Abstract
Increasing numbers of Americans are reaching 85 years of age or older, yet there are no reliable biomarkers to predict who will live this long. The goal of this pilot study therefore was: (1) to identify a potential serum pattern that could identify proteins involved in longevity and (2) to determine if this pattern was a marker of longevity in an independent sample of individuals. Serum samples were analyzed in three cohorts of individuals (n = 12 in each) aged 20–34, 60–74, and ≥90 years who participated in The Louisiana Healthy Aging Study. The 12 most abundant proteins were removed and the remaining proteins separated by two-dimensional gel electrophoresis. Gels were matched and the intensity of each spot quantified. Multivariate discriminant analysis was used to identify a serum pattern that could separate these three age cohorts. Seven protein spots were found that correctly distinguished the subjects into the three groups. However, these spots were not as successful in discriminating the ages in a second set of 15 individuals as only eight of these subjects were placed into their correct group. These preliminary results show that the proteomics approach can be used to identify potential proteins or markers that may be involved in the aging process and/or be important determinants of longevity.
Keywords: Aging, Serum, Proteomics, Biomarkers, Longevity
Introduction
Scientists have been interested in identifying processes that are associated with, and promote a healthy lifespan. A number of theories have been proposed to explain the aging process, including among others, lifetime cells and tissues’ exposure to reactive oxygen species (Biesalski 2002) resulting in oxidative stress and chronic up-regulation of pro-inflammatory mediators (Carter et al. 2007). Yet, besides a clear genetic determinant, we still do not understand why some individuals live longer and are healthier than others.
In 1987, Rowe and Kahn’s landmark paper on successful, usual, and pathological aging was published (Rowe and Kahn 1987) and defined a biomarker of aging as: “a biological parameter of an organism that either alone or in some multivariate composite will, in the absence of disease, better predict functional capacity at some later age than will chronological age”. Since then no consensus definition for a biomarker of aging has emerged and researchers have used this definition or other modifications. Much of the controversy has emerged due to a lack of standards for evaluating reliability and validity of candidate biomarkers (Ingram et al. 2001).
In the search for biomarkers, it is important to distinguish aging from longevity. Longevity provides little information about functioning, quality of life, or adaptation. We refer to biomarkers of longevity in this study, because, as described below, our study population included a group of nonagenarians.
A number of researchers have attempted to discover potential “biomarkers of aging.” For example, in a large longitudinal study, Nakamura and Miyao (2007) identified five candidate biomarkers of aging: systolic blood pressure, forced expiratory volume, hematocrit, albumin, and urea nitrogen. From a 27-year community-based cohort study of Japanese men, serum dehydroepiandrosterone sulfate was found to be a good predictor of longevity (Enomoto et al. 2008). Roth and colleagues (Roth et al. 2002) have identified insulin and body temperature as robust biomarkers of calorie restriction, a treatment known to increase life span in a number of animal models. Thus far, however, we have not been able to utilize these biomarkers to identify individuals that will live a longer and healthier life.
In the last couple of decades, new techniques in genomics, metabolomics, and proteomics have emerged that allow the simultaneous examination of a large number of variables. Currently these disciplines are being used to identify biomarkers for a variety of disease states, and to a lesser extent, for longevity. For example, genomic techniques recently were used to identify a genetic variation within the FOXO3A gene that is strongly associated with human longevity (Willcox et al. 2008). Metabolomic techniques identified a close association between free-fatty acids and hypertension in the salt-dependent hypertensive rodent, suggesting that this powerful approach may be useful in identifying potential biomarkers of hypertension and the physiological consequences of aging (Lu et al. 2008).
We are not aware of any studies that have used proteomic techniques to discover potential serum markers of longevity. We therefore undertook a pilot experiment to determine if a proteomic technique, two-dimensional (2-D) gel electrophoresis, could be used to identify a specific serum pattern that might segregate individuals into young, old, and elderly age cohorts and identify several potential serum proteins associated with longevity. We collected serum samples from subjects participating in the population-based Louisiana Healthy Aging Study (LHAS) and used multivariate statistical techniques to identify a combination of serum proteins that best sorted these subjects into their proper age groups. This combination was then tested in an independent group of subjects to see how well it performed as a potential indicator of longevity.
Materials and methods
Participant recruitment
This investigation represents one subset of The Louisiana Healthy Aging Study. Eight hundred and seventy-seven individuals participated in some aspect of the LHAS project, including >250 subjects who were nonagenarians (≥90 years old). Recruitment of subjects for the LHAS has been described elsewhere (Frisard et al. 2007). After individuals were screened and enrolled in the main project, eligibility was then determined for various ancillary projects. This pilot study is a subset of an ancillary project with 206 participants.
