and rats were euthanized at 8 wks for histological studies (H). Stars indicate recipients of NPC transplants. (B) Cresyl violet-stained coronal rat brain sections 8 wks after transplantation of vehicle (Veh) or NPCs (Cells) into young adult (3-mo-old, 3M) and aged (24-mo-old, 24M) rats. (C) Infarct volume (% of contralateral hemsiphere) was reduced after NPC transplantation in both age groups. Data are means ± SEM from 6–10 rats per group.
The reduction in infarct volume we observed previously in young adult rats given NPC transplants after MCAO was accompanied by improvement in a battery of neurobehavioral tests designed to detect long-term post-ischemic deficits (Jin et al. 2010). In this study we also assessed neurobehavioral outcome, using the cylinder test (Schallert et al. 2000) and elevated body swing test (EBST) (Borlongan & Sanberg 1995), which detect asymmetries in forelimb use and rotational behavior, respectively. In the cylinder test, 3-mo-old rats showed ~30% preferential use of the unaffected limb 6 wks post-transplant, which had essentially resolved by 8 wks (Figure 2). The preference observed at 6 wks was reduced to ~15% (p<0.05) in rats given NPC transplants. By comparison, 24-mo-old rats showed greater (~70%) preferential use of the unaffected limb at 6 wks post-transplant, which decreased only slightly (to ~50%) at 8 wks. NPC transplants reduced the preference for unaffected limb use, to ~30% (p<0.05) at 6 wks and ~15% (p<0.05) at 8 wks.
Figure 2. Behavioral testing in young-adult and aged rats after MCAO and NPC transplantation.
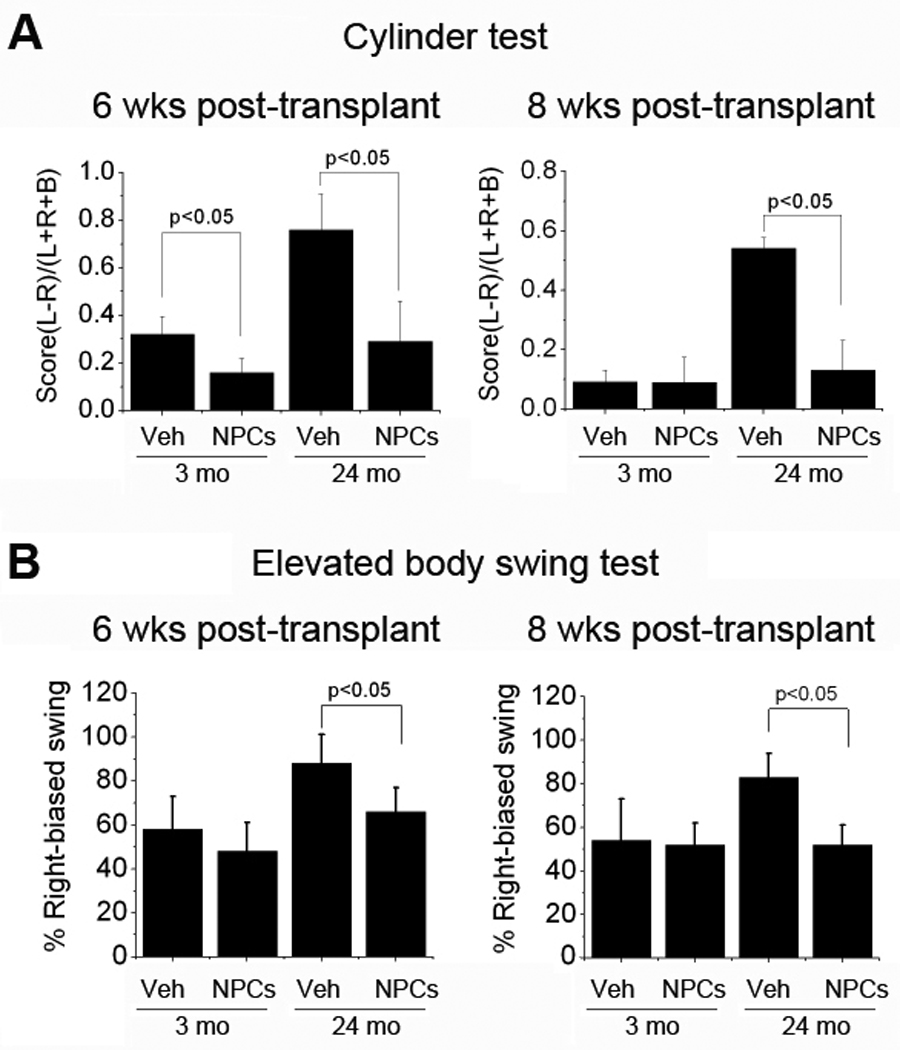
Testing was conducted 6 and 8 wks after transplantation of vehicle (Veh) or NPCs (Cells) into young adult (3-mo-old, 3M) and aged (24-mo-old, 24M) rats. (A) Cylinder test: asymmetry of forelimb use was scored as (L−R)/(L+R+B), where L=left, R=right, and B=both, with higher scores reflecting preferential use of the unaffected limb and, therefore, more severe impairment. (B) Elevated body swing test: The number of turns in each direction (L=left, R=right) was recorded, and the percentage of turns made away from the ischemic hemisphere (percent right-biased swing, normally 50%) was calculated as R/(R+L) × 100%. Data are means ± SEM from 6–10 rats per group.
In the EBST, 3-mo-old rats showed ~60% preferential rotation away from the ischemic hemisphere, which was similar at 6 and 8 wks post-transplant, and was not reduced by NPC transplants. In contrast, 24-mo rats exhibited greater rotational preference, but this preference was reduced significantly by transplantation, from ~90% to ~70% (p<0.05) at 6 wks and from ~80% to ~50% (p<0.05) at 8 wks post-transplant. Thus, despite more severe neurobehavioral deficits in 24- than in 3-mo-old rats under control (non-transplanted) conditions, 24-mo rats showed improved performance following NPC transplantation.
To investigate the survival and phenotypic fate of transplanted NPCs, brain sections from 24-mo-old rats who underwent MCAO, NPC transplantation 3 wks later, and bromodeoxyuridine (BrdU) administration followed by euthanasia 8 wks after that, were stained with antibodies against human and cell type-specific marker proteins. Figure 3 illustrates that HuN-immunopositive (transplanted human) cells were abundant at the transplant site, and that some HuN-positive cells were labeled with BrdU, suggesting that they had divided during the 24-hr period between BrdU administration and euthanasia. Other HuN-positive cells stained for caspase-3 cleavage product, indicating that they were undergoing caspase-dependent cell death.
Figure 3. Survival of transplanted NPCs in post-ischemic aged rat brain.