Subjects were excluded if they had diabetes (blood sugar >125 mg/dL); unstable cardiovascular disease; a myocardial infarction or a cerebrovascular accident in the last 3 months; severe high blood pressure; blood vessel aneurysm; took certain medications used for myasthenia gravis; had uncontrolled asthma, an asthma-like condition, or emphysema and/or chronic obstructive pulmonary disorder; thyroid disease; mental health problems requiring drug treatment; or a Mini-Mental State Examination Score <23. Blindness was the only functional exclusion criterion. All subjects were well enough to come to our study facility. Participants provided written informed consent. The Institutional Review Boards at the Pennington Biomedical Research Center, Louisiana State University Health Sciences Center and University of North Carolina at Charlotte approved the study.
A subset of 51 subjects participated in the present proteomics study. Each subject’s age, gender, and race (Caucasian, black, or other) were recorded. Height (cm) and weight (kg) were measured and body mass index (kg/m2) calculated. In addition, a serum sample was collected, allowed to clot for 20 min, processed to separate the serum and blood cells, and then frozen. Samples from the 51 subjects were collected over a 2.25-year period. The frozen serum was kept in a −80°C freezer until all the samples had been collected after which randomly selected samples were prepared for 2-D gel electrophoresis analysis.
Training and test groups
A training group of subjects was selected for use in identifying potential serum spots to segregate the three age groups and identify potentially important markers of longevity. At the time we designed the study and started sample collection, the variability in sample spot size among repeated gels had not been reported. So we used the literature reported standard deviation of albumin to calculate sample size. A statistical power analysis indicated that 12 subjects per age group would be adequate to ensure statistical power of 0.80 for discriminating differences among the three age groups that averaged 1/3 of a standard deviation. Twelve subjects from each of the three age group (young, 20–34 years; old, 60–74 years; and elderly, ≥90 years) were randomly selected by a research associate not associated with this study. The age of the subject was blinded to the study’s investigators who were told only that each subject belonged to group 1, 2, or 3; these numbers did not reflect a chronological age group. A second set of 15 subjects was randomly selected and referred to as the test group. Again, the ages of all subjects in this test group were not known to the investigators. The intent was to test how well the spots identified from the training group could predict the age group to which each individual in the test group belonged. The code for each group was broken after the study’s investigators had assigned the subject to a group and the dataset had been secured.
Sample preparation
For all 51 subjects, the 12 most abundant proteins were removed from the serum using the human IGY spin columns (formally Genway Biotech, Inc, San Diego, CA, now ProteomeLab™ IgY-12 High Capacity SC Spin Column kit, Beckman Coulter, Fullerton, CA, USA). These 12 proteins were albumin, IgG, fibrinogen, transferrin, IgA, IgM, Apo A-I, Apo A-II, haptoglobin, alpha 1-antitrypsin, alpha 1-acid glycoprotein, and alpha 2-macroglobulin. Ten microliters of serum was processed according to the directions provided by the manufacturer. Each sample was run in duplicate to generate enough protein for 2-D gel electrophoresis. The duplicates were combined and the volume reduced to 1 ml using the Micron YM-10 centrifugal filter devices (Amicon, Millipore, Bellerica, MA, USA) recommended by the IgY column manufacture. A sample from each age group was prepared on the same day to minimize day-to-day variation in sample preparation.
CHAPS re-suspension reagent (7 M urea, 2 M thiourea, 2% CHAPS; K10022, Proteome Systems, Woburn, MA, USA), 1 M Tris base (T-1503, Sigma, St Louis, MO, USA), 200 mM tributylphosphine (T-7567, Sigma-Aldrich, St Louis, MO, USA), and 1 M acrylamide (A-9099, Sigma-Aldrich, St Louis, MO, USA) was added to the 1 ml depleted serum sample to reduce and alkylate the cysteines. The sample was then incubated at room temperature for 90 min before stopping the akylation by the addition of 1 M dithiothreitol (43817, Sigma-Aldrich, St Louis, MO, USA). The sample volume was reduced to less than 200 µl and CHAPS re-suspension agent added to reduce the conductivity below 200 μS/cm. The volume was reduced to approximately 70 µl using a molecular weight cut-off filter (Micron YM-10 centrifugal filter device, Amicon, Millipore, Bellerica, MA, USA) and the sample was frozen at −80°C. Protein concentration was determined using the Bradford Protein assay. When all the samples in a set had been processed and the protein concentration determined, they were shipped frozen to the Proteomics Core at the Universityof Massachusetts Medical School for 2-D gel electrophoresis.