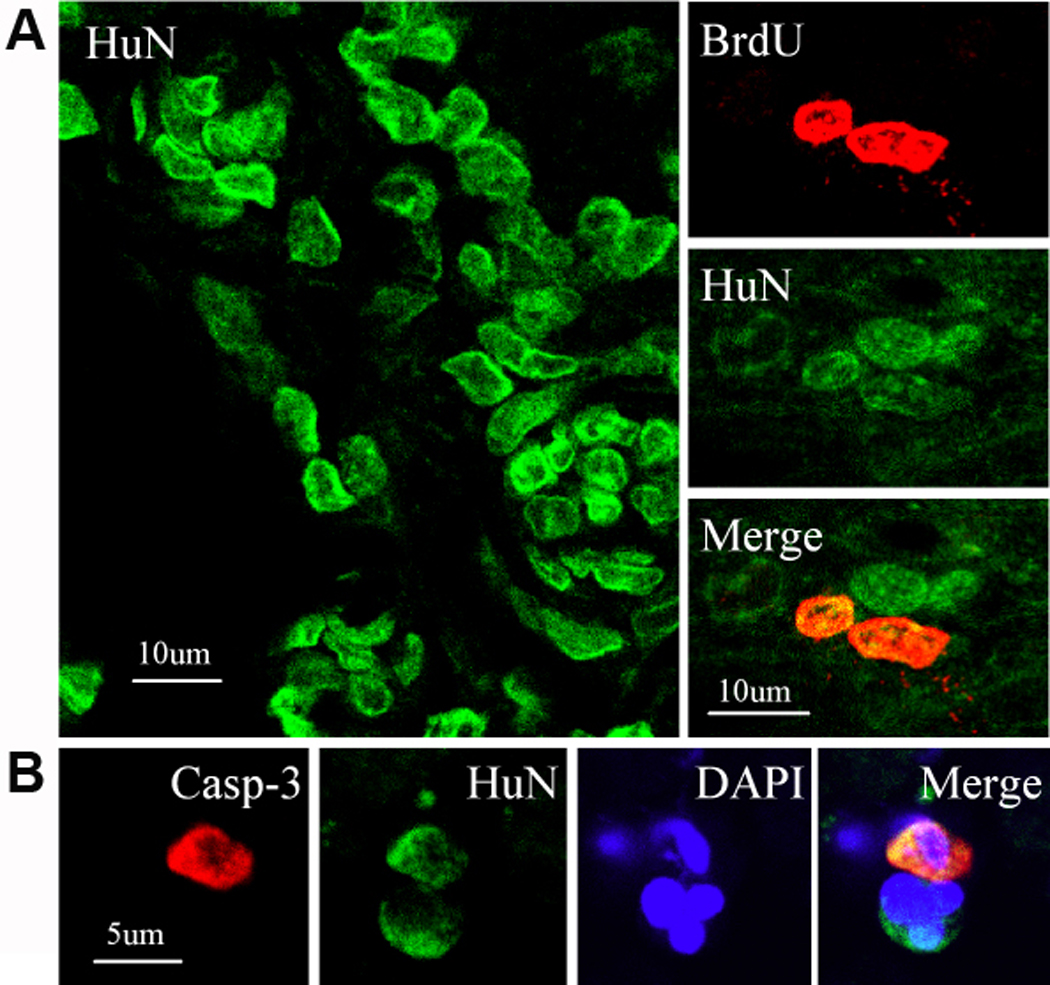
Sections were obtained 8 wks after transplantation of NPCs into aged (24-mo-old) rats. (A) Human nuclei (HuN)-positive (green) cells were present at the transplant site (left), and in some cases were labeled with BrdU (red), suggesting recent cell division (right). (B) Some HuN-positive (green) cells also expressed activated caspase-3 (red), consistent with ongoing caspase-dependent cell death. DAPI (blue) was used to counterstain nuclei.
In our previous study of NPC transplantation in young adult Sprague-Dawley rats, we found that transplanted cells went on to express markers of both neuronal and astroglial lineage (Jin et al. 2010). As shown in Figure 4, the same was true for 24-mo-old Fisher rats in the present study. Thus, we observed co-expression of the transplanted human cell marker HuN with three neuronal lineage markers—βIII tubulin, doubklecortin (DCX), and calbindin—and the astroglial marker glila fibrillary acidic protein (GFAP). DCX, which is associated most notably with migrating new neurons, was seen in cells that also exhibited features of migratory morphology.
Figure 4. Phenotypis fate of transplanted NPCs in post-ischemic aged rat brain.