2-D gel electrophoresis
Seventy-five micrograms of protein was loaded and separated by isoelectric focusing on 11-cm pH 3 to 10 and pH 4 to 7 immobilized pH gradient strips (Proteome Systems, Sydney, Australia). All gels were run in duplicate. After IEF, immobilized pH gradient strips were equilibrated in 6 M urea, 2% SDS, 50 mM tris-acetate buffer, pH 7.0, and 0.01% bromphenol blue and subjected to SDS-polyacrylamide gel electrophoresis on 6% to 15% Gel Chips (Proteome Systems). All gels were fixed and stained in SYPRO Ruby, 70 ml/gel (Invitrogen, Carlsbad, CA, USA) and imaged on a Typhoon scanner.
During a first discovery phase we ran a subject’s samples in triplicate to rule out artifact spots and to help evaluate the methods’ reproducibility. We found that most of the high abundance gel spots had a CV around 15%. Based on the Core’s experience, good laboratory practices, and because we had limited protein sample, we decided to run the gels in duplicate. To further reduce variation, the gels for each set were run on the same day by the same technician using the same manufactured lot of reagents.
Gel analysis
The 102 gels (duplicates for each of the 36 training group subjects and 15 test group subjects) were matched using Progenesis SameSpots software (Non-Linear, Research Triangle Park, NC, USA) and a total of 749 spots were identified. The intensity of each spot was normalized and both duplicate values in each individual were recorded in an Excel spreadsheet.
Mass spectrometry identification of spots
Gel spots were cut then digested with trypsin. The resulting peptide mixture was loaded on a Dionex PepMap C18 trap column and was separated by a New Objective reversed phase C18 Picofrit column/emitter. Peptide mass was determined by a Thermo-Fisher LTQ-XL linear ion trap mass spectrometer (Waltham, MA, USA) coupled with an Eksigent nanoLC (Dublin, CA, USA). The raw data were analyzed by the Mascot search engine V2.2 (Matrix Science Inc, Boston, MA, USA) against the human SwissProt database (false discovery rate <5%) to generate a list of possible proteins for that gel spot.
Statistical analysis
Prior to the serum spot analysis, we calculated basic statistics (means and standard errors) for height, weight, and BMI for subjects of each age group in both the training and test groups to characterize differences among groups. We used a one-way analysis of variance (ANOVA) to test for differences between the three age groups for the pooled values of each trait, and where significant, used Tukey’s post-hoc tests for pairwise comparisons of the age groups.
The serum spot analysis began with an estimation of the repeatability of the duplicate intensity values for each spot. This calculation was accomplished using the results of model II one-way ANOVAs for each spot conducted using all 51 subjects (pooled training and test groups). These ANOVAs yielded variance components between and within subjects, with repeatabilities being estimated by the ratio of the between-subject component over the total of the between- and within-subject components. To characterize these 749 repeated spots, we examined their distribution and calculated their means and standard errors. All subsequent analyses used the mean of the two replicate values.
Using the values for the training group, we ran a multivariate stepwise discriminant analysis on the values for each spot using the STEPDISC procedure in SAS (SAS Institute 1992). This procedure proceeds in a stepwise fashion by first entering the (spot) variable that makes the largest contribution to the separation of the three age groups, and then successively considers additional variables after conditional consideration of all variables already entered. Eventually, a final solution is reached in which only those variables remain that are most important in discriminating the groups. To reduce the large number of these variables, we lowered the significance levels for entry/exit in the model from the default value of 0.15 to 0.01. For comparative purposes, we also conducted another analysis with entry/exit values at the conventional 0.05 level.
Using the variables identified from the stepwise analysis, we next ran the DISCRIM procedure in SAS (SAS Institute 1992) to generate a discriminant function used to calculate a posteriori probability for each subject that expressed the likelihood of that subject belonging to each of the three age groups. This allowed each of the subjects (in both the training and test groups) to be classified into the age group with its highest probability. To evaluate the performance of the discriminant function, we focused on the percentage of the 15 subjects in the test group that were placed into their correct age group. The ages of subjects in the test group were made known only after the discriminant function was generated with the classification assignments completed.
Once the spots important in discriminating the age groups were resolved by discriminant analysis, we calculated their basic statistics over all pooled groups, including their pairwise correlations, and evaluated their significance with the sequential Bonferroni procedure (Rice 1989). In addition, we conducted a principal components analysis (Harmon 1960) of these correlations to further analyze covariation in the traits. Lastly, we plotted the scores of the first and second principal components to visualize the separation achieved among subjects of different ages in the training and test groups.
Results
Characterization of participants
Table 1 provides the characteristics of the subjects in both the training and test groups. Both genders were roughly equally represented in the different ages whereas the majority of the subjects were Caucasian. Analyses of variance suggested significant gender (but not race) differences for height, weight, and BMI, so we adjusted for differences between males and females and calculated basic statistics for these three traits over the pooled sexes. One-way ANOVAs showed that height significantly differed in subjects among the three age groups both in the training and test groups, with the youngest-aged subjects consistently taller than the elderly subjects. Subjects in the training group also exhibited significant differences in weight, with the elderly group being lighter. BMI differences were not significant for subjects in either group. All participants did not smoke except one subject in the young, old, elderly, and test group. The smoking participants were considered healthy based on the study selection criteria.
Table 1.
Gender (M = male, F = female), race (C = Caucasian, B = black, O = other) and mean ± standard errors for age, height, weight, and body mass index (BMI) for young, old, and elderly subjects in the training group and in the test group
| Age | Gender | Race | Height (cm) | Weight (kg) | BMI (kg/m2) | |
|---|---|---|---|---|---|---|
| Training group (n = 12 per group) | ||||||
| Young | 25 ± 0.4 (23–27) | 6M, 6F | 10C, 2B | 171.4 ± 1.5a | 78.9 ± 5.1a,b | 26.7 ± 1.4 |
| Old | 64 ± 0.6 (60–67) | 6M, 6F | 9C, 2B, 1O | 169.5 ± 1.7a | 88.1 ± 4.09a | 30.9 ± 1.6 |
| Elderly | 91 ± 0.1 (90–91) | 7M, 5F | 12C | 162.9 ± 1.2b | 70.0 ± 3.0b | 26.5 ± 1.2 |
| P value | 0.0005** | 0.0157* | 0.0608 | |||
| Test group (n = 5 per group) | ||||||
| Young | 31 ± 1.2 (28–35) | 2M, 3F | 3C, 2B | 173.7 ± 2.4a | 74.2 ± 5.6 | 24.5 ± 1.7 |
| Old | 72 ± 0.8 (70–74) | 4M, 1F | 5C, 0B | 169.4 ± 1.8a,b | 81.1 ± 3.7 | 28.3 ± 1.3 |
| Elderly | 94 ± 1.4 (90–97) | 3M, 2F | 4C, 1B | 163.3 ± 1.6b | 66.7 ± 2.5 | 25.1 ± 0.8 |
| P value | 0.0087** | 0.0863 | 0.1250 | |||
P values were obtained from ANOVAs testing for age effects; age groups sharing an a or b superscripts are not significantly different
Number in parenthesis are actual range
Repeatability of serum spot determinations
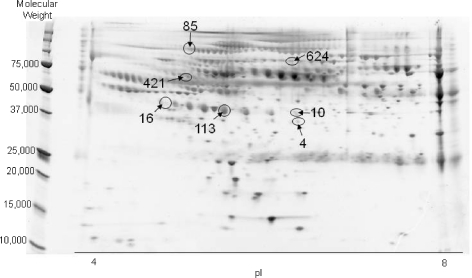
Figure 1 shows a representative serum 2-D gel electrophoresis map. Seven hundred and forty-nine spots were identified. Since each subject’s sample was run in duplicate, we calculated the repeatability of the two values for each of the 749 spots as previously described. These repeatabilities averaged 0.67 with a standard error of 0.0067, and their distribution was skewed on the high side with a noticeable drop in the number of repeatability below 0.35. We therefore considered any spots with a repeatability less than 0.35 (total of 37) to have unacceptable precision, and eliminated them from the analysis. This reduced the total number of spots used in the analysis from 749 to 712, the average repeatability for which increased slightly to 0.70 ± 0.005.
Fig. 1.
Representative 2-D gel electrophoresis of serum. The seven spots circled are the spots identified to discriminate among the three age groups
Discriminant analysis of serum spots
The discriminant analysis of all 712 serum spots from subjects in the training group (run with 0.01 entry and exit probabilities) identified seven spots as important in distinguishing among the three ages of these subjects (Table 2, Fig. 1). The discriminant function generated with these seven spots was successful in classifying 35 of the 36 subjects into their correct age group. Posterior probabilities were 1.0 for 13 of these subjects, with the highest values for each subject all being at least 0.89 or above. Generalized squared distances for the young/old, young/elderly, and old/elderly comparisons were 11.2, 73.5, and 42.6, respectively, suggesting that this function achieved the greatest discrimination between the young versus the elderly participants. However, this function only classified eight of the 15 subjects (53%) in the test group into their proper age category
Table 2.