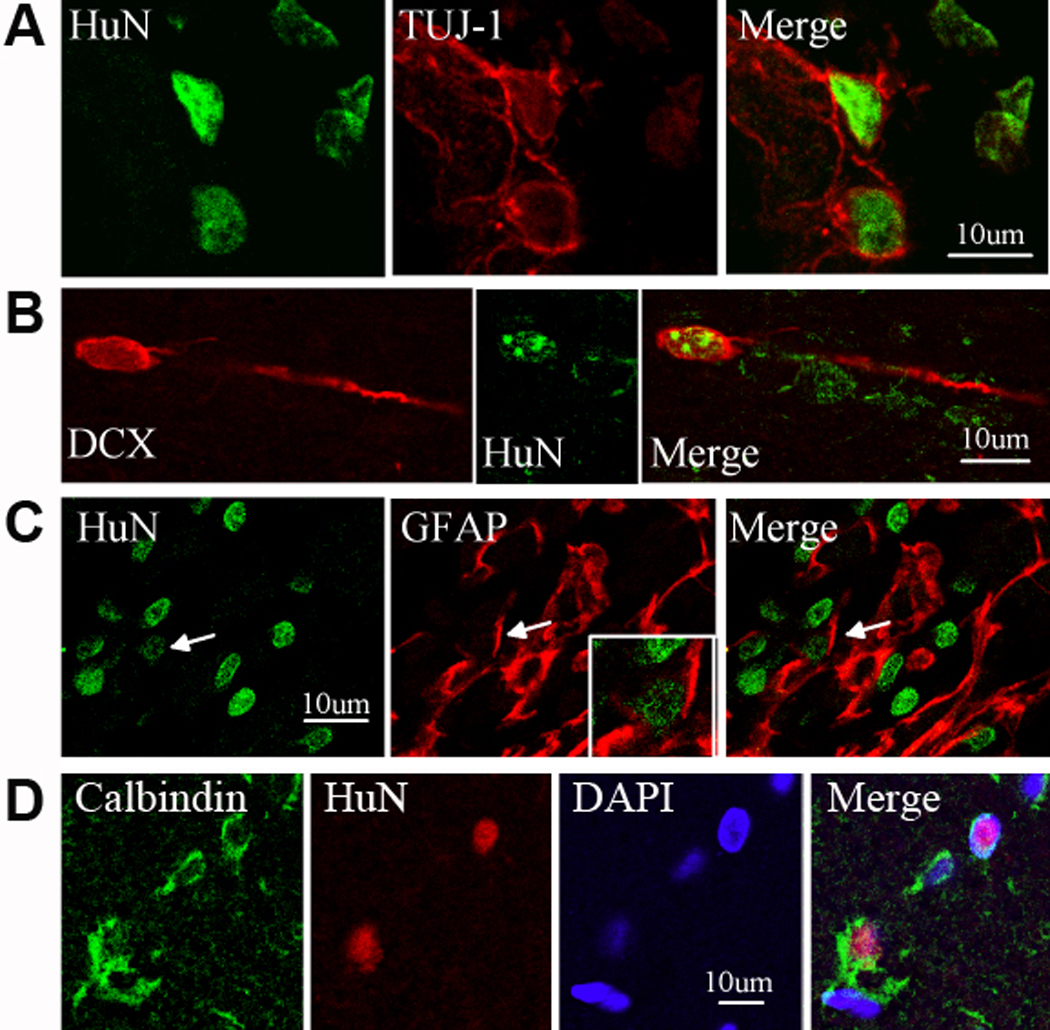
Sections were obtained 8 wks after transplantation of NPCs into aged (24-mo-old) rats. Transplanted (HuN-positive, green) cells co-expressed the neuronal lineage markers βIII tubulin (TUJ-1 antibody) (A) or DCX (B), the astroglial marker GFAP (C), or the mature neuronal marker calbindin (D). DAPI (blue) was used to counterstain nuclei.