Multivariate discriminant analysis results using 712 serum spots for trials with entry/exit probabilities 0.01/0.01
| Entry P | Exit P | No. of spots | Spot no. (in order of importance) | F values | Training group Y, O, E subjects correctly classified | Test group Y, O, E subjects correctly classified |
|---|---|---|---|---|---|---|
| 0.01 | 0.01 | 7 | 16, 85, 10, 113, 4, 421, 624 | 14.56, 8.91, 12.57, 11.32, 9.70, 6.32, 6.32 | 12/12, 11/12, 12/12 35/36 = 97% | 3/5, 2/5, 3/5 8/15 = 53% |
The spots important in discriminating the three age groups are given in order of their importance with their associated F statistics. Also shown are the number and % of subjects correctly classified into young (Y), old (O), and elderly (E) ages in both the training group and the test group
Analysis of seven serum spots
Basic statistics for the seven spots identified in the discriminant analysis are given in Table 3. The means, in arbitrary units, vary from nearly 1.5 to over 3 while the standard deviations range from 0.077 (spot 624) to 0.264 (spot 4). The 21 pairwise correlations of these seven spots (not shown) varied from −0.47 to +0.55 for spot 85 vs. spot 421, only this single value reaching statistical significance when adjusted by the sequential Bonferroni procedure (Rice 1989).
Table 3.
Basic statistics for the seven spots over all subjects in the training and test groups
| Spot | Mean | Standard deviation | PC1 | PC2 |
|---|---|---|---|---|
| 4 | 1.92 | 0.264 | 0.25 | 0.25 |
| 10 | 2.79 | 0.173 | −0.41 | −0.26 |
| 16 | 1.57 | 0.187 | 0.49 | 0.43 |
| 85 | 1.81 | 0.126 | 0.36 | −0.27 |
| 113 | 3.13 | 0.084 | −0.14 | 0.60 |
| 421 | 1.92 | 0.111 | 0.57 | −0.16 |
| 624 | 2.82 | 0.077 | 0.23 | −0.48 |
The first two components (PC1 and PC2) from a principal components analysis of the correlations also are given
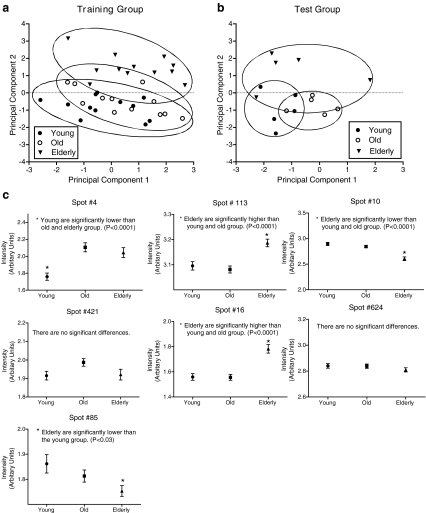
The first principal component (PC1) generated in the analysis of these correlations is a contrast especially of spots 16 and 421 with 10 (Table 3) and accounted for almost 28% of the total variance. The second PC (PC2) also is a contrast, with the highest loadings on spots 113 and 624. It accounted for roughly 22% of the variation and therefore the first two principal components accounted for approximately 50% of the total variation in intensity of the seven spots.
A plot of the scores for these components (Fig. 2a and b) shows that PC2 serves to separate elderly from young/old participants, and to some extent young from old. PC1 further disperses the subjects, although it is not clear that it has separated any particular age group from another. There appears to be weak congruence between subjects of the same age in both the training and test groups, although the test group subjects of each age are somewhat less dispersed. In general, this pattern reflects the somewhat poor percentage of correct classification for the test subjects generated from the discriminant analysis.
Fig. 2.
Graphical representation of the first two components from the principal component analysis of the serum spots (a and b) and the average intensity of the seven spots selected by discriminant analysis (c). Individual subjects from each age group (filled circle young, filled triangle old, unfilled circle elderly) are shown in the training group (a) and test group (b). For c, the average ± SEM are shown for each group for each spot
Means and standard errors of the spot variables in each of the three age groups are shown in Fig. 2c. Confirming the tendency seen in the principal components results, the elderly group tended to differ more often from the young and old groups. Two spots (4, 16, and 113) were higher in the elderly group compared to the young group. Two spots (10 and 85) were lower in the elderly group compared to the young and old groups.
The identity of the seven spots is shown in Table 4. There were multiple hits for each spot using the guidelines recommended by Matrix Science for the Mascot program. We have included those hits with a MOWSE score greater than 200. The first hit score for spots 85 and 421 is much greater than the score for subsequent hits, so we are relatively confident spot number 85 is inter-alpha-trypsin inhibitor heavy chain H4 and spot number 421 is kininogen-1.
Table 4.