DISCUSSION
Transplantation of several tissues—including kidney (Saxena et al. 2009), heart (Aliabadi et al. 2007), liver (Keswani et al. 2004), and bone marrow (Jantunen 2006)—can provide therapeutic benefit in elderly patients. The prospect of cell transplantation for stroke is also appealing, but what special problems the aged recipient may present are unknown (Bliss et al. 2010). One concern is that the aged brain may be less receptive to the survival, proliferation, and function of transplanted neural cells. This arises partly because of the reduced capacity for endogenous neurogenesis observed in the aged brain (Seki & Arai 1991; Kempermann et al. 1998). To the extent that this impairment is due to age-related changes in the brain environment, as opposed to cell-autonomous defects in neural precursor cells, it might affect transplanted cells as well. For example, levels of several growth factors that stimulate neurogenesis decline with aging in neurogenic brain regions (Shetty et al. 2005). If transplanted NPCs are similarly dependent on these factors, neurotransplantation into the aged brain might prove futile.
We found previously that transplantation of hESC-derived, nestin+/SOX1+/calbindin−/GFAP−/OX4− NPCs, together with Matrigel scaffolding, into young adult Sprague-Dawley rat brain 3 wks after MCAO reduced infarct volume and improved neurobehavioral performance (Jin et al. 2010). The major finding of the present study is that transplantation of the same cells and scaffolding confers benefit in both young adult (3-mo-old) and aged (24-mo-old) Fisher 344 rats. Infarct volume was larger and behavioral function more impaired in older rats. However, the magnitude of reduction in infarct volume afforded by NPC transplantation was comparable (~50% in 3- and ~40% in 24-mo-old animals). In addition, although older rats also showed more severe behavioral deficits, they also demonstrated more marked improvement following transplantation, perhaps because behavioral deficits were largely resolved by 6–8 wks post-transplant in younger animals.
A notable feature of the response to NPC transplantation is that it occurred even though transplantation was delayed until 3 wks after ischemia. The option for delayed treatment is clinically important because current therapy for stroke is only effective for a few hours after the event. Most previous studies of NPC transplantation in rodents have employed post-ischemic intervals of 1 wk or less, an exception being a study in which medial ganglionic eminence cells from E15 rat embryos were transplanted 2 wks post-MCAO (Daadi et al. 2009a). Some growth factors can also be administered up to several days after MCAO in rodents and produce improvement in infarct volume, behavior or both (Greenberg & Jin 2006). In one case, transforming growth factor-α given to rats 4 wks after MCAO was still capable of improving behavioral outcome (Guerra-Crespo et al. 2009). However, none of these studies involved aged animals.
In this and our prior study (Jin et al. 2010) of NPC transplantation after cerebral ischemia in rats, we did not treat the recipients with immunosuppressive drugs. Some (Ishibashi et al. 2004; Kelly et al. 2004; Daadi et al. 2009b) but not other (Chu et al. 2004; Chu et al. 2005; Daadi et al. 2010) previous studies have employed immunosuppression with cyclosporine A, although none has compared immunocompetent and immunosuppressed animals. Whether outcome would have been even better had our rats been immunosuppressed is impossible to say, but several factors argue against the need for immune suppression in this setting (Barker & Widner 2004). First, the brain is a relatively immunoprivileged site, due partly to the blood-brain barrier, a paucity of resident antigen-presenting cells, and sparse lymphatic drainage. Second, cell-suspension—as opposed to than solid tissue—grafts tend to evoke lesser immune responses. Third, intaparenchymal transplants (and perhaps especially transplants into poorly vascularized brain tissue) appear less immunogenic than, e.g., periventricular transplants. Fourth, transplantation into sites of pre-existing astro- and microglial activation may be associated with enhanced trophic support and improved graft survival (Duan et al. 1998). Finally, graft rejection in brain may occur over a longer time course than is typically monitored in rodents (Brevig et al. 2000). The role of aging in transplant rejection is complex, as observed in clinical studies. Immune function is impaired by aging, and elderly transplant recipients have a reduced incidence of acute rejection, although the risk of chronic rejection is increased and the adverse effects of immunosuppressive therapy are more common (Saxena et al. 2009). It is, therefore, possible that immunosenescence contributed to the success of NPC transplantation in our aged rats.