Protein identification of each spot
| Spot number | Protein | Score | Coverage (%) | Mass (kDa) | pI |
|---|---|---|---|---|---|
| 85 | Inter-alpha-trypsin inhibitor heavy chain H4 | 2,904 | 23 | 103,552 | 6.51 |
| Inter-alpha-trypsin inhibitor heavy chain H1 | 518 | 12 | 101,844 | 6.31 | |
| 421 | Kininogen-1 | 1,313 | 18 | 73,041 | 6.34 |
| Angiotensinogen | 464 | 15 | 53,439 | 5.87 | |
| 16 | Haptoglobin | 524 | 26 | 39,521 | 6.13 |
| Haptoglobin | 376 | 20 | 45,889 | 6.42 | |
| Complement C4 | 248 | 3 | 194,367 | 6.65 | |
| 113 | Haptoglobin | 925 | 36 | 45,889 | 6.13 |
| Haptoglobin | 767 | 24 | 39,521 | 6.42 | |
| apoA | 279 | 35 | 45,398 | 5.28 | |
| 10 | Haptoglobin | 507 | 26 | 45,889 | 6.13 |
| Complement C4 | 478 | 2 | 194,367 | 6.65 | |
| Haptoglobin | 381 | 22 | 39,521 | 6.42 | |
| 4 | Haptoglobin | 391 | 26 | 45,889 | 6.13 |
| Haptoglobin-related protein | 284 | 22 | 39,521 | 6.42 | |
| 624 | Complement C3 (fragment?) | 697 | 12 | 188,686 | 6.02 |
| Gelsolin | 413 | 10 | 86,096 | 5.90 | |
| Serum albumin | 222 | 17 | 71,363 | 5.92 | |
| Ig mu chain C region | 216 | 12 | 49,991 | 6.35 |
Each spot was excised from the 2-D electrophoresis gel and the protein reduced to peptides by trypsin. The mass of each peptide was determine by LCMS and matched to known proteins in the Mascot database using the guidelines recommended by Matrix Science (Boston, MA, USA). The molecular weight of several proteins is greater than the position of that spot on the gel. These may be a fragment of the higher molecular weight protein
Discussion
The goal of our pilot study was to determine if a proteomic approach is a useful technique to identify serum markers that may be involved in longevity. Our experimental design included a wide age range, from 20-year-old to nonagenarian subjects, and the results of the serum marker analysis would seem to be most relevant to longevity considerations. Other studies have sought biomarkers of aging which has different implications and criteria (Ingram et al. 2001).
Many profiles of healthy aging have been developed and published but currently there is no single marker or combination of biochemical or physiological markers that can predict longevity among individuals. Such a marker or markers clearly would be an important gerontological tool that would assist scientists and clinicians in understanding the aging process and the potential molecular mechanisms of longevity. However, efforts to identify a single screening molecular marker have not been fruitful because most biomarkers have poor sensitivity or specificity. To overcome this difficulty, one approach has been to measure several biomarkers simultaneously with genomics, metabolomics, and/or proteomics techniques. In this study, we used 2-D gel electrophoresis, a technique that allowed us to evaluate simultaneously many serum proteins and their isoforms that are not routinely available in clinical laboratory analyses.
Our study involved a cross-sectional analysis of 51 subjects in three age groups: young (20–34 years), old (60–74 years), and elderly (≥90 years). Ideally, it would have been preferable to use longitudinal data collected from the same individuals over a >70-year period; but this has not been feasible. Many longitudinal studies have been conducted, although most are done over a shorter period of time. For example, Ueno et al. (2003) developed a biological aging score for Japanese men and women using longitudinal data collected over a 4–7-year period. For our cross-sectional study, we selected age groups that did not overlap and were clearly separated. Healthy individuals in each age group were recruited although given the current average life expectancy of 75.2 years for men and 80.4 years for women (US Department of Health and Human Services et al. 2007), we would not expect a large number of these individuals to live to 90 years of age and beyond. We recognize that age-associated disease is not only a common occurrence but an integral part of aging (Masoro 2001).
One intent of this study was to uncover serum protein patterns that might be reliable markers of longevity in human populations. To this end, we quantified over 700 serum proteins in 36 subjects in a training group, and used multivariate discriminant analysis to identify those proteins most useful in separating these subjects into their appropriate age group (young, old, or elderly). The preliminary results were encouraging in generating a function based on seven proteins that classified 35 of the 36 subjects into their correct age group. When applied to a different (test) set of 15 subjects representing the same three age groups, however, this function misclassified seven of these subjects (i.e., 47%). Therefore, we were not successful in finding a serum protein pattern effective in discriminating age groups, at least among this small set of participants.
Our results, however, do not suggest that all attempts at investigating serum samples to classify human age differences should be abandoned. The number of training subjects we assayed was necessarily limited in this preliminary study, and a greater sample size would provide a more comprehensive picture of the inherent variation in these serum proteins. Such variation would include gender and race differences that preliminary analyses suggested were significant for many of the serum proteins in our sample. Although the protein values were not statistically adjusted for these factors, we conducted a non-parametric discriminant analysis using the same seven protein values previously identified as well as gender and race. The classification results were identical to those obtained from the parametric discriminant analysis, so potential gender/race differences in some of the spots did not alter the discrimination we achieved. Nonetheless, it would seem reasonable to include a larger number of subjects of each gender and race in each of several age groups in future serum protein analyses.
An analysis of a large number of serum proteins in 600 or more human subjects would be a labor-intensive task, especially if all proteins were measured twice. However, double measurement may not be necessary as our analysis showed that repeatability of these serum proteins averaged 70%. With this reasonably high level of repeatability, the expected variation of the mean of two measurements is 85% of that for just one measurement; so with two measurements, the overall phenotypic variation is reduced by only 15% (Falconer and Mackay 1996). On the other hand, using the mean of the two repeat measures for each spot as we did in our analysis reduces by one-half the measurement error contribution to the total phenotypic variation. Where possible, therefore, it would be advisable to obtain repeat measures to increase the precision of the data and reduce error variability. Genetic and environmental variation in serum spot values among the subjects is distinct from that due to measurement error, and therefore presumably contributed the bulk of the total phenotypic variation in these spots.
The serum profile we identified from this small number of subjects would not be clinically useful. A clinical test should have a very high degree of sensitivity and specificity and this was not achieved when we applied our discriminant function to the test group subjects. While presumably not of clinical value, however, the serum profile may prove useful in epidemiological and/or population studies.
Identification of the seven spots is shown in Table 4. Several of the identified proteins are involved in metabolic pathways affected by the aging process. For example, inter-alpha-trypsin inhibitor heavy chain H4 is an acute-phase protein that is induced in several pathological conditions (e.g., cancer) associated with age (Zhuo and Kimata 2008). Kininogen is a cysteine proteinase inhibitor linked to inflammation and blood clotting. It has been suggested as a biomarker of aging in Fisher 344 rats (Acuna-Castillo et al. 2006). ApoA4 is a lipoprotein that has been implicated in Alzheimer’s disease (Carter 2007) and aging (Garasto et al. 2003). Gelsolin is a calcium-activated actin filament also linked to a number of pathological conditions such as cancer (Spinardi and Witke 2007). Haptoglobin is a protein associated with inflammation (Quaye 2008).
Given the large number of spots analyzed in this study, it might be argued that most or all of these seven proteins identified as best discriminating the subjects in our three age groups may represent false positives. Thus, from simple probability considerations using 720 total spots, we might expect about seven to reach significance at the 1% level by chance alone, just as we found. The 0.01 entry/exit probability level used in the multivariate discriminant analysis, however, is reached only when certain combinations of these variables separate the three groups, and is quite different from probabilities for spots obtained from individual ANOVAs. As in fact was previously seen (see Fig. 2c), these individual probabilities for each spot differed considerably, some not even reaching significance (p > 0.05). To assess the individual spots and appropriately adjust for potential false positives, one approach is to calculate the Bonferroni threshold for significance, which for 720 spots is calculated as 0.05/720 = 0.0000694. In fact two spots, 16 (0.0000000894) and 10 (0.000037428), have probabilities from the individual ANOVAs that are less than this value, suggesting that they are unlikely to be false positives. Both of these spots are haptoglobins, and it will be interesting to see whether future studies also suggest a role for these particular proteins in the aging process.
Conclusions
In conclusion, we identified seven serum spots using the 2-D gel electrophoresis technique that successfully classified young, old, and elderly training group subjects into their appropriate ages, but the function derived from these spots was not successful in discriminating the ages of another unknown (test) group of subjects. However, these preliminary results suggest that these or other serum spots may be importance in the biological aging process and that further investigations are warranted.
Acknowledgments
This work was supported by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund (HEF (2001-06) 02) and by the National Institute on Aging (PO1 AGO22064).
We would like to acknowledge the efforts of Dr. Doug Hinerfeld at the Proteomics Core at the University of Massachusetts Medical School who prepared the 2-D gels.