The mechanism through which NPC transplantation reduces infarct size and improves functional outcome after experimental cerebral ischemia is elusive. Cell replacement may be a contributing factor, but transplanted cells also release factors with neuroprotective or neurotrophic effects. Vascular endothelial growth factor (VEGF), for example, has been implicated in the protective effect of murine NPCs against MCAO in mouse brain, which is blocked by a VEGFR2 receptor antagonist and by a soluble VEGFR2 decoy receptor (Harms et al. 2010). In another study (Zhu et al. 2005), overexpression of VEGF in transplanted rat NPCs enhanced the effect of transplants on neurological function. Overexpression of other growth factors—including neurotrophin-3 (Zhang et al. 2008), fibroblast growth factor-2 (FGF-2) (Jenny et al. 2009), and glial cell line-derived neurotrophic factor (Chen et al. 2009)—in rat NPCs has also been reported to potentiate the effects of NPC transplantation after MCAO in rats.
EXPERIMENTAL PROCEDURES
Distal MCAO
Aged (24-mo-old) and young adult (3-mo-old) male Fisher 344 rats were obtained from the National Institute on Aging aged rodent colony. All animal procedures were conducted in accordance with National Institutes of Health (NIH) guidelines and with the approval of the Institutional Animal Care and Use Committee. Male rats were anesthetized with 4% isoflurane in 70% N2O/30% O2 using a mask. Permanent distal MCAO was performed as previously described (Nawashiro et al. 1997; Won et al. 2006). In brief, a 2-cm incision was made between the left orbit and tragus under the surgical microscope. The temporal muscle was retracted laterally and a 3-mm diameter craniotomy was made just rostral to the foramen ovale. The dura was incised with a 26–gauge needle and the distal MCA was exposed. The left MCA was occluded by electrocoagulation without damaging the brain surface. Interruption of blood flow was confirmed visually under the microscope, the temporal muscle was repositioned, and the skin was closed. Rectal temperature was measured and maintained at 37±0.2°C with a heating blanket. In sham-operated controls the MCA was visualized but not occluded.
Cell culture
NPCs derived from hESC line BG01 were obtained from Aruna Biomedical Inc. (Athens, GA). Cells were seeded on polyornithine- and laminin-coated plastic dishes and cultured in proliferation medium, consisting of Neurobasal medium with B27 supplementation containing 2 mM L-glutamine and 50 µg/ml Pen Strep (all from Invitrogen), and 10 ng/ml leukemia inhibitory factor and 20 ng/ml FGF-2 (both from R&D Systems) (Shin et al. 2006). Cells were propagated further in proliferation medium and, upon reaching 90–100% confluence, were triturated to detach them from dishes. After centrifugation at 200×g for 4 min, cells were resuspended in fresh medium and replated.
Cell transplantation
Cells were transplanted 3 wks after induction of focal ischemia. Rats (n=6–10 per group) were re-anesthetized with 4% isoflurane in 70% N2O/30% O2 and placed in stereotaxic frames with a rat head holder. Burr holes were drilled with a dental drill, which was irrigated continuously with saline at room temperature to prevent overheating of the underlying cortex. NPCs (1.2 × 105 cells/µl) were mixed with 15 µl of Matrigel suspension and immediately injected with a Hamilton syringe into the cortical infarct cavity over 5 min. The needle was then left in place for an additional 15 min before being slowly withdrawn. Control rats received injections of aCSF vehicle. Bone wounds were closed with bone wax, anesthesia was discontinued, and rats were returned to their cages. Eight weeks post-transplant, they were perfused with 0.9% saline and 4% paraformaldehyde in PBS (pH 7.5) to measure infarct volume.
Measurement of infarct volume
Brains were removed and 40-µm coronal sections were prepared and stained with cresyl violet. Infarct area was measured by a blinded observer using the NIH Image program, and areas were multiplied by the distance between sections to obtain the respective volumes. Infarct volume was calculated as a percentage of the volume of the contralateral hemisphere, as previously described (Swanson et al. 1990).
Cylinder test
The cylinder test (Schallert et al. 2000) was used to measure asymmetry of forelimb use 6 and 8 wks post-transplant (n=6–10 rats per group). Twenty movements were recorded by a blinded examiner during a 10-min testing session, and rated as involving the affected (right, R), unaffected (left, L) or both (B) limbs. Asymmetry of forelimb use was scored as (L−R)/(L+R+B), with higher scores reflecting preferential use of the unaffected limb and, therefore, more severe impairment.
Elevated body swing test (EBST)
Asymmetric motor behavior was also measured 6 and 8 wks post-transplant (n=6–10 rats per group) using the EBST (Borlongan & Sanberg 1995). Rats were held by the base of the tail and raised 10 cm above the testing surface. The initial direction of body swing (turning of the upper body by >10° to either side) was recorded by a blinded examiner in three sets of ten trials, performed over 5 min. The number of turns in each direction (right, R or left, L) was recorded, and the percentage of turns made away from the ischemic hemisphere (percent right-biased swing, normally 50%) was calculated as R/(R+L) × 100%.
BrdU administration
BrdU (50 mg/kg in saline) was given by the intraperitoneal route twice daily 24 hr before rats were euthanized (8 wks post-transplant). Brains were perfused with saline and 4% paraformaldehyde in PBS and embedded in paraffin.
Immunohistochemistry
Brain sections were embedded in paraffin and 6-µm coronal and sagittal sections were prepared. Immunohistochemistry and double immunostaining were performed as described previously (Jin et al. 2002; Jin et al. 2003). To detect BrdU-labeled cells, paraffin sections were treated with 50% formamide, 280 mM NaCl, and 30 mM sodium citrate at 65°C for 2 h, incubated in 2 M HCl at 37°C for 30 min, and rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min. Primary antibodies were affinity-purified goat polyclonal anti-doublecortin (DCX; Santa Cruz Biotechnology; 1:500), sheep polyclonal anti-BrdU (Biodesign; 1:1000), mouse monoclonal anti-human nuclei (HuN, Chemicon; 1;300), rabbit polyclonal anti-βIII tubulin (TUJ-1, Covance, Berkeley; 1:1000), rabbit anti-cleaved caspase-3 (Cell Signaling Technology; 1:200), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP, Sigma-Aldrich; 1:400), and rabbit polyclonal anti-calbindin (Upstate; 1:400). Secondary antibodies were Alexa Fluro 488-, or 594- or 647-conjugated donkey anti- goat, anti- mouse or anti- rabbit IgG (Molecular Probes; 1:200–500). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd.) equipped with a two-photon Chameleon laser (Coherent Inc.). Images were acquired using LSM 510 Imaging Software (Carl Zeiss Ltd). Controls included omitting or preabsorbing primary or omitting secondary antibody.
Statistical Analyses
Quantitative data were expressed as mean ± SEM from the indicated number of experiments. Behavioral data were analyzed by two-way analysis of variance (ANOVA) with repeated measures, followed by post-hoc multiple comparison tests (Fisher PLSD or Student’s paired t test with the Bonferroni correction). Infarct volume data were analyzed by one-way ANOVA followed by Fisher PLSD post-hoc tests. p values <0.05 were considered significant.
ACKNOWLEDGEMENTS
This work was supported by NIH grants NS44921 (to D.A.G) and AG21980 and NS057186 (to K.J.).
Contributor Information
Kunlin Jin, Email: kjin@buckinstitute.org.
XiaoOu Mao, Email: xmao@buckinstitute.org.
Lin Xie, Email: lxie@buckinstitute.org.
Rose B. Greenberg, Email: rosegreenberg@aol.com.
Botao Peng, Email: bobpeng13@gmail.com.
Alexander Moore, Email: alexneedsmoore@gmail.com.