We acknowledge and thank everyone working on the Louisiana Healthy Aging Study from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge; Louisiana State University Health Sciences Center, Tulane University, New Orleans; and University of Alabama at Birmingham: S. Michal Jazwinski, PhD, Mark Batzer, PhD, Pauline Callinan, Jerilyn Walker, Scott W. Herke, DeQuindra Rouzan, Jennifer Arceneaux, RN, Andrew Pellett, PhD, Henry Rothschild, MD, PhD, Crystal Traylor, APRN, MSN, WHNP, David Welsh, MD, Joseph Su, PhD, Yu-wen Chiu, Elizabeth Fontham, PhD, Cruz Velasco-Gonzalez, PhD, Jennifer Hayden, Matthew Leblanc, Li Li, MD, Sangkyu Kim, Hui-Yi Lin, PhD, Beth Schmidt, Jessi Thomspon, PhD, Valentina Greco, PhD, Beth Kimball, Meghan Allen, Donald Scott, PhD, John Mountz, PhD, MD, Hui-Chen Hsu, PhD, Pili Zhang, Kim Pederson, Juling Zhou, PhD, Tiffany Hall, Kim Landry, Mandy Shipp, Anita Smith, Lauri Byerley, PhD, James P DeLany, PhD, Robert Schwartz, PhD, Evest Broussard, Michael Businelle, Paula Geiselman, PhD, Darla Kendzor, Vijay Hegde, PhD, Robert Wood, PhD, Michael Welsch, PhD, Iina E. Antikainen, Fernanada Holton, Carl Lavie, MD, Artie Brown, Ryan Russell, Devon Dobrosielski, Arturo Ace, Katie Cherry, PhD, Karri Hawley, PhD, Emily Olinde, Jenny Denver, and Kay Lopez, DSN.
References
- Acuna-Castillo C, Leiva-Salcedo E, Gomez CR, Perez V, Li M, Torres C, Walter R, Murasko DM, Sierra F. T-kininogen: a biomarker of aging in Fisher 344 rats with possible implications for the immune response. J Gerontol A Biol Sci Med Sci. 2006;61:641–649. doi: 10.1093/gerona/61.7.641. [DOI] [PubMed] [Google Scholar]
- Biesalski HK. Free radical theory of aging. Curr Opin Clin Nutr Metab Care. 2002;5:5–10. doi: 10.1097/00075197-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Carter CJ. Convergence of genes implicated in Alzheimer’s disease on the cerebral cholesterol shuttle: APP, cholesterol, lipoproteins, and atherosclerosis. Neurochem Int. 2007;50:12–38. doi: 10.1016/j.neuint.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954–966. doi: 10.1139/H07-085. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Adachi H, Fukami A, Furuki K, Satoh A, Otsuka M, Kumagae S, Nanjo Y, Shigetoh Y, Imaizumi T. Serum dehydroepiandrosterone sulfate levels predict longevity in men: 27-year follow-up study in a community-based cohort (Tanushimaru study) J Am Geriatr Soc. 2008;56:994–998. doi: 10.1111/j.1532-5415.2008.01692.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4. Essex: Longman; 1996. [Google Scholar]
- Frisard MI, Fabre JM, Russell RD, King CM, Delany JP, Wood RH, Ravussin E. Physical activity level and physical functionality in nonagenarians compared to individuals aged 60–74 years. J Gerontol A Biol Sci Med Sci. 2007;62:783–788. doi: 10.1093/gerona/62.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garasto S, Rose G, DeRango F, Berardelli M, Corsonello A, Feraco E, Mari V, Maletta R, Bruni A, Franceschi C, Carotenuto L, De BG. The study of APOA1, APOC3 and APOA4 variability in healthy ageing people reveals another paradox in the oldest old subjects. Ann Hum Genet. 2003;67:54–62. doi: 10.1046/j.1469-1809.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Harmon H. Modern factor analysis. 3. Chicago: University of Chicago Press; 1960. [Google Scholar]
- Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/S0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Lu YAJ, Wang G, Hao H, Huang Q, Yan B, Zha W, Gu S, Ren H, Zhang Y, Fan X, Zhang M, Hao K. Gas chromatography/time-of-flight mass spectrometry based metabonomic approach to differentiating hypertension- and age-related metabolic variation in spontaneously hypertensive rats. Rapid Commun Mass Spectrom. 2008;22:2882–2888. doi: 10.1002/rcm.3670. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Physiology of aging. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S218–S222. doi: 10.1123/ijsnem.11.s1.s218. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A Biol Sci Med Sci. 2007;62:1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102:735–742. doi: 10.1016/j.trstmh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Rice W. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.2307/2409177. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- SAS® Technical Report, SAS/STAT Software: changes and enhancements, release 6.07 edn. Cary: SAS Institute, Inc; 1992. [Google Scholar]
- Spinardi L, Witke W. Gelsolin and diseases. Subcell Biochem. 2007;45:55–69. doi: 10.1007/978-1-4020-6191-2_3. [DOI] [PubMed] [Google Scholar]
- Ueno LM, Yamashita Y, Moritani T, Nakamura E. Biomarkers of aging in women and the rate of longitudinal changes. J Physiol Anthropol Appl Hum Sci. 2003;22:37–46. doi: 10.2114/jpa.22.37. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Center for Disease Control, and National Center for Health Statistics (2007) Health, United States, 27, 1–551. 2007. Washington D.C., U. S. Government Printing Office. Ref Type: Report
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Kimata K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res. 2008;49:311–320. doi: 10.1080/03008200802325458. [DOI] [PubMed] [Google Scholar]