Maeve B. Greenberg, Email: maevegr@aol.com.
REFERENCES
- Aliabadi AZ, Zuckermann AO, Grimm M. Immunosuppressive therapy in older cardiac transplant patients. Drugs Aging. 2007;24:913–932. doi: 10.2165/00002512-200724110-00004. [DOI] [PubMed] [Google Scholar]
- Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30:2985–2994. [Google Scholar]
- Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevig T, Holgersson J, Widner H. Xenotransplantation for CNS repair: immunological barriers and strategies to overcome them. Trends Neurosci. 2000;23:337–344. doi: 10.1016/s0166-2236(00)01605-2. [DOI] [PubMed] [Google Scholar]
- Chen B, Gao XQ, Yang CX, Tan SK, Sun ZL, Yan NH, Pang YG, Yuan M, Chen GJ, Xu GT, Zhang K, Yuan QL. Neuroprotective effect of grafting GDNF genemodified neural stem cells on cerebral ischemia in rats. Brain Res. 2009;1284:1–11. doi: 10.1016/j.brainres.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, Sinn DI, Lee YS, Kim SU, Kim M, Roh JK. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neurosci Res. 2005;53:384–390. doi: 10.1016/j.neures.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, Schaar B, Malenka RC, Palmer TD, Steinberg GK. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009a;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009b;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan WM, Widner H, Cameron RM, Brundin P. Quinolinic acid-induced inflammation in the striatum does not impair the survival of neural allografts in the rat. Eur J Neurosci. 1998;10:2595–2606. doi: 10.1046/j.1460-9568.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Growth factors and stroke. NeuroRx. 2006;3:458–465. doi: 10.1016/j.nurx.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Crespo M, Gleason D, Sistos A, Toosky T, Solaroglu I, Zhang JH, Bryant PJ, Fallon JH. Transforming growth factor-alpha induces neurogenesis and behavioral improvement in a chronic stroke model. Neuroscience. 2009;160:470–483. doi: 10.1016/j.neuroscience.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, Shizuko I, Shimazaki T, Onodera M, Okano H, Mizusawa H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res. 2004;78:215–223. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- Jantunen E. Autologous stem cell transplantation beyond 60 years of age. Bone Marrow Transplant. 2006;38:715–720. doi: 10.1038/sj.bmt.1705514. [DOI] [PubMed] [Google Scholar]
- Jenny B, Kanemitsu M, Tsupykov O, Potter G, Salmon P, Zgraggen E, Gascon E, Skibo G, Dayer AG, Kiss JZ. Fibroblast growth factor-2 overexpression in transplanted neural progenitors promotes perivascular cluster formation with a neurogenic potential. Stem Cells. 2009;27:1309–1317. doi: 10.1002/stem.46. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, Gorostiza O, Wang X, Greenberg DA. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani RN, Ahmed A, Keeffe EB. Older age and liver transplantation: a review. Liver Transpl. 2004;10:957–967. doi: 10.1002/lt.20155. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997;17:229–232. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]
- Saxena R, Yu X, Giraldo M, Arenas J, Vazquez M, Lu CY, Vaziri ND, Silva FG, Zhou XJ. Renal transplantation in the elderly. Int Urol Nephrol. 2009;41:195–210. doi: 10.1007/s11255-008-9489-6. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci Res. 1991;12:503–513. doi: 10.1016/s0168-0102(09)80003-5. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–138. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM, Jr, Yu SP, Choi DW. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006 doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Wang RZ, Li GL, Wei JJ, Li ZJ, Feng M, Kang J, Du WC, Ma WB, Li YN, Yang, Kong YG. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci Lett. 2008;444:227–230. doi: 10.1016/j.neulet.2008.08.049. [DOI] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010 doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, Zhu Y, Yang GY. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325–333. doi: 10.1227/01.neu.0000166682.50272.bc. discussion 325–333. [DOI] [PubMed] [Google Scholar]